Antibiotic-Loaded Nano-Sized Delivery Systems: An Insight into Gentamicin and Vancomycin
Abstract
1. Introduction
Overview of the Significance of Anti-Infective Drug Delivery
2. Challenges Associated with Infection Therapies Based on Traditional Drug Formulations
3. Gentamicin and Vancomycin
3.1. Gentamicin

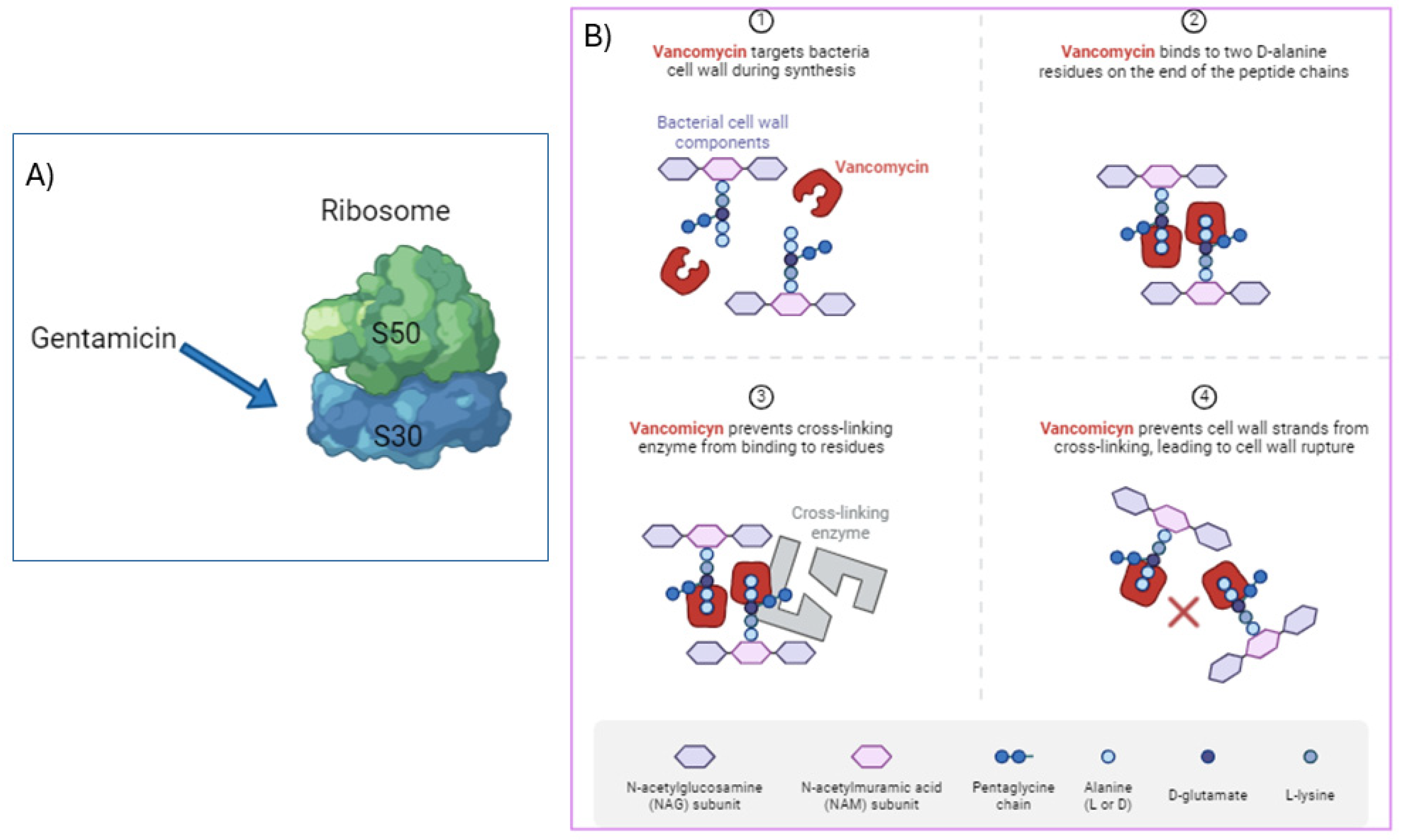
3.2. Vancomycin
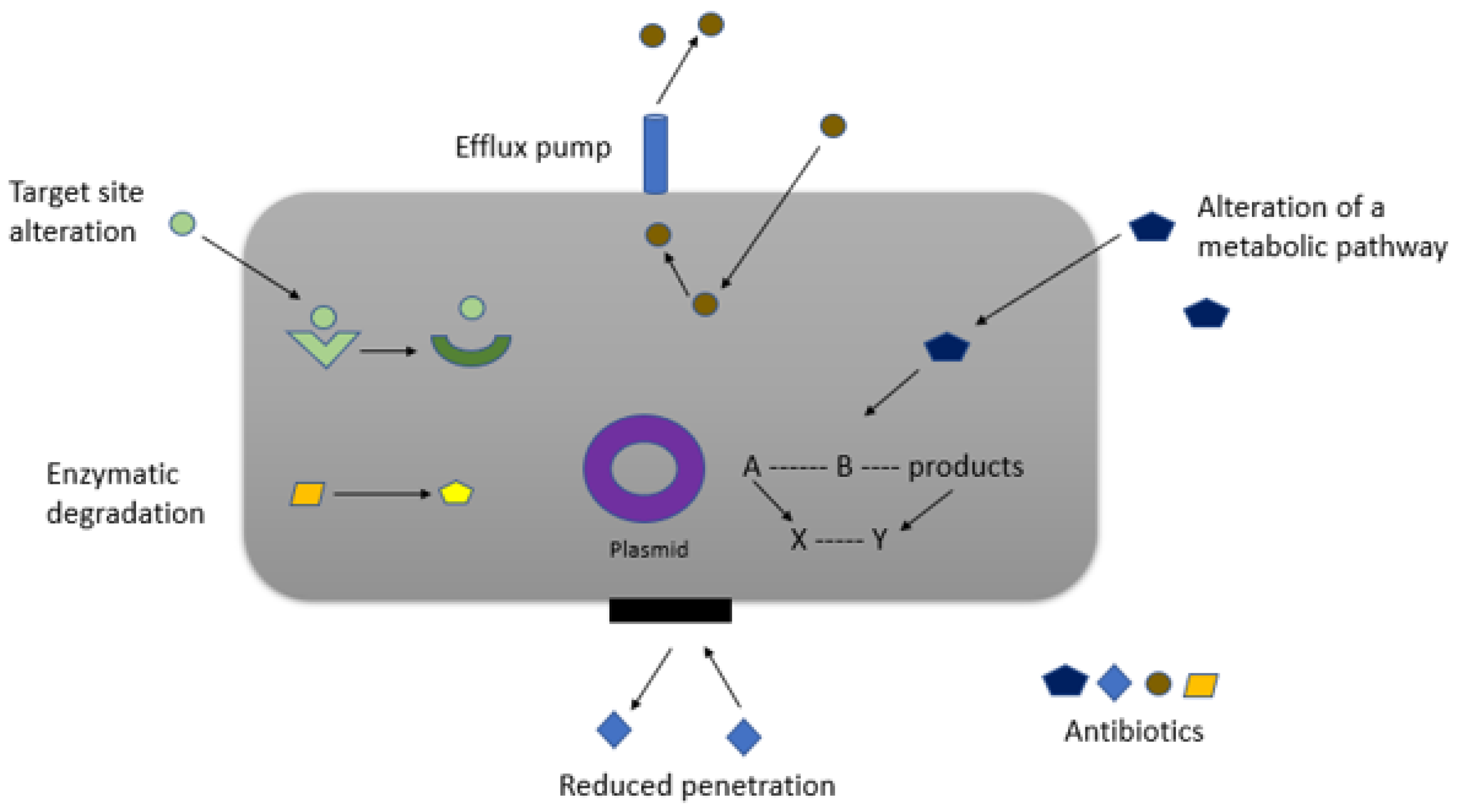
3.3. Beyond Resistance: Other Concerns and Strategies for Sustainable Use
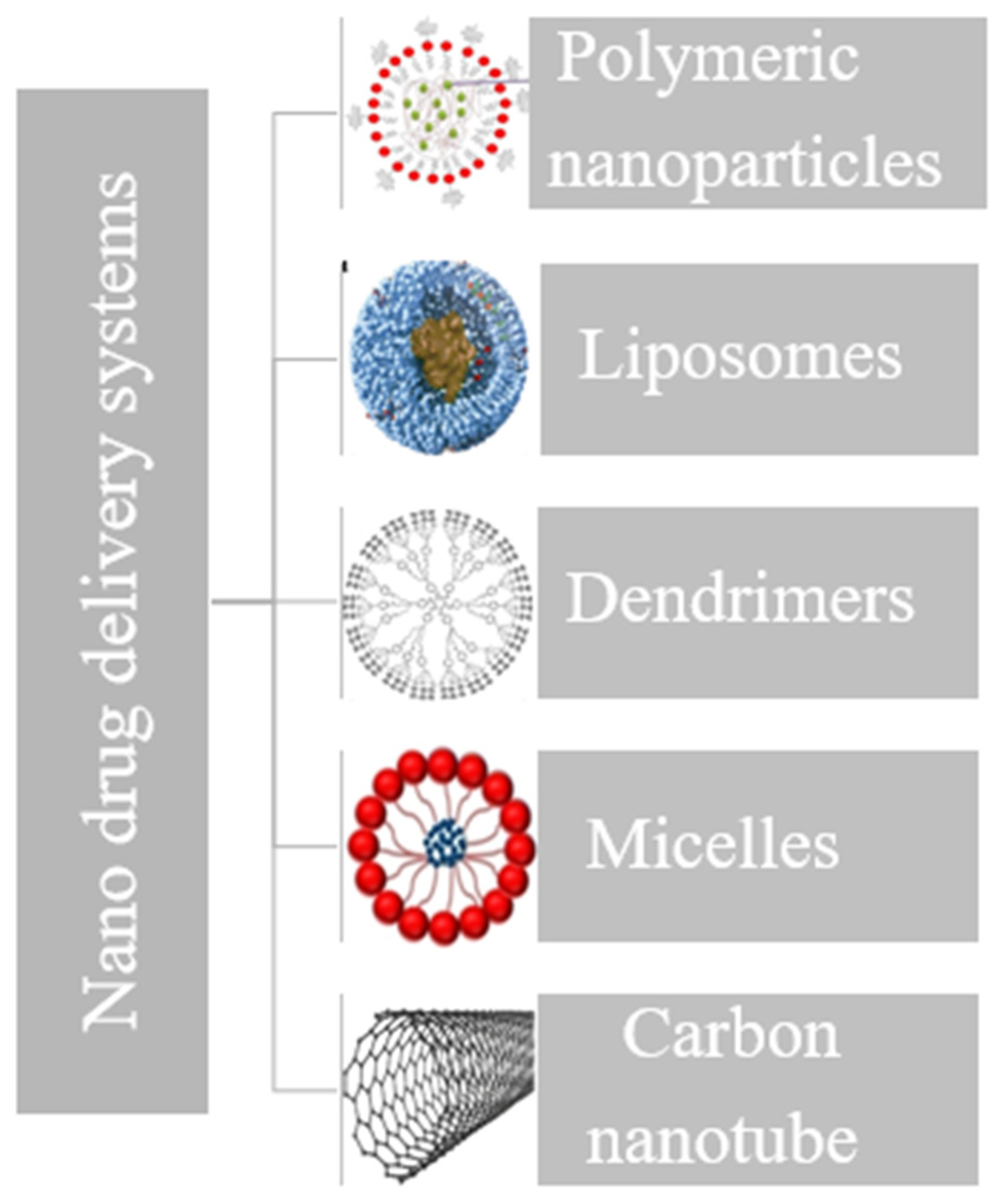
4. Rationale for Nanoparticle-Based Drug Delivery
5. Gentamicin (GS) and Vancomycin (VM) Nanosized Delivery System Case Studies
5.1. Polymeric Nanoparticles
5.2. Inorganic Nanoparticles
5.3. Liposomes
5.4. Dendrimers
5.5. Micelle-Based Drug Delivery
5.6. Carbon-Nanotube (CNT)-Based Drug Delivery
6. Nanosized DDS Interactions with Bacterial Membranes
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO Bacterial Priority Pathogens List 2024; Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024.
- Loretz, B.; Oh, Y.-K.; Hudson, S.; Gu, Z.; Lehr, C.-M. Drug delivery for fighting infectious diseases: A global perspective. Drug Deliv. Transl. Res. 2021, 11, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Graef, F.; Gordon, S.; Lehr, C.-M. Anti-infectives in drug delivery—Overcoming the gram-negative bacterial cell envelope. In How to Overcome the Antibiotic Crisis: Facts, Challenges, Technologies and Future Perspectives; Springer: Cham, Switzerland, 2016; pp. 475–496. [Google Scholar]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, P.C.; Ezeako, E.C.; Okpara, J.O.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- De Rubis, G.; Paudel, K.R.; Corrie, L.; Mehndiratta, S.; Patel, V.K.; Kumbhar, P.S.; Manjappa, A.S.; Disouza, J.; Patravale, V.; Gupta, G. Applications and advancements of nanoparticle-based drug delivery in alleviating lung cancer and chronic obstructive pulmonary disease. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 397, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Strugnell, R.A.; Newton, H.J.; Kupz, A. Survival strategies of intracellular bacterial pathogens. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 457–488. [Google Scholar]
- Chen, Y.; Jiang, Y.; Xue, T.; Cheng, J. Strategies for the eradication of intracellular bacterial pathogens. Biomater. Sci. 2024, 12, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Thakuria, A.; Kataria, B.; Gupta, D. Nanoparticle-based methodologies for targeted drug delivery—An insight. J. Nanoparticle Res. 2021, 23, 87. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnology 2018, 16, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, S.M.; Quinn, D.J.; Ingram, R.J.; Gilmore, B.F.; Donnelly, R.F.; Taggart, C.C.; Scott, C.J. Gentamicin-loaded nanoparticles show improved antimicrobial effects towards Pseudomonas aeruginosa infection. Int. J. Nanomed. 2012, 7, 4053–4063. [Google Scholar]
- European Pharmacopoeia 11th Edition. Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph.-eur.-11th-edition (accessed on 1 May 2024).
- Athauda, I.; Shetty, M.; Pai, P.; Hegde, M.; Gurumurthy, S.; Babitha, K. Enhanced Bactericidal Effects and Drug Delivery with Gentamicin-Conjugated Nanoparticles. J. Clust. Sci. 2024, 35, 371–390. [Google Scholar] [CrossRef]
- Buabeng, K.O.; Mackenzie, A.R.; Laing, R.B.S.; Cook, I.; Jappy, B.; Gould, I.M. Assessment of the efficacy, safety and quality of gentamicin use in Aberdeen Royal Infirmary. J. Antimicrob. Chemother. 1999, 44, 843–845. [Google Scholar] [CrossRef][Green Version]
- Hodiamont, C.J.; van den Broek, A.K.; de Vroom, S.L.; Prins, J.M.; Mathôt, R.A.; van Hest, R.M. Clinical pharmacokinetics of gentamicin in various patient populations and consequences for optimal dosing for gram-negative infections: An updated review. Clin. Pharmacokinet. 2022, 61, 1075–1094. [Google Scholar] [CrossRef]
- Siber, G.R.; Echeverria, P.; Smith, A.L.; Paisley, J.W.; Smith, D.H. Pharmacokinetics of gentamicin in children and adults. J. Infect. Dis. 1975, 132, 637–651. [Google Scholar] [CrossRef]
- Dowding, J. Mechanisms of gentamicin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1977, 11, 47–50. [Google Scholar] [CrossRef]
- Peterlini, M.; Barbosa, A.; Pedreira, M. Stability of vancomycin hydrochloride solutions in high concentration and extended time of infusion. Intensive Care Med. Exp. 2015, 3, A717. [Google Scholar] [CrossRef]
- Tsutsuura, M.; Moriyama, H.; Kojima, N.; Mizukami, Y.; Tashiro, S.; Osa, S.; Enoki, Y.; Taguchi, K.; Oda, K.; Fujii, S. The monitoring of vancomycin: A systematic review and meta-analyses of area under the concentration-time curve-guided dosing and trough-guided dosing. BMC Infect. Dis. 2021, 21, 153. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Alqurashi, M.K.; Althaqafi, A.S.; Alsharif, J.M.; Faidah, H.S.; Bushyah, M.; Alotaibi, A.F.; Elrggal, M.E.; Mahrous, A.J.; Abuhussain, S.S.A. A systematic review on clinical safety and efficacy of vancomycin loading dose in critically ill patients. Antibiotics 2022, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.P. Vancomycin: A History. Clin. Infect. Dis. 2006, 42 (Suppl. S1), S5–S12. [Google Scholar] [CrossRef]
- Boneca, I.G.; Chiosis, G. Vancomycin resistance: Occurrence, mechanisms and strategies to combat it. Expert Opin. Ther. Targets 2003, 7, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Azzam, A.; Elkafas, H.; Khaled, H.; Ashraf, A.; Yousef, M.; Elkashef, A.A. Prevalence of Vancomycin-resistant enterococci (VRE) in Egypt (2010–2022): A systematic review and meta-analysis. J. Egypt. Public Health Assoc. 2023, 98, 8. [Google Scholar] [CrossRef]
- Sparo, M.; Delpech, G.; García Allende, N. Impact on public health of the spread of high-level resistance to gentamicin and vancomycin in enterococci. Front. Microbiol. 2018, 9, 410861. [Google Scholar] [CrossRef]
- Zeb, A.; Gul, M.; Nguyen, T.-T.-L.; Maeng, H.-J. Controlled release and targeted drug delivery with poly (lactic-co-glycolic acid) nanoparticles: Reviewing two decades of research. J. Pharm. Investig. 2022, 52, 683–724. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: Research progress, challenges, and prospects. World J. Microbiol. Biotechnol. 2021, 37, 1–30. [Google Scholar]
- Liu, W.-B.; Gao, R.-T.; Zhou, L.; Liu, N.; Chen, Z.; Wu, Z.-Q. Combination of vancomycin and guanidinium-functionalized helical polymers for synergistic antibacterial activity and biofilm ablation. Chem. Sci. 2022, 13, 10375–10382. [Google Scholar] [CrossRef]
- Mishra, M.; Patole, S.; Mohapatra, H. Nanoparticles: Powerful tool to mitigate antibiotic resistance. In Sustainable Agriculture Reviews 49: Mitigation of Antimicrobial Resistance Vol 2. Natural and Synthetic Approaches; Springer: Cham, Switzerland, 2021; pp. 171–204. [Google Scholar]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.-H.; Adibkia, K. Physicochemical characterization and antimicrobial evaluation of gentamicin-loaded CaCO3 nanoparticles prepared via microemulsion method. J. Drug Deliv. Sci. Technol. 2016, 35, 16–23. [Google Scholar] [CrossRef]
- Mosselhy, D.A.; Ge, Y.; Gasik, M.; Nordström, K.; Natri, O.; Hannula, S.-P. Silica-gentamicin nanohybrids: Synthesis and antimicrobial action. Materials 2016, 9, 170. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Neogi, S. Gentamicin coated iron oxide nanoparticles as novel antibacterial agents. Mater. Res. Express 2017, 4, 095005. [Google Scholar] [CrossRef]
- Alhariri, M.; Majrashi, M.A.; Bahkali, A.H.; Almajed, F.S.; Azghani, A.O.; Khiyami, M.A.; Alyamani, E.J.; Aljohani, S.M.; Halwani, M.A. Efficacy of neutral and negatively charged liposome-loaded gentamicin on planktonic bacteria and biofilm communities. Int. J. Nanomed. 2017, 12, 6949–6961. [Google Scholar] [CrossRef]
- Jiang, L.; Greene, M.K.; Insua, J.L.; Pessoa, J.S.; Small, D.M.; Smyth, P.; McCann, A.P.; Cogo, F.; Bengoechea, J.A.; Taggart, C.C. Clearance of intracellular Klebsiella pneumoniae infection using gentamicin-loaded nanoparticles. J. Control. Release 2018, 279, 316–325. [Google Scholar] [CrossRef]
- Pan, X.; Chen, S.; Li, D.; Rao, W.; Zheng, Y.; Yang, Z.; Li, L.; Guan, X.; Chen, Z. The synergistic antibacterial mechanism of gentamicin-loaded CaCO3 nanoparticles. Front. Chem. 2018, 5, 130. [Google Scholar] [CrossRef] [PubMed]
- Razei, A.; Cheraghali, A.M.; Saadati, M.; Ramandi, M.F.; Panahi, Y.; Hajizade, A.; Siadat, S.D.; Behrouzi, A. Gentamicin-loaded chitosan nanoparticles improve its therapeutic effects on brucella-infected J774A. 1 murine cells. Galen Med. J. 2019, 8, e1296. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, J.; Zhang, B.; Zheng, Y.; Yang, L.; Shen, Y.; Zuo, B.; Zhang, F. Gentamicin-loaded silk/nanosilver composite scaffolds for MRSA-induced chronic osteomyelitis. R. Soc. Open Sci. 2019, 6, 182102. [Google Scholar] [CrossRef]
- Dhal, C.; Mishra, R. In vitro and in vivo evaluation of gentamicin sulphate-loaded PLGA nanoparticle-based film for the treatment of surgical site infection. Drug Deliv. Transl. Res. 2020, 10, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Viquez, E.; Esquivel-Alvarado, D.; Madrigal-Carballo, S.; Krueger, C.G.; Reed, J.D. Antimicrobial proanthocyanidin-chitosan composite nanoparticles loaded with gentamicin. Int. J. Biol. Macromol. 2020, 162, 1500–1508. [Google Scholar] [CrossRef]
- Sun, Y.; Bhattacharjee, A.; Reynolds, M.; Li, Y.V. Synthesis and characterizations of gentamicin-loaded poly-lactic-co-glycolic (PLGA) nanoparticles. J. Nanoparticle Res. 2021, 23, 1–15. [Google Scholar] [CrossRef]
- Kamal, R.A.; Kahdhum, Q.A.; Mohammed, A.I.; Essa, A.J.; Mohamad, E.A.; Abod, S.A.; Jasim, G.H. Characterization and Evaluation The Biological Activity of Prepared Nano-Gentamicin Nanoparticles. IOP Conf. Ser. Earth Environ.Sci. 2021, 910, 012078. [Google Scholar] [CrossRef]
- Kazeminava, F.; Javanbakht, S.; Nouri, M.; Gholizadeh, P.; Nezhad-Mokhtari, P.; Ganbarov, K.; Tanomand, A.; Kafil, H.S. Gentamicin-loaded chitosan/folic acid-based carbon quantum dots nanocomposite hydrogel films as potential antimicrobial wound dressing. J. Biol. Eng. 2022, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hakeem, M.A.; Abdel Maksoud, A.I.; Aladhadh, M.A.; Almuryif, K.A.; Elsanhoty, R.M.; Elebeedy, D. Gentamicin–Ascorbic Acid Encapsulated in Chitosan Nanoparticles Improved In Vitro Antimicrobial Activity and Minimized Cytotoxicity. Antibiotics 2022, 11, 1530. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Heacock, J.; Chen, C.; Qiu, K.; Zou, L.; Liu, J.; Li, Y.V. Incorporation of gentamicin-encapsulated poly (lactic-co-glycolic acid) nanoparticles into polyurethane/poly (ethylene oxide) nanofiber scaffolds for biomedical applications. ACS Appl. Nano Mater. 2023, 6, 16096–16105. [Google Scholar] [CrossRef]
- Dorati, R.; DeTrizio, A.; Spalla, M.; Migliavacca, R.; Pagani, L.; Pisani, S.; Chiesa, E.; Conti, B.; Modena, T.; Genta, I. Gentamicin sulfate PEG-PLGA/PLGA-H nanoparticles: Screening design and antimicrobial effect evaluation toward clinic bacterial isolates. Nanomaterials 2018, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Pichavant, L.; Carrié, H.; Nguyen, M.N.; Plawinski, L.; Durrieu, M.-C.; Héroguez, V. Vancomycin functionalized nanoparticles for bactericidal biomaterial surfaces. Biomacromolecules 2016, 17, 1339–1346. [Google Scholar] [CrossRef]
- Hur, Y.E.; Park, Y. Vancomycin-functionalized gold and silver nanoparticles as an antibacterial nanoplatform against methicillin-resistant Staphylococcus aureus. J. Nanosci. Nanotechnol. 2016, 16, 6393–6399. [Google Scholar] [CrossRef]
- Cerchiara, T.; Abruzzo, A.; Palomino, R.A.Ñ.; Vitali, B.; De Rose, R.; Chidichimo, G.; Ceseracciu, L.; Athanassiou, A.; Saladini, B.; Dalena, F. Spanish Broom (Spartium junceum L.) fibers impregnated with vancomycin-loaded chitosan nanoparticles as new antibacterial wound dressing: Preparation, characterization and antibacterial activity. Eur. J. Pharm. Sci. 2017, 99, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, R.-J.; Xu, J.-J.; Shen, L.-F.; Gao, J.-Q.; Wang, X.-P.; Wang, N.-N.; Shou, D.; Hu, Y. Efficient induction of antimicrobial activity with vancomycin nanoparticle-loaded poly (trimethylene carbonate) localized drug delivery system. Int. J. Nanomed. 2017, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Bhise, K.; Sau, S.; Kebriaei, R.; Rice, S.A.; Stamper, K.C.; Alsaab, H.O.; Rybak, M.J.; Iyer, A.K. Combination of vancomycin and cefazolin lipid nanoparticles for overcoming antibiotic resistance of MRSA. Materials 2018, 11, 1245. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.M.d.A.C.; Dórea, A.C.S.; Droppa-Almeida, D.; de Mélo Silva, I.S.; Montoro, F.E.; Alves, L.L.; Macedo, M.L.H.; Padilha, F.F. Development and characterization of PLGA nanoparticles containing antibiotics. J. Nanoparticle Res. 2018, 20, 289. [Google Scholar] [CrossRef]
- Kaur, A.; Preet, S.; Kumar, V.; Kumar, R.; Kumar, R. Synergetic effect of vancomycin loaded silver nanoparticles for enhanced antibacterial activity. Colloids Surf. B Biointerfaces 2019, 176, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Li, T.-J.; Tsai, P.-J. Vancomycin-loaded nanoparticles enhance sporicidal and antibacterial efficacy for Clostridium difficile infection. Front. Microbiol. 2019, 10, 451735. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Moreira, M.L.A.; Costa, I.F.d.J.B.; de Sousa, V.P.; Rodrigues, C.R.; Sisnande, T.; do Carmo, F.A.; Leal, I.C.R.; Dos Santos, K.R.N.; da Silva, L.C.R.P. Vancomycin-loaded nanoparticles against vancomycin intermediate and methicillin resistant Staphylococcus aureus strains. Nanotechnology 2020, 31, 375101. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kumar, R. Formulation of biocompatible vancomycin conjugated gold nanoparticles for enhanced antibacterial efficacy. ES Energy Environ. 2021, 15, 34–44. [Google Scholar] [CrossRef]
- Nader, D.; Yousef, F.; Kavanagh, N.; Ryan, B.K.; Kerrigan, S.W. Targeting internalized Staphylococcus aureus using vancomycin-loaded nanoparticles to treat recurrent bloodstream infections. Antibiotics 2021, 10, 581. [Google Scholar] [CrossRef]
- Hagbani, T.A.; Yadav, H.; Moin, A.; Lila, A.S.A.; Mehmood, K.; Alshammari, F.; Khan, S.; Khafagy, E.-S.; Hussain, T.; Rizvi, S.M.D. Enhancement of vancomycin potential against pathogenic bacterial strains via gold nano-formulations: A nano-antibiotic approach. Materials 2022, 15, 1108. [Google Scholar] [CrossRef]
- Hafizi, T.; Shahriari, M.H.; Abdouss, M.; Kahdestani, S.A. Synthesis and characterization of vancomycin-loaded chitosan nanoparticles for drug delivery. Polym. Bull. 2023, 80, 5607–5621. [Google Scholar] [CrossRef]
- Nouruzi, E.; Hosseini, S.M.; Asghari, B.; Mahjoub, R.; Zare, E.N.; Shahbazi, M.-A.; Kalhori, F.; Arabestani, M.R. Effect of poly (lactic-co-glycolic acid) polymer nanoparticles loaded with vancomycin against Staphylococcus aureus biofilm. BMC Biotechnol. 2023, 23, 39. [Google Scholar] [CrossRef]
- Zong, T.-X.; Silveira, A.P.; Morais, J.A.V.; Sampaio, M.C.; Muehlmann, L.A.; Zhang, J.; Jiang, C.-S.; Liu, S.-K. Recent advances in antimicrobial nano-drug delivery systems. Nanomaterials 2022, 12, 1855. [Google Scholar] [CrossRef]
- Pisani, S.; Dorati, R.; Chiesa, E.; Genta, I.; Modena, T.; Bruni, G.; Grisoli, P.; Conti, B. Release profile of gentamicin sulfate from polylactide-co-polycaprolactone electrospun nanofiber matrices. Pharmaceutics 2019, 11, 161. [Google Scholar] [CrossRef]
- Simpson, E.; Sarwar, H.; Jack, I.; Lowry, D. Evaluation of the Potential of Chitosan Nanoparticles as a Delivery Vehicle for Gentamicin for the treatment of Osteomyelitis. Antibiotics 2024, 13, 208. [Google Scholar] [CrossRef]
- Ural, M.S.; Menéndez-Miranda, M.; Salzano, G.; Mathurin, J.; Aybeke, E.N.; Deniset-Besseau, A.; Dazzi, A.; Porcino, M.; Martineau-Corcos, C.; Gref, R. Compartmentalized Polymeric Nanoparticles Deliver Vancomycin in a pH-Responsive Manner. Pharmaceutics 2021, 13, 1992. [Google Scholar] [CrossRef]
- Xu, J.; Xu, B.; Shou, D.; Xia, X.; Hu, Y. Preparation and evaluation of vancomycin-loaded N-trimethyl chitosan nanoparticles. Polymers 2015, 7, 1850–1870. [Google Scholar] [CrossRef]
- Pranantyo, D.; Zhang, K.; Si, Z.; Hou, Z.; Chan-Park, M.B. Smart multifunctional polymer systems as alternatives or supplements of antibiotics to overcome bacterial resistance. Biomacromolecules 2022, 23, 1873–1891. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Sikwal, D.R.; Rambharose, S.; Mocktar, C.; Singh, S.; Bester, L.; Oh, J.K.; Renukuntla, J.; Govender, T. Enhancing targeted antibiotic therapy via pH responsive solid lipid nanoparticles from an acid cleavable lipid. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Harris, M.A.; LeVine, D.; Ghimire, M.; Jennings, J.A.; Morshed, B.I.; Haggard, W.O.; Bumgardner, J.D.; Mishra, S.R.; Fujiwara, T. Magnetic stimulus responsive vancomycin drug delivery system based on chitosan microbeads embedded with magnetic nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Mas, N.; Galiana, I.; Mondragón, L.; Aznar, E.; Climent, E.; Cabedo, N.; Sancenon, F.; Murguía, J.R.; Martínez–Máñez, R.; Marcos, M.D. Enhanced efficacy and broadening of antibacterial action of drugs via the use of capped mesoporous nanoparticles. Chem. Eur. J. 2013, 19, 11167–11171. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Zhou, D.; Xu, J.; Hong, S.; Wei, W.; Zhao, T.; Huang, H.; Fang, W. One-pot synthesis of vancomycin-encapsulated ZIF-8 nanoparticles as multivalent and photocatalytic antibacterial agents for selective-killing of pathogenic gram-positive bacteria. J. Mater. Sci. 2021, 56, 9434–9444. [Google Scholar] [CrossRef]
- Rashid, M.; Rabbi, M.A.; Ara, T.; Hossain, M.M.; Islam, M.S.; Elaissari, A.; Ahmad, H.; Rahman, M.M. Vancomycin conjugated iron oxide nanoparticles for magnetic targeting and efficient capture of Gram-positive and Gram-negative bacteria. RSC Adv. 2021, 11, 36319–36328. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Chaudhary, A. One pot synthesis of gentamicin conjugated gold nanoparticles as an efficient antibacterial agent. J. Clust. Sci. 2021, 32, 995–1002. [Google Scholar] [CrossRef]
- Mugabe, C.; Azghani, A.O.; Omri, A. Liposome-mediated gentamicin delivery: Development and activity against resistant strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J. Antimicrob. Chemother. 2005, 55, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Atashbeyk, D.G.; Khameneh, B.; Tafaghodi, M.; Fazly Bazzaz, B.S. Eradication of methicillin-resistant Staphylococcus aureus infection by nanoliposomes loaded with gentamicin and oleic acid. Pharm. Biol. 2014, 52, 1423–1428. [Google Scholar] [CrossRef]
- Nicolosi, D.; Scalia, M.; Nicolosi, V.M.; Pignatello, R. Encapsulation in fusogenic liposomes broadens the spectrum of action of vancomycin against Gram-negative bacteria. Int. J. Antimicrob. Agents 2010, 35, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, M.; Shariati, M.M.; Malaekeh-Nikouei, B.; Tajani, A.S.; Mahmoudi, A.; Abrishami, M.; Khameneh, B. Preparation and in vivo evaluation of nanoliposomes containing vancomycin after intravitreal injection in albino rabbits. Iran. J. Basic Med. Sci. 2020, 23, 551. [Google Scholar] [PubMed]
- Joshi, M.D.; Iacoban, P.; Scheetz, M.H. Pharmacokinetic and biomarker quantification studies on vancomycin-loaded PEGylated liposomes and its potential to reduce vancomycin-induced kidney injury: A rat study. Pharmaceutics 2023, 15, 1582. [Google Scholar] [CrossRef]
- Obuobi, S.; Julin, K.; Fredheim, E.G.; Johannessen, M.; Škalko-Basnet, N. Liposomal delivery of antibiotic loaded nucleic acid nanogels with enhanced drug loading and synergistic anti-inflammatory activity against S. aureus intracellular infections. J. Control. Release 2020, 324, 620–632. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Fréchet, J.M. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Sheykhloo, H.; Milani, M.; Najafi, F.; Bani, F.; Zarebkohan, A. Conjugation of Gentamicin to Polyamidoamine Dendrimers Improved Anti-bacterial Properties against Pseudomonas aeruginosa. Adv. Pharm. Bull. 2021, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Chosy, M.B.; Sun, J.; Rahn, H.P.; Liu, X.; Brčić, J.; Wender, P.A.; Cegelski, L. Vancomycin-Polyguanidino Dendrimer Conjugates Inhibit Growth of Antibiotic-Resistant Gram-Positive and Gram-Negative Bacteria and Eradicate Biofilm-Associated S. aureus. ACS Infect. Dis. 2024, 10, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Chang, J.; Lin, J.; Zhu, J. The pH-controlled dual-drug release from mesoporous bioactive glass/polypeptide graft copolymer nanomicelle composites. Eur. J. Pharm. Biopharm. 2008, 69, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xie, S.; Wei, J.; Song, X.; Ding, Z.; Li, X. Antibacterial micelles with vancomycin-mediated targeting and pH/lipase-triggered release of antibiotics. ACS Appl. Mater. Interfaces 2018, 10, 36814–36823. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Dutta, D.; Sarkar, A.; Chattopadhyay, P. Functionalized carbon nanotubes: Synthesis, properties and applications in water purification, drug delivery, and material and biomedical sciences. Nanoscale Adv. 2021, 3, 5722–5744. [Google Scholar] [CrossRef] [PubMed]
- Rosen, Y.; Gurman, P. Carbon nanotubes for drug delivery applications. In Nanotechnology and Drug Delivery; CRC Press: Boca Raton, FL, USA, 2014; pp. 233–248. [Google Scholar]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon nanotubes: Smart drug/gene delivery carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shi, H.; Yang, H.; Yan, S.; Luan, S.; Li, Y.; Teng, M.; Khan, A.F.; Yin, J. Fabrication of antibacterial electrospun nanofibers with vancomycin-carbon nanotube via ultrasonication assistance. Mater. Des. 2017, 120, 128–134. [Google Scholar] [CrossRef]
- Al Thaher, Y.; Khalil, R.; Abdelghany, S.; Salem, M.S. Antimicrobial PMMA bone cement containing long releasing multi-walled carbon nanotubes. Nanomaterials 2022, 12, 1381. [Google Scholar] [CrossRef]
- Radaic, A.; de Jesus, M.B.; Kapila, Y.L. Bacterial anti-microbial peptides and nano-sized drug delivery systems: The state of the art toward improved bacteriocins. J. Control. Release 2020, 321, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Sangiorgio, G.; Nicitra, E.; Bivona, D.; Bonomo, C.; Bonacci, P.; Santagati, M.; Musso, N.; Bongiorno, D.; Stefani, S. Interactions of Gram-Positive Bacterial Membrane Vesicles and Hosts: Updates and Future Directions. Int. J. Mol. Sci. 2024, 25, 2904. [Google Scholar] [CrossRef] [PubMed]
- Saxena, D.; Maitra, R.; Bormon, R.; Czekanska, M.; Meiers, J.; Titz, A.; Verma, S.; Chopra, S. Tackling the outer membrane: Facilitating compound entry into Gram-negative bacterial pathogens. Npj Antimicrob. Resist. 2023, 1, 17. [Google Scholar] [CrossRef]
- Rohde, M. The Gram-positive bacterial cell wall. Microbiol. Spectr. 2019, 7, 10.1128. [Google Scholar] [CrossRef] [PubMed]
- Hurdle, J.G.; O’neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Joly, H.; Omri, A. Liposomes as a carrier for gentamicin delivery: Development and evaluation of the physicochemical properties. Int. J. Pharm. 2008, 359, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Lutwyche, P.; Cordeiro, C.; Wiseman, D.J.; St-Louis, M.; Uh, M.; Hope, M.J.; Webb, M.S.; Finlay, B.B. Intracellular delivery and antibacterial activity of gentamicin encapsulated in pH-sensitive liposomes. Antimicrob. Agents Chemother. 1998, 42, 2511–2520. [Google Scholar] [CrossRef]
- Rukholm, G.; Mugabe, C.; Azghani, A.O.; Omri, A. Antibacterial activity of liposomal gentamicin against Pseudomonas aeruginosa: A time–kill study. Int. J. Antimicrob. Agents 2006, 27, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Serri, A.; Mahboubi, A.; Zarghi, A.; Moghimi, H.R. Investigating the Antimicrobial Efficacy of Liposomal Vancomycin in Gram-positive and Gram-negative bacteria-A Preliminary Mechanistic Study: Antimicrobial Effects of Liposomal Vancomycin. Iran. J. Pharm. Sci. 2018, 14, 13–24. [Google Scholar]
- Muppidi, K.; Pumerantz, A.S.; Wang, J.; Betageri, G. Development and stability studies of novel liposomal vancomycin formulations. Int. Sch. Res. Not. 2012, 2012, 636743. [Google Scholar] [CrossRef]
- Lankalapalli, S.; Tenneti, V.; Nimmali, S.K. Design and development of vancomycin liposomes. Indian J. Pharm. Educ. Res 2015, 49, 208–215. [Google Scholar] [CrossRef]
- Sande, L.; Sanchez, M.; Montes, J.; Wolf, A.J.; Morgan, M.A.; Omri, A.; Liu, G.Y. Liposomal encapsulation of vancomycin improves killing of methicillin-resistant Staphylococcus aureus in a murine infection model. J. Antimicrob. Chemother. 2012, 67, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Omri, A.; Suntres, Z.E.; Shek, P.N. Enhanced activity of liposomal polymyxin B against Pseudomonas aeruginosa in a rat model of lung infection. Biochem. Pharmacol. 2002, 64, 1407–1413. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]

| Nanosized Delivery System | NP Preparation Method | Target Bacterial Strain | Reference |
|---|---|---|---|
| Gentamicin-loaded CaCO3 nanoparticles | Microemulsion | Staphylococcus aureus | [28] |
| Silica–gentamicin nanohybrids | Base-catalyzed precipitation | Bacillus subtilis, Pseudomonas fluorescens, E. coli | [29] |
| Gentamicin coated iron oxide nanoparticles | Co-precipitation | S. aureus, E. coli, P. aeruginosa, Bacillus subtilis | [30] |
| Gentamicin-loaded liposomes | Dehydration–rehydration | P. aeruginosa, K. oxytoca | [31] |
| Gentamicin nanoparticles | water-in-oil-in-water | K. pneumoniae | [32] |
| Gentamicin-loaded CaCO3 nanoparticles | Carbonization | B. subtillis | [33] |
| Gentamicin-loaded chitosan nanoparticles | Ionic gelation | Brucella melitensis | [34] |
| Gentamicin-loaded silk/nanosilver composite | Chemical synthesis | Methicillin-resistant S. aureus (MRSA) | [35] |
| Gentamicin sulfate-loaded PLGA nanoparticle | Double emulsion solvent removal | P. aeruginosa, S. aureus | [36] |
| Gentamicin-loaded proanthocyanidin–chitosan composite nanoparticles | Ionic gelation | E.coli, S. aureus, P.aeruginosa | [37] |
| Gentamicin-loaded PLGA nanoparticles | Double emulsion evaporation | E. coli | [38] |
| Gentamicin nano gel | Sol-gel application | E.coli, St. epidermidis | [39] |
| Gentamicin-loaded chitosan/folic acid-based carbon quantum dots | Hydrothermal technique | E. faecalis, P. aeruginosa, S. mutans, S. aureus, K. pneumoniae, E. coli | [40] |
| Gentamicin–ascorbic acid-loaded chitosan nanoparticles | Ionotropic gelation | S. aureus, P. aeruginosa | [41] |
| Gentamicin-loaded PLGA/polyurethane/poly(ethylene oxide) nanoparticles | Double emulsion solvent evaporation | E. coli | [42] |
| Gentamicin-coupled gold nanoparticles (G-GNPs) | Sol-gel method | E. fergusonii | [12] |
| Gentamicin-loaded PEG-PLGA/PLGAH nanoparticles | Solvent precipitation | P.aeruginosa, S. aureus clinical strains | [43] |
| Nanosized Delivery System | NP Preparation Method | Target Bacterial Strain | Reference |
|---|---|---|---|
| Functionalized nanoparticles of α-norbornenyl-ωvancomycin poly(ethylene oxide) macromonomers | Ring-opening metathesis polymerization | Methicillin resistant S. aureus | [44] |
| Vancomycin-functionalized gold and silver nanoparticles | Chemical synthesis | Methicillin-resistant S. aureus (MRSA) | [45] |
| Vancomycin-loaded chitosan nanoparticles | Ionic gelation | S. aureus | [46] |
| Vancomycin-loaded N-trimethyl chitosan nanoparticles | Chemical synthesis | S. aureus | [47] |
| Vancomycin and Cefazolin-loaded lipid nanoparticles | Reverse phase evaporation | Methicillin-resistant S. aureus (MRSA) | [48] |
| Vancomycin–PLGA-conjugated nanoparticles | Double emulsification-solvent evaporation | S. aureus, P. aeruginosa | [49] |
| Vancomycin-loaded silver nanoparticles | Chemical synthesis (reduction) | S. aureus, E. coli | [50] |
| Vancomycin-loaded iron oxide nanoparticles | Thermal decomposition | Clostridium difficile | [51] |
| Vancomycin-loaded PLGA nanoparticles | Double emulsion solvent evaporation | S. aureus | [52] |
| Vancomycin-conjugated gold nanoparticles | Chemical synthesis | S. aureus, E. coli | [53] |
| Vancomycin-loaded PLGA nanoparticle | Water-in-oil double emulsion | S. aureus | [54] |
| Vancomycin-functionalized gold nanoparticles (V-GNPs) | One pot synthesis | E.coli, Klebsiella oxytoca, S. aureus, P. aeruginosa | [55] |
| Vancomycin-loaded PLGA nanoparticles | Emulsification-solvent evaporation | Enterococcus faecalis | [22] |
| Vancomycin-loaded chitosan nanoparticles | Ionotropic gelation | S. aureus | [56] |
| PLGA nanoparticles loaded with vancomycin and conjugated with lysostaphin (PLGA-VAN-LYS) | Double emulsion evaporation | S. aureus | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisani, S.; Tufail, S.; Rosalia, M.; Dorati, R.; Genta, I.; Chiesa, E.; Conti, B. Antibiotic-Loaded Nano-Sized Delivery Systems: An Insight into Gentamicin and Vancomycin. J. Funct. Biomater. 2024, 15, 194. https://doi.org/10.3390/jfb15070194
Pisani S, Tufail S, Rosalia M, Dorati R, Genta I, Chiesa E, Conti B. Antibiotic-Loaded Nano-Sized Delivery Systems: An Insight into Gentamicin and Vancomycin. Journal of Functional Biomaterials. 2024; 15(7):194. https://doi.org/10.3390/jfb15070194
Chicago/Turabian StylePisani, Silvia, Shafia Tufail, Mariella Rosalia, Rossella Dorati, Ida Genta, Enrica Chiesa, and Bice Conti. 2024. "Antibiotic-Loaded Nano-Sized Delivery Systems: An Insight into Gentamicin and Vancomycin" Journal of Functional Biomaterials 15, no. 7: 194. https://doi.org/10.3390/jfb15070194
APA StylePisani, S., Tufail, S., Rosalia, M., Dorati, R., Genta, I., Chiesa, E., & Conti, B. (2024). Antibiotic-Loaded Nano-Sized Delivery Systems: An Insight into Gentamicin and Vancomycin. Journal of Functional Biomaterials, 15(7), 194. https://doi.org/10.3390/jfb15070194











