Antibacterial Size Effect of ZnO Nanoparticles and Their Role as Additives in Emulsion Waterborne Paint
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Synthesis of ZnO Nanoparticles
2.3. ZnO Nanoparticles’ Characterization
2.4. Minimal Inhibitory Concentration (MIC)
2.5. Minimal Bactericidal Concentration (MBC)
2.6. Kinetic Growth of Bacteria
2.7. Paint Formulation with ZnO Nanoparticles
2.8. Measurement of the Antibacterial Activity of Paints Containing Nanoparticles
3. Results
3.1. Characterization of ZnO Nanoparticles
3.2. Correlation between ZnO Nanoparticle Size and Minimal Inhibitory Concentration (MIC)
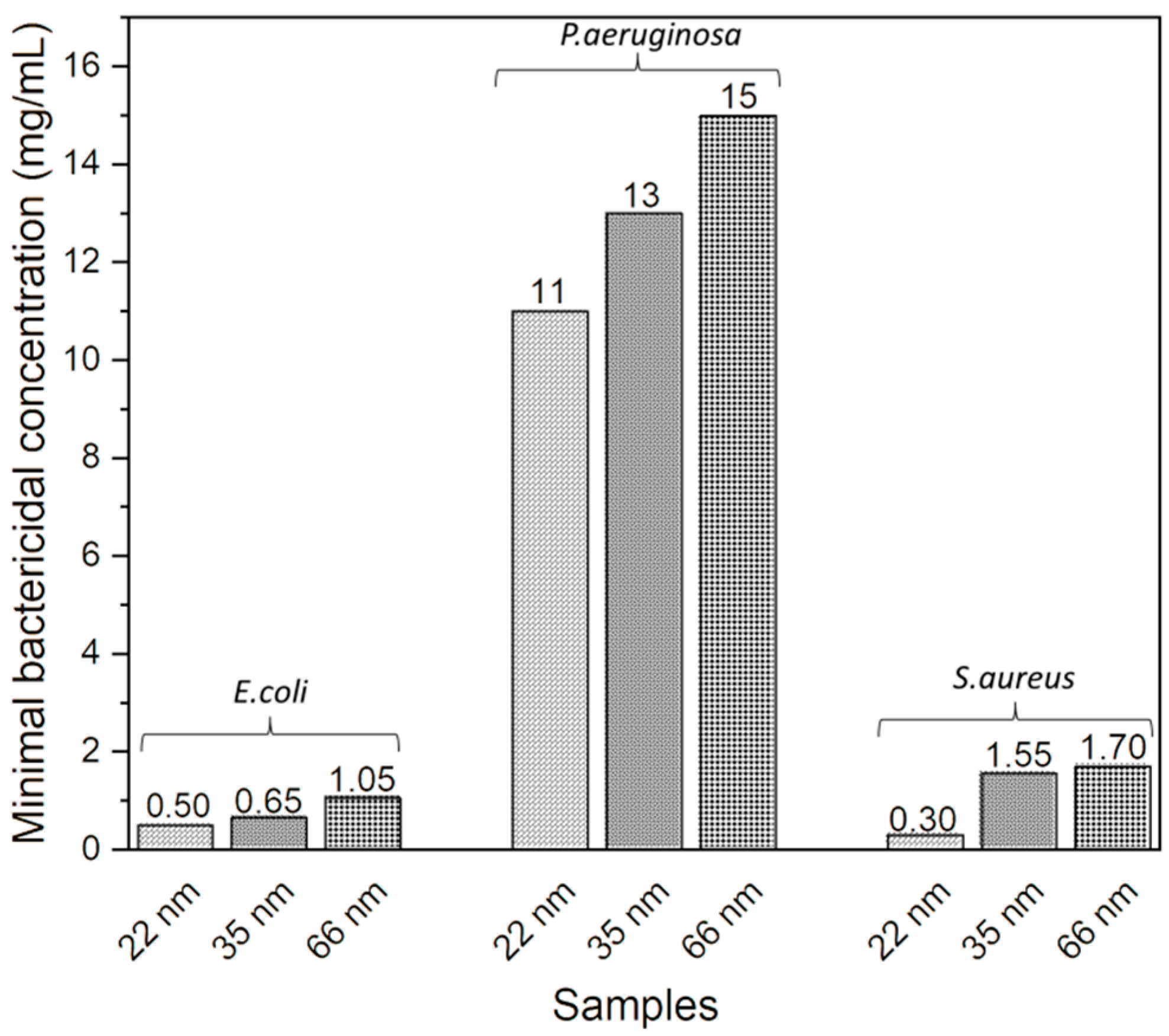
3.3. Correlation between ZnO NP Size and Minimal Bactericidal Concentration (MBC)
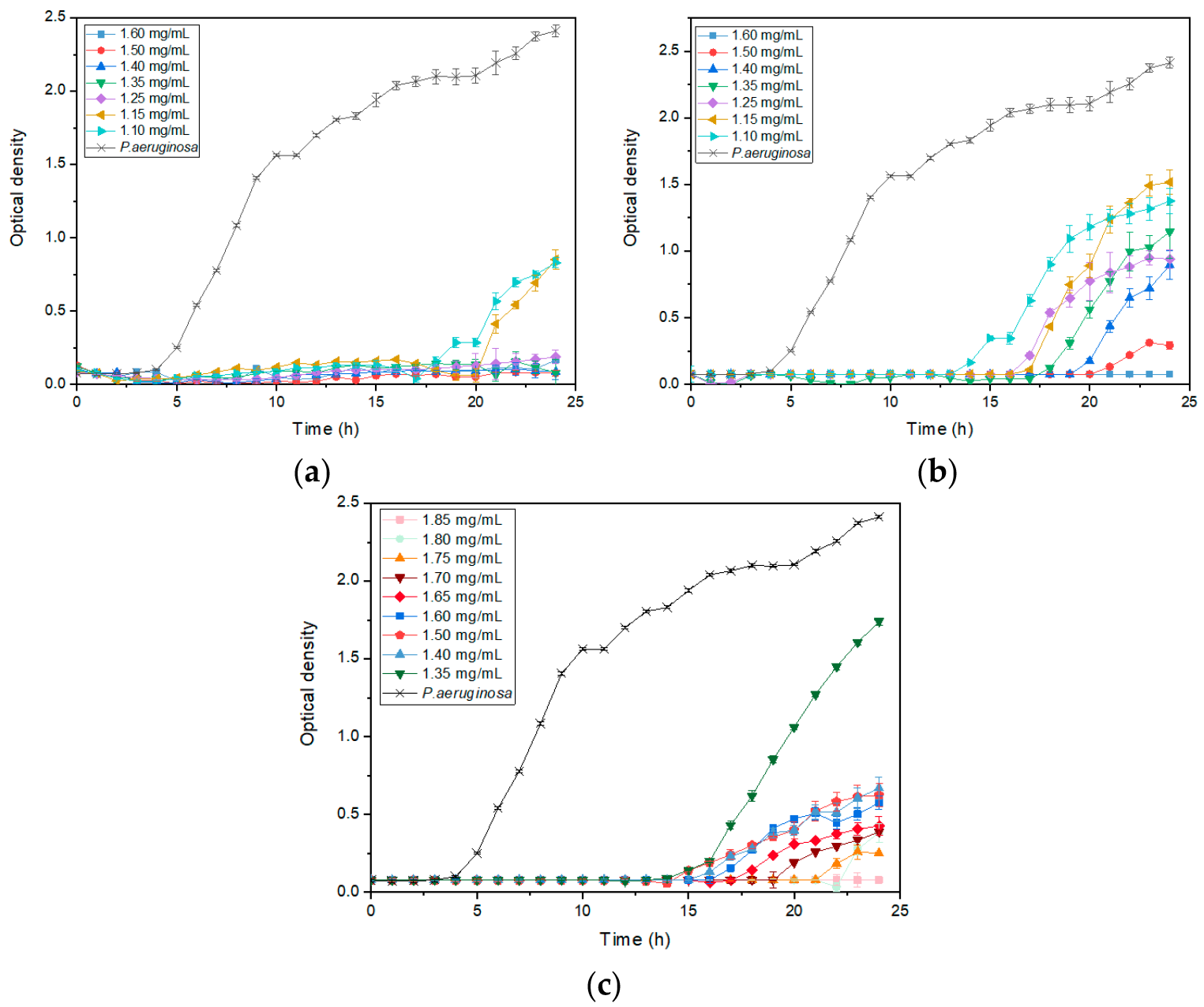
3.4. ZnO NP Size Effect on Bacterial Growth Kinetics
3.5. Antibacterial Activity of ZnO Nanoparticles on Emulsion Waterborne Paint
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baviskar, A.S.; Khatib, K.I.; Rajpal, D.; Dongare, H.C. Nosocomial Infections in Surgical Intensive Care Unit: A Retrospective Single-Center Study. Int. J. Crit. Illn. Inj. Sci. 2019, 9, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Ajulo, S.; Awosile, B. Global Antimicrobial Resistance and Use Surveillance System (GLASS 2022): Investigating the Relationship between Antimicrobial Resistance and Antimicrobial Consumption Data across the Participating Countries. PLoS ONE 2024, 19, e0297921. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.; Zembower, T.R. Antimicrobial Resistance. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 619–635. [Google Scholar] [CrossRef] [PubMed]
- Strengthening the EU Response to Prevention and Control of Antimicrobial Resistance (AMR): Policy Priorities for Effective Implementation Strengthening the EU Response to Prevention and Control of Antimicrobial Resistance (AMR): Policy Priorities for Effective Implementation. Available online: https://eurohealthobservatory.who.int/publications/i/strengthening-the-eu-response-to-prevention-and-control-of-antimicrobial-resistance-(amr)-policy-priorities-for-effective-implementation (accessed on 1 July 2024).
- Raghunath, A.; Perumal, E. Metal Oxide Nanoparticles as Antimicrobial Agents: A Promise for the Future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Pachaiappan, R.; Rajendran, S.; Show, P.L.; Manavalan, K.; Naushad, M. Metal/Metal Oxide Nanocomposites for Bactericidal Effect: A Review. Chemosphere 2021, 272, 128607. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Mishra, N.; Gadani, K.; Solanki, P.S.; Shah, N.A.; Tiwari, M. Mechanism of Anti-Bacterial Activity of Zinc Oxide Nanoparticle Against Carbapenem-Resistant Acinetobacter baumannii. Front. Microbiol. 2018, 9, 1218. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Das, D.K.; Kaur, J.; Kumar, A.; Ubaidullah, M.; Hasan, M.; Yadav, K.K.; Gupta, R.K. Transition Metal-Based Nanoparticles as Potential Antimicrobial Agents: Recent Advancements, Mechanistic, Challenges, and Future Prospects. Discov. Nano 2023, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO Nanostructured Materials and Their Potential Applications: Progress, Challenges and Perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Sarojini, S.; Jayaram, S. An Impact of Antibacterial Efficacy of Metal Oxide Nanoparticles: A Promise for Future. In Bio-Manufactured Nanomaterials: Perspectives and Promotion; Pal, K., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 393–406. ISBN 978-3-030-67223-2. [Google Scholar]
- Jiang, S.; Lin, K.; Cai, M. ZnO Nanomaterials: Current Advancements in Antibacterial Mechanisms and Applications. Front. Chem. 2020, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Izzi, M.; Sportelli, M.C.; Torsi, L.; Picca, R.A.; Cioffi, N. Synthesis and Antimicrobial Applications of ZnO Nanostructures: A Review. ACS Appl. Nano Mater. 2023, 6, 10881–10902. [Google Scholar] [CrossRef]
- Dutta, G.; Sugumaran, A. Bioengineered Zinc Oxide Nanoparticles: Chemical, Green, Biological Fabrication Methods and Its Potential Biomedical Applications. J. Drug Deliv. Sci. Technol. 2021, 66, 102853. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.d.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial Action and Target Mechanisms of Zinc Oxide Nanoparticles against Bacterial Pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Alshahrani, M.Y.; Wahab, S.; Al-Harbi, A.I.; Nisar, N.; Alraey, Y.; Alqahtani, A.; Mir, M.A.; Irfan, S.; Saeed, M. Zinc Oxide Nanoparticle: An Effective Antibacterial Agent against Pathogenic Bacterial Isolates. J. King Saud. Univ. Sci. 2022, 34, 102110. [Google Scholar] [CrossRef]
- Perveen, R.; Shujaat, S.; Qureshi, Z.; Nawaz, S.; Khan, M.I.; Iqbal, M. Green versus Sol-Gel Synthesis of ZnO Nanoparticles and Antimicrobial Activity Evaluation against Panel of Pathogens. J. Mater. Res. Technol. 2020, 9, 7817–7827. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO Size and Shape Effect on Antibacterial Activity and Cytotoxicity Profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef] [PubMed]
- Girma, A.; Abera, B.; Mekuye, B.; Mebratie, G. Antibacterial Activity and Mechanisms of Action of Inorganic Nanoparticles against Foodborne Bacterial Pathogens: A Systematic Review. IET Nanobiotechnol. 2024, 2024, 5417924. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Dey, A.; Neogi, S. An Insight into the Mechanism of Antibacterial Activity by Magnesium Oxide Nanoparticles. J. Mater. Chem. B 2021, 9, 5329–5339. [Google Scholar] [CrossRef]
- Kessler, A.; Hedberg, J.; Blomberg, E.; Odnevall, I. Reactive Oxygen Species Formed by Metal and Metal Oxide Nanoparticles in Physiological Media—A Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization. Nanomaterials 2022, 12, 1922. [Google Scholar] [CrossRef] [PubMed]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A Review on Enhancing the Antibacterial Activity of ZnO: Mechanisms and Microscopic Investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Menon, S.; Venkat Kumar, S.; Rajeshkumar, S. Mechanistic Study on Antibacterial Action of Zinc Oxide Nanoparticles Synthesized Using Green Route. Chem. Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.K.; Gaur, S.; Sengupta, M.; Singh, M.S. Mechanistic Insights into Nanoparticle Surface-Bacterial Membrane Interactions in Overcoming Antibiotic Resistance. Front. Microbiol. 2023, 14, 1135579. [Google Scholar] [CrossRef] [PubMed]
- Raghav, A.; Kaur, S.; Setia, G.; Kumar, S. Nanomaterials Induced Cell Disruption: An Insight into Mechanism. In Biogenic Nanomaterials for Environmental Sustainability: Principles, Practices, and Opportunities; Shah, M.P., Bharadvaja, N., Kumar, L., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 227–249. ISBN 978-3-031-45956-6. [Google Scholar]
- Thakur, S.; Neogi, S. Effect of Doped ZnO Nanoparticles on Bacterial Cell Morphology and Biochemical Composition. Appl. Nanosci. 2021, 11, 159–171. [Google Scholar] [CrossRef]
- Xin, Z.; He, Q.; Wang, S.; Han, X.; Fu, Z.; Xu, X.; Zhao, X. Recent Progress in ZnO-Based Nanostructures for Photocatalytic Antimicrobial in Water Treatment: A Review. Appl. Sci. 2022, 12, 7910. [Google Scholar] [CrossRef]
- Kaur, H.; Rauwel, P.; Rauwel, E. Chapter 6—Antimicrobial Nanoparticles: Synthesis, Mechanism of Actions. In Antimicrobial Activity of Nanoparticles; Guisbiers, G., Ed.; Advanced Topics in Biomaterials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 155–202. ISBN 978-0-12-821637-8. [Google Scholar]
- Ghaffari, S.-B.; Sarrafzadeh, M.-H.; Salami, M.; Alvandi, A. A Comparative Study of the Action Mechanisms and Development Strategies of Different ZnO-Based Nanostructures in Antibacterial and Anticancer Applications. J. Drug Deliv. Sci. Technol. 2024, 91, 105221. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, K.; Thakur, N.; Chauhan, S.; Chauhan, M.S. The Effect of Shape and Size of ZnO Nanoparticles on Their Antimicrobial and Photocatalytic Activities: A Green Approach. Bull. Mater. Sci. 2020, 43, 20. [Google Scholar] [CrossRef]
- Jaiswal, P.B.; Jejurikar, S.; Mondal, A.; Pushkar, B.; Mazumdar, S. Antibacterial Effects of ZnO Nanodisks: Shape Effect of the Nanostructure on the Lethality in Escherichia coli. Appl. Biochem. Biotechnol. 2023, 195, 3067–3095. [Google Scholar] [CrossRef]
- Motelica, L.; Oprea, O.-C.; Vasile, B.-S.; Ficai, A.; Ficai, D.; Andronescu, E.; Holban, A.M. Antibacterial Activity of Solvothermal Obtained ZnO Nanoparticles with Different Morphology and Photocatalytic Activity against a Dye Mixture: Methylene Blue, Rhodamine B and Methyl Orange. Int. J. Mol. Sci. 2023, 24, 5677. [Google Scholar] [CrossRef]
- Zubair, N.; Akhtar, K. Morphology Controlled Synthesis of ZnO Nanoparticles for In-Vitro Evaluation of Antibacterial Activity. Trans. Nonferrous Met. Soc. China 2020, 30, 1605–1614. [Google Scholar] [CrossRef]
- Gharpure, S.; Ankamwar, B. Synthesis and Antimicrobial Properties of Zinc Oxide Nanoparticles. J. Nanosci. Nanotechnol. 2020, 20, 5977–5996. [Google Scholar] [CrossRef] [PubMed]
- Droepenu, E.K.; Amenyogbe, E.; Boatemaa, M.A.; Opoku, E. Study of the Antimicrobial Activity of Zinc Oxide Nanostructures Mediated by Two Morphological Structures of Leaf Extracts of Eucalyptus robusta Sm. Heliyon 2024, 10, e25590. [Google Scholar] [CrossRef] [PubMed]
- Lallo da Silva, B.; Caetano, B.L.; Chiari-Andréo, B.G.; Pietro, R.C.L.R.; Chiavacci, L.A. Increased Antibacterial Activity of ZnO Nanoparticles: Influence of Size and Surface Modification. Colloids Surf. B Biointerfaces 2019, 177, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Bouttier-Figueroa, D.C.; Cortez-Valadez, M.; Flores-Acosta, M.; Robles-Zepeda, R.E. Green Synthesis of Zinc Oxide Nanoparticles Using Plant Extracts and Their Antimicrobial Activity. BioNanoSci 2024. [Google Scholar] [CrossRef]
- Aldeen, T.S.; Ahmed Mohamed, H.E.; Maaza, M. ZnO Nanoparticles Prepared via a Green Synthesis Approach: Physical Properties, Photocatalytic and Antibacterial Activity. J. Phys. Chem. Solids 2022, 160, 110313. [Google Scholar] [CrossRef]
- Awasthi, A.; Sharma, P.; Jangir, L.; Kamakshi; Awasthi, G.; Awasthi, K.K.; Awasthi, K. Dose Dependent Enhanced Antibacterial Effects and Reduced Biofilm Activity against Bacillus Subtilis in Presence of ZnO Nanoparticles. Mater. Sci. Eng. C 2020, 113, 111021. [Google Scholar] [CrossRef]
- Dadi, R.; Kerignard, E.; Traoré, M.; Mielcareck, C.; Kanaev, A.; Azouani, R. Evaluation of Antibacterial Efficiency of Zinc Oxide Thin Films Nanoparticles against Nosocomial Bacterial Strains. Chem. Eng. Trans. 2021, 84, 13–18. [Google Scholar] [CrossRef]
- Wang, F.; Qi, J.; Zhu, L. Ag/MoS2 Nanozyme-Modified ZnO Nanopillar Surface for Enhanced Synergistic Mechanical and Chemical Antibacterial Activity. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133494. [Google Scholar] [CrossRef]
- Zaman, Y.; Ishaque, M.Z.; Waris, K.; Shahzad, M.; Siddique, A.B.; Arshad, M.I.; Zaman, H.; Ali, H.M.; Kanwal, F.; Aslam, M.; et al. Modified Physical Properties of Ni Doped ZnO NPs as Potential Photocatalyst and Antibacterial Agents. Arab. J. Chem. 2023, 16, 105230. [Google Scholar] [CrossRef]
- Xiang, E.; Moran, C.S.; Ivanovski, S.; Abdal-hay, A. Nanosurface Texturing for Enhancing the Antibacterial Effect of Biodegradable Metal Zinc: Surface Modifications. Nanomaterials 2023, 13, 2022. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liang, H.-W.; Yang, Y.; Yu, S.-H. Stability and Reactivity: Positive and Negative Aspects for Nanoparticle Processing. Chem. Rev. 2018, 118, 3209–3250. [Google Scholar] [CrossRef]
- Amodeo, J.; Pizzagalli, L. Modeling the Mechanical Properties of Nanoparticles: A Review. C. R. Phys. 2021, 22, 35–66. [Google Scholar] [CrossRef]
- Mirhosseini, F.; Amiri, M.; Daneshkazemi, A.; Zandi, H.; Javadi, Z.S. Antimicrobial Effect of Different Sizes of Nano Zinc Oxide on Oral Microorganisms. Front. Dent. 2019, 16, 105–112. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- El-Habib, I.; Roynette, A.; Morakchi-Goudjil, H.; Lemarchand, A.; Christine, M.; Azouani, R.; Traore, M. Synthesis by Soft Chemistry of Size-Controlled Zinc Oxide (ZnO) Nanocrystals for Antimicrobial Applications. MATEC Web Conf. 2023, 379, 06003. [Google Scholar] [CrossRef]
- Luo, Z.; Zhu, M.; Guo, M.; Lian, Z.; Tong, W.; Wang, J.; Zhang, B.; Wei, W. Ultrasonic-Assisted Dispersion of ZnO Nanoparticles and Its Inhibition Activity to Trichoderma viride. J. Nanosci. Nanotechnol. 2018, 18, 2352–2360. [Google Scholar] [CrossRef]
- ISO 22196:2011(En); Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2011. Available online: https://www.iso.org/obp/ui/#iso:std:iso:22196:ed-2:v1:en (accessed on 18 March 2024).
- Pasquet, J.; Chevalier, Y.; Couval, E.; Bouvier, D.; Noizet, G.; Morlière, C.; Bolzinger, M.-A. Antimicrobial Activity of Zinc Oxide Particles on Five Micro-Organisms of the Challenge Tests Related to Their Physicochemical Properties. Int. J. Pharm. 2014, 460, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial Activity of ZnO Nanoparticle Suspensions on a Broad Spectrum of Microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef]
- Palanikumar, L.; Ramasamy, S.N.; Balachandran, C. Size-Dependent Antimicrobial Response of Zinc Oxide Nanoparticles. IET Nanobiotechnol. 2014, 8, 111–117. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.; Memic, A. Size-Dependent Antimicrobial Properties of CuO Nanoparticles against Gram-Positive and -Negative Bacterial Strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- Lallo da Silva, B.; Abuçafy, M.P.; Berbel Manaia, E.; Oshiro Junior, J.A.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship Between Structure And Antimicrobial Activity Of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced Antibacterial Activity of Nanocrystalline ZnO Due to Increased ROS-Mediated Cell Injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Álvarez-Chimal, R.; García-Pérez, V.I.; Álvarez-Pérez, M.A.; Tavera-Hernández, R.; Reyes-Carmona, L.; Martínez-Hernández, M.; Arenas-Alatorre, J.Á. Influence of the Particle Size on the Antibacterial Activity of Green Synthesized Zinc Oxide Nanoparticles Using Dysphania Ambrosioides Extract, Supported by Molecular Docking Analysis. Arab. J. Chem. 2022, 15, 103804. [Google Scholar] [CrossRef]
- Aldin, K.S.; Al-Hariri, S.; Ali-Nizam, A. Effectiveness of ZnO Nano Particles against the Foodborne Microbial Pathogens E. coli and S. aureus. Jordan J. Chem. (JJC) 2020, 15, 87–94. [Google Scholar] [CrossRef]
- Grossich, R.; Lemos Vilches, M.; Costa, C.S.; Pezzoni, M. Role of Pel and Psl Polysaccharides in the Response of Pseudomonas aeruginosa to Environmental Challenges: Oxidative Stress Agents (UVA, H2O2, Sodium Hypochlorite) and Its Competitor Staphylococcus aureus. Microbiology 2023, 169, 001301. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic Resistance in Pseudomonas Aeruginosa: Mechanisms and Alternative Therapeutic Strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Joo, S.H.; Kumar, N.; Toborek, M. Antibacterial Effect and Toxicity Pathways of Industrial and Sunscreen ZnO Nanoparticles on Escherichia coli. J. Environ. Chem. Eng. 2017, 5, 3024–3032. [Google Scholar] [CrossRef]
- Fiori, J.; Silva, L.; Picolli, K.C.; Ternus, R.; Ilha, J.; Decalton, F.; de Mello, J.M.; Riella, H.; Fiori, M. Zinc Oxide Nanoparticles as Antimicrobial Additive for Acrylic Paint. Mater. Sci. Forum 2017, 899, 248–253. [Google Scholar] [CrossRef]
- Agustin, W.; Albari, M.T.; Ghifari, M.A.; Ghifari, M.R.; Purnamasari, D.; Mandeli, R.S. The Antibacterial Properties of Paint with the Addition of ZnO Nanoparticles. AIP Conf. Proc. 2024, 3001, 030004. [Google Scholar] [CrossRef]
- Foudi, H.; Soukeur, A.; Rekhila, G.; Trari, M.; Amara, M. Synthesis and Characterization of ZnO Nanoparticles for Antibacterial Paints. Chem. Pap. 2023, 77, 1489–1496. [Google Scholar] [CrossRef]
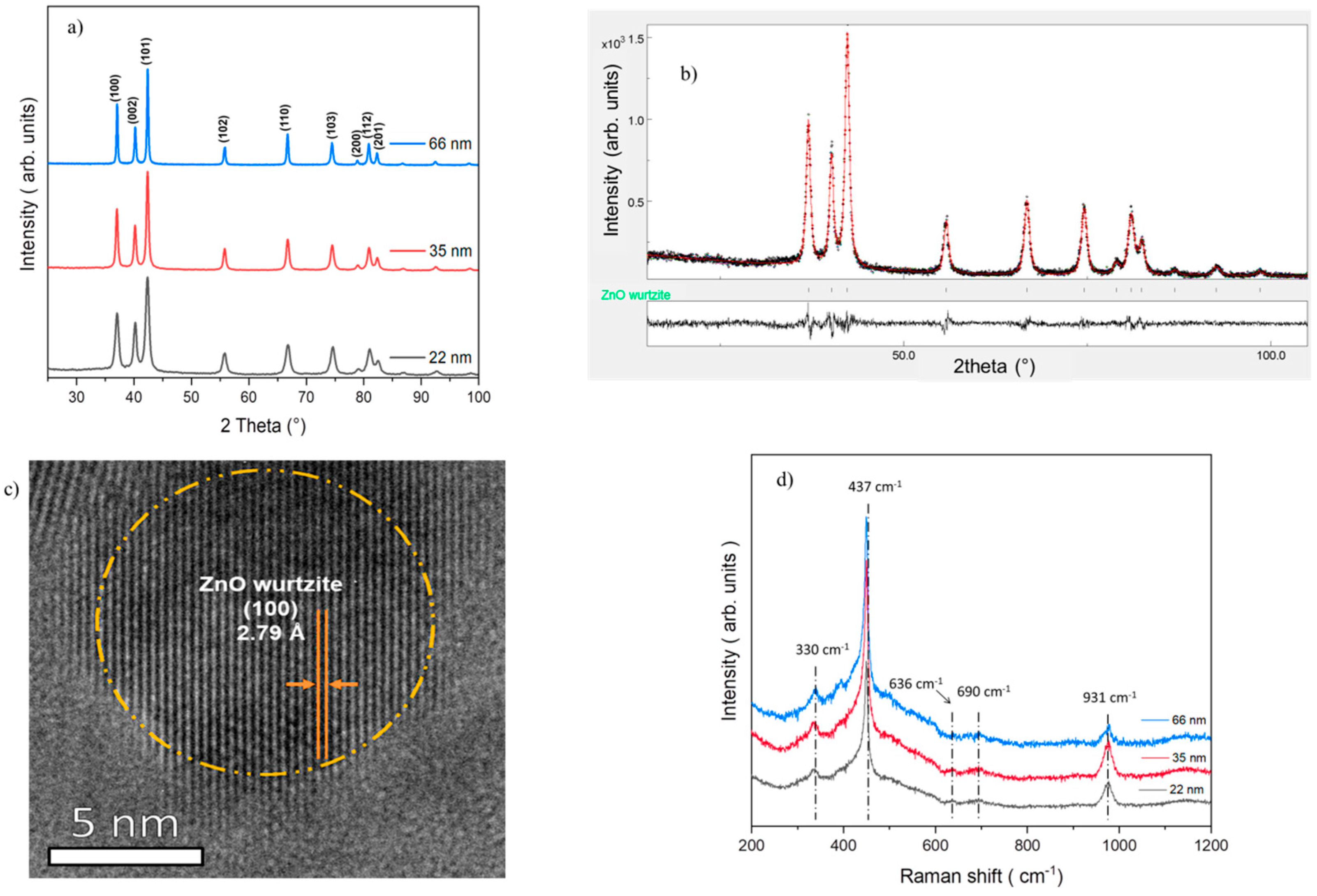




| Sample Size | [Zn2+]/[MEA] | [Zn2+]/[H2O] | Agitation Time | Temperature |
|---|---|---|---|---|
| 22 nm | 1 | 5 | 22 h | 80 °C |
| 35 nm | 1 | 10 | 2 h | 110 °C |
| 66 nm | 2 | 10 | 22 h | 110 °C |
| Crystallite Size (nm) | Lattice Parameters (Å) | Microstrain (%) | Sig = GoF | |
|---|---|---|---|---|
| a = b | c | |||
| 22 | 3.25 | 5.20 | 0.002 | 1.18 |
| 35 | 3.25 | 5.21 | 0.001 | 1.21 |
| 66 | 3.25 | 5.21 | 0.001 | 1.60 |
| Bacteria | Control at 24 h | Sample at 24 h |
|---|---|---|
| S. aureus | 4.55 ± 0.32 Log CFU/cm2 | <1 Log10 CFU/cm2 |
| E. coli | 3.90 ± 0.02 Log10 CFU/cm2 | <1 Log10 CFU/cm2 |
| P. aeruginosa | 6.41 ± 0.11 Log10 CFU/cm2 | 6.18 ± 0.08 Log10 CFU/cm2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Habib, I.; Maatouk, H.; Lemarchand, A.; Dine, S.; Roynette, A.; Mielcarek, C.; Traoré, M.; Azouani, R. Antibacterial Size Effect of ZnO Nanoparticles and Their Role as Additives in Emulsion Waterborne Paint. J. Funct. Biomater. 2024, 15, 195. https://doi.org/10.3390/jfb15070195
El-Habib I, Maatouk H, Lemarchand A, Dine S, Roynette A, Mielcarek C, Traoré M, Azouani R. Antibacterial Size Effect of ZnO Nanoparticles and Their Role as Additives in Emulsion Waterborne Paint. Journal of Functional Biomaterials. 2024; 15(7):195. https://doi.org/10.3390/jfb15070195
Chicago/Turabian StyleEl-Habib, Imroi, Hassan Maatouk, Alex Lemarchand, Sarah Dine, Anne Roynette, Christine Mielcarek, Mamadou Traoré, and Rabah Azouani. 2024. "Antibacterial Size Effect of ZnO Nanoparticles and Their Role as Additives in Emulsion Waterborne Paint" Journal of Functional Biomaterials 15, no. 7: 195. https://doi.org/10.3390/jfb15070195






