Hybrid Bone Substitute Containing Tricalcium Phosphate and Silver Modified Hydroxyapatite–Methylcellulose Granules
Abstract
:1. Introduction
2. Materials and Methods
2.1. αTCP
2.2. Hybrid Granules
2.3. Biomicroconcretes
2.4. Chemical and Phase Composition
2.5. Setting Times
2.6. Compressive Strength
2.7. Microstructure
2.8. Chemical Stability and Bioactive Potential in SBF
3. Results
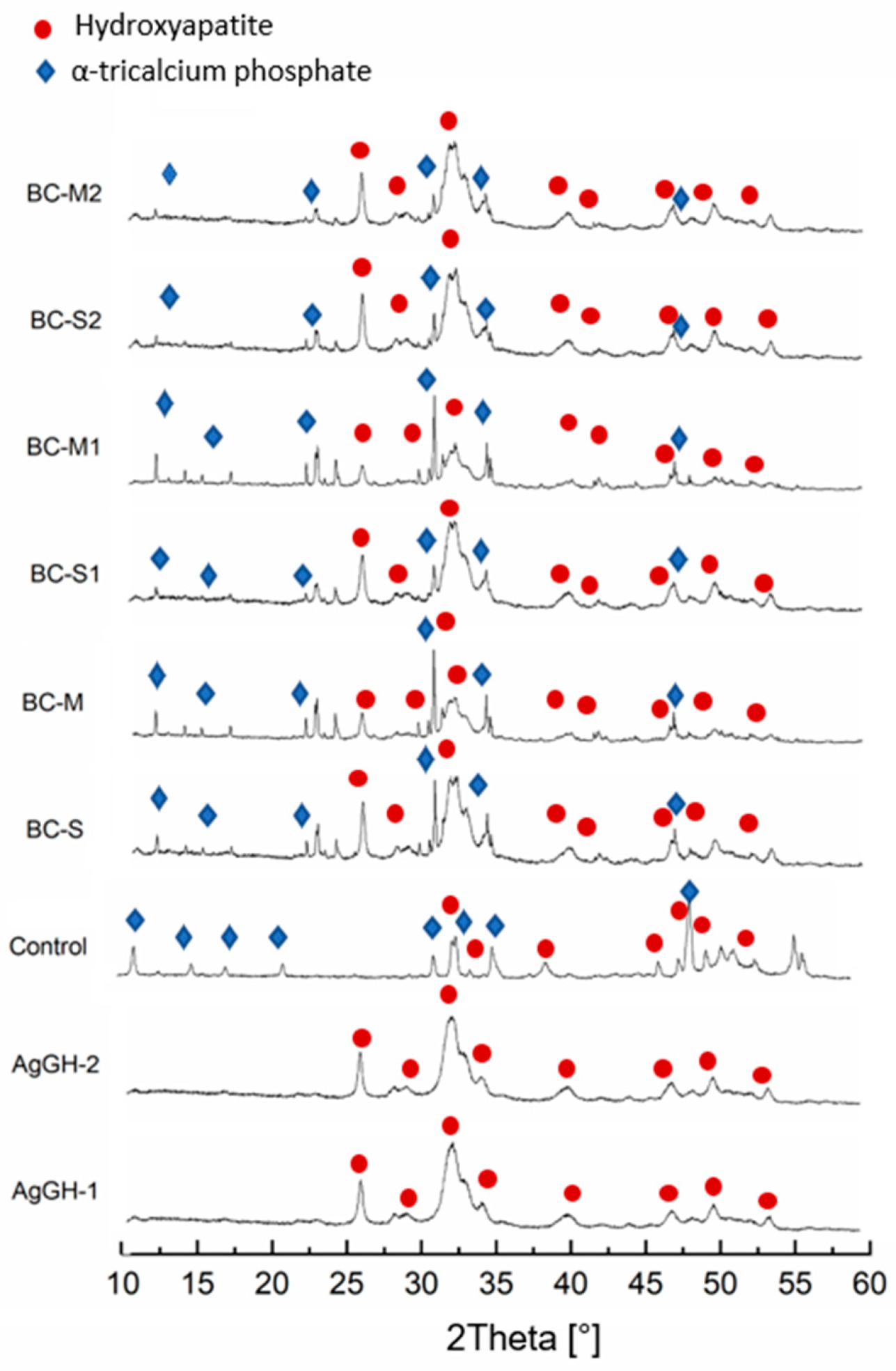
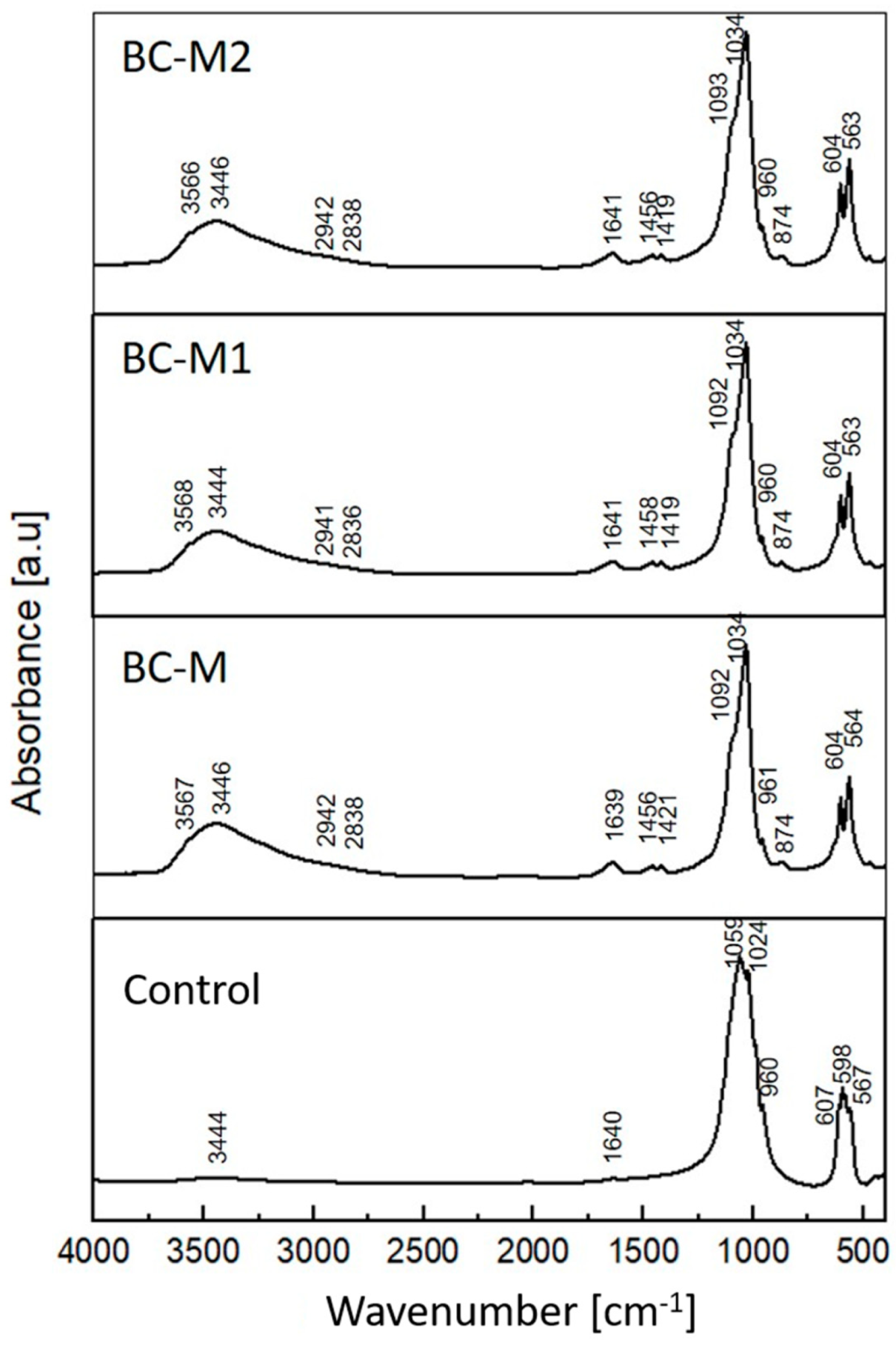
3.1. Chemical and Phase Composition
3.2. Setting Times
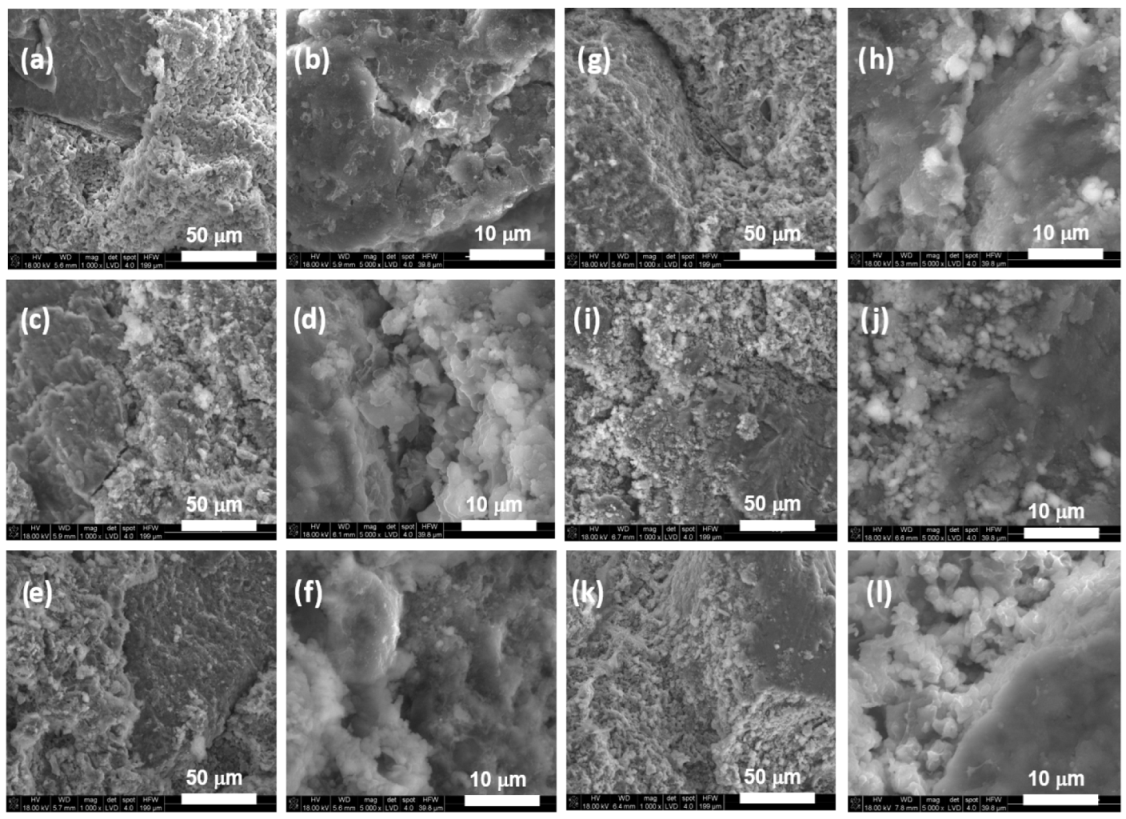
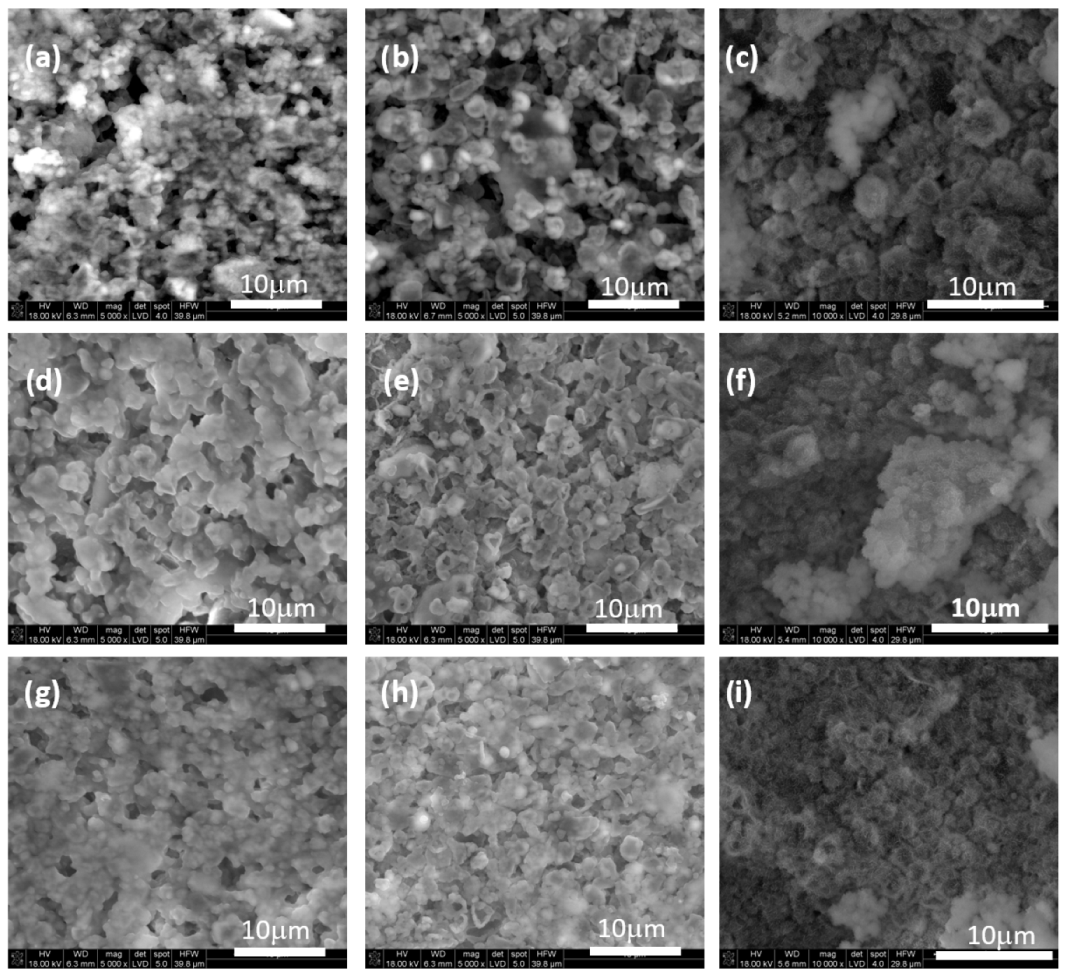
3.3. Microstructure
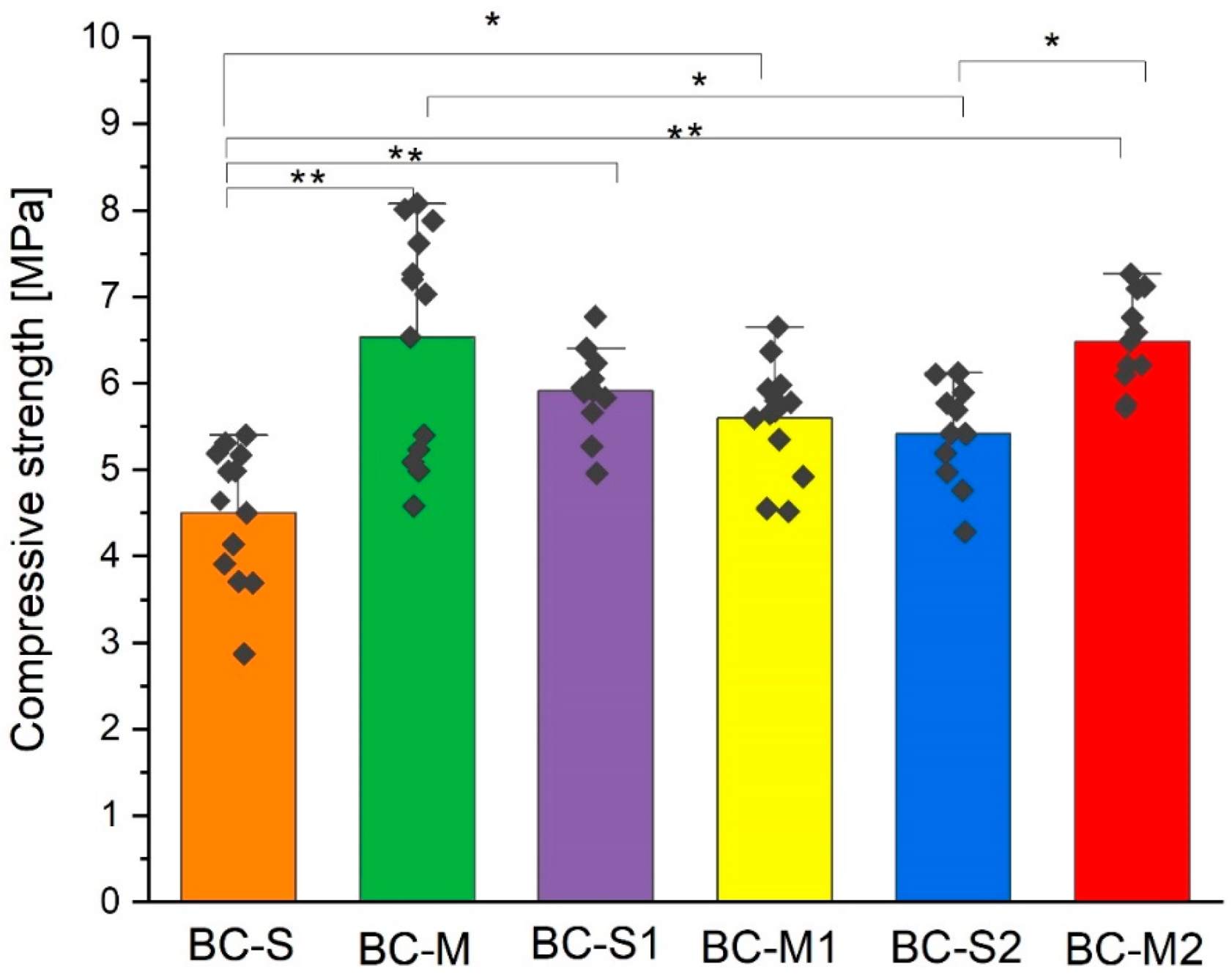
3.4. Compressive Strength
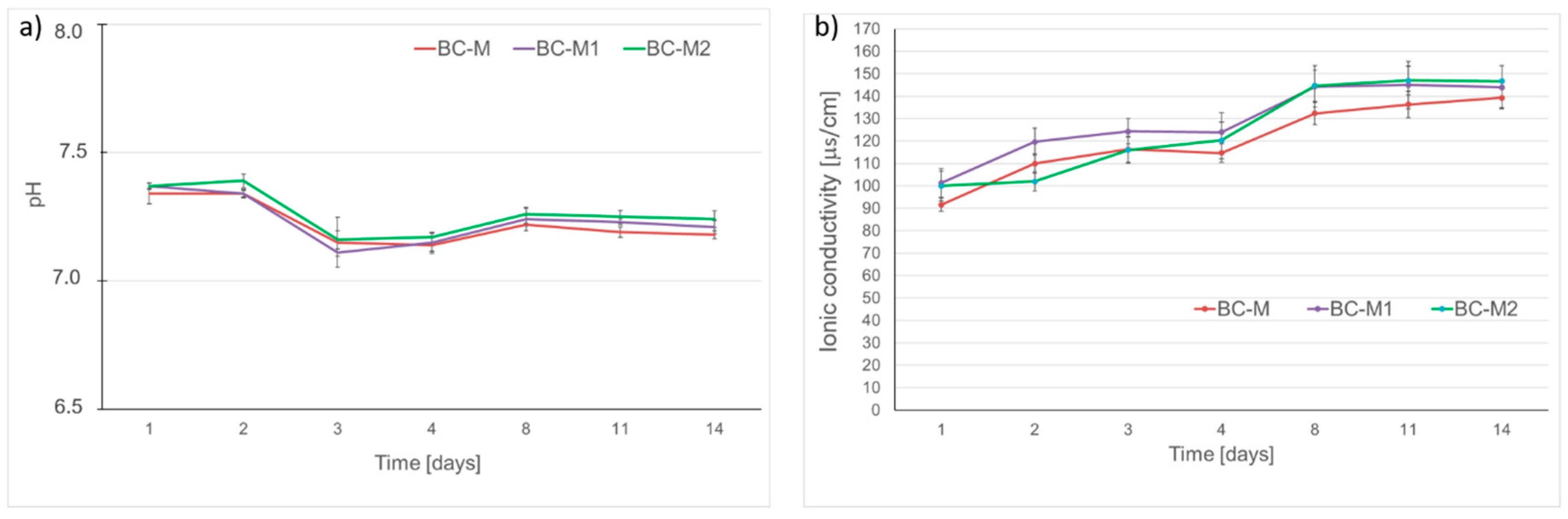
3.5. Chemical Stability and Bioactive Potential
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vezenkova, A.; Locs, J. Sudoku of porous, injectable calcium phosphate cements–Path to osteoinductivity. Bioact. Mater. 2022, 17, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Jia, S.J.; Hao, D.J. Advances in the modification of injectable calcium-phosphate-based bone cements for clinical application. Chin. Med. J. 2020, 133, 2610–2612. [Google Scholar] [CrossRef] [PubMed]
- Demir-Oğuz, Ö.; Boccaccini, A.R.; Loca, D. Injectable bone cements: What benefits the combination of calcium phosphates and bioactive glasses could bring? Bioact. Mater. 2023, 19, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yang, F.; Wolke, J.G.; Li, Y.; Jansen, J.A. Incorporation of biodegradable electrospun fibers into calcium phosphate cement for bone regeneration. Acta Biomater. 2010, 6, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Kucko, N.W.; de Lacerda Schickert, S.; Sobral Marques, T.; Herber, R.P.; Van den Beuken, J.J.; Zuo, Y.; Leeuwenburgh, S.C. Tough and osteocompatible calcium phosphate cements reinforced with poly (vinyl alcohol) fibers. ACS Biomater. Sci. Eng. 2019, 5, 2491–2505. [Google Scholar] [CrossRef]
- Paknahad, A.; Goudarzi, M.; Kucko, N.W.; Leeuwenburgh, S.C.; Sluys, L.J. Calcium phosphate cement reinforced with poly (vinyl alcohol) fibers: An experimental and numerical failure analysis. Acta Biomater. 2021, 119, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Weichhold, J.; Pfeiffle, M.; Kade, J.C.; Hurle, K.; Gbureck, U. Aqueous calcium phosphate cement inks for 3D printing. Adv. Eng. Mater. 2023, 25, 2300789. [Google Scholar] [CrossRef]
- Xiong, X.; Chen, Y.; Yuan, R.; Qiu, G.; Weir, M.D.; Xu, H.H.K.; Liu, J.; Ruan, J.; Chang, X.; Qu, S. 3D-printed mechanically strong calcium phosphate cement scaffold with metformin/stem cell-encapsulating alginate microbeads for bone tissue engineering. J. Bionic Eng. 2022, 19, 1658–1670. [Google Scholar] [CrossRef]
- Huang, S.M.; Chen, W.C.; Wu, C.C.; Liu, S.M.; Ko, C.L.; Chen, J.C.; Shih, C.J. Synergistic effect of drug/antibiotic-impregnated micro/nanohybrid mesoporous bioactive glass/calcium phosphate composite bone cement on antibacterial and osteoconductive activities. Biomater. Adv. 2023, 152, 213524. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Huang, M.; Liu, J.; Wang, P.; Schneider, A.; Ren, K.; Oates, T.W.; Weir, M.D.; Xu, H.H.K.; Zhao, L. Antibacterial calcium phosphate cement with human periodontal ligament stem cell-microbeads to enhance bone regeneration and combat infection. J. Tissue Eng. Regen. Med. 2021, 15, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Olhero, S.M.; Gheduzzi, S.; Miles, A.W.; Ferreira, J.M.F. Influence of setting liquid composition and liquid-to-powder ratio on properties of a Mg-substituted calcium phosphate cement. Acta Biomater. 2009, 5, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Monma, H.; Kanazawa, T. The hydration of α-tricalcium phosphate. J. Ceram. Assoc. 1976, 84, 209–213. [Google Scholar] [CrossRef]
- Tronco, M.C.; Cassel, J.B.; Dos Santos, L.A. α-TCP-based calcium phosphate cements: A critical review. Acta Biomater. 2022, 151, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Siek, D.; Ślósarczyk, A.; Przekora, A.; Belcarz, A.; Zima, A.; Ginalska, G.; Czechowska, J. Evaluation of antibacterial activity and cytocompatibility of α-TCP based bone cements with silver-doped hydroxyapatite and CaCO3. Ceram. Int. 2017, 43, 13997–14007. [Google Scholar] [CrossRef]
- Fernández, E.; Vlad, M.D.; Hamcerencu, M.; Darie, A.; Torres, R.; Lopez, J. Effect of iron on the setting properties of α-TCP bone cements. J. Mater. Sci. 2005, 40, 3677–3682. [Google Scholar] [CrossRef]
- Dziadek, M.; Zima, A.; Cichoń, E.; Czechowska, J.; Ślósarczyk, A. Biomicroconcretes based on the hybrid HAp/CTS granules, α-TCP and pectins as a novel injectable bone substitutes. Mater. Lett. 2020, 265, 127457. [Google Scholar] [CrossRef]
- Wu, S.; Lei, L.; Bao, C.; Liu, J.; Weir, M.D.; Ren, K.; Schneider, A.; Oates, T.W.; Liu, J.; Xu, H.H. An injectable and antibacterial calcium phosphate scaffold inhibiting Staphylococcus aureus and supporting stem cells for bone regeneration. Mater. Sci. Eng. C 2021, 120, 111688. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.H.; Chu, P.Y.; Huang, S.M.; Shih, B.S.; Ko, C.L.; Hu, J.J.; Chen, W.C. Injectability, processability, drug loading, and antibacterial activity of gentamicin-impregnated mesoporous bioactive glass composite calcium phosphate bone cement in vitro. Biomimetics 2022, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Nuzulia, N.A.; Mart, T.; Ahmed, I.; Sari, Y.W. The Use of Microspheres for Cancer Embolization Therapy: Recent Advancements and Prospective. ACS Biomater. Sci. Eng. 2024, 10, 637–656. [Google Scholar] [CrossRef]
- Kamitakahara, M.; Asahara, K.; Matsubara, H. Calcium phosphate cements comprising spherical porous calcium phosphate granules: Synthesis, structure, and properties. J. Asian Ceram. Soc. 2022, 10, 731–738. [Google Scholar] [CrossRef]
- Meng, D.; Dong, L.; Wen, Y.; Xie, Q. Effects of adding resorbable chitosan microspheres to calcium phosphate cements for bone regeneration. Mater. Sci. Eng. C 2015, 47, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Ye, J. Construction and properties of poly (lactic-co-glycolic acid)/calcium phosphate cement composite pellets with microspheres-in-pellet structure for bone repair. Ceram. Int. 2016, 42, 5587–5592. [Google Scholar] [CrossRef]
- Zima, A.; Czechowska, J.; Siek, D.; Olkowski, R.; Noga, M.; Lewandowska-Szumieł, M.; Ślósarczyk, A. How calcite and modified hydroxyapatite influence physicochemical properties and cytocompatibility of alpha-TCP based bone cements. J. Mater. Sci. Mater. Med. 2017, 28, 117. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.A.; Lee, G.S.; Park, J.H.; Kim, H.W. Osteoclastic cell behaviors affected by the α-tricalcium phosphate based bone cements. J. Mater. Sci. Mater. Med. 2010, 21, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Fosca, M.; Graziani, V.; Egorov, A.A.; Zobkov, Y.V.; Fedotov, A.Y.; Ortenzi, M.; Caminiti, R.; Baranchikov, A.E.; Komlev, V.S. Silver-doped calcium phosphate bone cements with antibacterial properties. J. Funct. Biomater. 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cummins, E. Hazard characterization of silver nanoparticles for human exposure routes. J. Environ. Sci. Health Part A 2020, 55, 704–725. [Google Scholar] [CrossRef] [PubMed]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B. A pharmacological and toxicological profile of silver as an antimicrobial agent in medical devices. Adv. Pharmacol. Pharm. Sci. 2010, 2010, 910686. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, J.A.; Webster, D.A.; Becker, R.O. O. Silver polymethyl methacrylate antibacterial bone cement. Clin. Orthop. Relat. Res. 1979, 143, 266–270. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Tang, Z.; Liu, H.; Zhang, R.; Ge, J.; Yang, H.; Ni, X.; Lin, X.; Yang, L. Study on injectable silver-incorporated calcium phosphate composite with enhanced antibacterial and biomechanical properties for fighting bone cement-associated infections. Colloids Surf. B Biointerfaces 2023, 227, 113382. [Google Scholar] [CrossRef]
- Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). PAC 2007, 79, 1801.
- Mir, S.H.; Nagahara, L.A.; Thundat, T.; Mokarian-Tabari, P.; Furukawa, H.; Khosla, A. Organic-inorganic hybrid functional materials: An integrated platform for applied technologies. J. Electrochem. Soc. 2018, 165, B3137–B3156. [Google Scholar] [CrossRef]
- Sanchez, C.; Ribot, F.; Lebeau, B. Molecular design of hybrid organic-inorganic nanocomposites synthesized via sol-gel chemistry. J. Mater. Chem. 1999, 9, 35–44. [Google Scholar] [CrossRef]
- Park, W.; Shin, H.; Choi, B.; Rhim, W.K.; Na, K.; Han, D.K. Advanced hybrid nanomaterials for biomedical applications. Prog. Mater. Sci. 2020, 114, 100686. [Google Scholar] [CrossRef]
- Zima, A. Hydroxyapatite-chitosan based bioactive hybrid biomaterials with improved mechanical strength. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 193, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Czechowska, J.; Cichoń, E.; Belcarz, A.; Ślósarczyk, A.; Zima, A. Effect of gold nanoparticles and silicon on the bioactivity and antibacterial properties of hydroxyapatite/chitosan/tricalcium phosphate-based biomicroconcretes. Materials 2021, 14, 3854. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.R.; de Souza Maguerroski Castilho, M.; de Lima Veeck, A.P.; da Rosa, C.G.; Noronha, C.M.; Maciel, M.V.O.B.; Barreto, P.M. Antioxidant and antimicrobial methylcellulose films containing Lippia alba extract and silver nanoparticles. Carbohydr. Polym. 2018, 192, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, L.; Fiorati, A.; D’Agostino, A.; Pelacani, C.M.; Chiesa, R.; Farè, S.; De Nardo, L. Smart methylcellulose hydrogels for pH-triggered delivery of silver nanoparticles. Gels 2022, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Doyle, P.S. Design and use of a thermogelling methylcellulose nanoemulsion to formulate nanocrystalline oral dosage forms. Adv. Mater. 2021, 33, 2008618. [Google Scholar] [CrossRef] [PubMed]
- Bhui, D.K.; Pyne, S.; Sarkar, P.; Bar, H.; Sahoo, G.P.; Misra, A. Temperature controlled synthesis of silver nanostructures of variable morphologies in aqueous methyl cellulose matrix. J. Mol. Liq. 2011, 158, 170–174. [Google Scholar] [CrossRef]
- Nishikawa, H.; Nakata, E.; Nakano, S.; Nakajima, T.; Morii, T. Influence of polymer molecular weight on the properties of in situ synthesized silver–methylcellulose nanocomposite films with a CO2 laser. J. Mater. Sci. 2020, 55, 2090–2100. [Google Scholar] [CrossRef]
- Czechowska, J. Self-assembling, hybrid hydroxyapatite-methylcellulose granules, modified with nano-silver. Mater. Lett. 2021, 300, 130156. [Google Scholar] [CrossRef]
- ASTM C266-21; Standard Test Method for Time Setting of Hydraulic-Cement Paste by Gillmore Needles in ASTM Annual Book of Standards. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- Kokubo, T.; Kim, H.M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Gil, F.J.; Ginebra, M.P.; Driessens, F.C.M.; Planell, J.A.; Best, S.M. Calcium phosphate bone cements for clinical applications. Part I: Solution chemistry. J. Mater. Sci. Mater. Med. 1999, 10, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Carrodeguas, R.G.; De Aza, S. α-Tricalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef] [PubMed]
- Hurle, K.; Neubauer, J.; Bohner, M.; Doebelin, N.; Goetz-Neunhoeffer, F. Effect of amorphous phases during the hydraulic conversion of α-TCP into calcium-deficient hydroxyapatite. Acta Biomater. 2014, 10, 3931–3941. [Google Scholar] [CrossRef] [PubMed]
- Pańtak, P.; Czechowska, J.P.; Zima, A. The influence of silane coupling agents on the properties of α-TCP-based ceramic bone substitutes for orthopaedic applications. RSC Adv. 2023, 13, 34020–34031. [Google Scholar] [CrossRef] [PubMed]
- Maity, D.; Mollick, M.M.R.; Mondal, D.; Bhowmick, B.; Bain, M.K.; Bankura, K.; Sarkar, J.; Acharya, K.; Chattopadhyay, D. Synthesis of methylcellulose–silver nanocomposite and investigation of mechanical and antimicrobial properties. Carbohydr. Polym. 2012, 90, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.L.; Vieira, J.G.; Barud, H.S.; Assunção, R.; Filho, G.R.; Ribeiro, S.J.L.; Messadeqq, Y. Synthesis and characterization of methylcellulose produced from bacterial cellulose under heterogeneous condition. J. Braz. Chem. Soc. 2015, 26, 1861–1870. [Google Scholar] [CrossRef]
- Rangelova, N.; Radev, L.; Nenkova, S.; Miranda Salvado, I.M.; Vas Fernandes, M.H.; Herzog, M. Methylcellulose/SiO2 hybrids: Sol-gel preparation characterization by XRD, FTIR and AFM. Cent. Eur. J. Chem. 2011, 9, 112–118. [Google Scholar] [CrossRef]
- Viera, R.G.; Rodrigues Filho, G.; de Assunção, R.M.; Meireles, C.D.S.; Vieira, J.G.; de Oliveira, G.S. Synthesis and characterization of methylcellulose from sugar cane bagasse cellulose. Carbohydr. Polym. 2007, 67, 182–189. [Google Scholar] [CrossRef]
- Nezafati, N.; Farokhi, M.; Heydari, M.; Hesaraki, S.; Nasab, N.A. In vitro bioactivity and cytocompatablity of an injectable calcium phosphate cement/silanated gelatin microsphere composite bone cement. Compos. Part B Eng. 2019, 175, 107146. [Google Scholar] [CrossRef]
- Misch, C.E.; Qu, Z.; Bidez, M.W. Mechanical properties of trabecular bone in the human mandible: Implications for dental implant treatment planning and surgical placement. J. Oral Maxillofac. Surg. 1999, 57, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.L.; Kim, B.; Padalhin, A.R.; Faruq, O.; Sultana, T.; Lee, B.T. In vitro and in vivo evaluation of bioglass microspheres incorporated brushite cement for bone regeneration. Mater. Sci. Eng. C 2019, 103, 109775. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Brauer, D.S.; Wilson, R.M.; Hill, R.G.; Hing, K.A. Influence of cell culture medium composition on in vitro dissolution behavior of a fluoride-containing bioactive glass. J. Biomed. Mater. Res. Part A 2014, 102, 647–654. [Google Scholar] [CrossRef]
- Zalewska, J.; Przekora, A.; Pałka, K.; Belcarz, A. Gypsum-related compensation of ions uptake by highly porous hydroxyapatite ceramics–Consequences for osteoblasts growth and proliferation. Biomater. Adv. 2022, 133, 112665. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, A.; Schamel, M.; Gbureck, U.; Gelinsky, M. Osteoclastic differentiation and resorption is modulated by bioactive metal ions Co2+, Cu2+ and Cr3+ incorporated into calcium phosphate bone cements. PLoS ONE 2017, 12, e0182109. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A.; Czechowska, J.; Pijocha, D.; Ślósarczyk, A.; Ginalska, G. Do novel cement-type biomaterials reveal ion reactivity that affects cell viability in vitro? Open Life Sci. 2014, 9, 277–289. [Google Scholar] [CrossRef]

| Material | Solid Phase * | Liquid Phase | L/P [g/g] | |
|---|---|---|---|---|
| Granules | Powder | |||
| BC-S | GH 300–400 µm | αTCP | 0.5 wt.% methylcellulose in 2.0 wt.% Na2HPO4 | 0.64 |
| BC-M | GH 400–600 µm | |||
| BC-S1 | AgGH-1 (0.1 wt.% Ag) 300–400 µm | αTCP | 0.5 wt.% methylcellulose in 2.0 wt.% Na2HPO4 | 0.64 |
| BC-M1 | AgGH-1 (0.1 wt.% Ag) 400–600 µm | |||
| BC- S2 | AgGH-2 (1.0 wt.% Ag) 300–400 µm | αTCP | 0.5 wt.% methylcellulose in 2.0 wt.% Na2HPO4 | 0.64 |
| BC-M2 | AgGH-2 (1.0 wt.% Ag) 400–600 µm | |||
| Control | -- | αTCP | 0.5 wt.% methylcellulose in 2.0 wt.% Na2HPO4 | 0.48 |
| Material | Hydroxyapatite [wt.%] | αTCP [wt.%] |
|---|---|---|
| BC-S | 73 ± 5 | 27 ± 5 |
| BC-M | 55 ± 4 | 45 ± 4 |
| BC-S1 | 89 ± 1 | 11 ± 1 |
| BC-M1 | 62 ± 4 | 38 ± 4 |
| BC- S2 | 92 ± 1 | 8 ± 1 |
| BC-M2 | 93 ± 1 | 7 ± 1 |
| Control | 40 ± 4 | 60 ± 4 |
| Cement | Initial ti [min] | Final tf [min] |
|---|---|---|
| BC-S | 8 ± 1 | 22 ± 2 |
| BC-M | 10 ± 1 | 24 ± 2 |
| BC-S1 | 13 ± 1 | 28 ± 2 |
| BC-M1 | 14 ± 1 | 29 ± 2 |
| BC-S2 | 12 ± 1 | 32 ± 2 |
| BC-M2 | 13 ± 1 | 30 ± 3 |
| Control | 4 ± 1 | 9 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czechowska, J.P.; Dorner-Reisel, A.; Zima, A. Hybrid Bone Substitute Containing Tricalcium Phosphate and Silver Modified Hydroxyapatite–Methylcellulose Granules. J. Funct. Biomater. 2024, 15, 196. https://doi.org/10.3390/jfb15070196
Czechowska JP, Dorner-Reisel A, Zima A. Hybrid Bone Substitute Containing Tricalcium Phosphate and Silver Modified Hydroxyapatite–Methylcellulose Granules. Journal of Functional Biomaterials. 2024; 15(7):196. https://doi.org/10.3390/jfb15070196
Chicago/Turabian StyleCzechowska, Joanna P., Annett Dorner-Reisel, and Aneta Zima. 2024. "Hybrid Bone Substitute Containing Tricalcium Phosphate and Silver Modified Hydroxyapatite–Methylcellulose Granules" Journal of Functional Biomaterials 15, no. 7: 196. https://doi.org/10.3390/jfb15070196






