Enhancement of Biocompatibility of High-Transparency Zirconia Abutments with Human Gingival Fibroblasts via Cold Atmospheric Plasma Treatment: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Zirconia Disks
2.2. Helium CAP Jet
2.3. Surface Roughness
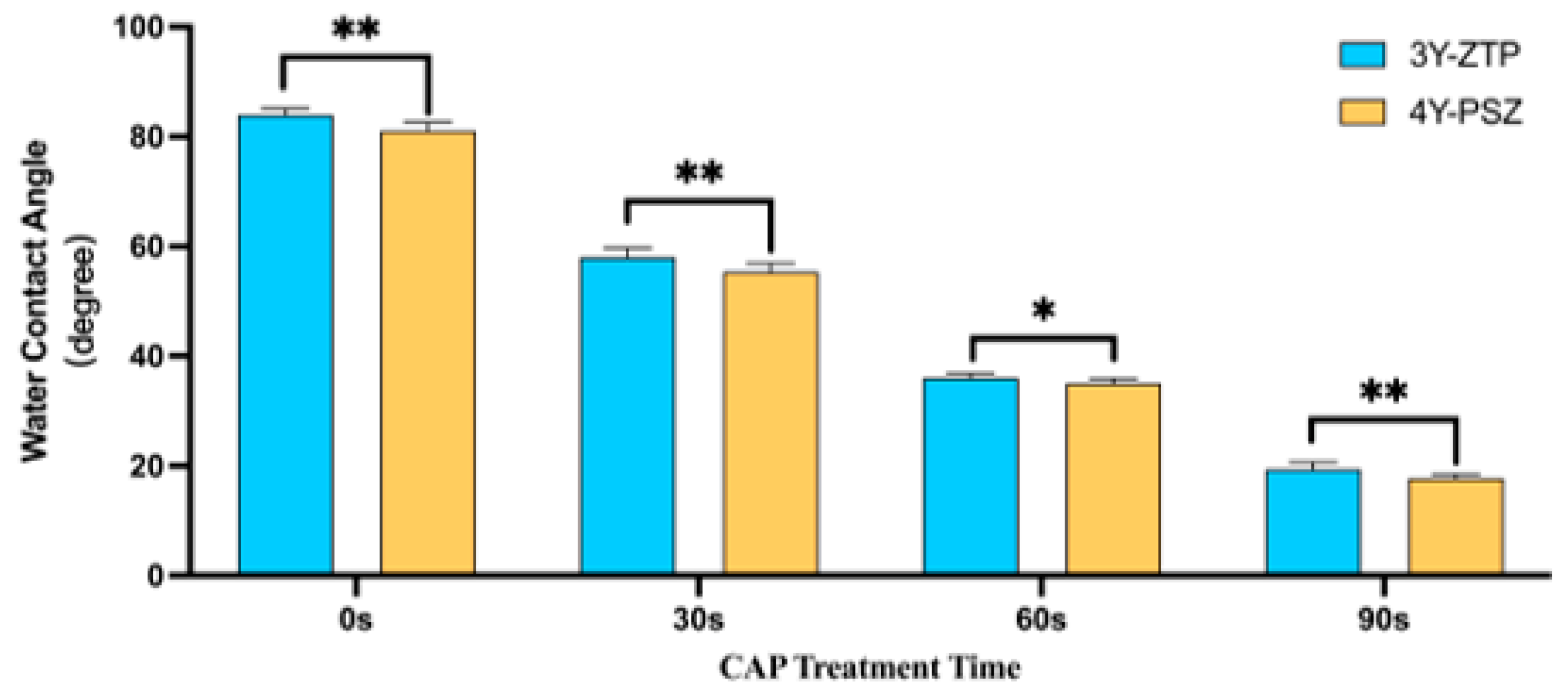
2.4. Surface Wettability
2.5. XPS Analysis
2.6. Cell Culture
2.7. Cell Adhesion and Proliferation Assays (CCK-8 Analysis)
2.8. Confocal Laser Scanning Microscopic Analysis
2.9. Gene Expression Analysis
2.10. Statistical Analysis
3. Results
3.1. Surface Characteristics
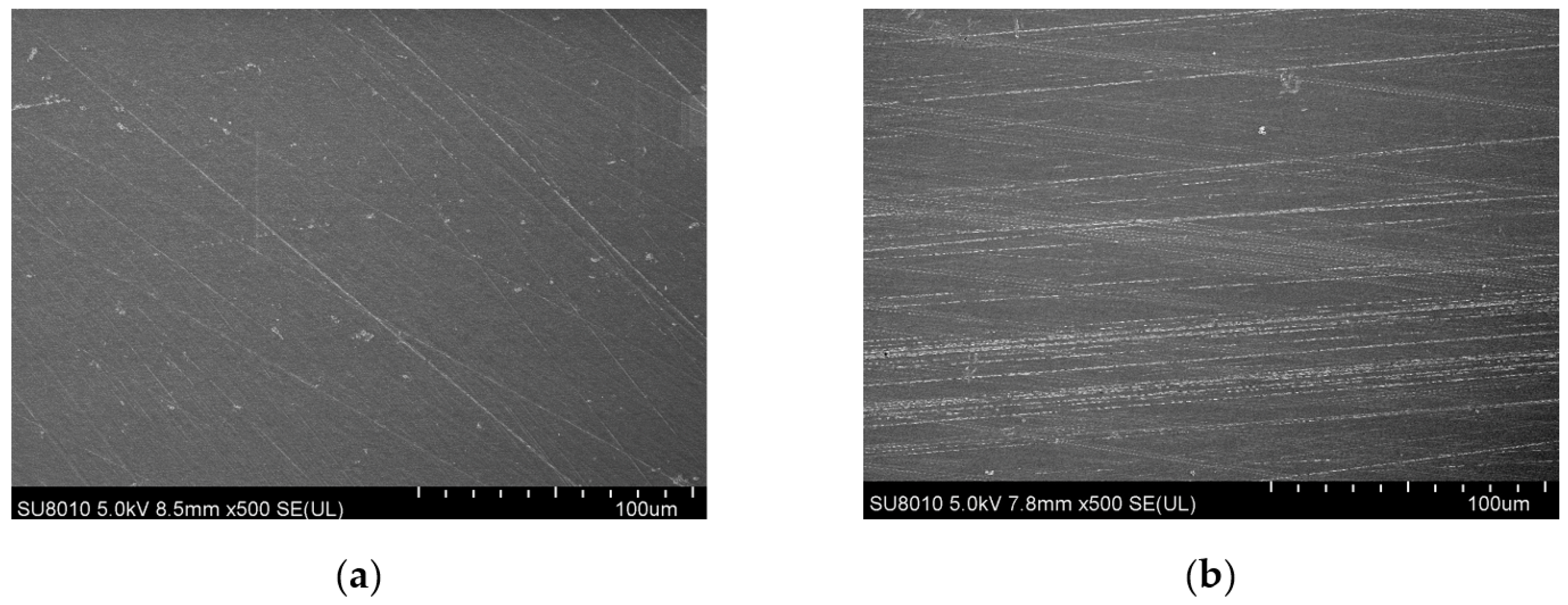
3.1.1. Surface Topography
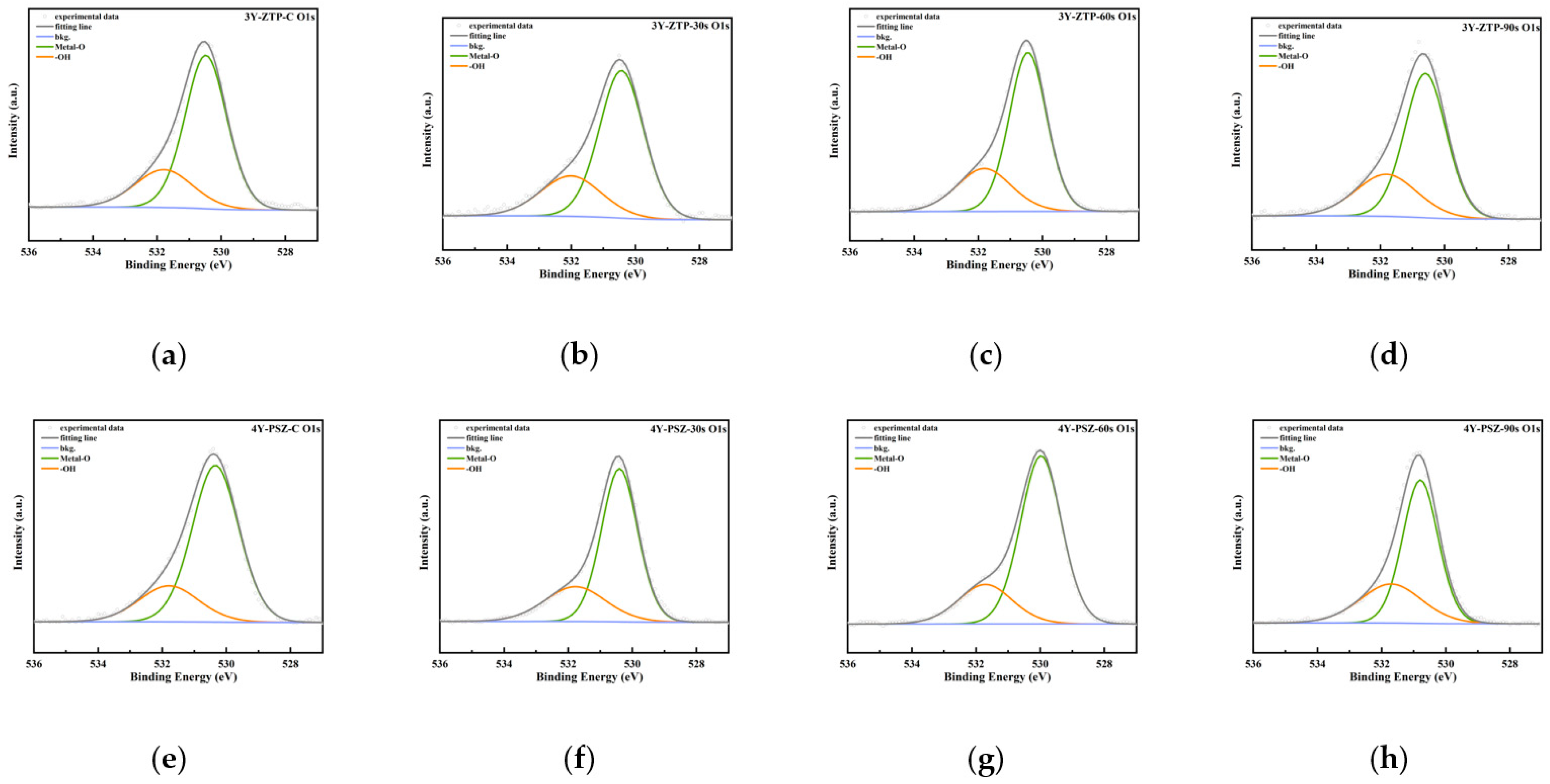
3.1.2. Surface Chemical Elemental Composition
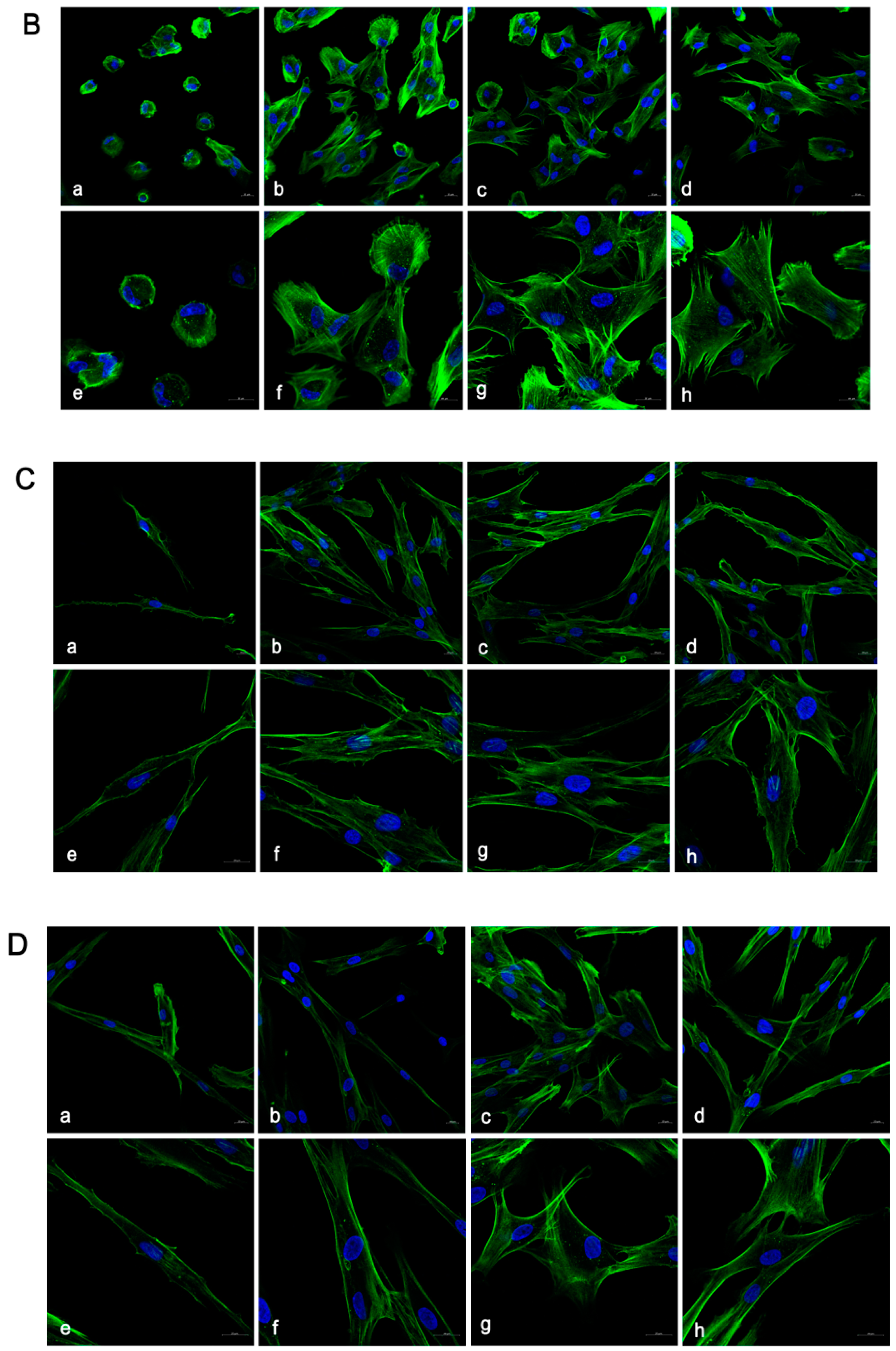
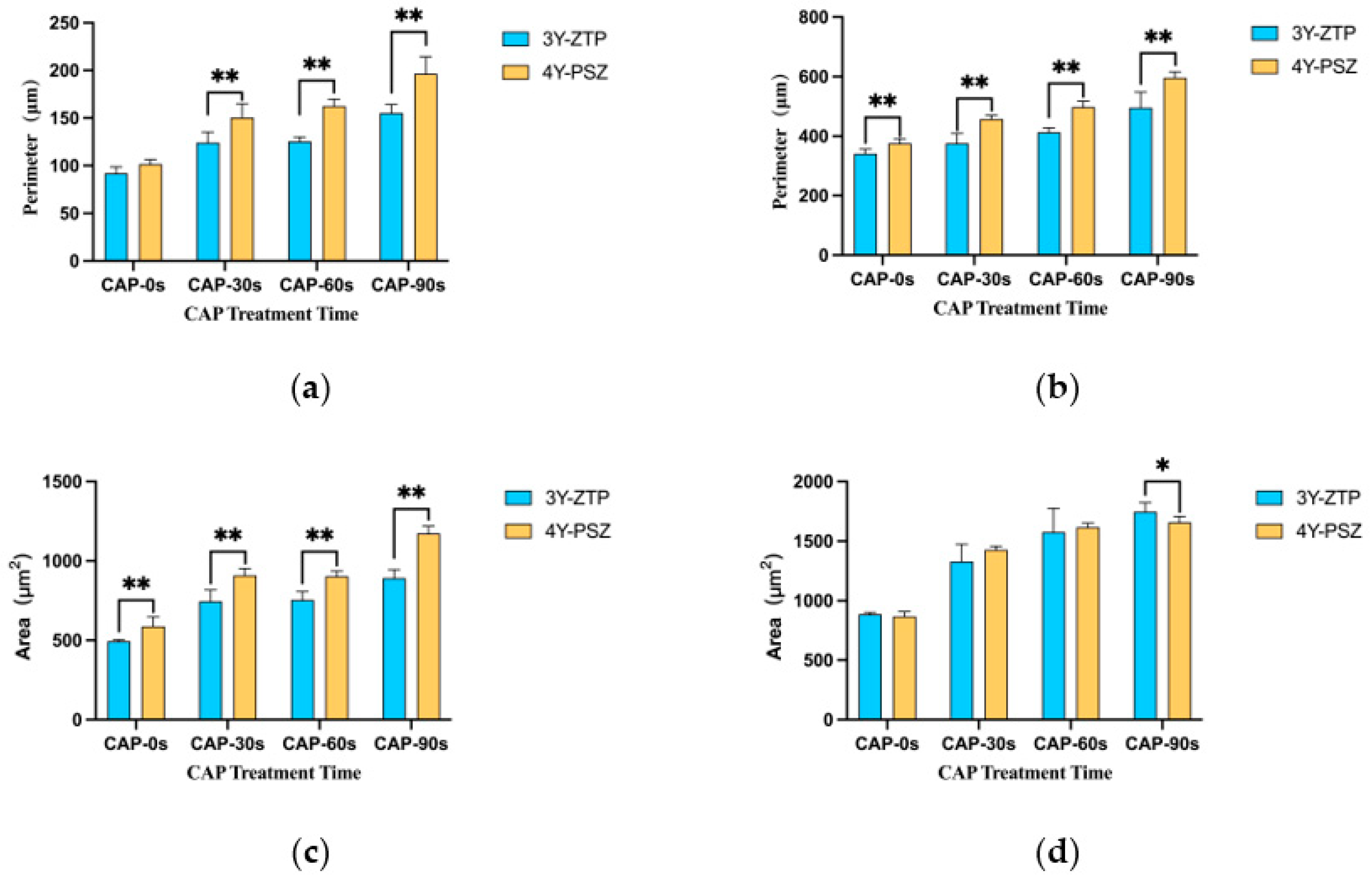
3.2. Cell Adhesion and Proliferation Morphology
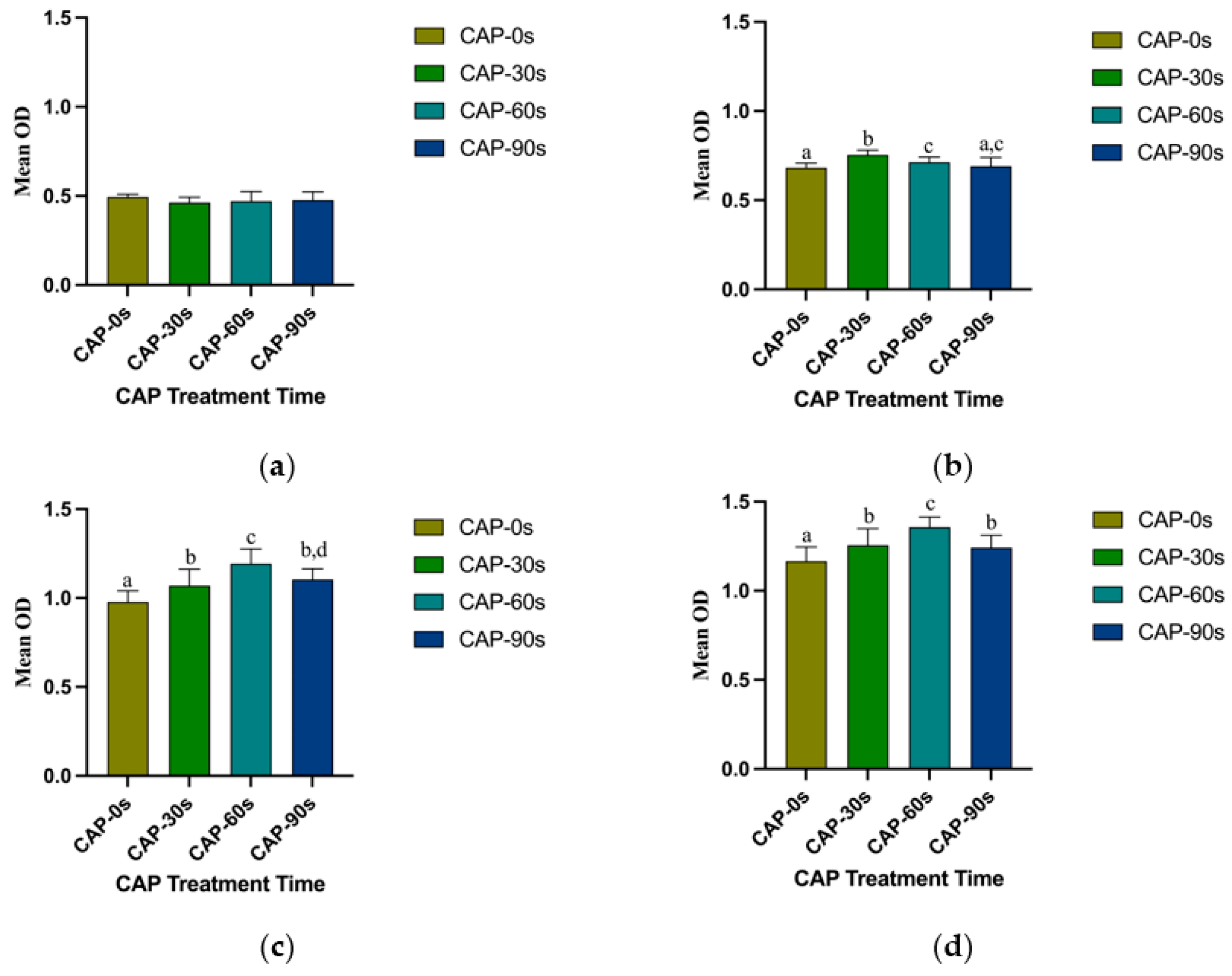
3.3. Cell Adhesion and Proliferation Ability
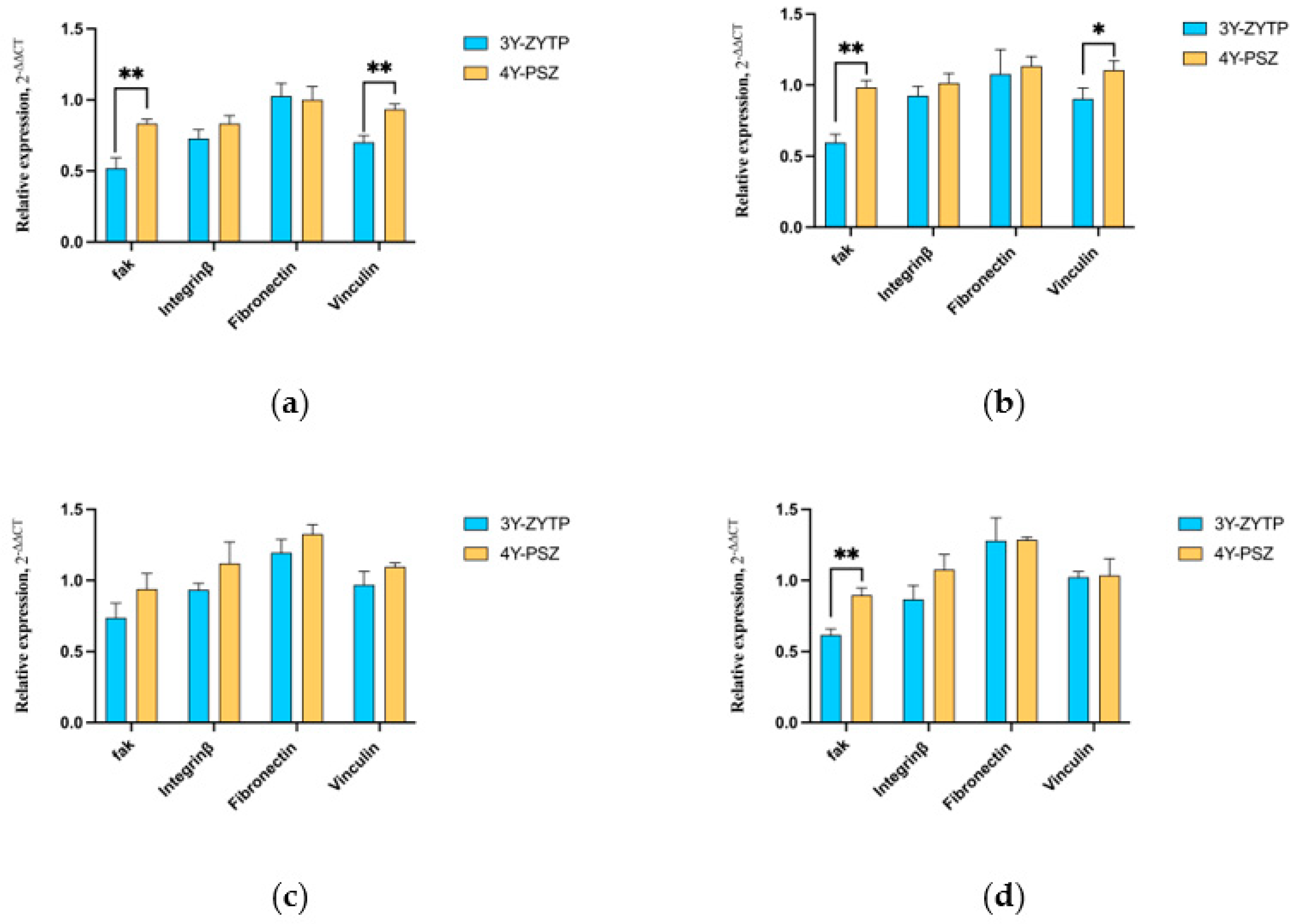
3.4. Expression of Adhesion and Proliferation-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buser, D.; Janner, S.F.; Wittneben, J.G.; Bragger, U.; Ramseier, C.A.; Salvi, G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant. Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef]
- Romandini, M.; Lima, C.; Pedrinaci, I.; Araoz, A.; Soldini, M.C.; Sanz, M. Prevalence and risk/protective indicators of peri-implant diseases: A university-representative cross-sectional study. Clin. Oral Implant. Res. 2021, 32, 112–122. [Google Scholar] [CrossRef]
- Palombo, D.; Rahmati, M.; Vignoletti, F.; Sanz-Esporrin, J.; Haugen, H.J.; Sanz, M. Hard and soft tissue healing around implants with a modified implant neck configuration: An experimental in vivo preclinical investigation. Clin. Oral Implant. Res. 2021, 32, 1127–1141. [Google Scholar] [CrossRef]
- Borie, M.; Lecloux, G.; Bosshardt, D.; Barrantes, A.; Haugen, H.J.; Lambert, F.; Bacevic, M. Peri-implant soft tissue integration in humans—Influence of materials: A study protocol for a randomised controlled trial and a pilot study results. Contemp. Clin. Trials Commun. 2020, 19, 100643. [Google Scholar] [CrossRef]
- Chai, W.L.; Moharamzadeh, K.; Brook, I.M.; Van Noort, R. A review of histomorphometric analysis techniques for assessing implant-soft tissue interface. Biotech. Histochem. 2011, 86, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Dib-Zaitum, I.; Guadilla-Gonzalez, Y.; Flores-Fraile, J.; Dib-Zakkour, J.; Benito-Garzon, L.; Montero, J. Effect Morphology and Surface Treatment of the Abutments of Dental Implants on the Dimension and Health of Peri-Implant Biological Space. Materials 2022, 15, 4422. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Campbell, S.D.; Viana, M.A.; Knoernschild, K.L. Abutment Material Effect on Peri-implant Soft Tissue Color and Perceived Esthetics. J. Prosthodont. 2016, 25, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Lops, D.; Stellini, E.; Sbricoli, L.; Cea, N.; Romeo, E.; Bressan, E. Influence of abutment material on peri-implant soft tissues in anterior areas with thin gingival biotype: A multicentric prospective study. Clin. Oral Implant. Res. 2017, 28, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Vazouras, K.; Gholami, H.; Margvelashvili-Malament, M.; Kim, Y.J.; Finkelman, M.; Weber, H.P. An Esthetic Evaluation of Different Abutment Materials in the Anterior Maxilla: A Randomized Controlled Clinical Trial Using a Crossover Design. J. Prosthodont. 2022, 31, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Alqutaibi, A.Y.; Ghulam, O.; Krsoum, M.; Binmahmoud, S.; Taher, H.; Elmalky, W.; Zafar, M.S. Revolution of Current Dental Zirconia: A Comprehensive Review. Molecules 2022, 27, 1699. [Google Scholar] [CrossRef]
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Moussi, J.; Drury, J.L.; Wataha, J.C. Zirconia in biomedical applications. Expert. Rev. Med. Devices 2016, 13, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Ryu, J.S.; Shimono, M.; Lee, K.W.; Lee, J.M.; Jung, H.S. Differential Healing Patterns of Mucosal Seal on Zirconia and Titanium Implant. Front. Physiol. 2019, 10, 796. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.V.; Reche, A.; Paul, P.; Agarwal, S. Zirconia Facts and Perspectives for Biomaterials in Dental Implantology. Cureus 2023, 15, e46828. [Google Scholar] [CrossRef] [PubMed]
- Zarone, F.; Di Mauro, M.I.; Ausiello, P.; Ruggiero, G.; Sorrentino, R. Current status on lithium disilicate and zirconia: A narrative review. BMC Oral Health 2019, 19, 134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Inokoshi, M.; Batuk, M.; Hadermann, J.; Naert, I.; Van Meerbeek, B.; Vleugels, J. Strength, toughness and aging stability of highly-translucent Y-TZP ceramics for dental restorations. Dent. Mater. 2016, 32, e327–e337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lawn, B.R. Novel Zirconia Materials in Dentistry. J. Dent. Res. 2018, 97, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Luo, M.L.; Zhou, W.; Niu, L.N.; Chen, J.H.; Wang, F. The integration of peri-implant soft tissues around zirconia abutments: Challenges and strategies. Bioact. Mater. 2023, 27, 348–361. [Google Scholar] [CrossRef]
- Akiyama, Y.; Iwasa, F.; Hotta, Y.; Matsumoto, T.; Oshima, Y.; Baba, K. Effects of surface roughness of ceria-stabilized zirconia/alumina nanocomposite on the morphology and function of human gingival fibroblasts. Dent. Mater. J. 2021, 40, 472–480. [Google Scholar] [CrossRef]
- Rohr, N.; Zeller, B.; Matthisson, L.; Fischer, J. Surface structuring of zirconia to increase fibroblast viability. Dent. Mater. 2020, 36, 779–786. [Google Scholar] [CrossRef]
- Liang, Y.; Leng, Y.; Zhang, J. Influence of clinical zirconia surface treatments on microscopic characteristics and adhesion-proliferation behavior of human gingival fibroblasts. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101564. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, Y.; Hirota, M.; Hayakawa, T.; Ohkubo, C. Surrounding Tissue Response to Surface-Treated Zirconia Implants. Materials 2019, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Kobune, K.; Miura, T.; Sato, T.; Yotsuya, M.; Yoshinari, M. Influence of plasma and ultraviolet treatment of zirconia on initial attachment of human oral keratinocytes: Expressions of laminin gamma2 and integrin beta4. Dent. Mater. J. 2014, 33, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, T.; Matsuura, T.; Komatsu, K.; Sugita, Y.; Maeda, H.; Ogawa, T. Vacuum Ultraviolet (VUV) Light Photofunctionalization to Induce Human Oral Fibroblast Transmigration on Zirconia. Cells 2023, 12, 2542. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.B.D.; Marques, J.F.; Fernandes, B.F.; Costa, M.; Miranda, G.; Mata, A.; Carames, J.M.M.; Silva, F.S. Gingival fibroblasts behavior on bioactive zirconia and titanium dental implant surfaces produced by a functionally graded technique. J. Appl. Oral Sci. 2020, 28, e20200100. [Google Scholar] [CrossRef] [PubMed]
- Kalyoncuoglu, U.T.; Yilmaz, B.; Koc, S.G.; Evis, Z.; Arpaci, P.U.; Kansu, G. Investigation of surface structure and biocompatibility of chitosan-coated zirconia and alumina dental abutments. Clin. Implant. Dent. Relat. Res. 2018, 20, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Smeets, R.; Kluwe, L.; Hartjen, P.; Barbeck, M.; Cacaci, C.; Gosau, M.; Henningsen, A. Cytocompatibility of Titanium, Zirconia and Modified PEEK after Surface Treatment Using UV Light or Non-Thermal Plasma. Int. J. Mol. Sci. 2019, 20, 5596. [Google Scholar] [CrossRef]
- Canullo, L.; Genova, T.; Tallarico, M.; Gautier, G.; Mussano, F.; Botticelli, D. Plasma of Argon Affects the Earliest Biological Response of Different Implant Surfaces: An In Vitro Comparative Study. J. Dent. Res. 2016, 95, 566–573. [Google Scholar] [CrossRef]
- Bernhardt, T.; Semmler, M.L.; Schafer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxid. Med. Cell Longev. 2019, 2019, 3873928. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef]
- Moszczyńska, J.; Roszek, K.; Wiśniewski, M. Non-Thermal Plasma Application in Medicine-Focus on Reactive Species Involvement. Int. J. Mol. Sci. 2023, 24, 12667. [Google Scholar] [CrossRef] [PubMed]
- Von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.D. Plasma Medicine: A Field of Applied Redox Biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Han, I.; Choi, E.H. The role of non-thermal atmospheric pressure biocompatible plasma in the differentiation of osteoblastic precursor cells, MC3T3-E1. Oncotarget 2017, 8, 36399–36409. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Yang, Y.; Liu, X.Q.; Liu, M.Y.; Zhang, X.F.; Wang, X.; Li, H.P.; Tan, J.G. Enhanced Biological Behavior of In Vitro Human Gingival Fibroblasts on Cold Plasma-Treated Zirconia. PLoS ONE 2015, 10, e0140278. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, M.; Yang, Y.; Li, J.; Su, Y.F.; Li, H.P.; Tan, J.G. Inhibition of bacterial growth on zirconia abutment with a helium cold atmospheric plasma jet treatment. Clin. Oral Investig. 2020, 24, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, M.; Jia, Y.N.; Li, J.; Li, H.P.; Tan, J.G. Time-dependent reactive oxygen species inhibit Streptococcus mutans growth on zirconia after a helium cold atmospheric plasma treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111633. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Wang, C.; Wang, X.; Wang, Y.; Wang, N.; Chou, J.; Li, T.; Zhang, Z.; Ling, Y.; Chen, S. Effects of hydrogenated TiO2 nanotube arrays on protein adsorption and compatibility with osteoblast-like cells. Int. J. Nanomed. 2018, 13, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Rausch-fan, X.; Wieland, M.; Matejka, M.; Andrukhov, O.; Schedle, A. Initial attachment, subsequent cell proliferation/viability and gene expression of epithelial cells related to attachment and wound healing in response to different titanium surfaces. Dent. Mater. 2012, 28, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Matter, M.T.; Maliqi, L.; Keevend, K.; Guimond, S.; Ng, J.; Armagan, E.; Rottmar, M.; Herrmann, I.K. One-Step Synthesis of Versatile Antimicrobial Nano-Architected Implant Coatings for Hard and Soft Tissue Healing. ACS Appl. Mater. Interfaces 2021, 13, 33300–33310. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Lu, R.; Gao, S.; Ling, Y.; Chen, S. Responses of human gingival fibroblasts to superhydrophilic hydrogenated titanium dioxide nanotubes. Colloids Surf. B Biointerfaces 2021, 198, 111489. [Google Scholar] [CrossRef]
- Komatsu, K.; Matsuura, T.; Ogawa, T. Achieving Complete Human Gingival Fibroblast Collagen Coverage on Implant Abutments through Vacuum Ultraviolet (VUV) Photofunctionalization. Int. J. Oral Maxillofac. Implant. 2024, 0, 1–32. [Google Scholar] [CrossRef]
- Schwarz, F.; Ferrari, D.; Herten, M.; Mihatovic, I.; Wieland, M.; Sager, M.; Becker, J. Effects of surface hydrophilicity and microtopography on early stages of soft and hard tissue integration at non-submerged titanium implants: An immunohistochemical study in dogs. J. Periodontol. 2007, 78, 2171–2184. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L.; Obrecht, M.; Weber, K.; Dard, M.; Bosshardt, D.; Higginbottom, F.L.; Wilson, T.G., Jr.; Jones, A.A. Biologic width adjacent to loaded implants with machined and rough collars in the dog. Int. J. Periodontics Restor. Dent. 2014, 34, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Esfahanizadeh, N.; Motalebi, S.; Daneshparvar, N.; Akhoundi, N.; Bonakdar, S. Morphology, proliferation, and gene expression of gingival fibroblasts on Laser-Lok, titanium, and zirconia surfaces. Lasers Med. Sci. 2016, 31, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Schunemann, F.H.; Galarraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.M.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Chopra, D.; Guo, T.; Gulati, K.; Ivanovski, S. Load, unload and repeat: Understanding the mechanical characteristics of zirconia in dentistry. Dent. Mater. 2024, 40, e1–e17. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Sridhar, S.; Aghyarian, S.; Watkins-Curry, P.; Chan, J.Y.; Pozzi, A.; Rodrigues, D.C. Corrosion behavior of zirconia in acidulated phosphate fluoride. J. Appl. Oral Sci. 2016, 24, 52–60. [Google Scholar] [CrossRef]
- Manicone, P.F.; Rossi Iommetti, P.; Raffaelli, L. An overview of zirconia ceramics: Basic properties and clinical applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, M.; Yang, Y.; Zheng, M.; Yang, Y.; Liu, X.; Tan, J. Biofunctionalization of zirconia with cell-adhesion peptides via polydopamine crosslinking for soft tissue engineering: Effects on the biological behaviors of human gingival fibroblasts and oral bacteria. RSC Adv. 2020, 10, 6200–6212. [Google Scholar] [CrossRef]
- Razali, M.; Ngeow, W.C.; Omar, R.A.; Chai, W.L. An In-Vitro Analysis of Peri-Implant Mucosal Seal Following Photofunctionalization of Zirconia Abutment Materials. Biomedicines 2021, 9, 78. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, M.; Liao, Y.; Zhou, J.; Li, H.; Tan, J. Different behavior of human gingival fibroblasts on surface modified zirconia: A comparison between ultraviolet (UV) light and plasma. Dent. Mater. J. 2019, 38, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Hartjen, P.; Gosau, M.; Vollkommer, T.; Grust, A.L.C.; Fuest, S.; Kluwe, L.; Burg, S.; Smeets, R.; Henningsen, A. Effects of a Novel Cold Atmospheric Plasma Treatment of Titanium on the Proliferation and Adhesion Behavior of Fibroblasts. Int. J. Mol. Sci. 2021, 23, 420. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Rakic, M.; Corvino, E.; Burton, M.; Krumbeck, J.A.; Chittoor Prem, A.; Ravida, A.; Ignjatovic, N.; Sculean, A.; Menini, M.; et al. Effect of argon plasma pre-treatment of healing abutments on peri-implant microbiome and soft tissue integration: A proof-of-concept randomized study. BMC Oral. Health 2023, 23, 27. [Google Scholar] [CrossRef]
- Wagner, G.; Eggers, B.; Duddeck, D.; Kramer, F.J.; Bourauel, C.; Jepsen, S.; Deschner, J.; Nokhbehsaim, M. Influence of cold atmospheric plasma on dental implant materials—An in vitro analysis. Clin. Oral Investig. 2022, 26, 2949–2963. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zou, Z.; Smeets, R.; Kluwe, L.; Hartjen, P.; Cacaci, C.; Gosau, M.; Henningsen, A. Time Dependency of Non-Thermal Oxygen Plasma and Ultraviolet Irradiation on Cellular Attachment and mRNA Expression of Growth Factors in Osteoblasts on Titanium and Zirconia Surfaces. Int. J. Mol. Sci. 2020, 21, 8598. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Gorbanev, Y.; De Backer, J.; Van Loenhout, J.; Van Boxem, W.; Lemiere, F.; Cos, P.; Dewilde, S.; Smits, E.; Bogaerts, A. Non-Thermal Plasma as a Unique Delivery System of Short-Lived Reactive Oxygen and Nitrogen Species for Immunogenic Cell Death in Melanoma Cells. Adv. Sci. 2019, 6, 1802062. [Google Scholar] [CrossRef] [PubMed]
- Susin, C.; Fiorini, T.; Lee, J.; De Stefano, J.A.; Dickinson, D.P.; Wikesjo, U.M. Wound healing following surgical and regenerative periodontal therapy. Periodontology 2000 2015, 68, 83–98. [Google Scholar] [CrossRef]
- He, Y.; Gong, F.; Jin, T.; Liu, Q.; Fang, H.; Chen, Y.; Wang, G.; Chu, P.K.; Wu, Z.; Ostrikov, K.K. Dose-Dependent Effects in Plasma Oncotherapy: Critical In Vivo Immune Responses Missed by In Vitro Studies. Biomolecules 2023, 13, 707. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Park, D.; Choi, E.H. Altered antioxidant system stimulates dielectric barrier discharge plasma-induced cell death for solid tumor cell treatment. PLoS ONE 2014, 9, e103349. [Google Scholar] [CrossRef]
- Kaushik, N.; Uddin, N.; Sim, G.B.; Hong, Y.J.; Baik, K.Y.; Kim, C.H.; Lee, S.J.; Kaushik, N.K.; Choi, E.H. Responses of solid tumor cells in DMEM to reactive oxygen species generated by non-thermal plasma and chemically induced ROS systems. Sci. Rep. 2015, 5, 8587. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, P.; Yan, L.; Chen, X.; Wang, Z.; Liu, Q. Mechanism of non-thermal atmospheric plasma in anti-tumor: Influencing intracellular RONS and regulating signaling pathways. Free Radic. Res. 2024, 58, 333–353. [Google Scholar] [CrossRef] [PubMed]
- Keidar, M.; Yan, D.; Beilis, I.I.; Trink, B.; Sherman, J.H. Plasmas for Treating Cancer: Opportunities for Adaptive and Self-Adaptive Approaches. Trends Biotechnol. 2018, 36, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Adamovich, I.; Baalrud, S.D.; Bogaerts, A.; Bruggeman, P.J.; Cappelli, M.; Colombo, V.; Czarnetzki, U.; Ebert, U.; Eden, J.G.; Favia, P.; et al. The 2017 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2017, 50, 323001. [Google Scholar] [CrossRef]




| Gene | Sequences (5’—3’) | |
|---|---|---|
| Forward | Reverse | |
| Focal adhesion kinase | CTCCTA CTGCCAACCTGGAC | GCCGACTTCCTTCACCATAG |
| Integrin β3 | CAAAGGAACAGCAGAGAAGC | ATTGAGTAAGACAGGTCCATAA |
| Fibronectin | CGGAGAGACAGGAGGAAATAGCC | TTGCTGCTTGCGGGGCTGTC |
| Vinculin | CGAATCCCAACCATAAGCAC | CGCACAGTCTCCTTCACAGA |
| GAPDH | AAGGTCATCCCTGAGCTGAAC | ACGCCTGCTTCACCACCTTCT |
| Group | CAP-0 s | CAP-30 s | CAP-60 s | CAP-90 s | |
|---|---|---|---|---|---|
| 3Y-ZTP | Image |  |  |  |  |
| Water contact angle | 83.99 ± 1.22 | 58.00 ± 1.69 | 36.00 ± 0.85 | 19.37 ± 1.36 | |
| 4Y-PSZ | Image |  |  |  |  |
| Water contact angle | 81.04 ± 1.55 | 55.31 ± 1.65 | 35.04 ± 0.84 | 17.70 ± 0.78 |
| C (%) | O (%) | C/O Radio | ||
|---|---|---|---|---|
| CAP-0 s | 3Y-ZTP | 44.00 | 35.92 | 1.22 |
| 4Y-PSZ | 36.78 | 42.63 | 0.86 | |
| CAP-30 s | 3Y-ZTP | 36.20 | 43.15 | 0.84 |
| 4Y-PSZ | 38.65 | 49.23 | 0.79 | |
| CAP-60 s | 3Y-ZTP | 16.25 | 55.87 | 0.29 |
| 4Y-PSZ | 11.89 | 57.8 | 0.21 | |
| CAP-90 s | 3Y-ZTP | 17.19 | 57.04 | 0.30 |
| 4Y-PSZ | 13.07 | 56.97 | 0.23 |
| OH (%) | ||
|---|---|---|
| CAP-0 s | 3Y-ZTP | 22.72 |
| 4Y-PSZ | 25.36 | |
| CAP-30 s | 3Y-ZTP | 27.25 |
| 4Y-PSZ | 27.75 | |
| CAP-60 s | 3Y-ZTP | 28.30 |
| 4Y-PSZ | 28.71 | |
| CAP-90 s | 3Y-ZTP | 30.15 |
| 4Y-PSZ | 32.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Ma, X.; Tan, J.; Zhao, H.; Yang, Y.; Ye, X.; Liu, M.; Li, H. Enhancement of Biocompatibility of High-Transparency Zirconia Abutments with Human Gingival Fibroblasts via Cold Atmospheric Plasma Treatment: An In Vitro Study. J. Funct. Biomater. 2024, 15, 200. https://doi.org/10.3390/jfb15070200
Zheng M, Ma X, Tan J, Zhao H, Yang Y, Ye X, Liu M, Li H. Enhancement of Biocompatibility of High-Transparency Zirconia Abutments with Human Gingival Fibroblasts via Cold Atmospheric Plasma Treatment: An In Vitro Study. Journal of Functional Biomaterials. 2024; 15(7):200. https://doi.org/10.3390/jfb15070200
Chicago/Turabian StyleZheng, Miao, Xinrong Ma, Jianguo Tan, Hengxin Zhao, Yang Yang, Xinyi Ye, Mingyue Liu, and Heping Li. 2024. "Enhancement of Biocompatibility of High-Transparency Zirconia Abutments with Human Gingival Fibroblasts via Cold Atmospheric Plasma Treatment: An In Vitro Study" Journal of Functional Biomaterials 15, no. 7: 200. https://doi.org/10.3390/jfb15070200






