Osteoproductivity of Injectable Bone Grafts with and without Ostrich Eggshell Membrane Protein in Rabbit Femur
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Preparation of the OE Particles and OE Membrane Protein
2.3. Experimental Animals
2.4. Study Design
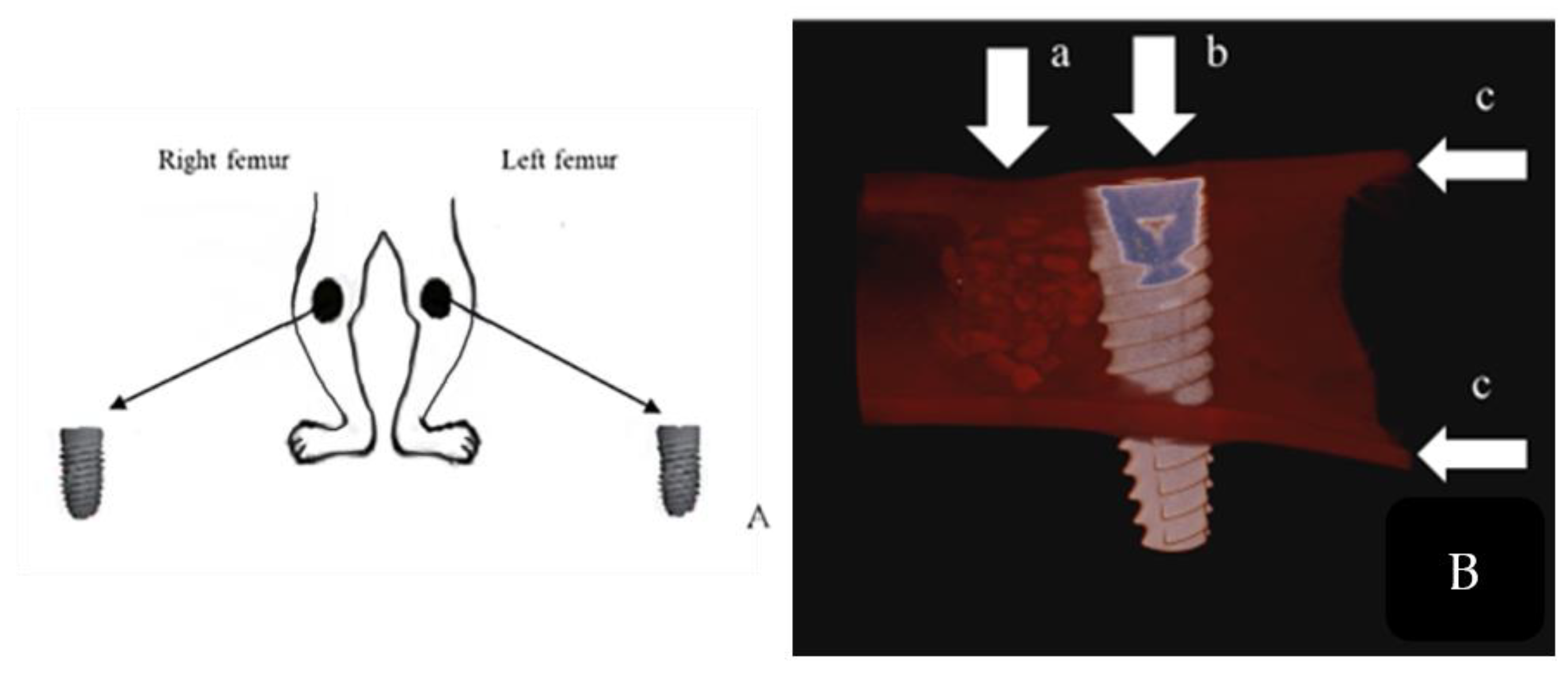
2.5. Surgical Procedure
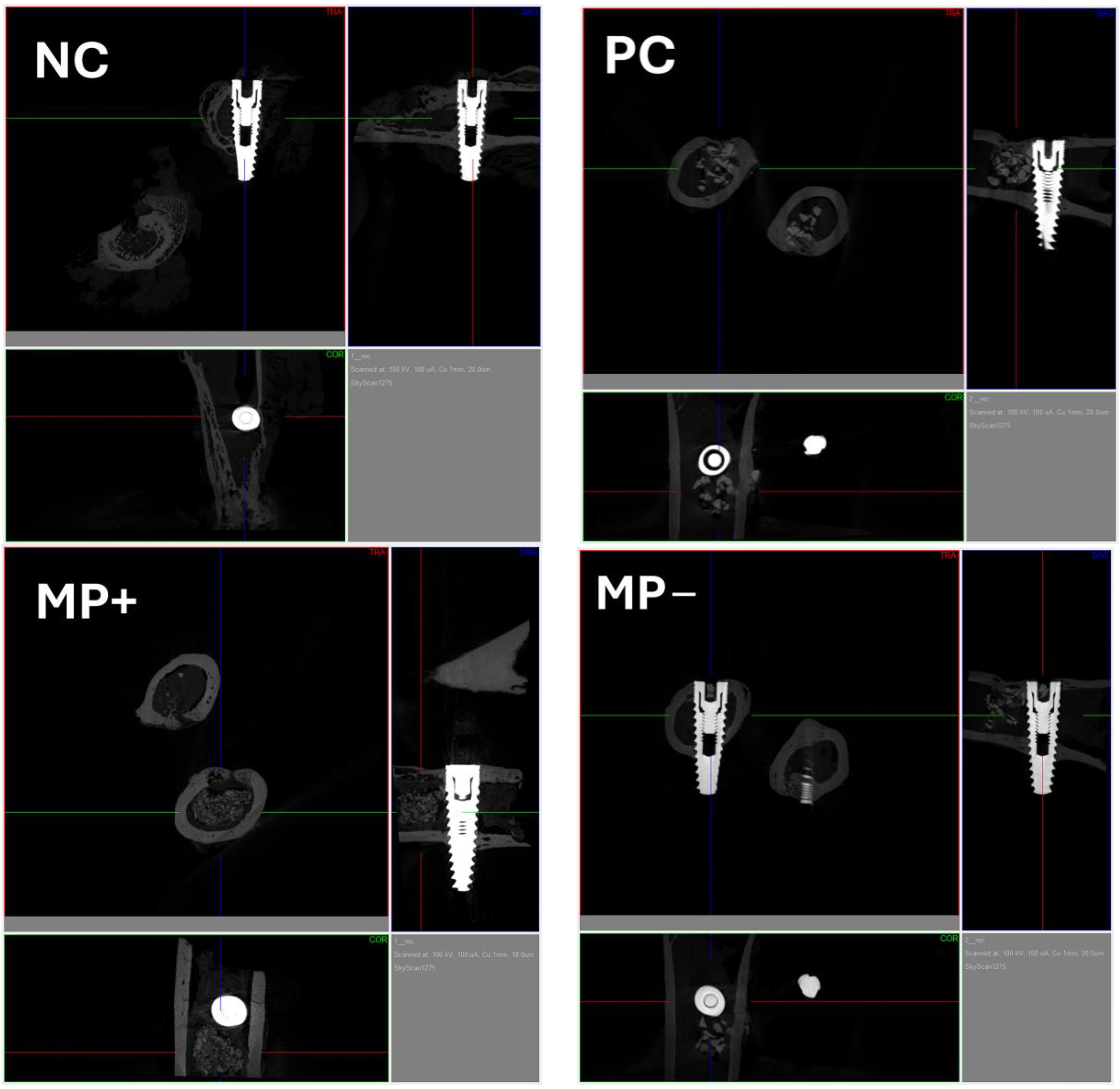
2.6. Micro-Computerized Tomographic (µ-CT) Evaluation
2.7. Histological Investigations and Histomorphometry
2.8. Statistical Analysis
3. Results
3.1. Clinical Results
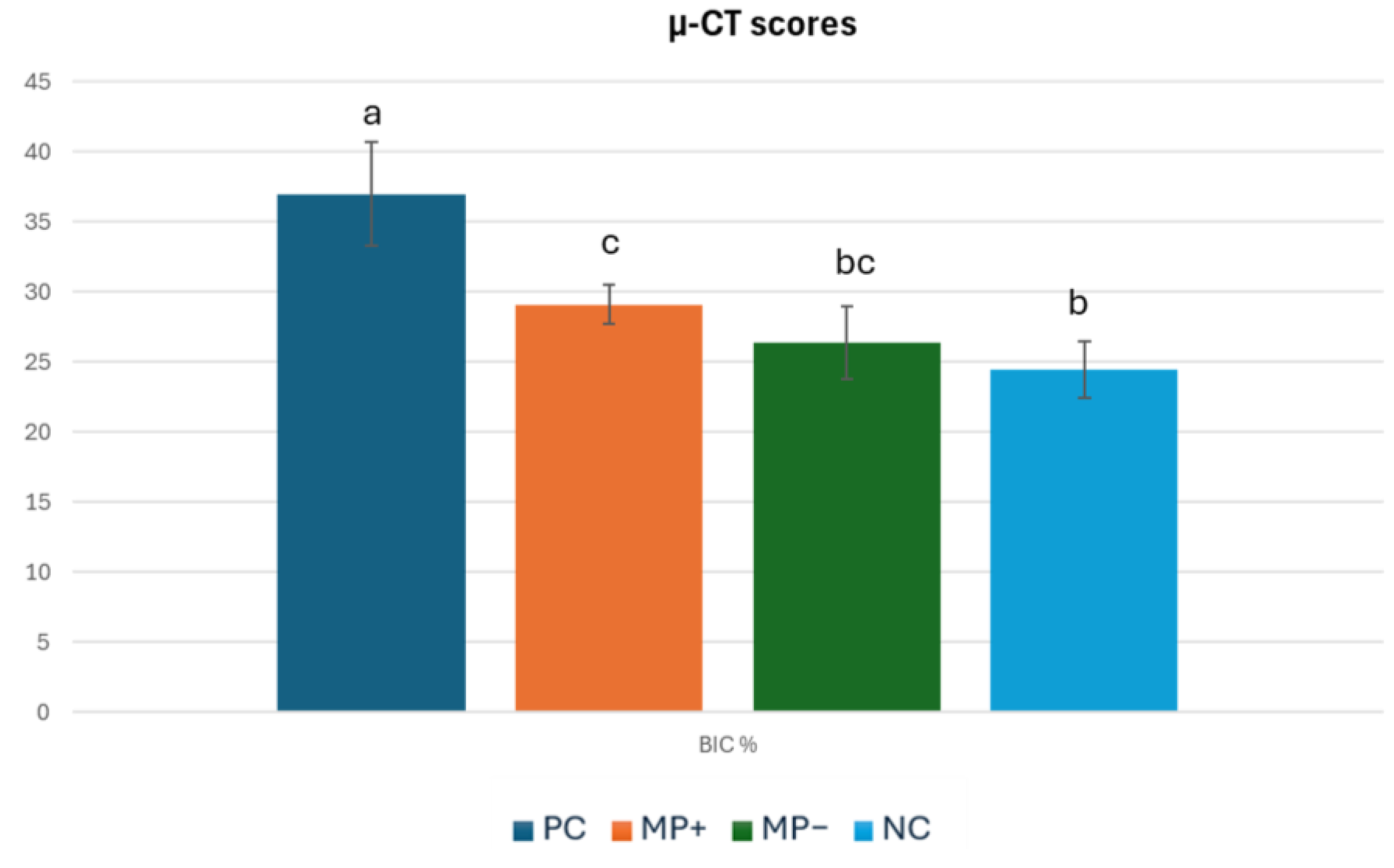
3.2. Micro-Computerized Tomographic (µ-CT) Results
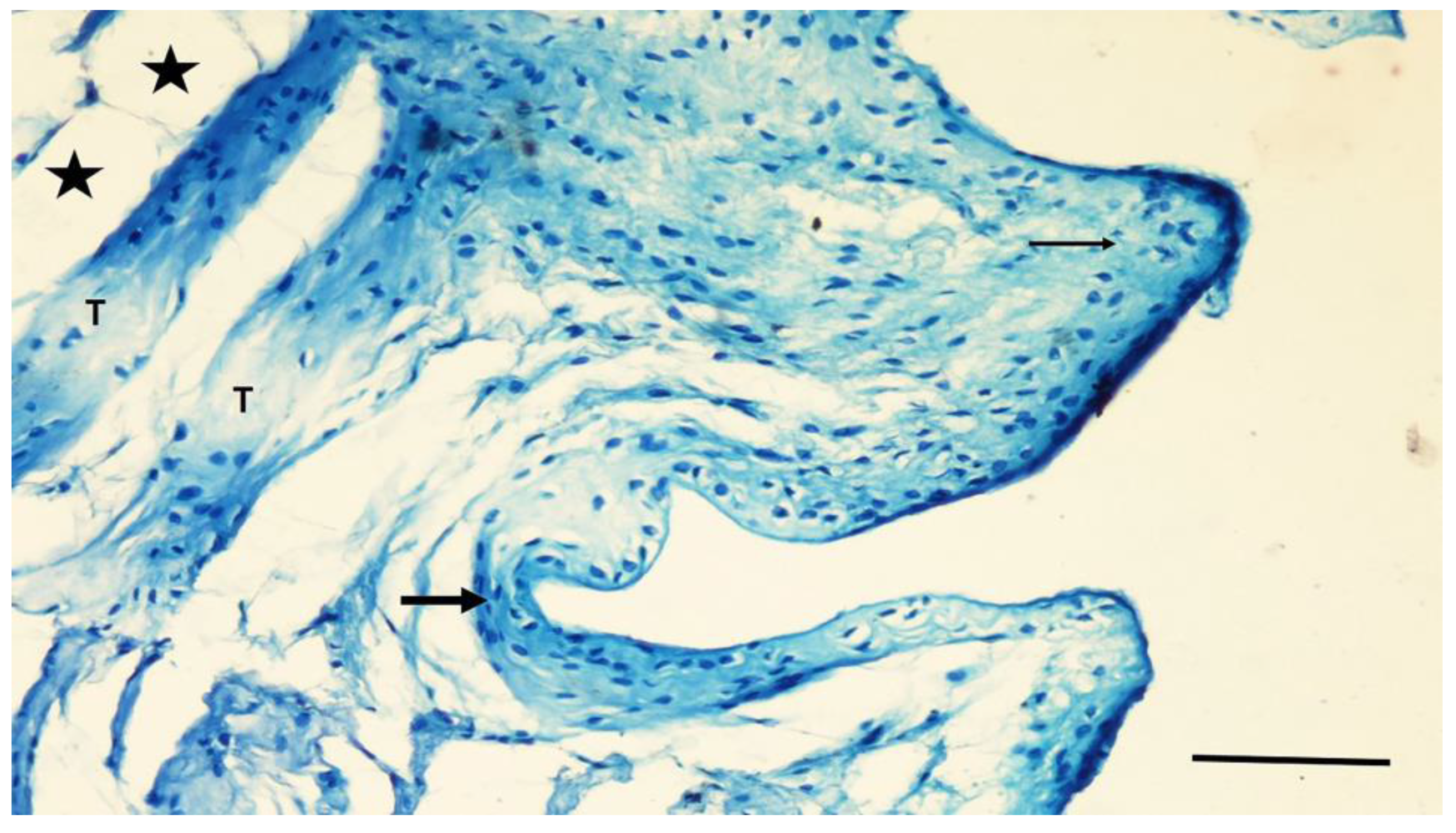
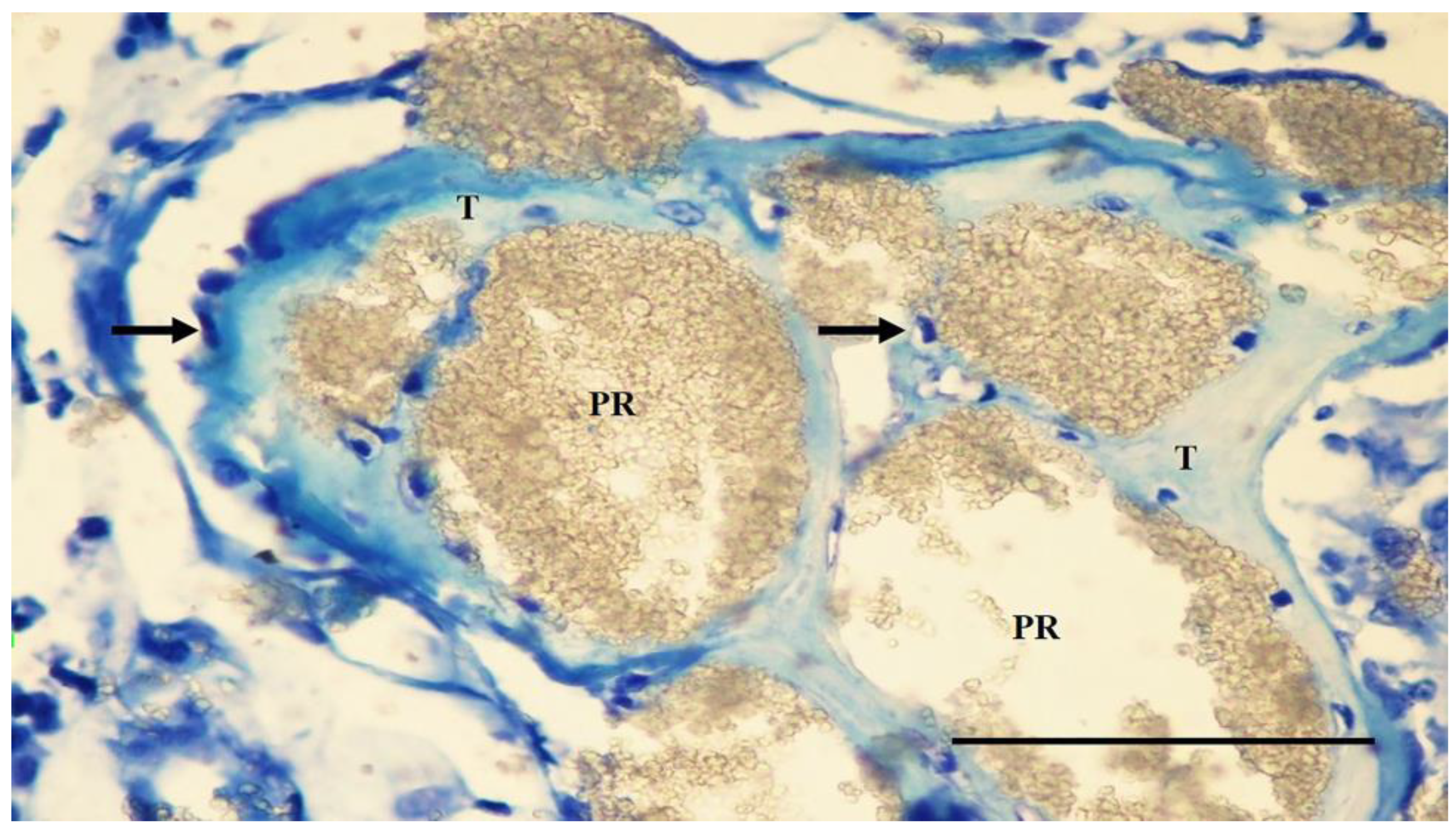
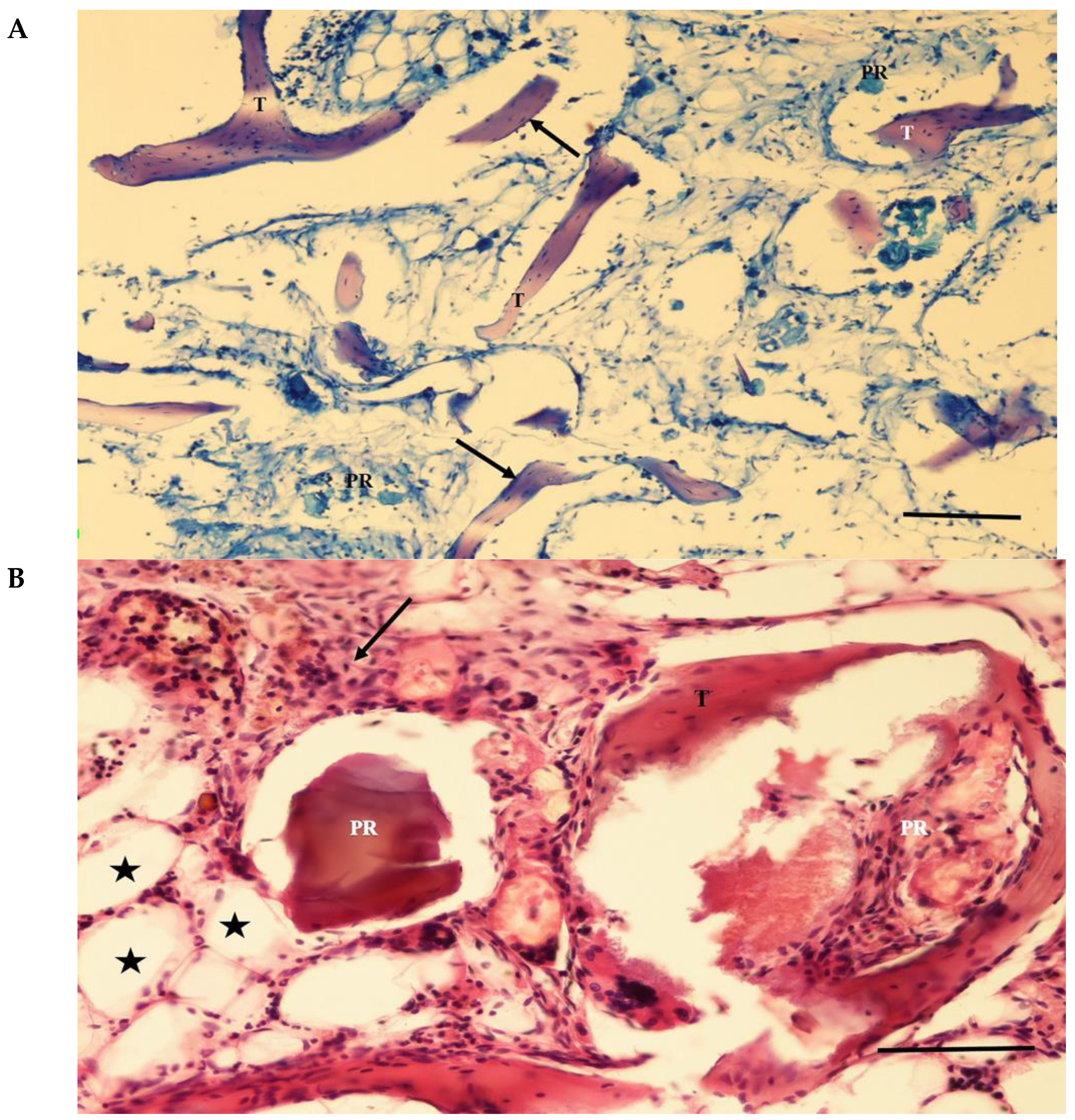
3.3. Histological Findings
3.4. Histomorphometric Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- de Lacerda Schickert, S.; Jansen, J.A.; Bronkhorst, E.M.; van den Beucken, J.J.; Leeuwenburgh, S.C. Stabilizing dental implants with a fiber-reinforced calcium phosphate cement: An in vitro and in vivo study. Acta Biomater. 2020, 110, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Hjalmarsson, L.; Gheisarifar, M.; Jemt, T.A. Systematic review of survival of single implants as presented in longitudinal studies with a follow-up of at least 10 years. Eur. J. Oral Implantol. 2016, 9 (Suppl. S1), S155–S162. [Google Scholar] [PubMed]
- Howe, M.-S.; Keys, W.; Richards, D. Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Tolstunov, L.; Hamrick, J.F.E.; Broumand, V.; Shilo, D.; Rachmiel, A. Bone Augmentation Techniques for Horizontal and Vertical Alveolar Ridge Deficiency in Oral Implantology. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Chiapasco, M.; Zaniboni, M.; Boisco, M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin. Oral Implants. Res. 2006, 17 (Suppl. S2), 136–159. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Zaniboni, M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: A systematic review. Clin. Oral Implants. Res. 2009, 20 (Suppl. S4), 113–123. [Google Scholar] [CrossRef] [PubMed]
- Juodzbalys, G.; Raustia, A.M.; Kubilius, R. A 5-year follow-up study on one-stage implants inserted concomitantly with localized alveolar ridge augmentation. J. Oral Rehabil. 2007, 34, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Torres-Mansilla, A.; Hincke, M.; Voltes, A.; López-Ruiz, E.; Baldión, P.A.; Marchal, J.A.; Álvarez-Lloret, P.; Gómez-Morales, J. Eggshell Membrane as a Biomaterial for Bone Regeneration. Polymers 2023, 15, 1342. [Google Scholar] [CrossRef]
- Oshida, Y.; Miyazaki, T. Bone-Grafting Biomaterials: Autografts, Hydroxyapatite, Calcium-Phosphates, and Biocomposites; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2024. [Google Scholar]
- Alghamdi, H.; Jansen, J. Dental Implants and Bone Grafts: Materials and Biological Issues; Woodhead Publishing: Cambridge, UK, 2019. [Google Scholar]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. S21), 92–102. [Google Scholar] [CrossRef]
- Uraz, A.; Gultekin, S.; Senguven, B.; Karaduman, B.; Sofuoglu, I.; Pehlivan, S.; Capan, Y.; Eren, K. Histologic and histomorphometric assessment of eggshell-derived bone graft substitutes on bone healing in rats. J. Clin. Exp. Dent. 2013, 5, e23. [Google Scholar] [CrossRef] [PubMed]
- Kavarthapu, A.; Malaiappan, S. Comparative evaluation of demineralized bone matrix and type II collagen membrane versus eggshell powder as a graft material and membrane in rat model. Indian J. Dent. Res. 2019, 30, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Uygur, S.; Ozmen, S.; Kandal, S.; Lortlar, N.; Omeroglu, S.; Arac, M.; Cenetoglu, S. Reconstruction of cranial bone defects using Struthio camelus eggshell. J. Craniofac. Surg. 2011, 22, 1843–1846. [Google Scholar] [CrossRef] [PubMed]
- Kattimani, V.S.; Lingamaneni, K.P.; Kreedapathi, G.E.; Kattappagari, K.K. Socket preservation using eggshell-derived nanohydroxyapatite with platelet-rich fibrin as a barrier membrane: A new technique. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Kattimani, V.S.; Chakravarthi, P.S.; Kanumuru, N.R.; Subbarao, V.V.; Sidharthan, A.; Kumar, T.S.S.; Prasad, L.K. Eggshell derived hydroxyapatite as bone graft substitute in the healing of maxillary cystic bone defects: A preliminary report. J. Int. Oral Health 2014, 6, 15–19. [Google Scholar] [PubMed]
- Kadhim, D.R.; Hamad, T.I.; Fatalla, A.A. Use of Eggshells as Bone Grafts around Commercially Pure Titanium Implant Screws Coated with Nano Calcium Sulfate. Int. J. Biomater. 2022, 2022, 8722283. [Google Scholar] [CrossRef] [PubMed]
- Qutieshat, A.; Mason, A.G.; Chadwick, R.G. Evaluation of Struthio camelus eggshell as an in vitro alternative to extracted human teeth in preliminary screening studies on dental erosion. Clin. Exp. Dent. Res. 2023, 9, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Pines, M.; Knopov, V.; Bar, A. Involvement of osteopontin in egg shell formation in the laying chicken. Matrix Biol. 1995, 14, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Panheleux, M.; Bain, M.; Fernandez, M.; Morales, I.; Gautron, J.; Arias, J.; Solomon, S.; Hincke, M.; Nys, Y. Organic matrix composition and ultrastructure of eggshell: A comparative study. Br. Poult. Sci. 1999, 40, 240–252. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; William, J.B.; Alejandra, C.; Innes, C.C.; Ulrich, D.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMJ Open Sci. 2020, 4, e100115. [Google Scholar]
- Carral, C.; Muñoz, F.; Permuy, M.; Liñares, A.; Dard, M.; Blanco, J. Mechanical and chemical implant decontamination in surgical peri-implantitis treatment: Preclinical “in vivo” study. J. Clin. Periodontol. 2016, 43, 694–701. [Google Scholar] [CrossRef]
- Kang, J.; Shibasaki, M.; Terauchi, M.; Oshibe, N.; Hyodo, K.; Marukawa, E. Comparative analysis of the kinetic properties of various bone substitutes filled into a peri-implant canine defect model. J. Periodontal. Implant Sci. 2024, 54, 96–107. [Google Scholar] [CrossRef]
- Pazzaglia, U.E.; Congiu, T.; Raspanti, M.; Ranchetti, F.; Quacci, D. Anatomy of the intracortical canal system: Scanning electron microscopy study in rabbit femur. Clin. Orthop. Relat. Res. 2009, 467, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kerns, D.G. Mechanisms of guided bone regeneration: A review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef]
- Kattimani, V.; Lingamaneni, K.P.; Yalamanchili, S.; Mupparapu, M. Use of eggshell-derived nano-hydroxyapatite as novel bone graft substitute-A randomized controlled clinical study. J. Biomater. Appl. 2019, 34, 597–614. [Google Scholar] [CrossRef] [PubMed]
- Onisor, F.; Opris, H.; Baciut, M.; Bran, S.; Dinu, C.; Opris, D.; Armencea, G.; Bumbu, B.; Baciut, G. Biocompatibility and histological responses of eggshell membrane for dental implant-guided bone regeneration. J. Med. Life 2023, 16, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Shibak, A.; Maghsoudi, A.; Rokouei, M.; Farhangfar, H.; Faraji-Arough, H. Investigation of egg production curve in ostrich using nonlinear functions. Poult. Sci. 2023, 102, 102333. [Google Scholar] [CrossRef]
- Lavelin, I.; Meiri, N.; Pines, M. New insight in eggshell formation. Poult. Sci. 2000, 79, 1014–1017. [Google Scholar] [CrossRef]
- Dupoirieux, L.; Pourquier, D.; Neves, M.; Téot, L. Resorption kinetics of eggshell: An in vivo study. J. Craniofac. Surg. 2001, 12, 53–58. [Google Scholar] [CrossRef]
- Durmuş, E.; Celik, I.; Ozturk, A.; Ozkan, Y.; Aydin, M.F. Evaluation of the potential beneficial effects of ostrich eggshell combined with eggshell membranes in healing of cranial defects in rabbits. J. Int. Med. Res. 2003, 31, 223–230. [Google Scholar] [CrossRef]
- Ünsal, A.; Durmuş, E.; Çelik, İ. Osteoproductivity in Experimentally Induced Cranial Bone Defects in Rabbits. Iran. Biomed. J. 2023, 26, 366–374. [Google Scholar] [PubMed]
- Kucukkolbasi, H.; Mutlu, N.; Isik, K.; Celik, I.; Oznurlu, Y. Histological evaluation of the effects of bioglass, hydroxyapatite, or demineralized freeze-dried bone, grafted alone or as composites, on the healing of tibial defects in rabbits. Saudi Med. J. 2009, 30, 329–333. [Google Scholar] [PubMed]
- Durmuş, E.; Celik, I.; Aydin, M.F.; Yildirim, G.; Sur, E. Evaluation of the biocompatibility and osteoproductive activity of ostrich eggshell powder in experimentally induced calvarial defects in rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 82–89. [Google Scholar] [CrossRef]
- Dupoirieux, L. Ostrich eggshell as a bone substitute: A preliminary report of its biological behaviour in animals—A possibility in facial reconstructive surgery. Br. J. Oral Maxillofac. Surg. 1999, 37, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Dupoirieux, L.; Pourquier, D.; Picot, M.C.; Neves, M. Comparative study of three different membranes for guided bone regeneration of rat cranial defects. Int. J. Oral Maxillofac. Surg. 2001, 30, 58–62. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Folkman, M.; Becker, A.; Meinster, I.; Masri, M.; Ormianer, Z. Comparison of bone-to-implant contact and bone volume around implants placed with or without site preparation: A histomorphometric study in rabbits. Sci. Rep. 2020, 10, 12446. [Google Scholar] [CrossRef]




| Gram | ||
|---|---|---|
| Contents | MP+ | MP− |
| Calcium Phosphate From Ostrich Eggshell Powder | 20 g | 20 g |
| Ostrich Eggshell Memrane Protein | 0.25 g | - |
| Calcium Chloride | 0.5 g | 0.5 g |
| Sodium Tri-Meta Phosphate | 1 g | 1 g |
| Carboxy Methyl Cellulose | 0.5 g | 0.5 g |
| Gum Arabic | 0.25 g | 0.25 g |
| Alginic Acid | 0.25 | 0.25 |
| PC (n = 8) | MP+ (n = 8) | MP− (n = 8) | NC (n = 8) | * p Value | |
|---|---|---|---|---|---|
| BIC % | 37.01 ± 3.7 a | 29.09 ± 1.4 c | 26.4 ± 2.6 bc | 24.45 ± 2.0 b | p < 0.01 |
| Groups (n = 8) | Connective Tissue Area% | Bone Tissue Area% | Residual Particle Area% | Adipose Tissue Area% | Blood Vessel Area% |
|---|---|---|---|---|---|
| PC | 12.00 ± 2.4 a | 27.31 ± 8 a | 25.53 ± 6.00 a | 14.7 ± 4.3 a | 14.34 ± 3.7 a |
| MP+ | 23.81 ± 2.1 b | 25.5 ± 8.00 a | 10.06 ± 2.4 b | 19.45 ± 2.2 b | 14.59 ± 5.6 a |
| MP− | 26.78 ± 5.4 b | 13.44 ± 4.2 b | 16.3 ± 4.4 c | 18.01 ± 3.4 ab | 20.32 ± 5.2 b |
| NC | 27.75 ± 1.1 b | 8.45 ± 0.9 b | null | 30.19 ± 1.5 c | 27.24 ± 1.5 c |
| * p values | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cengiz, Z.O.; Durmus, E.; Celik, I.; Aktı, A. Osteoproductivity of Injectable Bone Grafts with and without Ostrich Eggshell Membrane Protein in Rabbit Femur. J. Funct. Biomater. 2024, 15, 201. https://doi.org/10.3390/jfb15070201
Cengiz ZO, Durmus E, Celik I, Aktı A. Osteoproductivity of Injectable Bone Grafts with and without Ostrich Eggshell Membrane Protein in Rabbit Femur. Journal of Functional Biomaterials. 2024; 15(7):201. https://doi.org/10.3390/jfb15070201
Chicago/Turabian StyleCengiz, Ziya Ozan, Ercan Durmus, Ilhami Celik, and Ahmet Aktı. 2024. "Osteoproductivity of Injectable Bone Grafts with and without Ostrich Eggshell Membrane Protein in Rabbit Femur" Journal of Functional Biomaterials 15, no. 7: 201. https://doi.org/10.3390/jfb15070201



.jpg)



