Vaginal Ovule Loaded with Bismuth Lipophilic Nanoparticles and Cetylpyridinium Chloride Inhibits Human Cervical Carcinoma and Candida albicans Growth
Abstract
1. Introduction
2. Materials and Methods
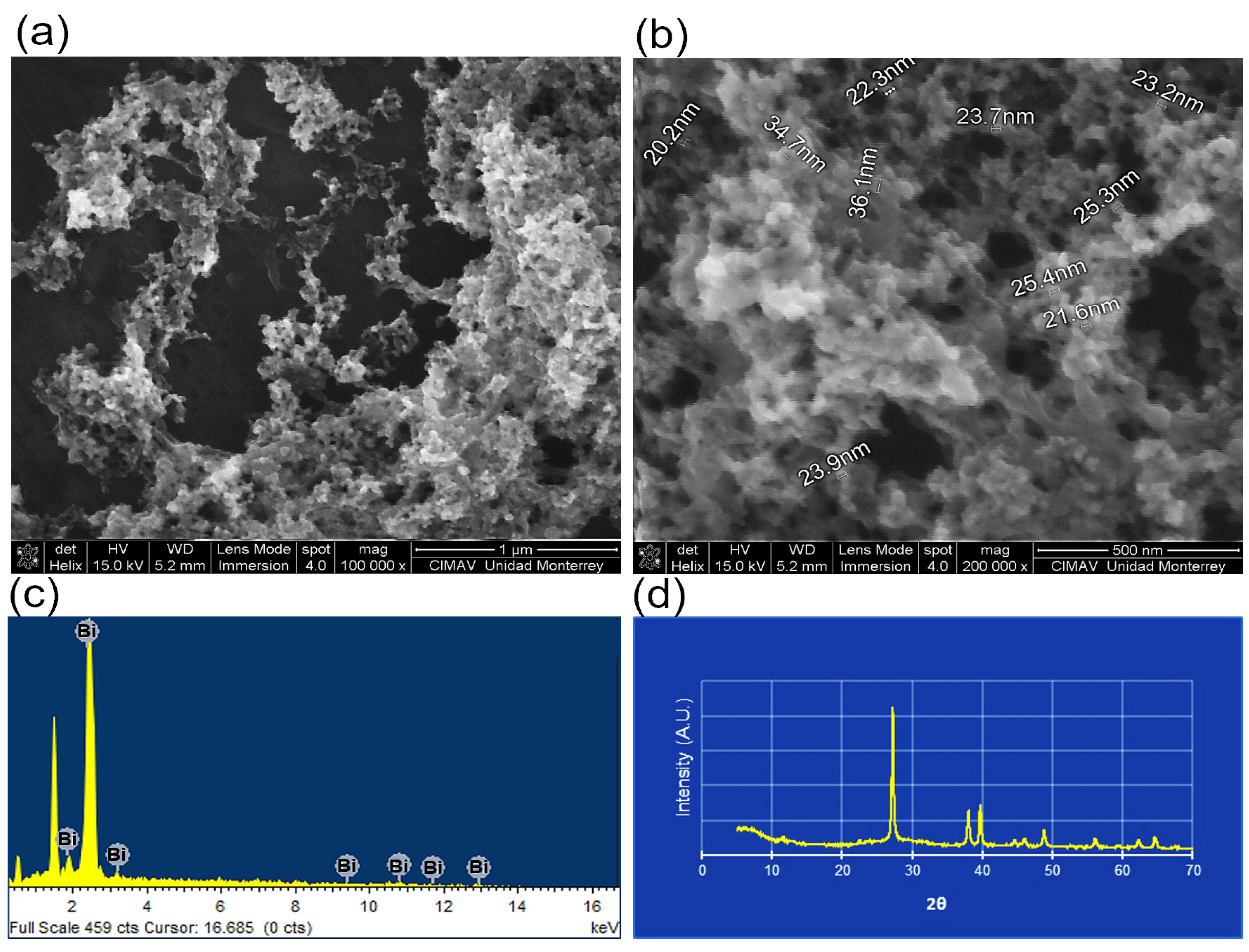
2.1. Synthesis and Characterization of BisBAL NPs
2.2. BisBAL NP-CPC Suspension
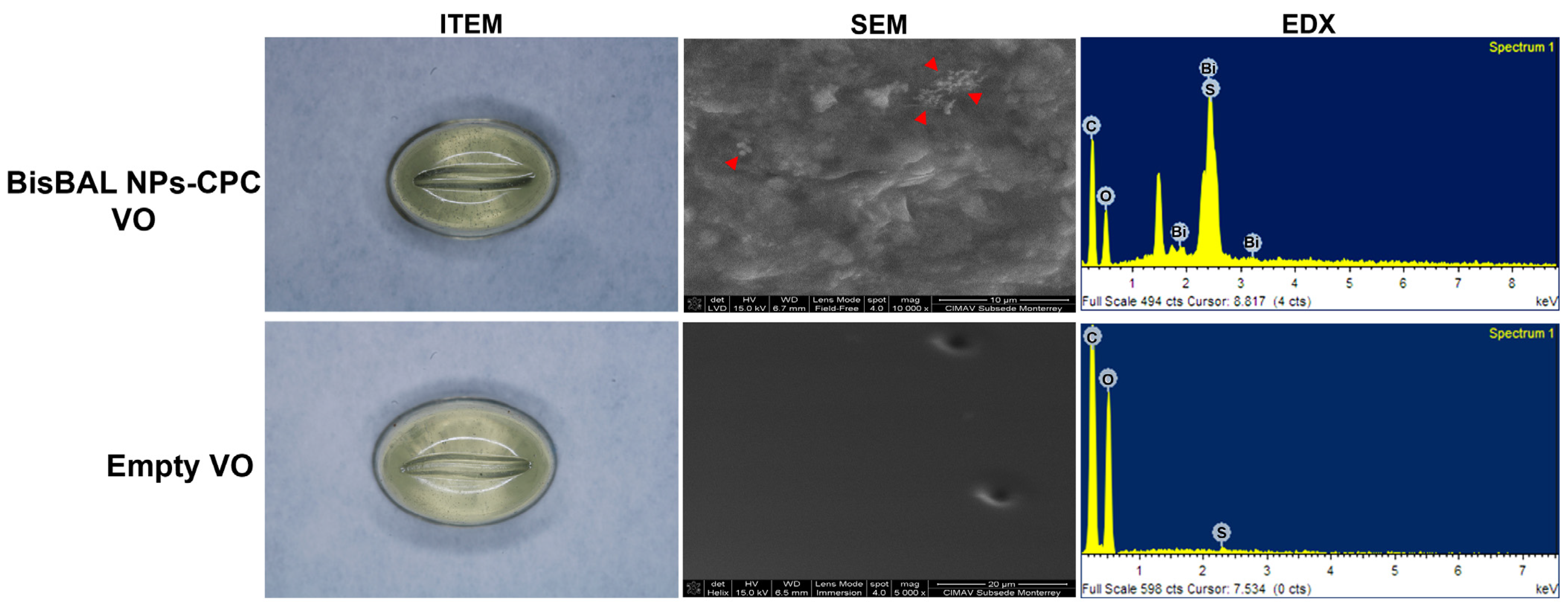
2.3. BisBAL NP-CPC Vaginal Ovule Development
2.4. Pharmacopeial Tests
2.4.1. Mass Uniformity
2.4.2. Disintegration Test and Bi Release Assay
2.4.3. Mechanical Compression Test
2.5. Ex Vivo Mucoadhesion Assay
2.6. Biological Tests
2.6.1. Microbial Culture
2.6.2. Disk Diffusion Assay
2.6.3. XTT Assay
2.6.4. Cell Culture
2.6.5. Cell Viability MTT Assay
2.7. Statistical Analysis
3. Results
3.1. BisBAL NP and BisBAL NP-CPC Vaginal Ovule Characterization
3.2. Mass Uniformity
3.3. Disintegration Test
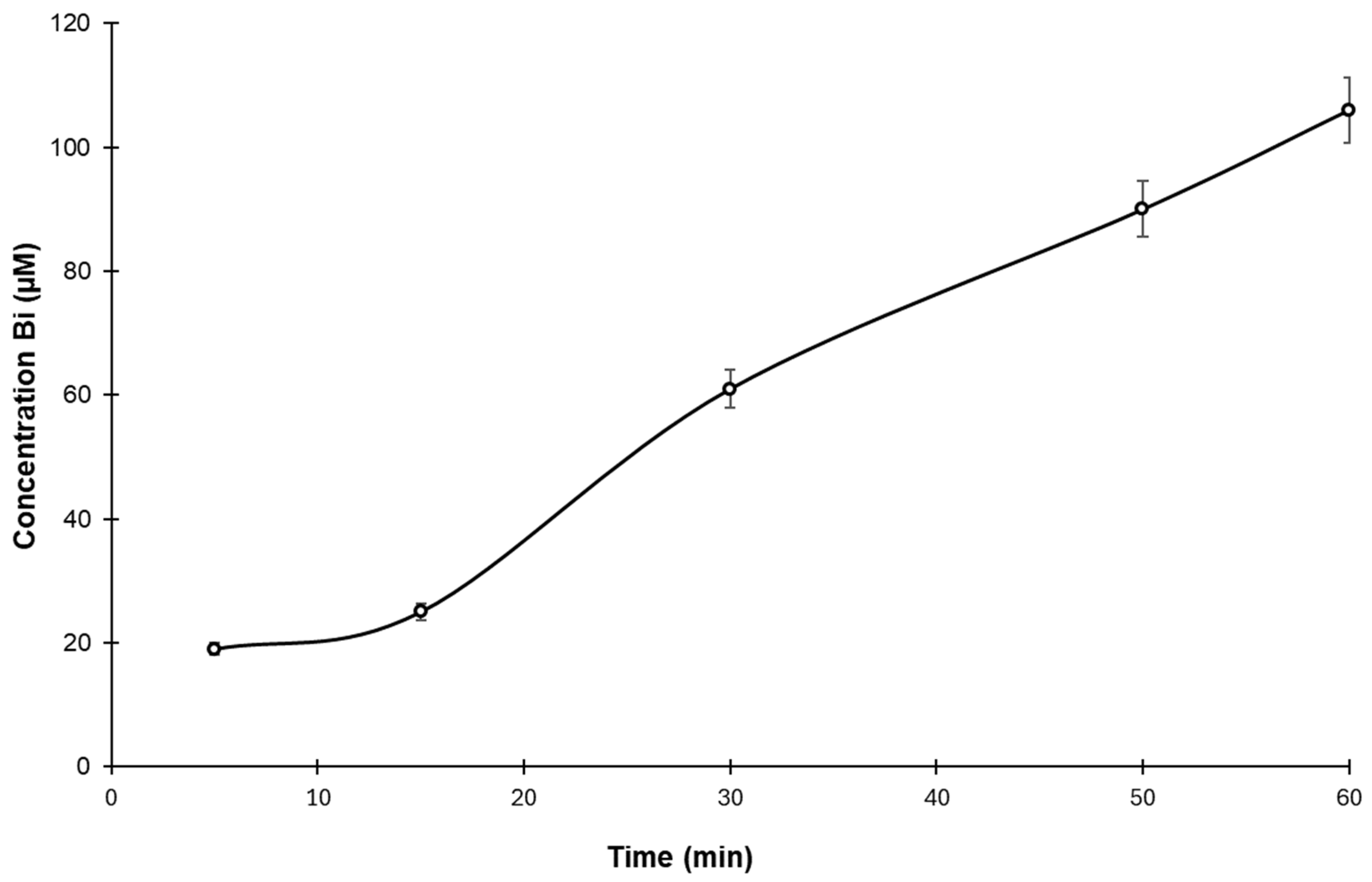
3.4. Drug Release Test
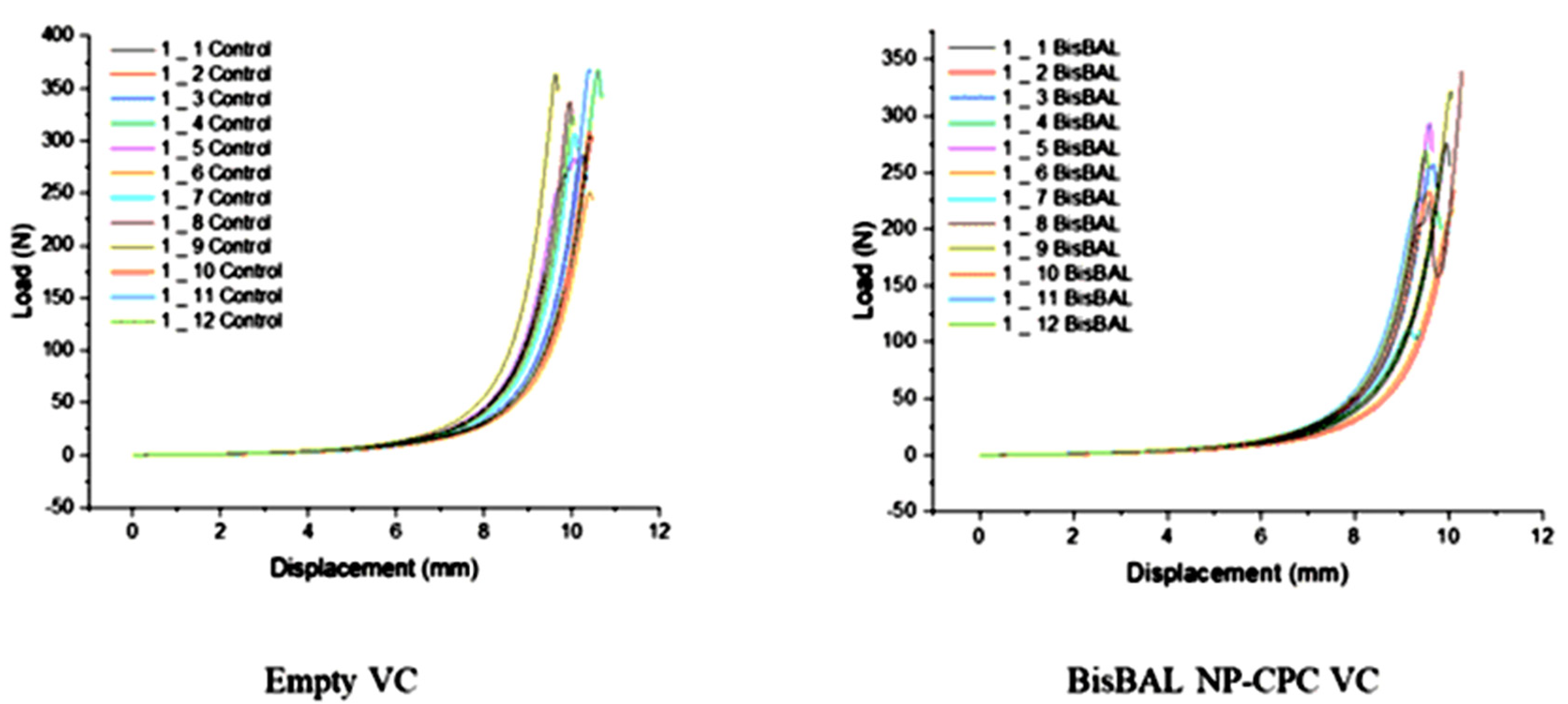
3.5. Mechanical Compression Test
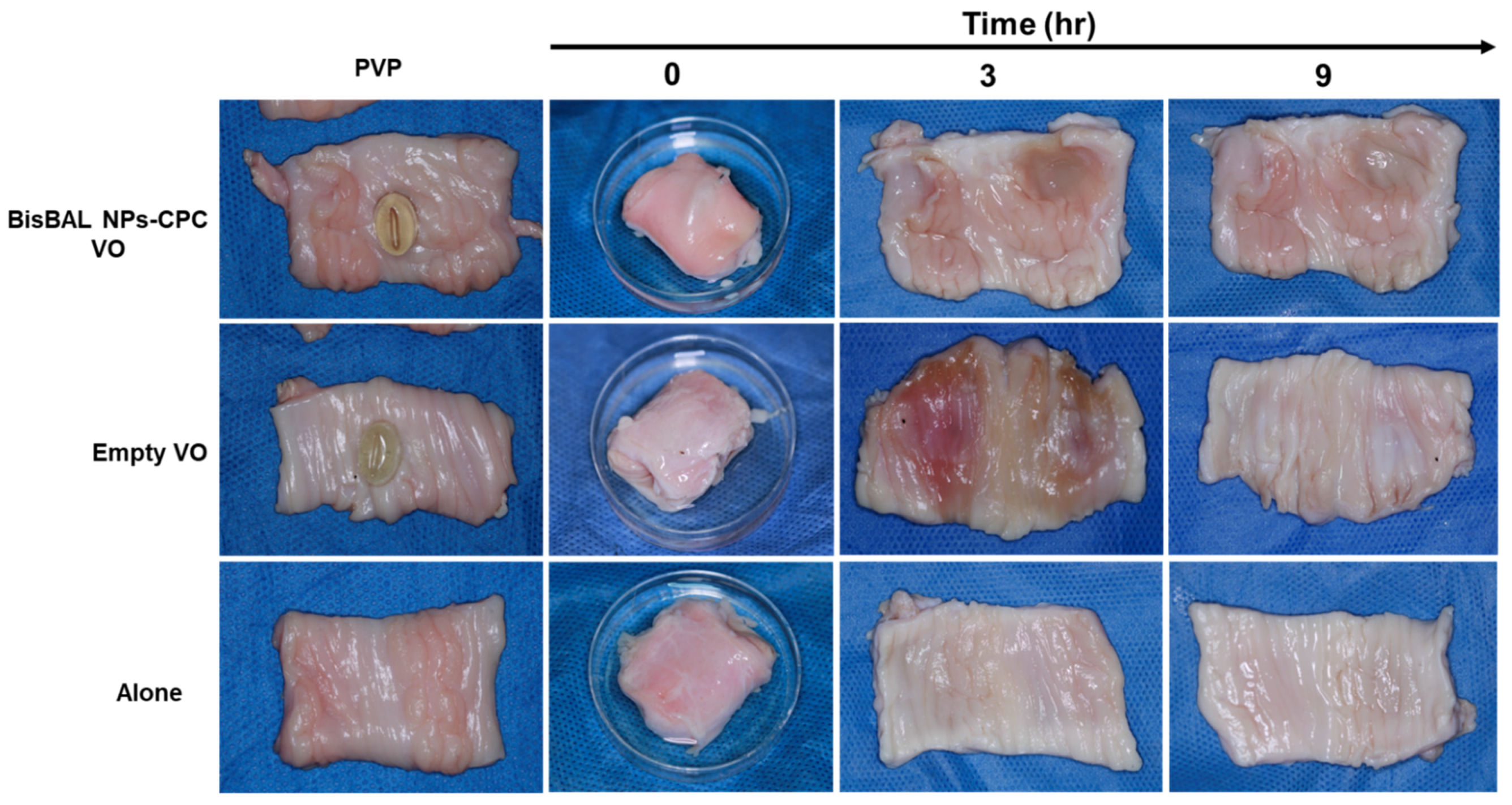
3.6. Ex Vivo Mucoadhesion Test
3.7. Antimycotic Activity
3.8. Antitumor Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Yadav, G.; Srinivasan, G.; Jain, A. Cervical cancer: Novel treatment strategies offer renewed optimism. Pathol. Res. Pract. 2024, 254, 155136. [Google Scholar] [CrossRef]
- Harro, C.D.; Pang, Y.Y.; Roden, R.B.; Hildesheim, A.; Wang, Z.; Reynolds, M.J.; Mast, T.C.; Robinson, R.; Murphy, B.R.; Karron, R.A.; et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 2001, 93, 284–292. [Google Scholar] [CrossRef]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.L. Cancer causes revisited: Human papillomavirus and cervical neoplasia. J. Natl. Cancer Inst. 1995, 87, 779–780. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Cetina-Pérez, L.; Luvián-Morales, J.; Delgadillo-González, M.; Castro-Eguiluz, D.; Galicia-Carmona, T.; Rely, K.; Vaca González, R.; Lugo-Martínez, G.; García-Barrientos, N.; Nateras, A. Sociodemographic characteristics and their association with survival in women with cervical cancer. BMC Cancer 2024, 24, 161. [Google Scholar] [CrossRef]
- Torreglosa-Hernández, S.; Grisales-Romero, H.; Morales-Carmona, E.; Hernández-Ávila, J.E.; Huerta-Gutiérrez, R.; Barquet-Muñoz, S.A.; Palacio-Mejía, L.S. Survival analysis and associated factors in patients with cervical cancer financed by the Seguro Popular in Mexico. Salud Publica Mex. 2022, 64, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Isla-Ortiz, D.; Palomares-Castillo, E.; Mille-Loera, J.E.; Ramírez-Calderón, N.; Mohar-Betancourt, A.; Meneses-García, A.A.; Reynoso-Noverón, N. Cervical Cancer in Young Women: Do They Have a Worse Prognosis? A Retrospective Cohort Analysis in a Population of Mexico. Oncologist 2020, 25, e1363–e1371. [Google Scholar] [CrossRef]
- Andrei, G.; Snoeck, R.; Piette, J.; Delvenne, P.; De Clercq, E. Antiproliferative effects of acyclic nucleoside phosphonates on human papillomavirus (HPV)-harboring cell lines compared with HPV-negative cell lines. Oncol. Res. 1998, 10, 523–531. [Google Scholar]
- Kim, K.Y.; Blatt, L.; Taylor, M.W. The effects of interferon on the expression of human papillomavirus oncogenes. J. Gen. Virol. 2000, 81, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, J.; Skwarczynski, M.; Stephenson, R.J.; Toth, I.; Hussein, W.M. Peptide-Based Nanovaccines in the Treatment of Cervical Cancer: A Review of Recent Advances. Int. J. Nanomed. 2022, 17, 869–900. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Z.; Feng, L.; Xia, Y.; Wei, G.; Lu, W. Advance in Nanomedicine for Improving Mucosal Penetration and Effective Therapy of Cervical Cancer. Small 2023, 19, e2303772. [Google Scholar] [CrossRef] [PubMed]
- García-Cuellar, C.M.; Cabral-Romero, C.; Hernández-Delgadillo, R.; Solis-Soto, J.M.; Meester, I.; Sánchez-Pérez, Y.; Nakagoshi-Cepeda, S.E.; Pineda-Aguilar, N.; Sánchez-Nájera, R.I.; Nakagoshi-Cepeda, M.A.A.; et al. Bismuth lipophilic nanoparticles (BisBAL NP) inhibit the growth of tumor cells in a mouse melanoma model. Anti-Cancer Agents Med. Chem. 2022, 22, 2548–2557. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi-Gahrouei, D.; Choghazardi, Y.; Kazemzadeh, A.; Naseri, P.; Shahbazi-Gahrouei, S. A review of bismuth-based nanoparticles and their applications in radiosensitising and dose enhancement for cancer radiation therapy. IET Nanobiotechnol./IET 2023, 17, 302–311. [Google Scholar] [CrossRef]
- Gomez, C.; Hallot, G.; Laurent, S.; Port, M. Medical Applications of Metallic Bismuth Nanoparticles. Pharmaceutics 2021, 13, 1793. [Google Scholar] [CrossRef] [PubMed]
- Badireddy, A.R.; Hernandez-Delgadillo, R.; Sánchez-Nájera, R.I.; Chellam, S.; Cabral-Romero, C. Synthesis and characterization of lipophilic bismuth dimercaptopropanol nanoparticles and their effects on oral microorganisms growth and biofilm formation. J. Nanoparticle Res. 2014, 16, 2456. [Google Scholar] [CrossRef]
- Cabral-Romero, C.; Solís-Soto, J.M.; Sánchez-Pérez, Y.; Pineda-Aguilar, N.; Meester, I.; Pérez-Carrillo, E.; Nakagoshi-Cepeda, S.E.; Sánchez-Nájera, R.I.; Nakagoshi-Cepeda, M.A.A.; Hernandez-Delgadillo, R.; et al. Antitumor activity of a hydrogel loaded with lipophilic bismuth nanoparticles on cervical, prostate, and colon human cancer cells. Anti-Cancer Drugs 2020, 31, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Pitten, F.A.; Kramer, A. Efficacy of cetylpyridinium chloride used as oropharyngeal antiseptic. Arzneim.-Forsch. 2001, 51, 588–595. [Google Scholar] [CrossRef]
- Teng, F.; He, T.; Huang, S.; Bo, C.P.; Li, Z.; Chang, J.L.; Liu, J.Q.; Charbonneau, D.; Xu, J.; Li, R.; et al. Cetylpyridinium chloride mouth rinses alleviate experimental gingivitis by inhibiting dental plaque maturation. Int. J. Oral Sci. 2016, 8, 182–190. [Google Scholar] [CrossRef]
- Johannes, L.; Billet, A. Glycosylation and raft endocytosis in cancer. Cancer Metastasis Rev. 2020, 39, 375–396. [Google Scholar] [CrossRef] [PubMed]
- García-Cuellar, C.M.; Hernández-Delgadillo, R.; Solis-Soto, J.M.; Meester, I.; Sánchez-Pérez, Y.; Nakagoshi-Cepeda, S.E.; Nakagoshi-Cepeda, M.A.A.; Chellam, S.; Cabral-Romero, C. Cetylpyridinium chloride inhibits human breast tumor cells growth in a no-selective way. J. Appl. Biomater. Funct. Mater. 2022, 20, 22808000221092157. [Google Scholar] [CrossRef] [PubMed]
- García-Cuellar, C.M.; Hernández-Delgadillo, R.; Torres-Betancourt, J.A.; Solis-Soto, J.M.; Meester, I.; Sánchez-Pérez, Y.; Pineda-Aguilar, N.; Nakagoshi-Cepeda, S.E.; Sánchez-Nájera, R.I.; Nakagoshi-Cepeda, M.A.A.; et al. Cumulative antitumor effect of bismuth lipophilic nanoparticles and cetylpyridinium chloride in inhibiting the growth of lung cancer. J. Appl. Biomater. Funct. Mater. 2023, 21, 22808000231161177. [Google Scholar] [CrossRef] [PubMed]
- Teworte, S.; Aleandri, S.; Weber, J.R.; Carone, M.; Luciani, P. Mucoadhesive 3D printed vaginal ovules to treat endometriosis and fibrotic uterine diseases. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2023, 188, 106501. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, H.d.O.; Medeiros, B.J.L.; Sá, B.M.d.; Araújo, J.T.C.d.; Kawakami, M.Y.M.; Favacho, H.A.S.; Carvalho, J.C.T. Study of dissolution profiles and desintegration of capsules containing the dried hydroethanolic extract of Calophyllum brasiliense. Rev. Bras. Farmacogn. 2013, 23, 194–199. [Google Scholar] [CrossRef][Green Version]
- Janeczko, M.; Kochanowicz, E. Biochanin A Inhibits the Growth and Biofilm of Candida Species. Pharmaceuticals 2024, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peterson, D.A.; Kimura, H.; Schubert, D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J. Neurochem. 1997, 69, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.A.; Pereira-da-Silva, G.; Silveira, R.; Mayer, P.C.M.; Zilly, A.; Lopes-Júnior, L.C. Safety, Efficacy, and Immunogenicity of Therapeutic Vaccines for Patients with High-Grade Cervical Intraepithelial Neoplasia (CIN 2/3) Associated with Human Papillomavirus: A Systematic Review. Cancers 2024, 16, 672. [Google Scholar] [CrossRef] [PubMed]
- Brun, J.L. Human papillomavirus vaccines. J. Gynecol. Obstet. Biol. Reprod. 2008, 37 (Suppl. S1), S155–S166. [Google Scholar] [CrossRef]
- Adams, M.; Jasani, B.; Fiander, A. Human papilloma virus (HPV) prophylactic vaccination: Challenges for public health and implications for screening. Vaccine 2007, 25, 3007–3013. [Google Scholar] [CrossRef]
- Farr, A.; Effendy, I.; Frey Tirri, B.; Hof, H.; Mayser, P.; Petricevic, L.; Ruhnke, M.; Schaller, M.; Schaefer, A.P.A.; Sustr, V.; et al. Guideline: Vulvovaginal candidosis (AWMF 015/072, level S2k). Mycoses 2021, 64, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.R.; Ribeiro Godoy, J.S.; Stivalet Svidzinski, T.I.; Bruschi, M.L. Preparation and characterization of mucoadhesive thermoresponsive systems containing propolis for the treatment of vulvovaginal candidiasis. J. Pharm. Sci. 2013, 102, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.; Holmes, A.; Kwok, P.C.L.; Kumeria, T.; Namjoshi, S.; Imran, M.; Matteucci, L.; Ali, M.; Tai, W.; Benson, H.A.E.; et al. Advances and future perspectives in epithelial drug delivery. Adv. Drug Deliv. Rev. 2022, 186, 114293. [Google Scholar] [CrossRef] [PubMed]
- Palmeira-de-Oliveira, R.; Duarte, P.; Palmeira-de-Oliveira, A.; das Neves, J.; Amaral, M.H.; Breitenfeld, L.; Martinez-de-Oliveira, J. Women’s experiences, preferences and perceptions regarding vaginal products: Results from a cross-sectional web-based survey in Portugal. Eur. J. Contracept. Reprod. Health Care Off. J. Eur. Soc. Contracept. 2015, 20, 259–271. [Google Scholar] [CrossRef]
- Palmeira-de-Oliveira, R.; Oliveira, A.S.; Rolo, J.; Tomás, M.; Palmeira-de-Oliveira, A.; Simões, S.; Martinez-de-Oliveira, J. Women’s preferences and acceptance for different drug delivery routes and products. Adv. Drug Deliv. Rev. 2022, 182, 114133. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.S.; Chandran, R.; Abrahamse, H. Overcoming challenges in cancer treatment: Nano-enabled photodynamic therapy as a viable solution. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1942. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Kumar, M.; Bharti, K.; Manjit, M.; Mishra, B. Biopolymer-based tumor microenvironment-responsive nanomedicine for targeted cancer therapy. Nanomedicine 2024, 19, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Shahab, U.; Alshammari, A.; Alyahyawi, A.R.; Akasha, R.; Alharazi, T.; Ahmad, R.; Khanam, A.; Habib, S.; Kaur, K.; et al. Nano-therapeutics: The upcoming nanomedicine to treat cancer. IUBMB Life 2024. [Google Scholar] [CrossRef] [PubMed]
- Avgoustakis, K.; Angelopoulou, A. Biomaterial-Based Responsive Nanomedicines for Targeting Solid Tumor Microenvironments. Pharmaceutics 2024, 16, 179. [Google Scholar] [CrossRef]
- Ragelle, H.; Rahimian, S.; Guzzi, E.A.; Westenskow, P.D.; Tibbitt, M.W.; Schwach, G.; Langer, R. Additive manufacturing in drug delivery: Innovative drug product design and opportunities for industrial application. Adv. Drug Deliv. Rev. 2021, 178, 113990. [Google Scholar] [CrossRef]
- Mao, X.; Calero-Pérez, P.; Montpeyó, D.; Bruna, J.; Yuste, V.J.; Candiota, A.P.; Lorenzo, J.; Novio, F.; Ruiz-Molina, D. Intranasal Administration of Catechol-Based Pt(IV) Coordination Polymer Nanoparticles for Glioblastoma Therapy. Nanomaterials 2022, 12, 1221. [Google Scholar] [CrossRef] [PubMed]
- Petre, I.; Sirbu, D.T.; Petrita, R.; Toma, A.D.; Peta, E.; Dimcevici-Poesina, F. Real-world study of Cerviron(®) vaginal ovules in the treatment of cervical lesions of various etiologies. Biomed. Rep. 2023, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Calvo, N.L.; Svetaz, L.A.; Alvarez, V.A.; Quiroga, A.D.; Lamas, M.C.; Leonardi, D. Chitosan-hydroxypropyl methylcellulose tioconazole films: A promising alternative dosage form for the treatment of vaginal candidiasis. Int. J. Pharm. 2019, 556, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Dobaria, N.B.; Mashru, R.C.; Badhan, A.C.; Thakkar, A.R. A novel intravaginal delivery system for itraconazole: In vitro and in vivo evaluation. Curr. Drug Deliv. 2009, 6, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alba, J.; Valle-Gay, A.; Dibildox, M.; Vargas, J.A.; González, J.; García, M.; López, L.H.; Fentimex Mexican Study, G. Fenticonazole nitrate for treatment of vulvovaginitis: Efficacy, safety, and tolerability of 1-gram ovules, administered as ultra-short 2-day regimen. J. Chemother. 2004, 16, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, X.; Guo, X.; Quan, H. Mitofusin-2 Triggers Cervical Carcinoma Cell Hela Apoptosis via Mitochondrial Pathway in Mouse Model. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 46, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiang, D.; Lu, X.; Fang, L.; Cui, C.; Shi, Q.; Yang, X. Human serum albumin-bound paclitaxel nanoparticle inhibits cervical carcinoma cell proliferation and oxidative damage through CYP3A4 and CYP2C8. Heliyon 2024, 10, e24460. [Google Scholar] [CrossRef]
- Sharma, V.; Sinha, E.S.; Singh, J. Investigation of in-vitro Anti-Cancer and Apoptotic Potential of Garlic-Derived Nanovesicles against Prostate and Cervical Cancer Cell Lines. Asian Pac. J. Cancer Prev. APJCP 2024, 25, 575–585. [Google Scholar] [CrossRef]




| Dissolution Medium | Disintegration Time (min) * |
|---|---|
| Acetate buffer (pH, 4.2) | 19 ± 0.482 |
| PBS (pH, 7.4) | 23 ± 0.788 |
| Distilled water | 14 ± 0.842 |
| Empty VO | 1 mM BisBAL NP-CPC VO | 24 mM Miconazole |
|---|---|---|
| 0 ± 0 | 23 ± 0.968 | 20.35 ± 0.899 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral-Romero, C.; Hernández-Delgadillo, R.; Torres-Betancourt, J.A.; García-Cuellar, C.M.; Sánchez-Pérez, Y.; Solis-Soto, J.M.; Meester, I.; Pineda-Aguilar, N.; Nakagoshi-Cepeda, S.E.; Cauich-Rodríguez, J.V.; et al. Vaginal Ovule Loaded with Bismuth Lipophilic Nanoparticles and Cetylpyridinium Chloride Inhibits Human Cervical Carcinoma and Candida albicans Growth. J. Funct. Biomater. 2024, 15, 206. https://doi.org/10.3390/jfb15080206
Cabral-Romero C, Hernández-Delgadillo R, Torres-Betancourt JA, García-Cuellar CM, Sánchez-Pérez Y, Solis-Soto JM, Meester I, Pineda-Aguilar N, Nakagoshi-Cepeda SE, Cauich-Rodríguez JV, et al. Vaginal Ovule Loaded with Bismuth Lipophilic Nanoparticles and Cetylpyridinium Chloride Inhibits Human Cervical Carcinoma and Candida albicans Growth. Journal of Functional Biomaterials. 2024; 15(8):206. https://doi.org/10.3390/jfb15080206
Chicago/Turabian StyleCabral-Romero, Claudio, Rene Hernández-Delgadillo, Jesús Alejandro Torres-Betancourt, Claudia María García-Cuellar, Yesennia Sánchez-Pérez, Juan Manuel Solis-Soto, Irene Meester, Nayely Pineda-Aguilar, Sergio Eduardo Nakagoshi-Cepeda, Juan Valerio Cauich-Rodríguez, and et al. 2024. "Vaginal Ovule Loaded with Bismuth Lipophilic Nanoparticles and Cetylpyridinium Chloride Inhibits Human Cervical Carcinoma and Candida albicans Growth" Journal of Functional Biomaterials 15, no. 8: 206. https://doi.org/10.3390/jfb15080206
APA StyleCabral-Romero, C., Hernández-Delgadillo, R., Torres-Betancourt, J. A., García-Cuellar, C. M., Sánchez-Pérez, Y., Solis-Soto, J. M., Meester, I., Pineda-Aguilar, N., Nakagoshi-Cepeda, S. E., Cauich-Rodríguez, J. V., & Nakagoshi-Cepeda, M. A. A. (2024). Vaginal Ovule Loaded with Bismuth Lipophilic Nanoparticles and Cetylpyridinium Chloride Inhibits Human Cervical Carcinoma and Candida albicans Growth. Journal of Functional Biomaterials, 15(8), 206. https://doi.org/10.3390/jfb15080206







