Iron Oxide Nanoparticles: Parameters for Optimized Photoconversion Efficiency in Synergistic Cancer Treatment
Abstract
:1. Introduction
2. Iron Oxides in Medicine
3. Synthesis of IONPs
4. Parameters That Affect IONP-Mediated PTT
4.1. Laser Irradiation
4.2. IONPs’ Shape and Size
4.3. Form of Iron Oxide
4.4. Concentration of IONPs
4.5. Accumulation of IONPs
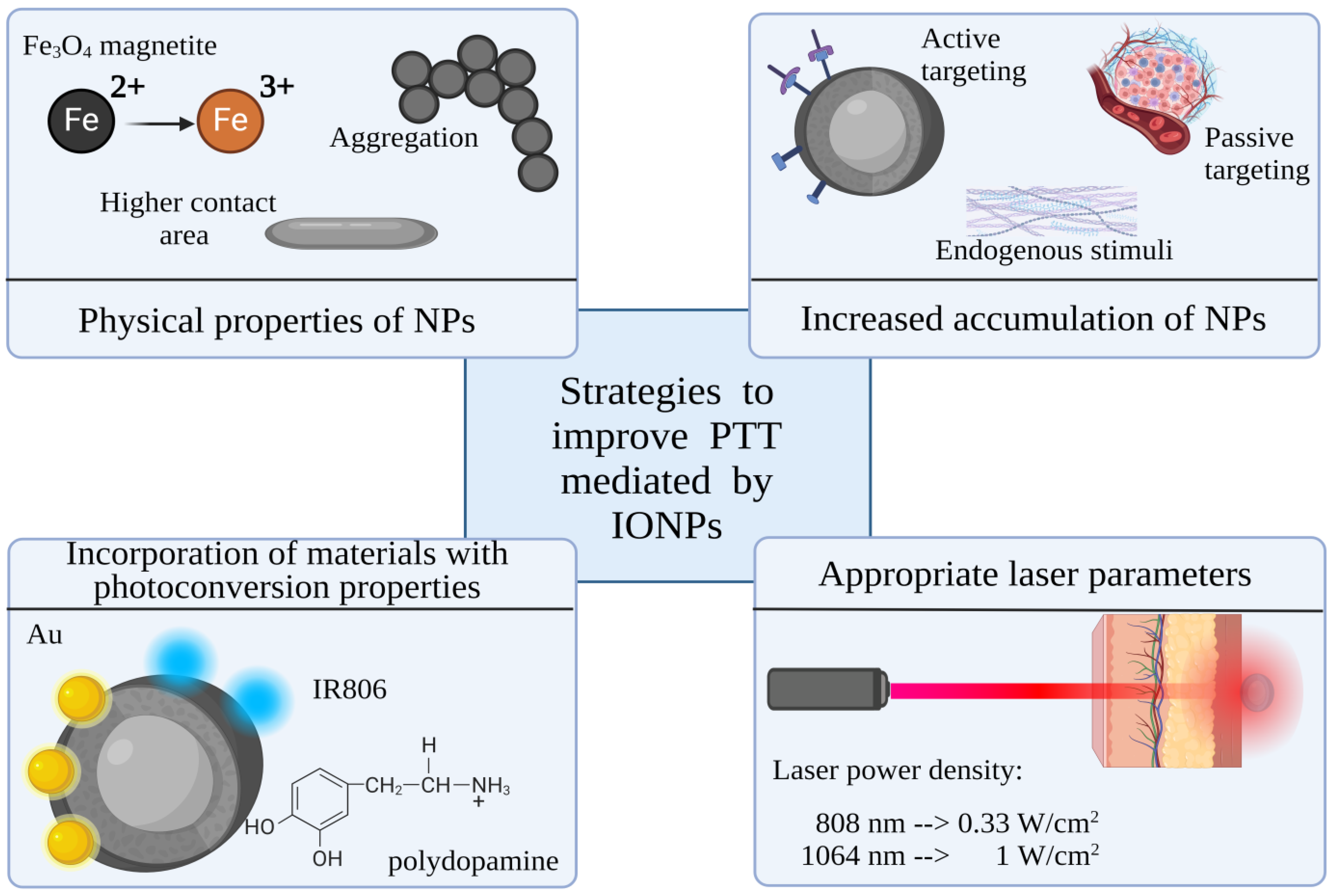
5. Strategies to Improve Photothermal Abilities of IONPs
6. IONPs in Theranostics
6.1. Iron Oxide as PTT and MRI Agent
6.2. IONP Composites as PTT and Fluorescence Agents
6.3. IONP Nanocomposites for PTT and SERS
6.4. IONP Nanocomposites for PTT and Computed Tomography Imaging
6.5. IONP Nanocomposites for PTT and PA Imaging
6.6. IONP Nanocomposites for PTT and Ultrasound Imaging
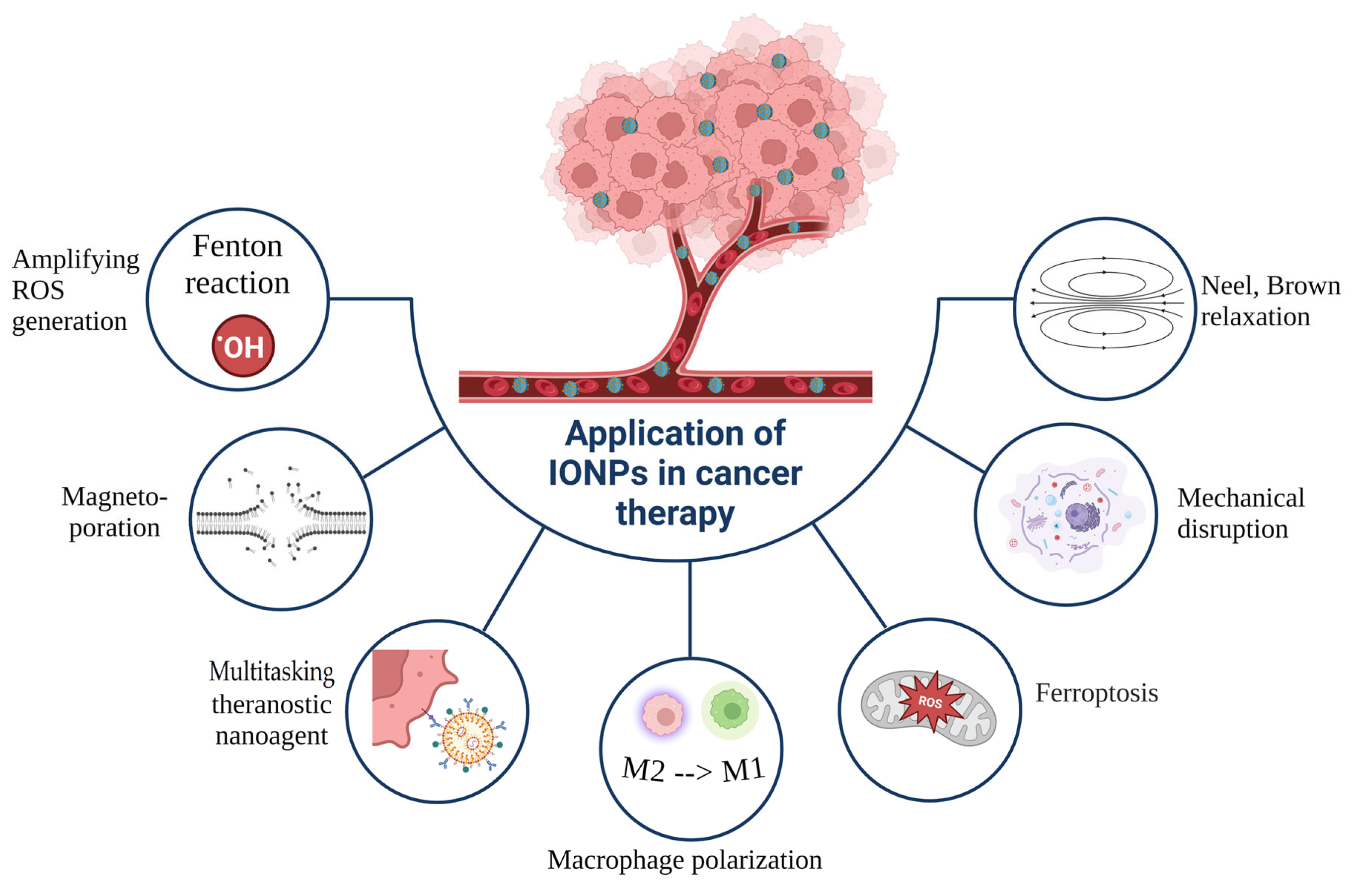
7. PTT in Combination with Other Treatment Modalities
7.1. Synergistic CT–PTT
7.2. Synergistic Radiation Therapy–PTT
7.3. Synergistic Photodynamic Therapy–PTT
7.4. Synergistic Ferroptosis–PTT
7.5. Synergistic MH–PTT
7.6. Synergistic Magneto-Mechanical Effect–PTT
7.7. Synergistic Immunotherapy–PTT
7.8. Synergistic Gene Therapy–PTT
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Su, J.X.; Li, S.J.; Zhou, X.F.; Zhang, Z.J.; Yan, Y.; Liu, S.L.; Qi, Q. Chemotherapy-induced metastasis: Molecular mechanisms and clinical therapies. Acta Pharmacol. Sin. 2023, 44, 1725–1736. [Google Scholar] [CrossRef]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for cancer: A trigger for metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, C.; Huang, Y.; He, M.; Xu, W.W.; Li, B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm 2021, 2, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Zein, R.; Sharrouf, W.; Selting, K. Physical Properties of Nanoparticles That Result in Improved Cancer Targeting. J. Oncol. 2020, 2020, 5194780. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Billone, P.S.; Mullett, W.M. Nanomedicine in action: An overview of cancer nanomedicine on the market and in clinical trials. J. Nanomater. 2013, 2013, 12. [Google Scholar] [CrossRef]
- Oei, A.L.; Kok, H.P.; Oei, S.B.; Horsman, M.R.; Stalpers, L.J.A.; Franken, N.A.P.; Crezee, J. Molecular and Biological Rationale of Hyperthermia as Radio- and Chemosensitizer. Adv. Drug Deliv. Rev. 2020, 163–164, 84–97. [Google Scholar] [CrossRef]
- Lin, F.C.; Hsu, C.H.; Lin, Y.Y. Nano-therapeutic cancer immunotherapy using hyperthermia-induced heat shock proteins: Insights from mathematical modeling. Int. J. Nanomed. 2018, 13, 3529–3539. [Google Scholar] [CrossRef]
- Roti Roti, J.L. Cellular responses to hyperthermia (40–46 °C): Cell killing and molecular events. Int. J. Hyperth. 2008, 24, 3–15. [Google Scholar] [CrossRef]
- Yi, G.Y.; Kim, M.J.; Kim, H.I.; Park, J.; Baek, S.H. Hyperthermia Treatment as a Promising Anti-Cancer Strategy: Therapeutic Targets, Perspective Mechanisms and Synergistic Combinations in Experimental Approaches. Antioxidants 2022, 11, 625. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, X.; Wang, D.; Qiao, Z.; Wang, D.; Huang, F.; Peng, H.; Hu, C. Designing high-efficiency light-to-thermal conversion materials for solar desalination and photothermal catalysis. J. Energy Chem. 2023, 79, 581–600. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Fan, K.; Yan, X. Iron Oxide Nanozyme: A Multifunctional Enzyme Mimetic for Biomedical Applications. Theranostics 2017, 7, 3207–3227. [Google Scholar] [CrossRef]
- Huang, Y.; Hsu, J.C.; Koo, H.; Cormode, D.P. Repurposing ferumoxytol: Diagnostic and therapeutic applications of an FDA-approved nanoparticle. Theranostics 2022, 12, 796–816. [Google Scholar] [CrossRef]
- Wang, S.; Luo, J.; Zhang, Z.; Dong, D.; Shen, Y.; Fang, Y.; Hu, L.; Liu, M.; Dai, C.; Peng, S.; et al. Iron and magnetic: New research direction of the ferroptosis-based cancer therapy. Am. J. Cancer Res. 2018, 8, 1933–1946. [Google Scholar]
- Kumari, S.; Raturi, S.; Kulshrestha, S.; Chauhan, K.; Dhingra, S.; András, K.; Thu, K.; Khargotra, R.; Singh, T. A comprehensive review on various techniques used for synthesizing nanoparticles. J. Mater. Res. Technol. 2023, 27, 1739–1763. [Google Scholar] [CrossRef]
- Luo, X.; Al-Antaki, A.H.M.; Alharbi, T.M.D.; Hutchison, W.D.; Zou, Y.-C.; Zou, J.; Sheehan, A.; Zhang, W.; Raston, C.L. Laser-Ablated Vortex Fluidic-Mediated Synthesis of Superparamagnetic Magnetite Nanoparticles in Water Under Flow. ACS Omega 2018, 3, 11172–11178. [Google Scholar] [CrossRef]
- Feng, K.; Xu, Z.; Wang, Y.; Wu, X.; Xiong, F.; Ruan, Y.; Sun, X. Renal-clearable porous hollow copper iron oxide nanoparticles for trimodal chemodynamic-photothermal-chemo anti-tumor therapy. Nanoscale 2023, 15, 3188–3198. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Zhou, J.; Zhao, X.; Cai, J. Flame spray pyrolyzed carbon-encapsulated Au/Fe3O4 nanoaggregates enabled efficient photothermal therapy and magnetic hyperthermia of esophageal cancer cells. Front. Bioeng. Biotechnol. 2024, 12, 1400765. [Google Scholar] [CrossRef] [PubMed]
- Byeon, J.H.; Park, J.H. Use of aerosol route to fabricate positively charged Au/Fe3O4 Janus nanoparticles as multifunctional nanoplatforms. Sci. Rep. 2016, 6, 35104. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, C. Magnetic iron oxide nanoparticle-loaded hydrogels for photothermal therapy of cancer cells. Front. Bioeng. Biotechnol. 2023, 11, 1130523. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, J.; Zhang, B.; Qu, J. Synthesis of Ag@Fe3O4 Nanoparticles for Photothermal Treatment of Ovarian Cancer. J. Nanomater. 2019, 2019, 6457968–6457976. [Google Scholar] [CrossRef]
- Shaw, S.K.; Kailashiya, J.; Gangwar, A.; Alla, S.K.; Gupta, S.K.; Prajapat, C.L.; Meena, S.S.; Dash, D.; Maiti, P.; Prasad, N.K. γ-Fe2O3 Nanoflowers as Efficient Magnetic Hyperthermia and Photothermal Agent. Appl. Surf. Sci. 2021, 560, 150025. [Google Scholar] [CrossRef]
- Kharey, P.; Goel, M.; Husain, Z.; Gupta, R.; Sharma, D.; Manikandan, M.; Palani, I.A.; Gupta, S. Green Synthesis of Biocompatible Superparamagnetic Iron Oxide-Gold Composite Nanoparticles for Magnetic Resonance Imaging, Hyperthermia and Photothermal Therapeutic Applications. Mater. Chem. Phys. 2023, 293, 126859. [Google Scholar] [CrossRef]
- Song, W.-R.; Yu, S.-X.; Jin, G.X.; Wang, X.; Chen, J.; Li, J.; Liu, G.; Yang, H.-H. Plant Polyphenol-Assisted Green Synthesis of Hollow CoPt Alloy Nanoparticles for Dual-Modality Imaging Guided Photothermal Therapy. Small 2016, 12, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- M Alahdal, H.; Ayad Abdullrezzaq, S.; Ibrahim M Amin, H.; F Alanazi, S.; Turki Jalil, A.; Khatami, M.; Mahmood Saleh, M. Trace elements-based Auroshell gold@ hematite nanostructure: Green synthesis and their hyperthermia therapy. IET Nanobiotechnology 2023, 17, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Le, W.; Mei, T.; Wang, Y.; Chen, B.; Liu, Z.; Xue, C. Cell membrane camouflaged nanoparticles: A new biomimetic platform for cancer photothermal therapy. Int. J. Nanomed. 2019, 14, 4431–4448. [Google Scholar] [CrossRef]
- Chen, M.; Sun, Y.; Liu, H. Cell membrane biomimetic nanomedicines for cancer phototherapy. Interdiscip. Med. 2023, 1, e20220012. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef]
- Wu, X.; Suo, Y.; Shi, H.; Liu, R.; Wu, F.; Wang, T.; Ma, L.; Liu, H.; Cheng, Z. Deep-tissue photothermal therapy using laser illumination at NIR-iia window. Nano-Micro Lett. 2020, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Bastiancich, C.; Da Silva, A.; Estève, M.-A. Photothermal Therapy for the Treatment of Glioblastoma: Potential and Preclinical Challenges. Front. Oncol. 2021, 10, 3095. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef]
- Soni, S.; Tyagi, H.; Taylor, R.A.; Kumar, A. The influence of tumour blood perfusion variability on thermal damage during nanoparticle-assisted thermal therapy. Int. J. Hyperth. 2015, 31, 615–625. [Google Scholar] [CrossRef]
- Freis, B.; Ramirez, M.D.L.A.; Kiefer, C.; Harlepp, S.; Iacovita, C.; Henoumont, C.; Affolter-Zbaraszczuk, C.; Meyer, F.; Mertz, D.; Boos, A.; et al. Effect of the Size and Shape of Dendronized Iron Oxide Nanoparticles Bearing a Targeting Ligand on MRI, Magnetic Hyperthermia, and Photothermia Properties—From Suspension to In Vitro Studies. Pharmaceutics 2023, 15, 1104. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Kolosnjaj-Tabi, J.; Abou-Hassan, A.; Plan Sangnier, A.; Curcio, A.; Silva, A.K.A.; Di Corato, R.; Neveu, S.; Pellegrino, T.; Liz-Marzán, L.M.; et al. Magnetic (Hyper)Thermia or Photothermia? Progressive Comparison of Iron Oxide and Gold Nanoparticles Heating in Water, in Cells, and In Vivo. Adv. Funct. Mater. 2018, 28, 1803660. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Z.; Li, W.; Wang, Z.; Li, Q.; Kong, F.; Zhang, H.; Zhu, X.; Du, Y.P.; Jin, Y.; et al. Appropriate size of magnetic nanoparticles for various bioapplications in cancer diagnostics and therapy. ACS Appl. Mater. Interfaces 2016, 8, 3092–3106. [Google Scholar] [CrossRef]
- Lozano-Pedraza, C.; Plaza-Mayoral, E.; Espinosa, A.; Sot, B.; Serrano, A.; Salas, G.; Blanco-Andujar, C.; Cotin, G.; Felder-Flesch, D.; Begin-Colin, S.; et al. Assessing the parameters modulating optical losses of iron oxide nanoparticles under near infrared irradiation. Nanoscale Adv. 2021, 3, 6490–6502. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.R. Photocatalytic activity of quantum dots. In Semiconductor Photocatalysis-Materials, Mechanisms and Applications; InTech: London, UK, 2016. [Google Scholar] [CrossRef]
- Peng, H.; Tang, S.; Tian, Y.; Zheng, R.; Zhou, L.; Yang, W. Highly Ligand-Directed and Size-Dependent Photothermal Properties of Magnetite Particles. Part. Part. Syst. Charact. 2016, 33, 332–340. [Google Scholar] [CrossRef]
- Guibert, C.; Dupuis, V.; Peyre, V.; Fresnais, J. Hyperthermia of magnetic nanoparticles: Experimental study of the role of aggregation. J. Phys. Chem. C 2015, 119, 28148–28154. [Google Scholar] [CrossRef]
- Shen, S.; Wang, S.; Zheng, R.; Zhu, X.; Jiang, X.; Fu, D.; Yang, W. Magnetic nanoparticle clusters for photothermal therapy with near-infrared irradiation. Biomaterials 2015, 39, 67–74. [Google Scholar] [CrossRef]
- Cabana, S.; Curcio, A.; Michel, A.; Wilhelm, C.; Abou-Hassan, A. Iron Oxide Mediated Photothermal Therapy in the Second Biological Window: A Comparative Study between Magnetite/Maghemite Nanospheres and Nanoflowers. Nanomaterials 2020, 10, 1548. [Google Scholar] [CrossRef]
- Gulzar, A.; Ayoub, N.; Mir, J.F.; Alanazi, A.M.; Shah, M.A.; Gulzar, A. In vitro and in vivo MRI imaging and photothermal therapeutic properties of Hematite (α-Fe2O3) Nanorods. J. Mater. Sci. Mater. Med. 2022, 33, 10. [Google Scholar] [CrossRef]
- Southern, P.; Pankhurst, Q.A. Commentary on the clinical and preclinical dosage limits of interstitially administered magnetic fluids for therapeutic hyperthermia based on current practice and efficacy models. Int. J. Hyperth. 2018, 34, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Liu, Y.; Liu, X.; Su, K.; Shi, K. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharm. Sin. B. 2021, 11, 2265–2285. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Jary, J.; Machnicka, B. Pharmacokinetics of magnetic iron oxide nanoparticles for medical applications. J. Nanobiotechnol. 2022, 20, 305. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Jain, R.K. Design considerations for nanotherapeutics in oncology. Nanomedicine 2015, 11, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Poudel, K.; Banstola, A.; Gautam, M.; Soe, Z.; Phung, C.D.; Pham, L.M.; Jeong, J.H.; Choi, H.G.; Ku, S.K.; Tran, T.H.; et al. Macrophage-membrane-camouflaged disintegrable and excretable nanoconstruct for deep tumor penetration. ACS Appl. Mater. Interfaces 2020, 12, 56767–56781. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Peng, Y.; Wen, Y.; Li, X.; Du, D.; Dai, W.; Tian, W.; Meng, Y. Recent progress in cancer cell membrane-based nanoparticles for biomedical applications. Beilstein J. Nanotechnol. 2023, 14, 262–279. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zheng, R.; Fang, X.; Wang, X.; Zhang, X.; Yang, W.; Sha, X. Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials 2016, 92, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Larson, T.A.; Bankson, J.; Aaron, J.; Sokolov, K. Hybrid plasmonic magnetic nanoparticles as molecular specific agents for MRI/optical imaging and photothermal therapy of cancer cells. Nanotechnology 2007, 18, 325101. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Lin, P.-Y.; Huang, P.-H.; Kuo, C.-Y.; Shalumon, K.T.; Chen, M.-Y.; Chen, J.-P. Magnetic Graphene Oxide for Dual Targeted Delivery of Doxorubicin and Photothermal Therapy. Nanomaterials 2018, 8, 193. [Google Scholar] [CrossRef]
- Yang, R.M.; Fu, C.P.; Fang, J.Z.; Xu, X.D.; Wei, X.H.; Tang, W.J.; Jiang, X.Q.; Zhang, L.M. Hyaluronan-modified superparamagnetic iron oxide nanoparticles for bimodal breast cancer imaging and photothermal therapy. Int. J. Nanomed. 2017, 12, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ghaznavi, H.; Hosseini-Nami, S.; Kamrava, S.K.; Irajirad, R.; Maleki, S.; Shakeri-Zadeh, A.; Montazerabadi, A. Folic acid conjugated peg coated gold–iron oxide core–shell nanocomplex as a potential agent for targeted photothermal therapy of cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Tang, J.; Zheng, R.; Guo, G.; Dong, A.; Wang, Y.; Yang, W. Nuclear-targeted multifunctional magnetic nanoparticles for photothermal therapy. Adv. Healthc. Mater. 2017, 6, 1601289. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Je, J.Y.; Moorthy, M.S.; Seo, H.; Cho, W.H. pH and NIR-light-responsive magnetic iron oxide nanoparticles for mitochondria-mediated apoptotic cell death induced by chemo-photothermal therapy. Int. J. Pharm. 2017, 531, 1–13. [Google Scholar] [CrossRef]
- Peng, H.; Yao, F.; Zhao, J.; Zhang, W.; Chen, L.; Wang, X.; Yang, P.; Tang, J.; Chi, Y. Unraveling mitochondria-targeting reactive oxygen species modulation and their implementations in cancer therapy by nanomaterials. Exploration 2023, 3, 20220115. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198. [Google Scholar] [CrossRef]
- Salvati, A.; Pitek, A.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.; Kelly, P.; Åberg, C.; Mahon, E.; Dawson, K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013, 8, 137–143. [Google Scholar] [CrossRef]
- Kale, S.; Burga, R.; Sweeney, E.; Zun, Z.; Sze, R.; Tuesca, A.; Subramony, A.; Fernandes, R. Composite Iron Oxide—Prussian Blue Nanoparticles for Magnetically Guided T1-Weighted Magnetic Resonance Imaging and Photothermal Therapy of Tumors. Int. J. Nanomedicine 2017, 12, 6413–6424. [Google Scholar] [CrossRef]
- Melancon, M.P.; Elliott, A.; Ji, X.; Shetty, A.; Yang, Z.; Tian, M.; Taylor, B.; Stafford, R.J.; Li, C. Theranostics with multifunctional magnetic gold nanoshells: Photothermal therapy and T2* magnetic resonance imaging. Invest. Radiol. 2011, 46, 132–140. [Google Scholar] [CrossRef]
- Wu, L.; Chen, L.; Liu, F.; Qi, X.; Ge, Y.; Shen, S. Remotely controlled drug release based on iron oxide nanoparticles for specific therapy of cancer. Colloids Surf. B Biointerfaces 2017, 152, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Wang, Z.; Ali, Z.; Zhang, H.; Wang, Y.; Xu, N.; Yin, H.; Sheng, F.; Hou, Y. A pH-responsive biomimetic drug delivery nanosystem for targeted chemo-photothermal therapy of tumors. Nano Res. 2022, 15, 4274–4284. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, Z.; Wang, X.; Deng, H.; Sun, L.; Lin, H.; Kang, F.; Zhang, Y.; Wang, Z.; Yang, W.; et al. Yolk–shell nanovesicles endow glutathione–responsive concurrent drug release and T1 MRI activation for cancer theranostics. Biomaterials 2020, 244, 119979. [Google Scholar] [CrossRef]
- Chen, A.; Lu, H.; Cao, R.; Zhu, Y.; Li, Y.; Ge, R.; Zhang, S.; Li, Y.; Xiao, L.; Su, L.; et al. A novel MMP-responsive nanoplatform with transformable magnetic resonance property for quantitative tumor bioimaging and synergetic chemo-photothermal therapy. Nano Today 2022, 45, 101524. [Google Scholar] [CrossRef]
- Cui, X.; Ruan, Q.; Zhuo, X.; Xia, X.; Hu, J.; Fu, R.; Li, Y.; Wang, J.; Xu, H. Photothermal Nanomaterials: A Powerful Light-to-Heat Converter. Chem. Rev. 2023, 123, 6891–6952. [Google Scholar] [CrossRef] [PubMed]
- Kasparis, G.; Sangnier, A.P.; Wang, L.; Efstathiou, C.; LaGrow, A.P.; Sergides, A.; Wilhelm, C.; Thanh, N.T.K. Zn doped iron oxide nanoparticles with high magnetization and photothermal efficiency for cancer treatment. J. Mater. Chem. B 2022, 11, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Boluda, A.N.; Vaquero, J.; Laurent, G.; Renault, G.; Bazzi, R.; Donnadieu, E.; Roux, S.; Fouassier, L.; Gazeau, F. Photothermal Depletion of Cancer-Associated Fibroblasts Normalizes Tumor Stiffness in Desmoplastic Cholangiocarcinoma. ACS Nano 2020, 14, 5738–5753. [Google Scholar] [CrossRef] [PubMed]
- Paściak, A.; Marin, R.; Abiven, L.; Pilch-Wróbel, A.; Misiak, M.; Xu, W.; Prorok, K.; Bezkrovnyi, O.; Marciniak, Ł.; Chanéac, C.; et al. Quantitative Comparison of the Light-to-Heat Conversion Efficiency in Nanomaterials Suitable for Photothermal Therapy. ACS Appl. Mater. Interfaces 2022, 14, 33555–33566. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, X.; Zhang, J.; Peng, C.; Shen, M.; Shi, X. Dendrimer-Stabilized Gold Nanoflowers Embedded with Ultrasmall Iron Oxide Nanoparticles for Multimode Imaging–Guided Combination Therapy of Tumors. Adv. Sci. 2018, 5, 1801612. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Zhou, B.; Zhou, F.; Murray, C.; Towner, R.A.; Smith, N.; Saunders, D.; Xie, G.; Chen, W.R. PEGylated reduced-graphene oxide hybridized with Fe3O4 nanoparticles for cancer photothermal-immunotherapy. J. Mater. Chem. B 2019, 7, 7406–7414. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, J.; Jin, H.; Yi, Y.; Shen, Y.; Zhang, X.; Liu, X.; Sun, Y.; Shi, W.; He, Y.; et al. Polypyrrole Nanosheets Prepared by Rapid In Situ Polymerization for NIR-II Photoacoustic-Guided Photothermal Tumor Therapy. Coatings 2023, 13, 1037. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, D.; Zeng, Y.; Wu, L.; Liu, X.; Liu, J. Nanocluster of superparamagnetic iron oxide nanoparticles coated with poly(dopamine) for magnetic field-targeting, highly sensitive MRI and photothermal cancer therapy. Nanotechnology 2015, 26, 115102. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Ding, Y.; Wang, K.; Xing, Z.; Sun, X.; Guo, W.; Hong, X.; Zhu, X.; Liu, Y. Enhanced photothermal-photodynamic therapy for glioma based on near-infrared dye functionalized Fe3O4 superparticles. Chem. Eng. J. 2020, 381, 122693. [Google Scholar] [CrossRef]
- Cao, Y.; Ren, Q.; Hao, R.; Sun, Z. Innovative strategies to boost photothermal therapy at mild temperature mediated by functional nanomaterials. Mater. Des. 2022, 214, 110391. [Google Scholar] [CrossRef]
- Huang, X.; Sheng, B.; Tian, H.; Chen, Q.; Yang, Y.; Bui, B.; Pi, J.; Cai, H.; Chen, S.; Zhang, J.; et al. Real-time SERS monitoring anticancer drug release along with SERS/MR imaging for pH-sensitive chemo-phototherapy. Acta Pharm. Sin. B 2023, 13, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Li, Z.; Ye, J.; Li, Z.; Fu, F.; Lin, S.-L.; Chang, C.A.; Yang, H.; Song, J. Magnetic targeted near–infrared II PA/MR imaging guided photothermal therapy to trigger cancer immunotherapy. Theranostics 2020, 10, 4997–5010. [Google Scholar] [CrossRef]
- Li, Y.; Kong, J.; Zhao, H.; Liu, Y. Synthesis of Multi-Stimuli Responsive Fe3O4 Coated with Diamonds Nanocomposite for Magnetic Assisted Chemo-Photothermal Therapy. Molecules 2023, 28, 1784. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hu, X.; Chen, L.; Wu, X.; Wang, D.; Wang, H.; Liang, C. Fe3O4@TiO2 Microspheres: Harnessing O2 Release and ROS Generation for Combination CDT/PDT/PTT/Chemotherapy in Tumours. Nanomaterials 2024, 14, 498. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Zhang, M.; Wu, F.; Zheng, T.; Sheng, B.; Liu, Y.; Shen, J.; Zhou, N.; Sun, Y. A theranostic nanocomposite with integrated black phosphorus nanosheet, Fe3O4@ MnO2-doped upconversion nanoparticles and chlorin for simultaneous multimodal imaging, highly efficient photodynamic and photothermal therapy. Chem. Eng. J. 2020, 391, 123525. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, Y.; Sun, L.; Wang, J.; Zhao, Z.; Huang, Z.; Mao, W.; Xue, R.; Chen, R.; Luo, J. Modulated ultrasmall γ-Fe2O3 nanocrystal assemblies for switchable magnetic resonance imaging and photothermal-ferroptotic-chemical synergistic cancer therapy. Adv. Funct. Mater. 2023, 33, 2211251. [Google Scholar] [CrossRef]
- Bao, J.; Guo, S.; Zu, X.; Zhuang, Y.; Fan, D.; Zhang, Y.; Shi, Y.; Ji, Z.; Cheng, J.; Pang, X. Polypyrrole-Coated Magnetite Vortex Nanoring for Hyperthermia-Boosted Photothermal/Magnetothermal Tumor Ablation Under Photoacoustic/Magnetic Resonance Guidance. Front. Bioeng. Biotechnol. 2021, 9, 721617. [Google Scholar] [CrossRef]
- Tsao, H.-Y.; Cheng, H.-W.; Kuo, C.-C.; Chen, S.-Y. Dual-Sensitive Gold-Nanocubes Platform with Synergistic Immunotherapy for Inducing Immune Cycle Using NIR-Mediated PTT/NO/IDO. Pharmaceuticals 2022, 15, 138. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Wang, M.; Liu, K.; Hoover, A.R.; Li, M.; Towner, R.A.; Mukherjee, P.; Zhou, F.; Qu, J.; et al. Synergistic interventional photothermal therapy and immunotherapy using an iron oxide nanoplatform for the treatment of pancreatic cancer. Acta Biomater. 2022, 138, 453–462. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, Q.; Wang, M.; Hoover, A.; Wang, X.; Zhou, F.; Towner, R.A.; Smith, N.; Saunders, D.; Song, J.; et al. Immunologically modified MnFe2O4 nanoparticles to synergize photothermal therapy and immunotherapy for cancer treatment. Chem. Eng. J. 2020, 396, 125239. [Google Scholar] [CrossRef]
- Daldrup-Link, H.E. Ten Things You Might Not Know about Iron Oxide Nanoparticles. Radiology 2017, 284, 616–629. [Google Scholar] [CrossRef]
- Ittrich, H.; Peldschus, K.; Raabe, N.; Kaul, M.; Adam, G. Superparamagnetic iron oxide nanoparticles in biomedicine: Applications and developments in diagnostics and therapy. Rofo 2013, 185, 1149–1166. [Google Scholar] [CrossRef]
- Alphandéry, E. Iron oxide nanoparticles as multimodal imaging tools. RSC Adv. 2019, 9, 40577–40587. [Google Scholar] [CrossRef]
- Kucheryavy, P.; He, J.; John, V.T.; Maharjan, P.; Spinu, L.; Goloverda, G.Z.; Kolesnichenko, V.L. Superparamagnetic Iron Oxide Nanoparticles with Variable Size and an Iron Oxidation State as Prospective Imaging Agents. Langmuir 2012, 29, 710–716. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, L.; Mo, C.; Zhou, X.; Chen, D.; He, Y.; He, H.; Kang, W.; Zhao, Y.; Jin, G. Polyethylene glycol-coated ultrasmall superparamagnetic iron oxide nanoparticles-coupled sialyl Lewis X nanotheranostic platform for nasopharyngeal carcinoma imaging and photothermal therapy. J. Nanobiotechnol. 2021, 19, 171. [Google Scholar] [CrossRef]
- Gu, L.; Ji, W. Recent progress on single-molecule localization microscopy. Biophys. Rep. 2021, 7, 365–376. [Google Scholar] [CrossRef]
- Shi, C.; Wu, J.B.; Pan, D. Review on Near-Infrared Heptamethine Cyanine Dyes as Theranostic Agents for Tumor Imaging, Targeting, and Photodynamic Therapy. J. Biomed. Opt. 2016, 21, 050901. [Google Scholar] [CrossRef]
- Chang, Y.T.; Li, X.; Kong, X.; Li, Y.; Liu, X.; Zhang, Y.; Tu, L.; Xue, B.; Wu, F.; Cao, D.; et al. A highly effective in vivo photothermal nanoplatform with dual imaging-guide therapy of cancer based on the charge reversal complex of dye and iron oxide. J. Mater. Chem. B 2015, 3, 8321–8327. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Pons, T.; Medintz, I.L.; Mattoussi, H. Biosensing with Luminescent Semiconductor Quantum Dots. Sensors 2006, 6, 925–953. [Google Scholar] [CrossRef]
- Lee, S.S.; Paliouras, M.; Trifiro, M.A. Functionalized Carbon Nanoparticles as Theranostic Agents and Their Future Clinical Utility in Oncology. Bioengineering 2023, 10, 108. [Google Scholar] [CrossRef]
- Wang, H.; Mu, Q.; Revia, R.; Wang, K.; Tian, B.; Lin, G.; Lee, W.; Hong, Y.-K.; Zhang, M. Iron oxide-carbon core-shell nanoparticles for dual-modal imaging-guided photothermal therapy. J. Control. Release 2018, 289, 70–78. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, M.Y.; Zhu, Q.; Chi, B.; Zeng, L.W.; Hu, J.M.; Shen, A.G. Monodispersed plasmonic prussian blue nanoparticles for zero-background SERS/mri-guided phototherapy. Nanoscale 2020, 12, 3292–3301. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Wang, H.; Hai, J.; Wang, B. Se Atom-Induced Synthesis of Concave Spherical Fe3O4@Cu2O Nanocrystals for Highly Efficient MRI–SERS Imaging-Guided NIR Photothermal Therapy. Part. Part. Syst. Charact. 2018, 35, 1800197. [Google Scholar] [CrossRef]
- Li, J.C.; Hu, Y.; Yang, J.; Wei, P.; Sun, W.J.; Shen, M.W.; Zhang, G.X.; Shi, X.Y. Hyaluronic acid-modified Fe3O4@Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomaterials 2015, 38, 10–21. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Deng, G.; Wang, Q.; Zhang, L.; Wang, Q.; Lu, J. Multifunctional PS@CS@Au-Fe3O4-FA Nanocomposites for CT, MR and Fluorescence Imaging Guided Targeted-Photothermal Therapy of Cancer Cells. J. Mater. Chem. B 2017, 5, 4221–4232. [Google Scholar] [CrossRef]
- Xia, J.; Yao, J.; Wang, L.V. Photoacoustic tomography: Principles and advances. Electromagn. Waves 2014, 147, 1–22. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Liu, X.-L.; Ma, H.; Li, G.; Li, Y.; Gao, F.; Peng, M.; Fan, H.M.; Liang, X.-J. Fe3O4–Pd Janus nanoparticles with amplified dual-mode hyperthermia and enhanced ROS generation for breast cancer treatment. Nanoscale Horiz. 2019, 4, 1450–1459. [Google Scholar] [CrossRef]
- Li, X.-D.; Liang, X.-L.; Yue, X.-L.; Wang, J.-R.; Li, C.-H.; Deng, Z.-J.; Jing, L.-J.; Lin, L.; Qu, E.-Z.; Wang, S.-M.; et al. Imaging guided photothermal therapy using iron oxide loaded poly(lactic acid) microcapsules coated with graphene oxide. J. Mater. Chem. B 2014, 2, 217–223. [Google Scholar] [CrossRef]
- Ke, H.; Wang, J.; Tong, S.; Jin, Y.; Wang, S.; Qu, E.; Bao, G.; Dai, Z. Gold nanoshelled liquid perfluorocarbon magnetic nanocapsules: A nanotheranostic platform for bimodal ultrasound/magnetic resonance imaging guided photothermal tumor ablation. Theranostics 2014, 4, 12–23. [Google Scholar] [CrossRef]
- Yang, Y.; Huangfu, L.; Li, H.; Yang, D. Research progress of hyperthermia in tumor therapy by influencing metabolic reprogramming of tumor cells. Int. J. Hyperth. 2023, 40, 2270654. [Google Scholar] [CrossRef]
- Urano, M. Invited Review: For the Clinical Application of Thermochemotherapy Given at Mild Temperatures. Int. J. Hyperth. 1999, 15, 79–107. [Google Scholar] [CrossRef]
- Jin, Z.; Dun, Y.; Xie, L.; Jiang, W.; Sun, X.; Hu, P.; Zheng, S.; Yu, Y. Preparation of doxorubicin-loaded porous iron Oxide@ polydopamine nanocomposites for MR imaging and synergistic photothermal-chemotherapy of cancer. Colloids Surf. B Biointerfaces 2021, 208, 112107. [Google Scholar] [CrossRef]
- Kwon, Y.M.; Je, J.; Cha, S.H.; Oh, Y.; Cho, W.H. Synergistic combination of chemo-phototherapy based on temozolomide/ICG-loaded iron oxide nanoparticles for brain cancer treatment. Oncol. Rep. 2019, 42, 1709–1724. [Google Scholar] [CrossRef]
- Hauser, A.K.; Mitov, M.I.; Daley, E.F.; McGarry, R.C.; Anderson, K.W.; Hilt, J.Z. Targeted iron oxide nanoparticles for the enhancement of radiation therapy. Biomaterials 2016, 105, 127–135. [Google Scholar] [CrossRef]
- Kwon, S.; Jung, S.; Baek, S.H. Combination Therapy of Radiation and Hyperthermia, Focusing on the Synergistic Anti-Cancer Effects and Research Trends. Antioxidants 2023, 12, 924. [Google Scholar] [CrossRef]
- Hu, R.; Zheng, M.; Wu, J.; Li, C.; Shen, D.; Yang, D.; Li, L.; Ge, M.; Chang, Z.; Dong, W. Core-Shell Magnetic Gold Nanoparticles for Magnetic Field-Enhanced Radio-Photothermal Therapy in Cervical Cancer. Nanomaterials 2017, 7, 111. [Google Scholar] [CrossRef]
- Fiorito, S.; Soni, N.; Silvestri, N.; Brescia, R.; Gavilán, H.; Conteh, J.S.; Mai, B.T.; Pellegrino, T. Fe3O4@Au@Cu2−xS Heterostructures Designed for Tri-Modal Therapy: Photo- Magnetic Hyperthermia and 64Cu Radio-Insertion. Small 2022, 18, 2200174. [Google Scholar] [CrossRef] [PubMed]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.-J.; Chen, Y.-C.; Huang, C.-C.; Yang, L.; Yu, J.; Huang, S.-W.; Lin, C.-H. Iron Hydroxide/Oxide-Reduced Graphene Oxide Nanocomposite for Dual-Modality Photodynamic and Photothermal Therapy In Vitro and In Vivo. Nanomaterials 2021, 11, 1947. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhou, J.Y. Chlorin e6-modified iron oxide nanoparticles for photothermal-photodynamic ablation of glioblastoma cells. Front. Bioeng. Biotechnol. 2023, 11, 1248283. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Ruan, L.; Wang, D.; Zhang, J.; Tang, J.; Guo, C.; Dou, R.; Zhou, M.; Hu, Y.; Chen, J. Boosting chemotherapy of bladder cancer cells by ferroptosis using intelligent magnetic targeting nanoparticles. Colloids Surf. B Biointerfaces 2024, 234, 113664. [Google Scholar] [CrossRef]
- Cun, J.-E.; Pan, Y.; Zhang, Z.; Lu, Y.; Li, J.; Pan, Q.; Gao, W.; Luo, K.; He, B.; Pu, Y. Photo-Enhanced Upcycling H2O2 into Hydroxyl Radicals by IR780-Embedded Fe3O4@MIL-100 for Intense Nanocatalytic Tumor Therapy. Biomaterials 2022, 287, 121687. [Google Scholar] [CrossRef]
- Anilkumar, T.S.; Lu, Y.-J.; Chen, J.-P. Optimization of the Preparation of Magnetic Liposomes for the Combined Use of Magnetic Hyperthermia and Photothermia in Dual Magneto-Photothermal Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 5187. [Google Scholar] [CrossRef]
- Lu, Q.; Dai, X.; Zhang, P.; Tan, X.; Zhong, Y.; Yao, C.; Song, M.; Song, G.; Zhang, Z.; Peng, G.; et al. Fe3O4@Au composite magnetic nanoparticles modified with cetuximab for targeted magneto-photothermal therapy of glioma cells. Int. J. Nanomed. 2018, 13, 2491–2505. [Google Scholar] [CrossRef]
- Naud, C.; Thébault, C.; Carrière, M.; Hou, Y.; Morel, R.; Berger, F.; Diény, B.; Joisten, H. Cancer treatment by magneto-mechanical effect of particles, a review. Nanoscale Adv. 2020, 2, 3632–3655. [Google Scholar] [CrossRef]
- Lopez, S.; Hallali, N.; Lalatonne, Y.; Hillion, A.; Antunes, J.C.; Serhan, N.; Clerc, P.; Fourmy, D.; Motte, L.; Carrey, J.; et al. Magneto-mechanical destruction of cancer-associated fibroblasts using ultra-small iron oxide nanoparticles and low frequency rotating magnetic fields. Nanoscale Adv. 2022, 4, 421–436. [Google Scholar] [CrossRef]
- Muzzi, B.; Albino, M.; Gabbani, A.; Omelyanchik, A.; Kozenkova, E.; Petrecca, M.; Innocenti, C.; Balica, E.; Lavacchi, A.; Scavone, F. Star-Shaped Magnetic-Plasmonic Au@Fe3O4 Nano-Heterostructures for Photothermal Therapy. ACS Appl. Mater. Interfaces 2022, 14, 29087–29098. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Mohsin, A.; Zaman, W.Q.; Zhang, Z.; Guan, W.; Chu, M.; Zhuang, Y.; Guo, M. Urchin-like Magnetic Microspheres for Cancer Therapy through Synergistic Effect of Mechanical Force, Photothermal and Photodynamic Effects. J. Nanobiotechnol. 2022, 20, 224. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Li, Z.; Chen, H.; Zeng, X.; Mei, L. An optimal portfolio of photothermal combined immunotherapy. Cell Rep. Phys. Sci. 2022, 3, 100898. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Gao, J. Biocompatible Iron Oxide Nanoparticles for Targeted Cancer Gene Therapy: A Review. Nanomaterials 2022, 12, 3323. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Prajapati, S.K.; Jain, A.; Singhal, R. Nano-formulated siRNA-based therapeutic approaches for cancer therapy. Nano Trends 2023, 1, 100006. [Google Scholar] [CrossRef]
- Wayteck, L.; Xiong, R.; Braeckmans, K.; De Smedt, S.C.; Raemdonck, K. Comparing photoporation and nucleofection for delivery of small interfering RNA to cytotoxic T cells. J. Control. Release 2017, 267, 154–162. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, G.; Xu, Y.; Gao, Y.; Mao, Q.; Zhang, Y.; Bai, L.; Li, W.; Wu, A.; Hu, W.; et al. Photoacoustic and magnetic resonance imaging-based gene and photothermal therapy using mesoporous nanoagents. Bioact. Mater. 2022, 9, 157–167. [Google Scholar] [CrossRef]

| Method | Nanoparticle | Size | Shape | Cell Line | Application | Ref. |
|---|---|---|---|---|---|---|
| Laser ablation | Fe3O4 | 15 nm | spherical, hexagonal | - | - | [16] |
| Galvanic reaction, etching | Copper–IONPs | 14 nm | porous, hollow | 4T1 cells L02 cells | chemodynamic, PTT, and chemotherapy | [17] |
| Flame spray pyrolysis | Carbon-encapsulated Au–Fe3O4 | 51.9–55.2 nm | spherical | esophageal cancer cells | MH and PTT | [18] |
| Single-pass aerosol route | Au–Fe3O4 | 24.7 nm | square-like | HEK 293 cells | CT—MRI dual-mode imaging, gene delivery, and PTT | [19] |
| Coprecipitation | Fe3O4-loaded alginate hydrogel | 61.3 nm | - | CT26 cells | PTT | [20] |
| Hydrothermal method | Ag@Fe3O4 | 250 nm | spherical | SKOV3 | PTT | [21] |
| Microwave-assisted polyol method | γ-Fe2O3 | 44–162 nm | nanoflowers | - | MH and PTT | [22] |
| Green synthesis | Au–IONP composite | 19 nm | spherical | HeLa cells | MH and PTT | [23] |
| Type of Modification | Mechanism of Action | Application in Cancer Therapy |
|---|---|---|
| Bandgap engineering | Narrowing of bandgap through doping-induced formation of energy states | Enhanced photothermal performance of NPs |
| Incorporation of elements that exhibit diverse conversion mechanisms | Combination of independent photothermal conversion mechanisms in hybrid NPs | Optimized absorption spectrum Multimodal imaging |
| Polymer coating on NPs with photothermal ability | Additional layer for light absorption and heat generation | Enhanced photothermal conversion efficiency; improved stability due to polymer shell, targeting, immune escape, drug loading, etc. |
| Photothermal organic dyes on NPs | Combination of two photothermal agents/photodynamic and photothermal agents | Optimized absorption spectrum; PTT–PDT therapy; fluorescence imaging |
| Nanocomposite | Functionalization | Responsiveness | Diagnostic Mode | Therapy Mode | Ref. |
|---|---|---|---|---|---|
| Fe3O4–GO | EGFR | magnetic targeting | - | chemo-PTT | [52] |
| MnFe2O4 | macrophage cell membrane, DOX | - | chemo-PTT | [63] | |
| PMP@USPIO | MMP9-sensitive peptides, DOX | MMP-9 | MRI | chemo-PTT | [65] |
| Au–USPIO | - | - | MRI/CT/ PA | PTT–RT | [70] |
| Fe3O4–rGO | - | - | MRI | PTT–immunotherapy | [71] |
| Fe3O4 | IR806 | - | - | PTT–PDT | [74] |
|
Fe3O4@Au@ Ag–GO | boronic ester DOX | pH-responsive | SERS/MRI | chemo-PTT | [76] |
| TiS2–IONPs | - | magnetic targeting | PA/MRI | PTT–immunotherapy | [77] |
| Fe3O4 |
nanodiamonds DOX | pH and NIR-responsive | MRI | chemo-PTT | [78] |
| Fe3O4@TiO2 | DOX |
magnetic targeting pH and NIR-responsive | - |
CDT/PDT/PTT/ chemotherapy | [79] |
| Fe3O4@MnO2 |
chlorin e6 black phosphorus | - | MRI/ fluorescence/PA/US | PTT–PDT | [80] |
| USPIO Fe2O3 | folic acid DOX | pH-responsive | MRI | PTT–chemotherapy–ferroptosis | [81] |
| Fe3O4@PPy–PEG | PPy | - | MRI/PA | MH–PTT | [82] |
| AuNPs–Fe3O4 |
GSNO/betanin 1-M-DT | pH-responsive | - | NO gas–PTT–immunotherapy | [83] |
| IMQ@IONs/ICG |
ICG/ imiquimod | - | MRI | PTT–immunotherapy | [84] |
| MnFe2O4 |
R837 ovalbumin | - | MRI | PTT–immunotherapy | [85] |
| Synergistic Therapy | Key Features | Mechanism of Action | Advantages |
|---|---|---|---|
| CT–PTT | Hyperthermia acts as a chemosensitizer, making chemotherapy more effective | Improves drug distribution by enhancing membrane fluidity and increasing vascular permeability | Better targeting of tumors, minimizing the damage to surrounding healthy tissue |
| Radiation Therapy–PTT | Reduced radiation resistance Potentially reduced radiation dose | IONPs amplify the generation of ROS within tumor cells, enhancing the efficacy of RT; hyperthermia increases blood flow and oxygenation | Enhances the effects of radiation, leading to more effective tumor control |
| Photodynamic Therapy–PTT | Improves the overall effectiveness of both therapies | Mild hyperthermia improves tumor oxygenation, enhancing PDT efficacy; ROS generated during PDT impair heat shock proteins, weakening their protective function against PTT | This synergy may result in fewer systemic side effects and lower doses than either therapy used alone |
| MH–PTT | The magnetic field penetrates deeper into the tissue compared to laser irradiation | Ensures more comprehensive tumor treatment by effectively targeting both surface and deeper tumor regions | Provides a dual thermal treatment that overcomes individual deficiencies |
| Immunotherapy–PTT | PTT enhances tumor immunogenicity | Iron overload triggers the polarization of macrophages towards an M1 phenotype | Enhances immune memory against tumor recurrence and metastasis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grancharova, T.; Zagorchev, P.; Pilicheva, B. Iron Oxide Nanoparticles: Parameters for Optimized Photoconversion Efficiency in Synergistic Cancer Treatment. J. Funct. Biomater. 2024, 15, 207. https://doi.org/10.3390/jfb15080207
Grancharova T, Zagorchev P, Pilicheva B. Iron Oxide Nanoparticles: Parameters for Optimized Photoconversion Efficiency in Synergistic Cancer Treatment. Journal of Functional Biomaterials. 2024; 15(8):207. https://doi.org/10.3390/jfb15080207
Chicago/Turabian StyleGrancharova, Tsenka, Plamen Zagorchev, and Bissera Pilicheva. 2024. "Iron Oxide Nanoparticles: Parameters for Optimized Photoconversion Efficiency in Synergistic Cancer Treatment" Journal of Functional Biomaterials 15, no. 8: 207. https://doi.org/10.3390/jfb15080207





