Enhancing Esthetics in Direct Dental Resin Composite: Investigating Surface Roughness and Color Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Contact Angle Measurements
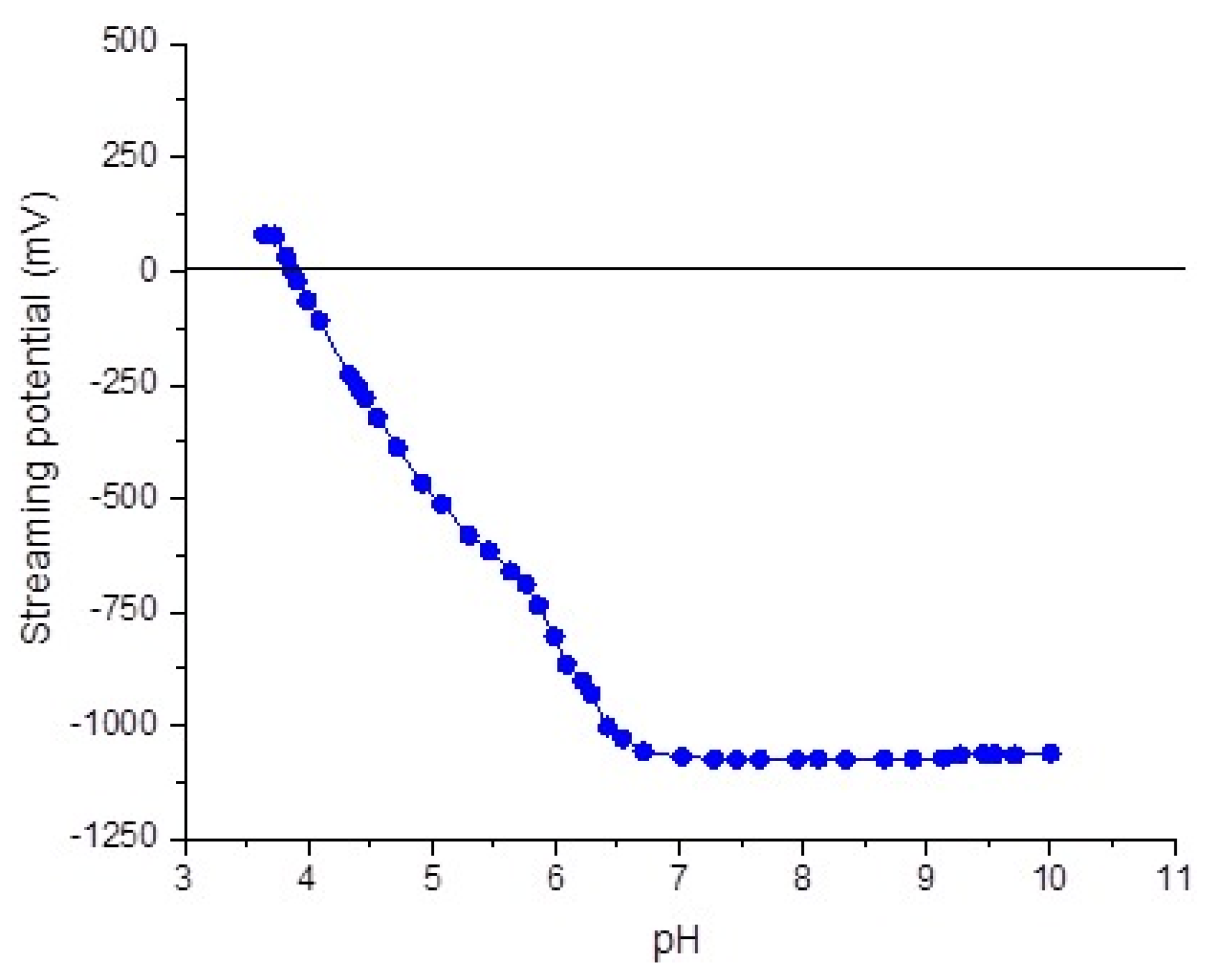
2.3. The Streaming Potential Measurements
2.4. Roughness Analysis
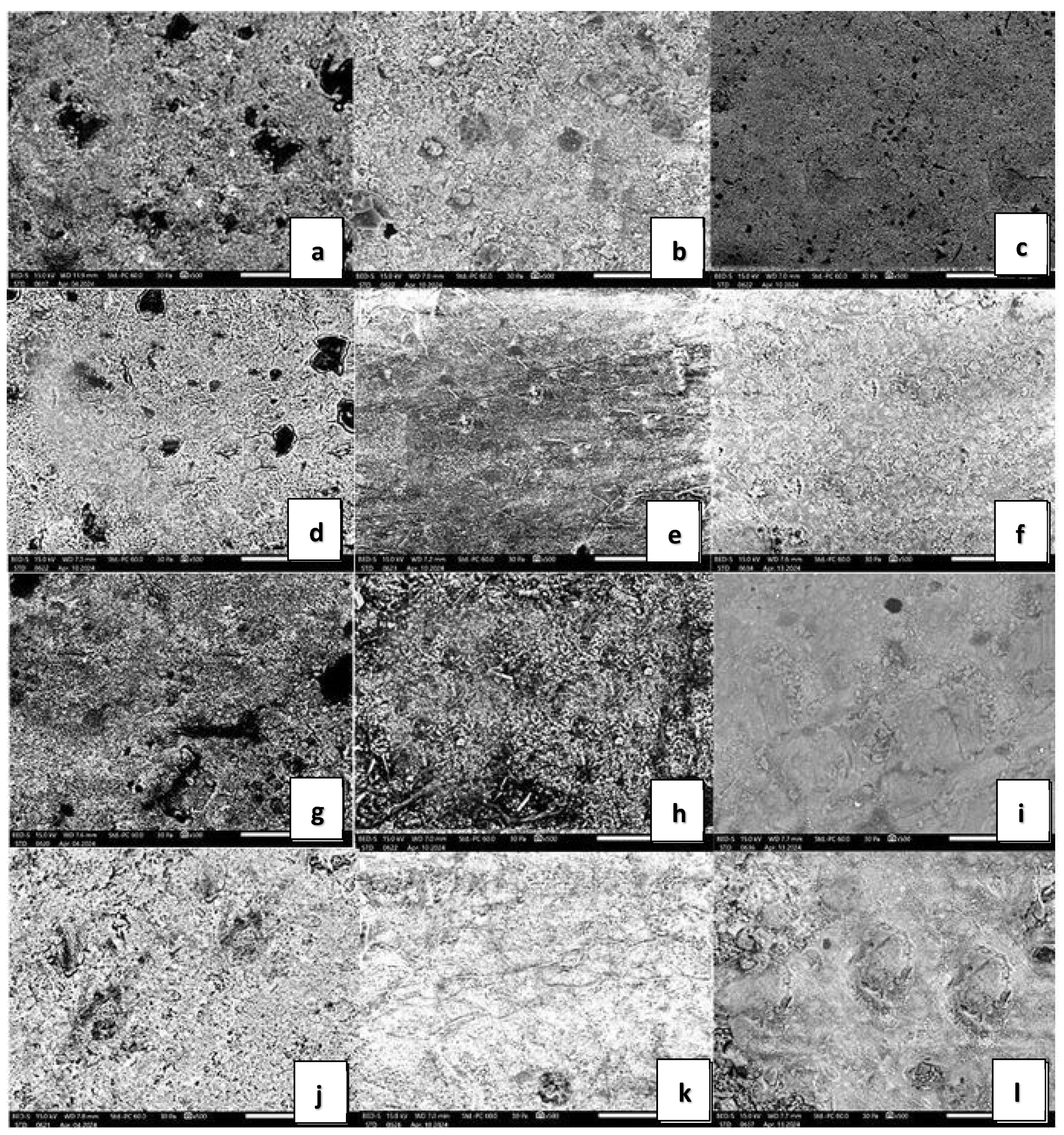
2.5. SEM Analysis
2.6. Color Measurement
2.7. Adherence of Red Wine, Black Coffee, and Coca-Cola
2.8. pH Measurement
2.9. Statistical Analysis
3. Results
3.1. Contact Angle
3.2. The Streaming Potential
3.3. Roughness
3.4. SEM Analysis
3.5. Color Metrics and the Impact of Various Substances (Red Wine, Black Coffee, and Coca-Cola)
3.6. pH Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cazzaniga, G.; Ottobelli, M.; Ionescu, A.; Garcia-Godoy, F.; Brambilla, E. Surface Properties of Resin-Based Composite Materials and Biofilm Formation: A Review of the Current Literature. Am. J. Dent. 2015, 28, 311–320. [Google Scholar]
- Sturz, C.R.C.; Faber, F.-J.; Scheer, M.; Rothamel, D.; Neugebauer, J. Effects of Various Chair-Side Surface Treatment Methods on Dental Restorative Materials with Respect to Contact Angles and Surface Roughness. Dent. Mater. J. 2015, 34, 796–813. [Google Scholar] [CrossRef]
- Azam, M.; Khan, A.; Muzzafar, D.; Faryal, R.; Siddiqi, S.; Ahmad, R.; Chauhdry, A.; Rehman, I. Structural, Surface, in Vitro Bacterial Adhesion and Biofilm Formation Analysis of Three Dental Restorative Composites. Materials 2015, 8, 3221–3237. [Google Scholar] [CrossRef]
- GBD 2017 Oral Disorders Collaborators; Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef]
- Sava-Rosianu, R.; Campus, G.; Matichescu, A.; Balean, O.; Dumitrache, M.A.; Lucaciu, P.O.; Daguci, L.; Barlean, M.C.; Maricutoiu, L.; Postolache, M.; et al. Caries Prevalence Associated with Oral Health-Related Behaviors among Romanian Schoolchildren. Int. J. Environ. Res. Public Health 2021, 18, 6515. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, Y.; Weir, M.D.; Xu, H.H.K.; Bai, Y.; Melo, M.A.S. Current Insights into the Modulation of Oral Bacterial Degradation of Dental Polymeric Restorative Materials. Materials 2017, 10, 507. [Google Scholar] [CrossRef]
- Elgezawi, M.; Haridy, R.; Abdalla, M.A.; Heck, K.; Draenert, M.; Kaisarly, D. Current Strategies to Control Recurrent and Residual Caries with Resin Composite Restorations: Operator- and Material-Related Factors. J. Clin. Med. 2022, 11, 6591. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Untreated Caries: A Systematic Review and Metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef]
- Yadav, R. Fabrication, Characterization, and Optimization Selection of Ceramic Particulate Reinforced Dental Restorative Composite Materials. Polym. Polym. Compos. 2022, 30, 096739112110627. [Google Scholar] [CrossRef]
- Yadav, R.; Meena, A. Mechanical and Two-body Wear Characterization of Micro-nano Ceramic Particulate Reinforced Dental Restorative Composite Materials. Polym. Compos. 2022, 43, 467–482. [Google Scholar] [CrossRef]
- Pietrokovski, Y.; Zeituni, D.; Schwartz, A.; Beyth, N. Comparison of Different Finishing and Polishing Systems on Surface Roughness and Bacterial Adhesion of Resin Composite. Materials 2022, 15, 7415. [Google Scholar] [CrossRef]
- Bohinc, K.; Tintor, E.; Kovačević, D.; Vidrih, R.; Zore, A.; Abram, A.; Kojić, Ž.; Obradović, M.; Veselinović, V.; Dolić, O. Bacterial Adhesion on Glass–Ionomer Cements and Micro/Nano Hybrid Composite Dental Surfaces. Coatings 2021, 11, 235. [Google Scholar] [CrossRef]
- Liber-Kneć, A.; Łagan, S. Surface Testing of Dental Biomaterials—Determination of Contact Angle and Surface Free Energy. Materials 2021, 14, 2716. [Google Scholar] [CrossRef]
- Guler, S.; Unal, M. The Evaluation of Color and Surface Roughness Changes in Resin Based Restorative Materials with Different Contents After Waiting in Various Liquids: An SEM and AFM Study. Microsc. Res Tech. 2018, 81, 1422–1433. [Google Scholar] [CrossRef]
- Rohym, S.; Tawfeek, H.E.M.; Kamh, R. Effect of Coffee on Color Stability and Surface Roughness of Newly Introduced Single Shade Resin Composite Materials. BMC Oral. Health 2023, 23, 236. [Google Scholar] [CrossRef] [PubMed]
- HerculiteTM XRV UltraTM. Available online: https://www.kerrdental.com/en-eu/dental-restoration-products/herculite-xrv-ultra-dental-composites (accessed on 26 May 2024).
- G-ænial A’CHORD|GC Europe N.V. Available online: https://www.gc.dental/europe/en/products/gaenialachord (accessed on 26 May 2024).
- OMNICHROMA Composite—Tokuyama Dental America. Available online: https://www.tokuyama-us.com/omnichroma-dental-composite/ (accessed on 26 May 2024).
- Nilsen, B.W.; Mouhat, M.; Haukland, T.; Örtengren, U.T.; Mercer, J.B. Heat Development in the Pulp Chamber During Curing Process of Resin-Based Composite Using Multi-Wave LED Light Curing Unit. Clin. Cosmet. Investig. Dent. 2020, 12, 271–280. [Google Scholar] [CrossRef]
- Choi, J.-W.; Lee, M.-J.; Oh, S.-H.; Kim, K.-M. Changes in the Physical Properties and Color Stability of Aesthetic Restorative Materials Caused by Various Beverages. Dent. Mater. J. 2019, 38, 33–40. [Google Scholar] [CrossRef]
- El Gezawi, M.; Kaisarly, D.; Al-Saleh, H.; ArRejaie, A.; Al-Harbi, F.; Kunzelmann, K. Degradation Potential of Bulk versus Incrementally Applied and Indirect Composites: Color, Microhardness, and Surface Deterioration. Oper. Dent. 2016, 41, e195–e208. [Google Scholar] [CrossRef]
- Magdy, N.; Kola, M.; Alqahtani, H.; Alqahtani, M.; Alghmlas, A. Evaluation of Surface Roughness of Different Direct Resin-Based Composites. J. Int. Soc. Prevent. Communit. Dent. 2017, 7, 104. [Google Scholar] [CrossRef]
- Sivtseva, P. Polishing Devices and Techniques on Resin-Based Composite Restorations—Systematic Review. Master’s Thesis, Universidade Fernando Pessoa, Porto, Portugal, 2021. [Google Scholar]
- Staehlke, S.; Brief, J.; Senz, V.; Eickner, T.; Nebe, J.B. Optimized Gingiva Cell Behavior on Dental Zirconia as a Result of Atmospheric Argon Plasma Activation. Materials 2023, 16, 4203. [Google Scholar] [CrossRef]
- Xu, J.; He, J.; Shen, Y.; Zhou, X.; Huang, D.; Gao, Y.; Haapasalo, M. Influence of Endodontic Procedure on the Adherence of Enterococcus Faecalis. J. Endod. 2019, 45, 943–949. [Google Scholar] [CrossRef]
- Staehlke, S.; Oster, P.; Seemann, S.; Kruse, F.; Brief, J.; Nebe, B. Laser Structured Dental Zirconium for Soft Tissue Cell Occupation—Importance of Wettability Modulation. Materials 2022, 15, 732. [Google Scholar] [CrossRef]
- Drop Shape Analyzer-DSA25E. Available online: https://www.kruss-scientific.com/en/products-services/products/dsa25e?gad_source=1&gclid=CjwKCAjwupGyBhBBEiwA0UcqaCFF6lTDfl_cmPOckBCExqhnQ0EBU0jj8Tx2FBujuWfIXqP8SGMbxBoCBX8QAvD_BwE (accessed on 26 May 2024).
- Infrastructure—Institute of Macromolecular Chemistry “Petru Poni” Iasi. Available online: https://icmpp.ro/laboratories/l4/infrastructure.php (accessed on 26 May 2024).
- Daniyan, I.; Tlhabadira, I.; Mpofu, K.; Adeodu, A. Development of Numerical Models for the Prediction of Temperature and Surface Roughness during the Machining Operation of Titanium Alloy (Ti6Al14V). Acta Polytech. 2020, 60, 369–390. [Google Scholar] [CrossRef]
- VITA. Easyshade® V—Perfect Shade Determination, Maximum Reliability. Available online: https://www.vita-zahnfabrik.com/pdb_GG2G50G200_en,98478.html (accessed on 26 May 2024).
- Colorimetry—Part 4: CIE 1976 L*a*b* Colour Space|CIE. Available online: https://cie.co.at/publications/colorimetry-part-4-cie-1976-lab-colour-space-0 (accessed on 26 May 2024).
- Backes, C.N.; França, F.M.G.; Turssi, C.P.; Amaral, F.L.B.D.; Basting, R.T. Color Stability of a Bulk-Fill Composite Resin Light-Cured at Different Distances. Braz. Oral. Res. 2020, 34, e119. [Google Scholar] [CrossRef]
- Poggio, C.; Vialba, L.; Marchioni, R.; Colombo, M.; Pietrocola, G. Esthetic Restorative Materials and Glass Ionomer Cements: Influence of Acidic Drink Exposure on Bacterial Adhesion. Eur. J. Dent. 2018, 12, 204–209. [Google Scholar] [CrossRef]
- Chakravarthy, Y.; Clarence, S. The Effect of Red Wine on Colour Stability of Three Different Types of Esthetic Restorative Materials: An in Vitro Study. J. Conserv. Dent. 2018, 21, 319. [Google Scholar] [CrossRef]
- Ishii, R.; Takamizawa, T.; Tsujimoto, A.; Suzuki, S.; Imai, A.; Barkmeier, W.; Latta, M.; Miyazaki, M. Effects of Finishing and Polishing Methods on the Surface Roughness and Surface Free Energy of Bulk-Fill Resin Composites. Oper. Dent. 2020, 45, E91–E104. [Google Scholar] [CrossRef]
- St-Pierre, L.; Martel, C.; Crépeau, H.; Vargas, M. Influence of Polishing Systems on Surface Roughness of Composite Resins: Polishability of Composite Resins. Oper. Dent. 2019, 44, E122–E132. [Google Scholar] [CrossRef]
- Alkhadim, Y.K.; Hulbah, M.J.; Nassar, H.M. Color Shift, Color Stability, and Post-Polishing Surface Roughness of Esthetic Resin Composites. Materials 2020, 13, 1376. [Google Scholar] [CrossRef] [PubMed]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial Adhesion on Orthopedic Implants. Adv. Colloid Interface Sci. 2020, 283, 102228. [Google Scholar] [CrossRef] [PubMed]
- Fugolin, A.P.P.; Pfeifer, C.S. New Resins for Dental Composites. J. Dent. Res. 2017, 96, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, J.; Yu, M.; Jin, C.; Huang, C. Effect of Aging and Bleaching on the Color Stability and Surface Roughness of a Recently Introduced Single-Shade Composite Resin. J. Dent. 2024, 143, 104917. [Google Scholar] [CrossRef] [PubMed]
- Hahnel, S.; Ionescu, A.C.; Cazzaniga, G.; Ottobelli, M.; Brambilla, E. Biofilm Formation and Release of Fluoride from Dental Restorative Materials in Relation to Their Surface Properties. J. Dent. 2017, 60, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Bilgili, D.; Dündar, A.; Barutçugil, Ç.; Tayfun, D.; Özyurt, Ö.K. Surface Properties and Bacterial Adhesion of Bulk-Fill Composite Resins. J. Dent. 2020, 95, 103317. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wang, X.; Gao, X.; Chen, F.; Liang, X.; Li, D. Effects of Surface Properties of Polymer-Based Restorative Materials on Early Adhesion of Streptococcus Mutans in Vitro. J. Dent. 2016, 54, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.G.; Soares, C.J.; Maia, T.S.; Bicalho, A.A.; Barbosa, T.P.; Costa, H.L.; Menezes, M.S. Effect of Acidic Drinks on Shade Matching, Surface Topography, and Mechanical Properties of Conventional and Bulk-Fill Composite Resins. J. Prosthet. Dent. 2019, 121, 868.e1–868.e8. [Google Scholar] [CrossRef] [PubMed]
- ÇeliK, N.; Sağsöz, Ö.; Gündoğdu, M. Farkli içeceklerin posterior kompozitlerin renk değişikliği ve yüzey pürüzlülüğü üzerine etkisinin değerlendirilmesi. Atatürk Üniv. Diş Hekim. Fakültesi Derg. 2017, 27, 27–33. [Google Scholar] [CrossRef]
- Barutcigil, Ç.; Yıldız, M. Intrinsic and Extrinsic Discoloration of Dimethacrylate and Silorane Based Composites. J. Dent. 2012, 40 (Suppl. S1), e57–e63. [Google Scholar] [CrossRef]
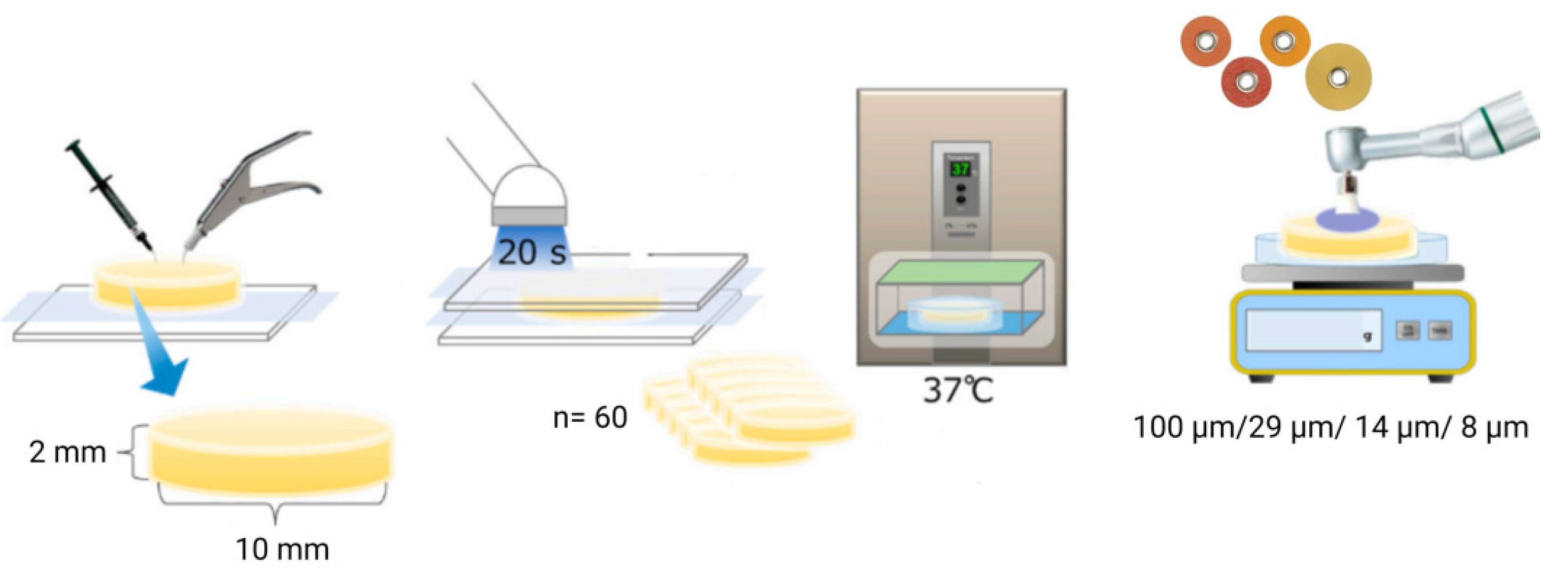



| Product Name | Type | Resin Matrix | Filler Content | Color | Lot Number | Manufacturer |
|---|---|---|---|---|---|---|
| Herculite Ultra XRV [16] | Nanohybrid resin composite | Bis-GMA, TEGDMA | 78% wt, 59% vol | A2 | 10198512 | Kerr, Italy |
| G-Aenial A’CHORD [17] | Hybrid resin composite | Bis-MEPP-based resin | 82% wt, 56% vol | Bleach | 230328C | Gc Tokyo, Japan |
| Omnichroma [18] | Supra-nanospherical resin composite | UDMA, TEGDMA | 79% wt, 68% vol | Universal | 123E83 | Tokuyama-Dental, Japan |
| Resin Composite | Mean Contact Angle | Standard Deviation |
|---|---|---|
| Herculite Ultra | 79.46 | 6.52 |
| G-ænial A’CHORD | 73.22 | 2.71 |
| Omnichroma | 64.94 | 3.08 |
| Type of Resin Composite | Initial SR (SD) | 10 Days Coffee Immersion (SD) | Paired t-Test | 10 Days Red Wine Immersion (SD) | Paired t-Test | 10 Days Coca-Cola Immersion (SD) | Paired t-Test |
|---|---|---|---|---|---|---|---|
| Herculite UltraXRV | 1.66 (±0.45) | 1.36 (±0.23) | t (14) = −4.28, p < 0.001 | 2.28 (±0.77) | t (14) = 3.42, p < 0.01 | 1.63 (±0.26) | t (14) = 1.75, p = 0.098 |
| G-ænial A’CHORD | 1.84 (±0.33) | 2.33 (±0.62) | t (14) = 4.12, p < 0.001 | 1.52 (±0.63) | t (14) = −2.31, p = 0.037 | 3.98 (±0.13) | t (14) = 9.53, p < 0.001 |
| Omnichroma | 1.66 (±0.09) | 1.53 (±0.38) | t (14) = 4.15, p < 0.001 | 1.15 (±0.08) | t (14) = 2.07, p = 0.055 | 1.21 (±0.31) | t (14) = 2.28, p = 0.039 |
| ∆E 10 Days Coffee Immersion | ∆E 10 Days Red Wine Immersion | ∆E 10 Days Coca-Cola Immersion | |
|---|---|---|---|
| Herculite UltraXRV | 4.82 (±1.30) | 5.95 (±0.34) | 3.99 (±0.52) |
| G-ænial A’CHORD | 15.28 (±0.74) | 12.51 (±0.38) | 2.79 (±0.16) |
| Omnichroma | 10.84 (±1.03) | 5.33 (±0.27) | 1.28 (±0.44) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajdu, A.I.; Dumitrescu, R.; Balean, O.; Lalescu, D.V.; Buzatu, B.L.R.; Bolchis, V.; Floare, L.; Utu, D.; Jumanca, D.; Galuscan, A. Enhancing Esthetics in Direct Dental Resin Composite: Investigating Surface Roughness and Color Stability. J. Funct. Biomater. 2024, 15, 208. https://doi.org/10.3390/jfb15080208
Hajdu AI, Dumitrescu R, Balean O, Lalescu DV, Buzatu BLR, Bolchis V, Floare L, Utu D, Jumanca D, Galuscan A. Enhancing Esthetics in Direct Dental Resin Composite: Investigating Surface Roughness and Color Stability. Journal of Functional Biomaterials. 2024; 15(8):208. https://doi.org/10.3390/jfb15080208
Chicago/Turabian StyleHajdu, Adrian Ioan, Ramona Dumitrescu, Octavia Balean, Dacian Virgil Lalescu, Berivan Laura Rebeca Buzatu, Vanessa Bolchis, Lucian Floare, Diana Utu, Daniela Jumanca, and Atena Galuscan. 2024. "Enhancing Esthetics in Direct Dental Resin Composite: Investigating Surface Roughness and Color Stability" Journal of Functional Biomaterials 15, no. 8: 208. https://doi.org/10.3390/jfb15080208
APA StyleHajdu, A. I., Dumitrescu, R., Balean, O., Lalescu, D. V., Buzatu, B. L. R., Bolchis, V., Floare, L., Utu, D., Jumanca, D., & Galuscan, A. (2024). Enhancing Esthetics in Direct Dental Resin Composite: Investigating Surface Roughness and Color Stability. Journal of Functional Biomaterials, 15(8), 208. https://doi.org/10.3390/jfb15080208













