Applications of Bioactive Strontium Compounds in Dentistry
Abstract
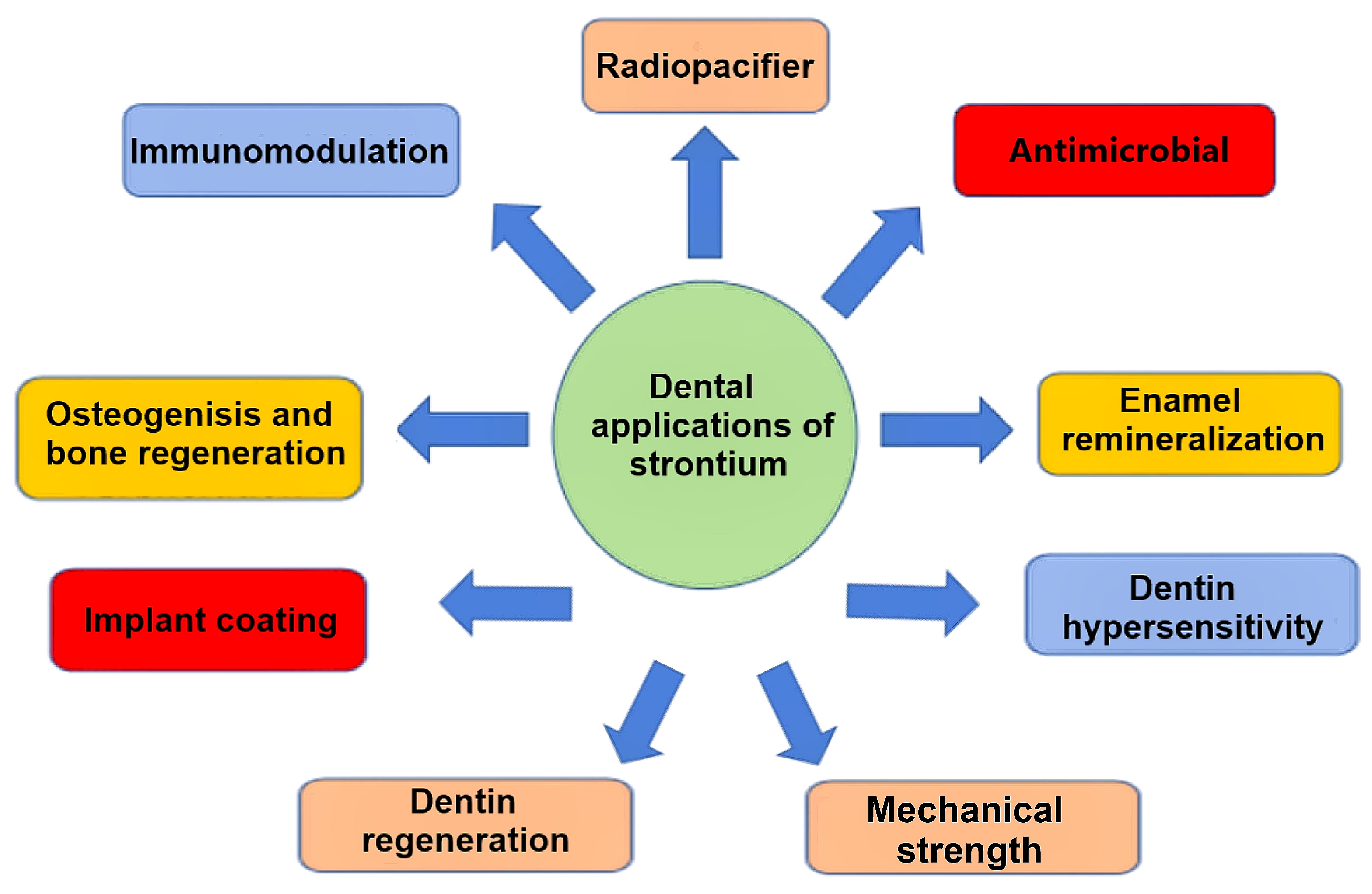
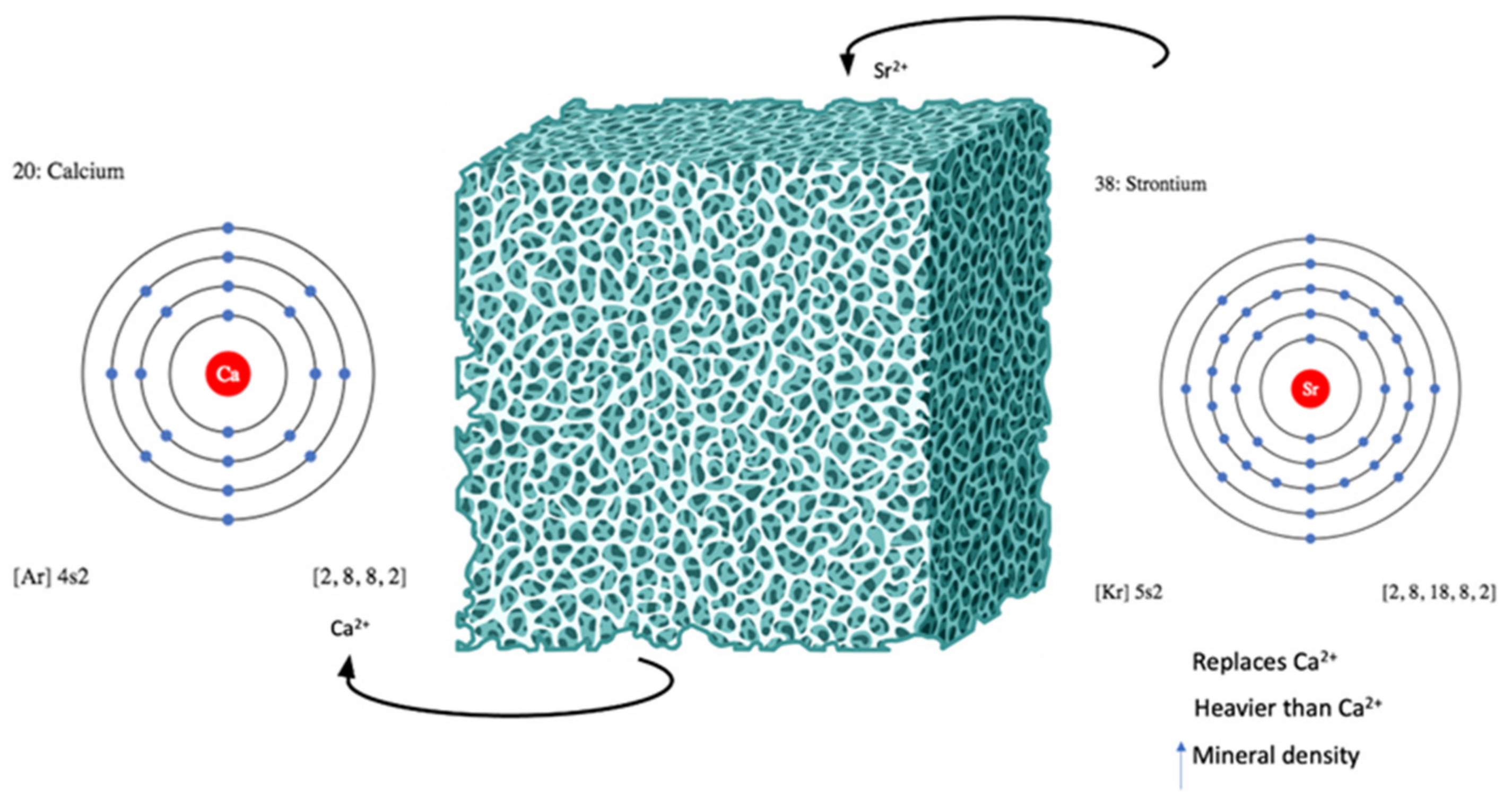
1. Introduction
2. Radiopacifying Properties of Strontium Compounds
3. Antimicrobial Effects of Strontium Compounds
4. Enamel Remineralization with Strontium Compounds
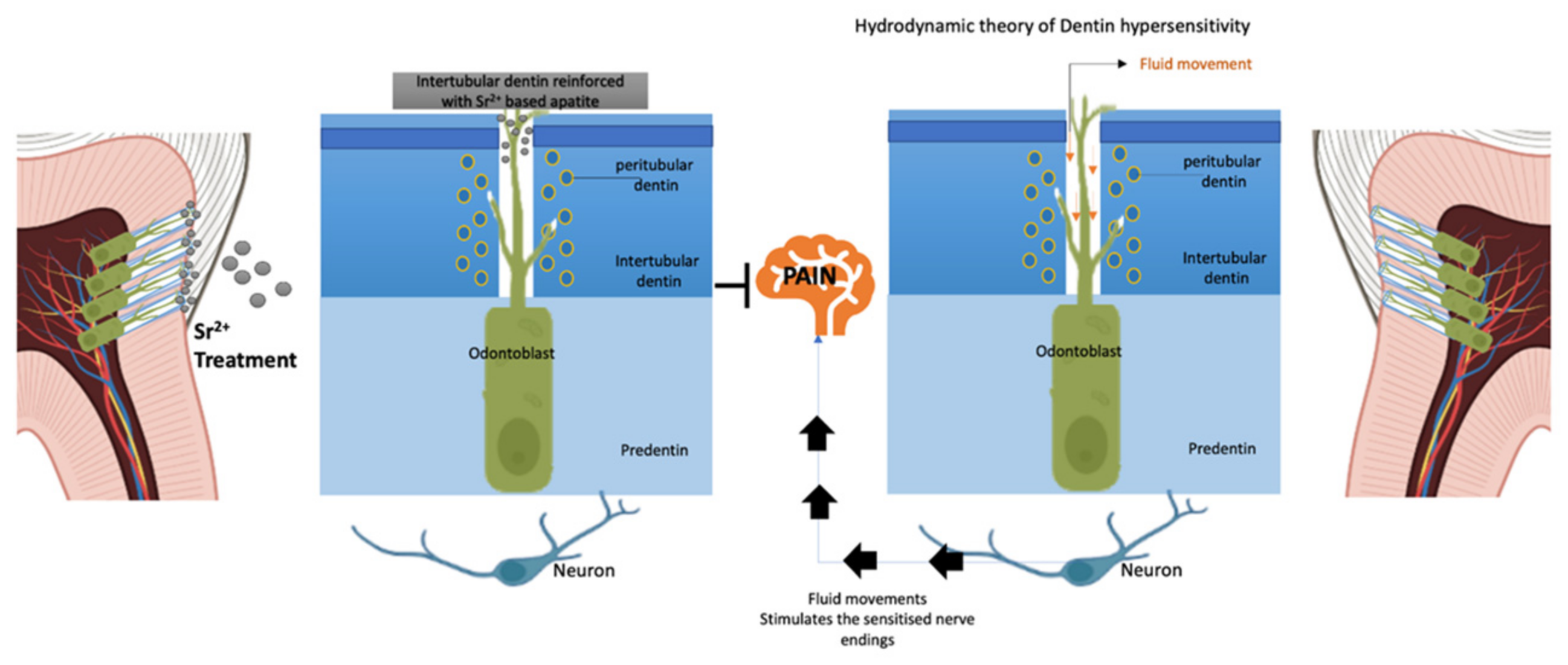
5. Managing Dentin Hypersensitivity with Strontium Compounds
| Study | Intervention | Outcomes of SEM Analysis |
|---|---|---|
| Kodaka et al., 2001 [69] | Sr chloride-based desensitizing toothpaste | 91.45% dentinal occlusion after 2 weeks. The occluding material contained artificial silica abrasive within the dentin sludge. |
| Arrais et al., 2003 [72] | Sr chloride-based desensitizing toothpaste | 80.1% dentinal occlusion after 7 days. Deposition of crystal-like structures within the dentinal tubules consisting of Ca carbonate, the abrasive system of the dentifrice. |
| Banfield et al., 2004 [70] | Sr acetate-based desensitizing toothpaste Sr chloride-based desensitizing toothpaste | 90% dentinal occlusion immediately. The occluding material was artificial silica abrasive. >70% dentinal occlusion immediately. The occluding material was artificial silica abrasive. |
| Oberg et al., 2009 [67] | 10% Sr chloride gel | Open and partially obliterated dentin tubules like the no treatment group. Only traces of Sr were detected in the peritubular dentin deposits. |
| Saeki et al., 2016 [66] | Sr acetate-based desensitizing toothpaste | Clear thin layer of silicon covered the dentine surface and openings of dentine tubules. |
| 10% Sr acetate solution | Thick Sr-containing layer reaching 20 µm into dentinal tubules. After specimens were soaked in DI water, Sr-containing layer could not be detected. | |
| Sr chloride-based desensitizing toothpaste | 50.54% dentinal occlusion after 7 days. The occlusion material was not reported. |
| Study | Efficacy of Sr-Containing Agents in DH Treatment |
|---|---|
| Martins et al., 2020 [81] | Sr was effective only for tactile stimulus relief. |
| Hu et al., 2019 [86] | Similar effects of Sr compared to fluoride, placebo, and potassium-containing toothpastes. |
| Cruz et al., 2019 [87] | Sr when not combined with potassium was no better than the negative control. |
| Hu et al., 2018 [85] | Sr when not combined with potassium had no desensitizing activity. |
| Bae et al., 2015 [84] | There was no statistically significant difference between Sr-containing toothpaste and placebo. |
| West et al., 2015 [83] | Sr acetate had equivocal pain-relieving effects when compared to arginine and was more effective than fluoride control. There is a lack of high-quality data supporting the use of Sr chloride salts for pain relief in dentine hypersensitivity; additional research is needed to determine whether this salt is useful. |
| Karim et al., 2013 [82] | There is inadequate evidence to state whether Sr salts per se are effective in reducing DH. |
6. Dentin Regeneration with Strontium Compounds
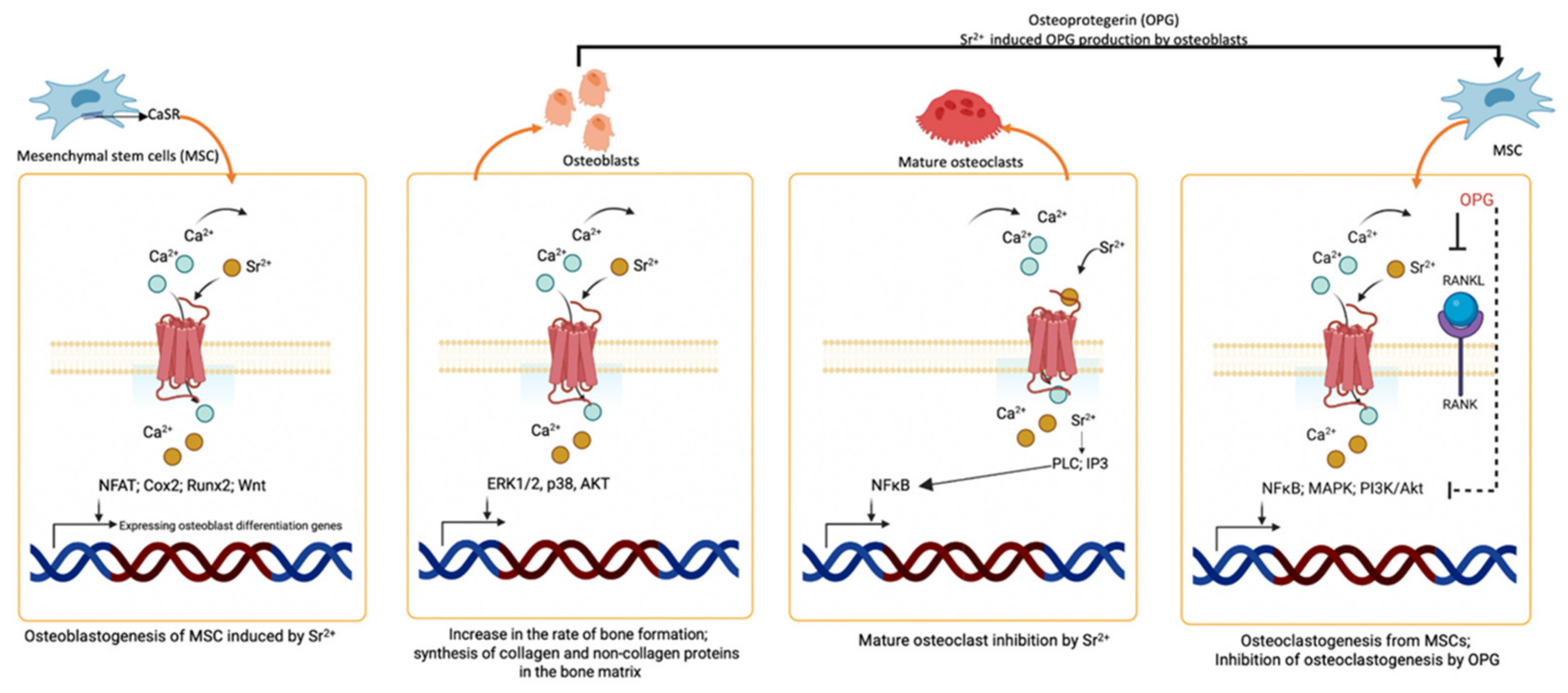
7. Osteogenic Effect and Bone Repair Potential of Strontium Compounds
8. Strontium and Implant Coatings
| Materials | Material Characteristics | Biological Characteristics | Reference |
|---|---|---|---|
| Sr-incorporated HA (SrHA) |
|
| Dai et al., 2021 [127] |
|
| Jiang et al., 2022 [98] | |
| SrHA with natural and synthetic polymers |
|
| Wang et al., 2020 [97] |
|
| Tsai et al., 2018 [104] | |
| Sr with bioactive glass |
|
| Jia et al., 2017 [94]; Zhang et al., 2014 [128] |
| Sr incorporation on dental implants |
|
| Zhao et al., 2021 [129] |
|
| Zhou et al., 2019 [130]; Choi et al., 2018 [131] | |
|
| Okuzu et al., 2021 [132] | |
|
| Offermanns et al., 2018 [133] | |
|
| Wang et al., 2019 [134] | |
|
| Chang et al., 2021 [135] | |
| Sr with hormone |
|
| Goker et al., 2018 [136] |
9. The Influence of Strontium on Mechanical Properties
10. Immunomodulatory Potential of Strontium
11. Future Perspectives
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davy, H. XXIII. Electro-chemical researches, on the decomposition of the earths; with observations on the metals obtained from the alkaline earths, and on the amalgam procured from ammonia. Philos. Trans. R. Soc. Lond. 1808, 98, 333–370. [Google Scholar]
- Partington, J. Early history of strontium. In Handbook of Stable Strontium; Springer: New York, NY, USA, 1981; pp. 1–9. [Google Scholar]
- Rana, R.; Höcker, M.; Myers, E.G. Atomic masses of strontium and ytterbium. Phys. Rev. A 2012, 86, 050502. [Google Scholar] [CrossRef]
- Amata, R.; Diamond, G.L.; Dorsey, A.; Fransen, M.E. Toxicological Profile for Strontium; U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2004. [Google Scholar]
- Fleischer, M. Recent Estimates of the Abundances of the Elements in the Earth’s Crust; US Department of the Interior, Geological Survey: Reston, VA, USA, 1953. [Google Scholar]
- Bronner, F. Dynamics and Function of Calcium in Mineral Metabolism; Comar, C.L., Bronner, F., Eds.; Academic Press: New York, NY, USA, 1964. [Google Scholar]
- Pan, H.-B.; Li, Z.-Y.; Wang, T.; Lam, W.; Wong, C.; Darvell, B.; Luk, K.; Hu, Y.; Lu, W. Nucleation of strontium-substituted apatite. Cryst. Growth Des. 2009, 9, 3342–3345. [Google Scholar] [CrossRef]
- Heurich, E.; Beyer, M.; Jandt, K.D.; Reichert, J.; Herold, V.; Schnabelrauch, M.; Sigusch, B.W. Quantification of dental erosion—A comparison of stylus profilometry and confocal laser scanning microscopy (CLSM). Dent. Mater. 2010, 26, 326–336. [Google Scholar] [CrossRef]
- Papillon, M. Recherches experimentales sur les modifications de la composition immediate des os. C. R. Acad. Sci. 1870, 71, 372–374. [Google Scholar]
- Schoenberg, H.P. Extent of strontium substitution for calcium in hydroxyapatite. Biochim. Biophys. Acta 1963, 75, 96–103. [Google Scholar] [CrossRef]
- Harrison, G.; Lumsden, E.; Raymond, W.; Sutton, A.; Boyd, J.; Neuman, W.; Hodge, H. On the mechanisms of skeletal fixation of strontium. Parts I and II. Arch. Biochem. Biophys. 1959, 80, 97–113. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Querido, W.; Caldas, R.J.; Campos, A.P.; Abraçado, L.G.; Farina, M. Strontium is incorporated in different levels into bones and teeth of rats treated with strontium ranelate. Calcif. Tissue Int. 2012, 91, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Dow, E.C.; Stanbury, J.B. Strontium and calcium metabolism in metabolic bone diseases. J. Clin. Investig. 1960, 39, 885–903. [Google Scholar] [CrossRef]
- D’Onofrio, A.; Kent, N.W.; Shahdad, S.A.; Hill, R.G. Development of novel strontium containing bioactive glass based calcium phosphate cement. Dent. Mater. 2016, 32, 703–712. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Sun, X.; Chen, X.; Xie, K.; Lin, M.; Yang, G.; Xu, S.; Xia, W.; Gou, Z. Preparation and in vitro evaluation of strontium-doped calcium silicate/gypsum bioactive bone cement. Biomed. Mater. 2014, 9, 045002. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-H.; Kao, C.-T.; Shen, Y.-F.; Lin, Y.-T.; Liu, Y.-T.; Yen, S.-Y.; Ho, C.-C. Substitutions of strontium in bioactive calcium silicate bone cements stimulate osteogenic differentiation in human mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2019, 30, 68. [Google Scholar] [CrossRef] [PubMed]
- Xuereb, M.; Sorrentino, F.; Damidot, D.; Camilleri, J. Development of novel tricalcium silicate-based endodontic cements with sintered radiopacifier phase. Clin. Oral Investig. 2016, 20, 967–982. [Google Scholar] [CrossRef] [PubMed]
- Lippert, F.; Hara, A.T. Strontium and caries: A long and complicated relationship. Caries Res. 2013, 47, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Femiano, F.; Femiano, R.; Femiano, L.; Nucci, L.; Minervini, G.; Antonelli, A.; Bennardo, F.; Barone, S.; Scotti, N.; Sorice, V.; et al. A New Combined Protocol to Treat the Dentin Hypersensitivity Associated with Non-Carious Cervical Lesions: A Randomized Controlled Trial. Appl. Sci. 2021, 11, 187. [Google Scholar] [CrossRef]
- Abdalla, M.M.; Lung, C.Y.K.; Bijle, M.N.; Yiu, C.K.Y. Physicochemical Properties and Inductive Effect of Calcium Strontium Silicate on the Differentiation of Human Dental Pulp Stem Cells for Vital Pulp Therapies: An In Vitro Study. Materials 2022, 15, 5854. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Hill, R.G.; Rawlinson, S.C. Strontium (Sr) elicits odontogenic differentiation of human dental pulp stem cells (hDPSCs): A therapeutic role for Sr in dentine repair? Acta Biomater. 2016, 38, 201–211. [Google Scholar] [CrossRef]
- Shahid, S.; Hassan, U.; Billington, R.; Hill, R.; Anderson, P. Glass ionomer cements: Effect of strontium substitution on esthetics, radiopacity and fluoride release. Dent. Mater. 2014, 30, 308–313. [Google Scholar] [CrossRef]
- ISO 9917-1; Dentistry-Water-Based Cements—Part 1: Powder/Liquid Acid–Base Cements. International Organization for Standardization: Geneva, Switzerland, 2007.
- You, J.; Yoo, J.-S.; Kum, K.-Y.; Hong, S.-H. Hydration behavior and radiopacity of strontium substituted Ca3SiO5 cement. J. Korean Ceram. Soc. 2021, 58, 330–336. [Google Scholar] [CrossRef]
- Pelepenko, L.E.; Marciano, M.A.; Francati, T.M.; Bombarda, G.; Antunes, T.B.M.; Sorrentino, F.; Martin, R.A.; Boanini, E.; Cooper, P.R.; Shelton, R.M. Can strontium replace calcium in bioactive materials for dental applications? J. Biomed. Mater. Res. Part A 2022, 110, 1892–1911. [Google Scholar] [CrossRef]
- Billington, R. Glass Fiber Sizing Agent. U.S. Patent US4067835A, 10 January 1978. [Google Scholar]
- Wu, C.; Ramaswamy, Y.; Kwik, D.; Zreiqat, H. The effect of strontium incorporation into CaSiO3 ceramics on their physical and biological properties. Biomaterials 2007, 28, 3171–3181. [Google Scholar] [CrossRef] [PubMed]
- Karpukhina, N.; Bushby, A. Incorporation of Strontium and Fluoride Containing Bioglass into Biodentine: Impact on Bioactivity, Radiopacity, Compressive Strength and Setting Time. Master’s Thesis, University of London, London, UK, 2012. [Google Scholar]
- Carvalho, E.; De Paula, D.; Neto, D.A.; Costa, L.; Dias, D.; Feitosa, V.; Fechine, P. Radiopacity and mechanical properties of dental adhesives with strontium hydroxyapatite nanofillers. J. Mech. Behav. Biomed. Mater. 2020, 101, 103447. [Google Scholar] [CrossRef] [PubMed]
- Höland, W.; Schweiger, M.; Dittmer, M.; Ritzberger, C. Radiopaque strontium fluoroapatite glass-ceramics. Front. Bioeng. Biotechnol. 2015, 3, 149. [Google Scholar] [CrossRef] [PubMed]
- Romieu, G.; Garric, X.; Munier, S.; Vert, M.; Boudeville, P. Calcium–strontium mixed phosphate as novel injectable and radio-opaque hydraulic cement. Acta Biomater. 2010, 6, 3208–3215. [Google Scholar] [CrossRef] [PubMed]
- Alkhraisat, M.H.; Rueda, C.; Cabrejos-Azama, J.; Lucas-Aparicio, J.; Mariño, F.T.; García-Denche, J.T.; Jerez, L.B.; Gbureck, U.; Cabarcos, E.L. Loading and release of doxycycline hyclate from strontium-substituted calcium phosphate cement. Acta Biomater. 2010, 6, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Guida, A.; Towler, M.; Wall, J.; Hill, R.; Eramo, S. Preliminary work on the antibacterial effect of strontium in glass ionomer cements. J. Mater. Sci. Lett. 2003, 22, 1401–1403. [Google Scholar] [CrossRef]
- Jayasree, R.; Kumar, T.; Mahalaxmi, S.; Abburi, S.; Rubaiya, Y.; Doble, M. Dentin remineralizing ability and enhanced antibacterial activity of strontium and hydroxyl ion co-releasing radiopaque hydroxyapatite cement. J. Mater. Sci. Mater. Med. 2017, 28, 95. [Google Scholar] [CrossRef] [PubMed]
- Brauer, D.S.; Karpukhina, N.; Kedia, G.; Bhat, A.; Law, R.V.; Radecka, I.; Hill, R.G. Bactericidal strontium-releasing injectable bone cements based on bioactive glasses. J. R. Soc. Interface 2013, 10, 20120647. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, H.; Neilands, J.; Svensäter, G.; Stavropoulos, A. Antimicrobial potential of strontium hydroxide on bacteria associated with peri-implantitis. Antibiotics 2021, 10, 150. [Google Scholar] [CrossRef]
- Forss, H. Release of fluoride and other elements from light-cured glass lonomers in neutral and acidic conditions. J. Dent. Res. 1993, 72, 1257–1262. [Google Scholar] [CrossRef]
- Mo, S.-S.; Bao, W.; Lai, G.-Y.; Wang, J.; Li, M.-Y. The microfloral analysis of secondary caries biofilm around Class I and Class II composite and amalgam fillings. BMC Infect. Dis. 2010, 10, 241. [Google Scholar] [CrossRef]
- Liu, J.; Rawlinson, S.C.; Hill, R.G.; Fortune, F. Strontium-substituted bioactive glasses in vitro osteogenic and antibacterial effects. Dent. Mater. 2016, 32, 412–422. [Google Scholar] [CrossRef]
- Dabsie, F.; Gregoire, G.; Sixou, M.; Sharrock, P. Does strontium play a role in the cariostatic activity of glass ionomer?: Strontium diffusion and antibacterial activity. J. Dent. 2009, 37, 554–559. [Google Scholar] [CrossRef]
- Yan, P.; Xia, J.S.; Chen, Y.P.; Liu, Z.P.; Guo, J.S.; Shen, Y.; Zhang, C.C.; Wang, J. Thermodynamics of binding interactions between extracellular polymeric substances and heavy metals by isothermal titration microcalorimetry. Bioresour. Technol. 2017, 232, 354–363. [Google Scholar] [CrossRef]
- Dai, L.L.; Mei, M.L.; Chu, C.H.; Lo, E.C.M. Remineralizing effect of a new strontium-doped bioactive glass and fluoride on demineralized enamel and dentine. J. Dent. 2021, 108, 103633. [Google Scholar] [CrossRef] [PubMed]
- Spets-Happonen, S.; Luoma, H.; Seppä, L.; Räisänen, J. The effect of different strontium concentrations on the efficacy of chlorhexidine-fluoride-strontium gel in preventing enamel softening in vitro. Arch. Oral Biol. 1993, 38, 107–112. [Google Scholar] [CrossRef]
- Spets-Happonen, S.; Luoma, H.; Seppä, L. High strontium addition to chlorhexidine-fluoride gel does not increase its caries-preventive effect in rats. Acta Odontol. Scand. 1996, 54, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Spets-Happonen, S.; Luoma, H.; Forss, H.; Kentala, J.; Alaluusua, S.; Luoma, A.R.; Grönroos, L.; Syvåoja, S.; Tapaninen, H.; Happonen, P. Effects of a chlorhexidine-fluoride-strontium rinsing program on caries, gingivitis and some salivary bacteria among Finnish school children. Eur. J. Oral Sci. 1991, 99, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Kytömaa, I.; Paakkola, O. Strontium-90 in Teeth: A Comparison of Methods. Acta Odontol. Scand. 1971, 29, 321–326. [Google Scholar] [CrossRef]
- Yassen, G.H.; Lippert, F.; Eckert, G.; Eder, J.; Zandoná, A.F. The effect of strontium and combinations of strontium and fluoride on the remineralization of artificial caries lesions in vitro. Quintessence Int. 2012, 43, e95–e103. [Google Scholar]
- Thuy, T.T.; Nakagaki, H.; Inukai, H.; Tsuboi, S.; Robinson, C. Effect of strontium on enamel remineralization in vitro. Caries Res. 2006, 40, 338. [Google Scholar]
- Wang, Y.-L.; Chang, H.-H.; Chiang, Y.-C.; Lin, C.-H.; Lin, C.-P. Strontium ion can significantly decrease enamel demineralization and prevent the enamel surface hardness loss in acidic environment. J. Formos. Med. Assoc. 2019, 118, 39–49. [Google Scholar] [CrossRef]
- Featherstone, J.; Shields, C.; Khademazad, B.; Oldershaw, M. Acid reactivity of carbonated apatites with strontium and fluoride substitutions. J. Dent. Res. 1983, 62, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.; Rodgers, B.; Smith, M. Physicochemical requirements for rapid remineralization of early carious lesions. Caries Res. 1981, 15, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.; Nakagaki, H.; Kato, K.; Hung, P.A.; Inukai, J.; Tsuboi, S.; Nakagaki, H.; Hirose, M.N.; Igarashi, S.; Robinson, C. Effect of strontium in combination with fluoride on enamel remineralisation in vitro. Arch. Oral Biol. 2008, 53, 1017–1022. [Google Scholar] [CrossRef]
- Ashrafi, M.; Spector, P.; Curzon, M. Pre-and posteruptive effects of low doses of strontium on dental caries in the rat. Caries Res. 1980, 14, 341–346. [Google Scholar] [CrossRef]
- Krishnan, V.; Bhatia, A.; Varma, H. Development, characterization and comparison of two strontium doped nano hydroxyapatite molecules for enamel repair/regeneration. Dent. Mater. 2016, 32, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Nair, K.R.; Sandhya, R.; Ashik, P.M.; Veedu, R.P.; Saleem, S. Evaluation of remineralization potential and cytotoxicity of a novel strontium-doped nanohydroxyapatite paste: An in vitro study. J. Conserv. Dent. (JCD) 2020, 23, 330. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.L.; Nudelman, F.; Chu, C.H.; Lo, E.C.M.; Mei, M.L. The effects of strontium-doped bioactive glass and fluoride on hydroxyapatite crystallization. J. Dent. 2021, 105, 103581. [Google Scholar] [CrossRef]
- Splieth, C.H.; Tachou, A. Epidemiology of dentin hypersensitivity. Clin. Oral Investig. 2013, 17, 3–8. [Google Scholar] [CrossRef]
- Yoshiyama, M.; Masada, J.; Uchida, A.; Ishida, H. Scanning electron microscopic characterization of sensitive vs. insensitive human radicular dentin. J. Dent. Res. 1989, 68, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Pawlowska, J. Strontium chloride—Its importance in dentistry and prophylaxis. Czas. Stomatol. 1956, 9, 353–357. [Google Scholar]
- Griffiths, H.; Morgan, G.; Williams, K.; Addy, M. Dentine hypersensitivity: The measurement in vitro of streaming potentials with fluid flow across dentine and hydroxyapatite. J. Periodontal Res. 1993, 28, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Absi, E.; Addy, M.; Adams, D. Dentine hypersensitivity: A study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J. Clin. Periodontol. 1987, 14, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Gillam, D.; Orchardson, R. Advances in the treatment of root dentine sensitivity: Mechanisms and treatment principles. Endod. Top. 2006, 13, 13–33. [Google Scholar] [CrossRef]
- Pashley, D.H. Dentin permeability, dentin sensitivity, and treatment through tubule occlusion. J. Endod. 1986, 12, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Hodge, H.C.; Gavett, E.; Thomas, I. The adsorption of strontium at forty degrees by enamel, dentin, bone, and hydroxyapatite as shown by the radioactive isotope. J. Biol. Chem. 1946, 163, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Porto, I.C.; Andrade, A.K.; Montes, M.A. Diagnosis and treatment of dentinal hypersensitivity. J. Oral Sci. 2009, 51, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Saeki, K.; Marshall, G.W.; Gansky, S.A.; Parkinson, C.R.; Marshall, S.J. Strontium effects on root dentin tubule occlusion and nanomechanical properties. Dent. Mater. 2016, 32, 240–251. [Google Scholar] [CrossRef]
- Oberg, C.; Pochapski, M.T.; Farago, P.V.; Granado, C.; Pilatti, G.L.; Santos, F.A. Evaluation of desensitizing agents on dentin permeability and dentinal tubule occlusion: An in vitro study. Gen. Dent. 2009, 57, 496–501. [Google Scholar]
- Olley, R.C.; Pilecki, P.; Hughes, N.; Jeffery, P.; Austin, R.S.; Moazzez, R.; Bartlett, D. An in situ study investigating dentine tubule occlusion of dentifrices following acid challenge. J. Dent. 2012, 40, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Kodaka, T.; Kuroiwa, M.; Kuroiwa, M.; Okumura, J.; Mori, R.; Hirasawa, S.; Kobori, M. Effects of brushing with a dentifrice for sensitive teeth on tubule occlusion and abrasion of dentin. J. Electron Microsc. 2001, 50, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Banfield, N.; Addy, M. Dentine hypersensitivity: Development and evaluation ofamodel in situ to study tubulepatency. J. Clin. Periodontol. 2004, 31, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.; Prange, M.; Naumova, E. Effectiveness of various toothpastes on dentine tubule occlusion. J. Dent. 2015, 43, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Arrais, C.A.G.; Micheloni, C.D.; Giannini, M.; Chan, D.C. Occluding effect of dentifrices on dentinal tubules. J. Dent. 2003, 31, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Addy, M.; West, N.X. The role of toothpaste in the aetiology and treatment of dentine hypersensitivity. Toothpastes 2013, 23, 75–87. [Google Scholar]
- Li, Y.; Lee, S.; Zhang, Y.P.; Delgado, E.; DeVizio, W.; Mateo, L.R. Comparison of clinical efficacy of three toothpastes in reducing dentin hypersensitivity. J. Clin. Dent. 2011, 22, 113. [Google Scholar]
- Dotta, T.C.; Hayann, L.; de Padua Andrade Almeida, L.; Nogueira, L.F.B.; Arnez, M.M.; Castelo, R.; Cassiano, A.F.B.; Faria, G.; Martelli-Tosi, M.; Bottini, M.; et al. Strontium Carbonate and Strontium-Substituted Calcium Carbonate Nanoparticles Form Protective Deposits on Dentin Surface and Enhance Human Dental Pulp Stem Cells Mineralization. J. Funct. Biomater. 2022, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Tirapelli, C.; Panzeri, H.; Soares, R.G.; Peitl, O.; Zanotto, E.D. A novel bioactive glass-ceramic for treating dentin hypersensitivity. Braz. Oral Res. 2010, 24, 381–387. [Google Scholar] [CrossRef]
- Hench, L.L. Bioactive materials: The potential for tissue regeneration. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1998, 41, 511–518. [Google Scholar] [CrossRef]
- Hench, L.L. Chronology of bioactive glass development and clinical applications. New J. Glas. Ceram. 2013, 3, 67–73. [Google Scholar] [CrossRef]
- Xia, W.; Qin, T.; Suska, F.; Engqvist, H. Bioactive spheres: The way of treating dentin hypersensitivity. ACS Biomater. Sci. Eng. 2016, 2, 734–740. [Google Scholar] [CrossRef]
- Acevedo, L.A.; Campos, L.A.; Dechandt, I.C.; Alegria, G.; Siqueira, R.L.; Zanotto, E.D.; Serbena, F.C.; Santos, F.A. Effect of bioactive glasses containing strontium and potassium on dentin permeability. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 517–526. [Google Scholar] [CrossRef]
- Martins, C.; Firmino, R.; Riva, J.; Ge, L.; Carrasco-Labra, A.; Brignardello-Petersen, R.; Colunga-Lozano, L.; Granville-Garcia, A.; Costa, F.; Yepes-Nuñez, J. Desensitizing toothpastes for dentin hypersensitivity: A network meta-analysis. J. Dent. Res. 2020, 99, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Karim, B.; Gillam, D. The efficacy of strontium and potassium toothpastes in treating dentine hypersensitivity: A systematic review. Int. J. Dent. 2013, 2013, 573258. [Google Scholar] [CrossRef]
- West, N.X.; Seong, J.; Davies, M. Management of dentine hypersensitivity: Efficacy of professionally and self-administered agents. J. Clin. Periodontol. 2015, 42, S256–S302. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Kim, Y.K.; Myung, S.K. Desensitizing toothpaste versus placebo for dentin hypersensitivity: A systematic review and meta-analysis. J. Clin. Periodontol. 2015, 42, 131–141. [Google Scholar] [CrossRef]
- Hu, M.-L.; Zheng, G.; Zhang, Y.-D.; Yan, X.; Li, X.-C.; Lin, H. Effect of desensitizing toothpastes on dentine hypersensitivity: A systematic review and meta-analysis. J. Dent. 2018, 75, 12–21. [Google Scholar] [CrossRef]
- Hu, M.-L.; Zheng, G.; Lin, H.; Yang, M.; Zhang, Y.-D.; Han, J.-M. Network meta-analysis on the effect of desensitizing toothpastes on dentine hypersensitivity. J. Dent. 2019, 88, 103170. [Google Scholar] [CrossRef]
- Cunha-Cruz, J.; Zeola, L.F. Limited evidence suggests that many types of desensitizing toothpaste may reduce dentin hypersensitivity, but not the ones with strontium or amorphous calcium phosphate. J. Evid. Based Dent. Pract. 2019, 19, 101337. [Google Scholar] [CrossRef]
- Barros, A.P.O.; de Melo Alencar, C.; de Melo Pingarilho Carneiro, A.; da Silva Pompeu, D.; Barbosa, G.M.; Araújo, J.L.N.; Silva, C.M. Combination of two desensitizing protocols to control dentin hypersensitivity in non-carious lesions: A randomized, double-blind clinical trial. Clin. Oral Investig. 2022, 26, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Pompeu, D.D.S.; de Paula, B.L.F.; Barros, A.P.O.; Nunes, S.C.; Carneiro, A.M.P.; Araújo, J.L.N.; Silva, C.M. Combination of strontium chloride and photobiomodulation in the control of tooth sensitivity post-bleaching: A split-mouth randomized clinical trial. PLoS ONE 2021, 16, e0250501. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Mandakhbayar, N.; El-Fiqi, A.; Kim, H.-W. Intracellular co-delivery of Sr ion and phenamil drug through mesoporous bioglass nanocarriers synergizes BMP signaling and tissue mineralization. Acta Biomater. 2017, 60, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Mandakhbayar, N.; El-Fiqi, A.; Lee, J.H.; Kim, H.W. Evaluation of Strontium-Doped Nanobioactive Glass Cement for Dentin-Pulp Complex Regeneration Therapy. ACS Biomater. Sci. Eng. 2019, 5, 6117–6126. [Google Scholar] [CrossRef] [PubMed]
- Basheer, N.; Madhubala, M.M.; Jayasree, R.; Mahalaxmi, S.; Ts, S.K. Effect of Strontium Substituted Tetracalcium Phosphate Cement on Proliferation and Mineralization Potential in Human Dental Pulp Stem Cells. Eur. Endod. J. 2021, 6, 295. [Google Scholar] [CrossRef] [PubMed]
- Mardas, N.; Dereka, X.; Stavropoulos, A.; Patel, M.; Donos, N. The role of strontium ranelate and guided bone regeneration in osteoporotic and healthy conditions. J. Periodontal Res. 2021, 56, 330–338. [Google Scholar] [CrossRef]
- Jia, X.; Long, Q.; Miron, R.J.; Yin, C.; Wei, Y.; Zhang, Y.; Wu, M. Setd2 is associated with strontium-induced bone regeneration. Acta Biomater. 2017, 53, 495–505. [Google Scholar] [CrossRef]
- Jia, X.; Miron, R.J.; Yin, C.; Xu, H.; Luo, T.; Wang, J.; Jia, R.; Wu, M.; Zhang, Y.; Li, Y. HnRNPL inhibits the osteogenic differentiation of PDLCs stimulated by SrCl2 through repressing Setd2. J. Cell. Mol. Med. 2019, 23, 2667–2677. [Google Scholar] [CrossRef]
- Bizelli-Silveira, C.; Pullisaar, H.; Abildtrup, L.A.; Andersen, O.Z.; Spin-Neto, R.; Foss, M.; Kraft, D.C. Strontium enhances proliferation and osteogenic behavior of periodontal ligament cells in vitro. J. Periodontal Res. 2018, 53, 1020–1028. [Google Scholar] [CrossRef]
- Wang, L.; Pathak, J.L.; Liang, D.; Zhong, N.; Guan, H.; Wan, M.; Miao, G.; Li, Z.; Ge, L. Fabrication and characterization of strontium-hydroxyapatite/silk fibroin biocomposite nanospheres for bone-tissue engineering applications. Int. J. Biol. Macromol. 2020, 142, 366–375. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, X.; Ma, Y.; Zhou, Y.; Liu, L.; Yu, F.; Fang, B.; Lin, K.; Xia, L.; Cai, M. Synergistic Effect of Micro-Nano-Hybrid Surfaces and Sr Doping on the Osteogenic and Angiogenic Capacity of Hydroxyapatite Bioceramics Scaffolds. Int. J. Nanomed. 2022, 17, 783. [Google Scholar] [CrossRef]
- Saidak, Z.; Marie, P.J. Strontium signaling: Molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol. Ther. 2012, 136, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Marx, D.; Yazdi, A.R.; Papini, M.; Towler, M. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020, 12, 100273. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG pathway: A mechanism involved in exercise-induced bone remodeling. BioMed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef] [PubMed]
- Lafzi, A.; Nejad, A.E.; Rad, M.R.; Namdari, M.; Sabetmoghaddam, T. In vitro release of silver ions and expression of osteogenic genes by MC3T3-E1 cell line cultured on nano-hydroxyapatite and silver/strontium-coated titanium plates. Odontology 2023, 111, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Montazerian, M.; Fiume, E.; Baino, F. Multiple and promising applications of strontium (Sr)-containing bioactive glasses in bone tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-W.; Yu, W.-X.; Hwang, P.-A.; Huang, S.-S.; Lin, H.-M.; Hsu, Y.-W.; Hsu, F.-Y. Fabrication and characterization of strontium-substituted hydroxyapatite-CaO-CaCO3 nanofibers with a mesoporous structure as drug delivery carriers. Pharmaceutics 2018, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.; Yu, W.; Hwang, P.; Hsu, Y.; Hsu, F. Fabrication and characteristics of PCL membranes containing strontium-substituted hydroxyapatite nanofibers for guided bone regeneration. Polymers 2019, 11, 1761. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-W.; Hsu, Y.-W.; Pan, W.-L.; Vadivelmurugan, A.; Hwang, P.-A.; Hsu, F.-Y. Influence of the Components and Orientation of Hydroxyapatite Fibrous Substrates on Osteoblast Behavior. J. Funct. Biomater. 2022, 13, 168. [Google Scholar] [CrossRef]
- Osman, R.B.; Swain, M.V. A critical review of dental implant materials with an emphasis on titanium versus zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef]
- Ting, M.; Jefferies, S.R.; Xia, W.; Engqvist, H.; Suzuki, J.B. Classification and effects of implant surface modification on the bone: Human cell–based in vitro studies. J. Oral Implantol. 2017, 43, 58–83. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface modifications and their effects on titanium dental implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C 2019, 98, 1294–1305. [Google Scholar] [CrossRef]
- Awasthi, S.; Pandey, S.K.; Arunan, E.; Srivastava, C. A review on hydroxyapatite coatings for the biomedical applications: Experimental and theoretical perspectives. J. Mater. Chem. B 2021, 9, 228–249. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A comprehensive review of bioactive glass coatings: State of the art, challenges and future perspectives. Coatings 2020, 10, 757. [Google Scholar] [CrossRef]
- Kim, J.; Kang, I.-G.; Cheon, K.-H.; Lee, S.; Park, S.; Kim, H.-E.; Han, C.-M. Stable sol–gel hydroxyapatite coating on zirconia dental implant for improved osseointegration. J. Mater. Sci. Mater. Med. 2021, 32, 81. [Google Scholar] [CrossRef] [PubMed]
- Helen, S.; Kumar, A.R. Electrical, mechanical and surface analysis of ion-doped hydroxyapatite for antibacterial activity. Appl. Phys. A 2018, 124, 535. [Google Scholar] [CrossRef]
- Rabiee, S.M.; Nazparvar, N.; Azizian, M.; Vashaee, D.; Tayebi, L. Effect of ion substitution on properties of bioactive glasses: A review. Ceram. Int. 2015, 41, 7241–7251. [Google Scholar] [CrossRef]
- Geng, Z.; Li, X.; Ji, L.; Li, Z.; Zhu, S.; Cui, Z.; Wang, J.; Cui, J.; Yang, X.; Liu, C. A novel snail-inspired bionic design of titanium with strontium-substituted hydroxyapatite coating for promoting osseointegration. J. Mater. Sci. Technol. 2021, 79, 35–45. [Google Scholar] [CrossRef]
- Newman, S.D.; Lotfibakhshaiesh, N.; O’Donnell, M.; Walboomers, X.F.; Horwood, N.; Jansen, J.A.; Amis, A.A.; Cobb, J.P.; Stevens, M.M. Enhanced osseous implant fixation with strontium-substituted bioactive glass coating. Tissue Eng. Part A 2014, 20, 1850–1857. [Google Scholar] [CrossRef]
- Capuccini, C.; Torricelli, P.; Sima, F.; Boanini, E.; Ristoscu, C.; Bracci, B.; Socol, G.; Fini, M.; Mihailescu, I.; Bigi, A. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: In vitro osteoblast and osteoclast response. Acta Biomater. 2008, 4, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, E.; Fredholm, Y.C.; Jell, G.; Lotfibakhshaiesh, N.; O’Donnell, M.D.; Hill, R.G.; Stevens, M.M. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials 2010, 31, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.S.; Zhou, W.-S.; He, X.W.; Liu, W.; Bai, B.L.; Zhou, Q.; Li, H.; Huang, Z.L.; Tu, K.; Hang, L.; et al. A comparative study of strontium-substituted hydroxyapatite coating on implant’s osseointegration for osteopenic rats. Med. Biol. Eng. Comput. 2016, 54, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Q.; Zhu, S.; Luo, E.; Li, J.; Feng, G.; Liao, Y.; Hu, J. The effect of strontium-substituted hydroxyapatite coating on implant fixation in ovariectomized rats. Biomaterials 2010, 31, 9006–9014. [Google Scholar] [CrossRef]
- Katunar, M.R.; Pastore, J.I.; Cisilino, A.; Merlo, J.; Alonso, L.S.; Baca, M.; Haddad, K.; Cere, S.; Ballarre, J. Early osseointegration of strontium-doped coatings on titanium implants in an osteoporotic rat model. Surf. Coat. Technol. 2022, 433, 128159. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, H.; Zhang, X.; Li, G.; Chang, Q.; Zhao, J.; Qiao, Y.; Ding, X.; Yang, G.; Liu, X. A strontium-incorporated nanoporous titanium implant surface for rapid osseointegration. Nanoscale 2016, 8, 5291–5301. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yao, L.; Wang, H.; Shen, X.; Lou, W.; Huang, C.; Wu, G. Magnetron sputtering of strontium nanolayer on zirconia implant to enhance osteogenesis. Mater. Sci. Eng. C 2021, 127, 112191. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.; Ammann, P.; Boivin, G.; Rey, C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif. Tissue Int. 2001, 69, 121. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, B.; Stępień, N.; Kolmas, J. The influence of strontium on bone tissue metabolism and its application in osteoporosis treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef]
- Dai, J.; Fu, Y.; Chen, D.; Sun, Z. A novel and injectable strontium-containing hydroxyapatite bone cement for bone substitution: A systematic evaluation. Mater. Sci. Eng. C 2021, 124, 112052. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, L.; Wu, C.; Miron, R.J. Periodontal regeneration using strontium-loaded mesoporous bioactive glass scaffolds in osteoporotic rats. PLoS ONE 2014, 9, e104527. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-W.; Zuo, K.-Q.; Wang, K.; Sun, Z.-Y.; Lu, Y.-P.; Cheng, L.; Xiao, G.-Y.; Liu, C. Interleukin-4 assisted calcium-strontium-zinc-phosphate coating induces controllable macrophage polarization and promotes osseointegration on titanium implant. Mater. Sci. Eng. C 2021, 118, 111512. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, Y.; Zhu, Y.; Lin, G.; Zhang, L.; Liu, X.; He, F. Antiadipogenesis and osseointegration of strontium-doped implant surfaces. J. Dent. Res. 2019, 98, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Park, J.W. Multifunctional effects of a modification of SLA titanium implant surface with strontium-containing nanostructures on immunoinflammatory and osteogenic cell function. J. Biomed. Mater. Res. Part A 2018, 106, 3009–3020. [Google Scholar] [CrossRef] [PubMed]
- Okuzu, Y.; Fujibayashi, S.; Yamaguchi, S.; Masamoto, K.; Otsuki, B.; Goto, K.; Kawai, T.; Shimizu, T.; Morizane, K.; Kawata, T.; et al. In vitro study of antibacterial and osteogenic activity of titanium metal releasing strontium and silver ions. J. Biomater. Appl. 2021, 35, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Offermanns, V.; Andersen, O.Z.; Riede, G.; Sillassen, M.; Jeppesen, C.S.; Almtoft, K.P.; Talasz, H.; Öhman-Mägi, C.; Lethaus, B.; Tolba, R.; et al. Effect of strontium surface-functionalized implants on early and late osseointegration: A histological, spectrometric and tomographic evaluation. Acta Biomater. 2018, 69, 385–394. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Li, Y.; Shi, J.; Zhou, J.; Zhang, L.; Deng, Y.; Yang, W. Strontium/adiponectin co-decoration modulates the osteogenic activity of nano-morphologic polyetheretherketone implant. Colloids Surf. B Biointerfaces 2019, 176, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-C.; Chung, C.-Y.; Chiu, C.-H.; Lin, M.H.-C.; Yang, J.-T. The Effect of Polybutylcyanoacrylate Nanoparticles as a Protos Delivery Vehicle on Dental Bone Formation. Int. J. Mol. Sci. 2021, 22, 4873. [Google Scholar] [CrossRef] [PubMed]
- Göker, F.; Ersanlı, S.; Arısan, V.; Cevher, E.; Güzel, E.E.; İşsever, H.; Ömer, B.; Altun, G.D.; Morina, D.; Yılmaz, T.E.; et al. Combined effect of parathyroid hormone and strontium ranelate on bone healing in ovariectomized rats. Oral Dis. 2018, 24, 1255–1269. [Google Scholar] [CrossRef]
- Arepalli, S.K.; Tripathi, H.; Hira, S.K.; Manna, P.P.; Pyare, R.; Singh, S.P. Enhanced bioactivity, biocompatibility and mechanical behavior of strontium substituted bioactive glasses. Mater. Sci. Eng. C 2016, 69, 108–116. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, S.; Wei, Z.; Bai, P. The Relationship between Residual Amount of Sr and Morphology of Eutectic Si Phase in A356 Alloy. Materials 2019, 12, 3222. [Google Scholar] [CrossRef]
- Saxena, K.; Ann, C.M.; Azwar, M.A.B.M.; Banavar, S.R.; Matinlinna, J.; Peters, O.A.; Daood, U. Effect of strontium fluoride on mechanical and remineralization properties of enamel: An in-vitro study on a modified orthodontic adhesive. Dent. Mater. 2024, 40, 811–823. [Google Scholar] [CrossRef]
- Amudha, S.; Ramya, J.R.; Arul, K.T.; Deepika, A.; Sathiamurthi, P.; Mohana, B.; Asokan, K.; Dong, C.-L.; Kalkura, S.N. Enhanced mechanical and biocompatible properties of strontium ions doped mesoporous bioactive glass. Compos. Part B Eng. 2020, 196, 108099. [Google Scholar] [CrossRef]
- Lunawat, K.; Kavitha, S.; Rajkumar, G.; Dhivya, V.; Kumar, N.R.; Mahalaxmi, S.; Shaik, F.A. Influence of strontium containing fluorophosphate glass onto structural and mechanical behavior of MTA network. J. Mech. Behav. Biomed. Mater. 2023, 140, 105750. [Google Scholar] [CrossRef]
- Rezaei, Y.; Moztarzadeh, F.; Shahabi, S.; Tahriri, M. Synthesis, characterization, and in vitro bioactivity of sol-gel-derived SiO2–CaO–P2O5–MgO-SrO bioactive glass. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2014, 44, 692–701. [Google Scholar] [CrossRef]
- Arron, J.R.; Choi, Y. Bone versus immune system. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef]
- Zhao, T.; Chu, Z.; Ma, J.; Ouyang, L. Immunomodulation effect of biomaterials on bone formation. J. Funct. Biomater. 2022, 13, 103. [Google Scholar] [CrossRef]
- Wei, Z.; Tian, P.; Liu, X.; Zhou, B. Hemocompatibility and selective cell fate of polydopamine-assisted heparinized PEO/PLLA composite coating on biodegradable AZ31 alloy. Colloids Surf. B Biointerfaces 2014, 121, 451–460. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Lee, C.-H.; Kim, Y.-J.; Jang, J.-H.; Park, J.-W. Modulating macrophage polarization with divalent cations in nanostructured titanium implant surfaces. Nanotechnology 2016, 27, 085101. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, W.; Liu, Z.; Ma, S.; Sun, Y.; Wu, X.; Zhang, X.; Gao, P. Application of a strontium-loaded, phase-transited lysozyme coating to a titanium surface to enhance osteogenesis and osteoimmunomodulation. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 2658. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, F.; Huang, D.; Fu, X.; Li, X.; Chen, X. Strontium-substituted submicrometer bioactive glasses modulate macrophage responses for improved bone regeneration. ACS Appl. Mater. Interfaces 2016, 8, 30747–30758. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Liu, C.; Kim, J.; Su, K.; Chen, T.; Zhao, L.; Lu, X.; Zhang, H.; Cui, Y.; et al. Spontaneous immunomodulation and regulation of angiogenesis and osteogenesis by Sr/Cu-borosilicate glass (BSG) bone cement to repair critical bone defects. Bioact. Mater. 2023, 23, 101–117. [Google Scholar] [CrossRef]
- Yuan, X.; Cao, H.; Wang, J.; Tang, K.; Li, B.; Zhao, Y.; Cheng, M.; Qin, H.; Liu, X.; Zhang, X. Immunomodulatory effects of calcium and strontium co-doped titanium oxides on osteogenesis. Front. Immunol. 2017, 8, 1196. [Google Scholar] [CrossRef]
- Cipriano, A.F.; Sallee, A.; Tayoba, M.; Alcaraz, M.C.C.; Lin, A.; Guan, R.-G.; Zhao, Z.-Y.; Liu, H. Cytocompatibility and early inflammatory response of human endothelial cells in direct culture with Mg-Zn-Sr alloys. Acta Biomater. 2017, 48, 499–520. [Google Scholar] [CrossRef]
- Graney, P.L.; Roohani-Esfahani, S.-I.; Zreiqat, H.; Spiller, K.L. In vitro response of macrophages to ceramic scaffolds used for bone regeneration. J. R. Soc. Interface 2016, 13, 20160346. [Google Scholar] [CrossRef]
- Marx, D.; Papini, M.; Towler, M. In vitro immunomodulatory effects of novel strontium and zinc-containing GPCs. Bio-Med. Mater. Eng. 2022, 33, 377–391. [Google Scholar] [CrossRef]
- Lourenço, A.H.; Torres, A.L.; Vasconcelos, D.P.; Ribeiro-Machado, C.; Barbosa, J.N.; Barbosa, M.A.; Barrias, C.C.; Ribeiro, C.C. Osteogenic, anti-osteoclastogenic and immunomodulatory properties of a strontium-releasing hybrid scaffold for bone repair. Mater. Sci. Eng. C 2019, 99, 1289–1303. [Google Scholar] [CrossRef]
- Xu, A.-T.; Xie, Y.-W.; Xu, J.-G.; Li, J.; Wang, H.; He, F.-M. Effects of strontium-incorporated micro/nano rough titanium surfaces on osseointegration via modulating polarization of macrophages. Colloids Surf. B Biointerfaces 2021, 207, 111992. [Google Scholar] [CrossRef]
- Fenbo, M.; Xingyu, X.; Bin, T. Strontium chondroitin sulfate/silk fibroin blend membrane containing microporous structure modulates macrophage responses for guided bone regeneration. Carbohydr. Polym. 2019, 213, 266–275. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, X.; Wang, Y.; Li, M.; Li, Y.; Liu, X.; Zhang, X.; Lan, Z.; Wang, J.; Du, Y.; et al. Zn/Sr dual ions-collagen co-assembly hydroxyapatite enhances bone regeneration through procedural osteo-immunomodulation and osteogenesis. Bioact. Mater. 2022, 10, 195–206. [Google Scholar] [CrossRef]
- Yu, D.; Guo, S.; Yu, M.; Liu, W.; Li, X.; Chen, D.; Li, B.; Guo, Z.; Han, Y. Immunomodulation and osseointegration activities of Na2TiO3 nanorods-arrayed coatings doped with different Sr content. Bioact. Mater. 2022, 10, 323–334. [Google Scholar] [CrossRef]
- Chantelle, L.; Kennedy, B.J.; de Oliveira, C.P.; Gouttefangeas, F.; Siu-Li, M.; Landers, R.; Ciorita, A.; Rostas, A.M.; dos Santos, I.M.G.; De Oliveira, A.L.M. Europium induced point defects in SrSnO3-based perovskites employed as antibacterial agents. J. Alloys Compd. 2023, 956, 170353. [Google Scholar] [CrossRef]
- Wu, T.; Lu, T.; Shi, H.; Wang, J.; Ye, J. Enhanced osteogenesis, angiogenesis and inhibited osteoclastogenesis of a calcium phosphate cement incorporated with strontium doped calcium silicate bioceramic. Ceram. Int. 2023, 49, 6630–6645. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalla, M.M.; Sayed, O.; Lung, C.Y.K.; Rajasekar, V.; Yiu, C.K.Y. Applications of Bioactive Strontium Compounds in Dentistry. J. Funct. Biomater. 2024, 15, 216. https://doi.org/10.3390/jfb15080216
Abdalla MM, Sayed O, Lung CYK, Rajasekar V, Yiu CKY. Applications of Bioactive Strontium Compounds in Dentistry. Journal of Functional Biomaterials. 2024; 15(8):216. https://doi.org/10.3390/jfb15080216
Chicago/Turabian StyleAbdalla, Mohamed Mahmoud, Osama Sayed, Christie Ying Kei Lung, Vidhyashree Rajasekar, and Cynthia Kar Yung Yiu. 2024. "Applications of Bioactive Strontium Compounds in Dentistry" Journal of Functional Biomaterials 15, no. 8: 216. https://doi.org/10.3390/jfb15080216
APA StyleAbdalla, M. M., Sayed, O., Lung, C. Y. K., Rajasekar, V., & Yiu, C. K. Y. (2024). Applications of Bioactive Strontium Compounds in Dentistry. Journal of Functional Biomaterials, 15(8), 216. https://doi.org/10.3390/jfb15080216







