Dihydromyricetin Nanoparticles Alleviate Lipopolysaccharide-Induced Acute Kidney Injury by Decreasing Inflammation and Cell Apoptosis via the TLR4/NF-κB Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Animal Experiments
2.3. Assessment of BUN and Cr
2.4. Measurements of Antioxidant Enzyme Activity
2.5. Measurement of Inflammatory Cytokines
2.6. Renal Histological Studies
2.7. qRT-PCR
2.8. TUNEL Staining
2.9. Immunohistochemistry
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
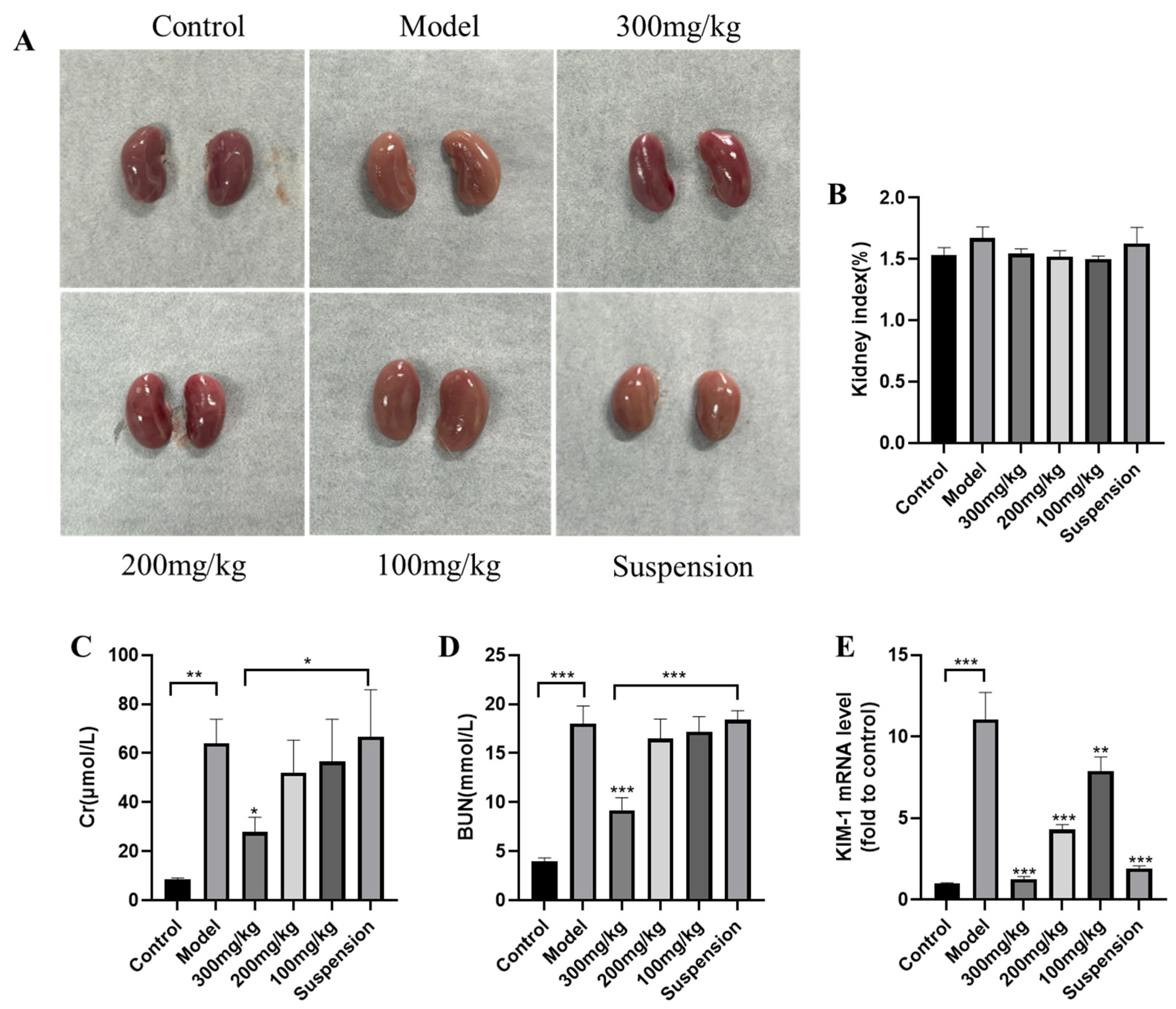
3.1. DMY Alleviates LPS-Induced AKI in Mice
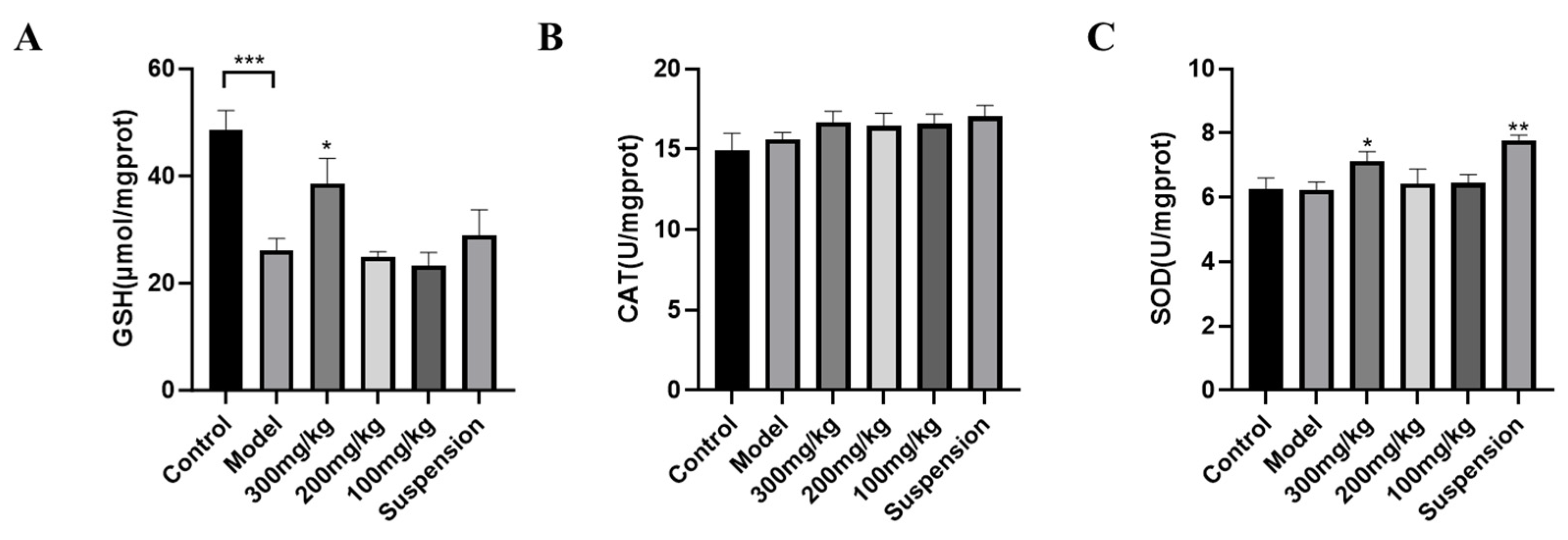
3.2. Antioxidant Enzyme Detection
3.3. Detection of Inflammatory Cytokines in Serum and Tissue
3.4. Histopathological Analysis
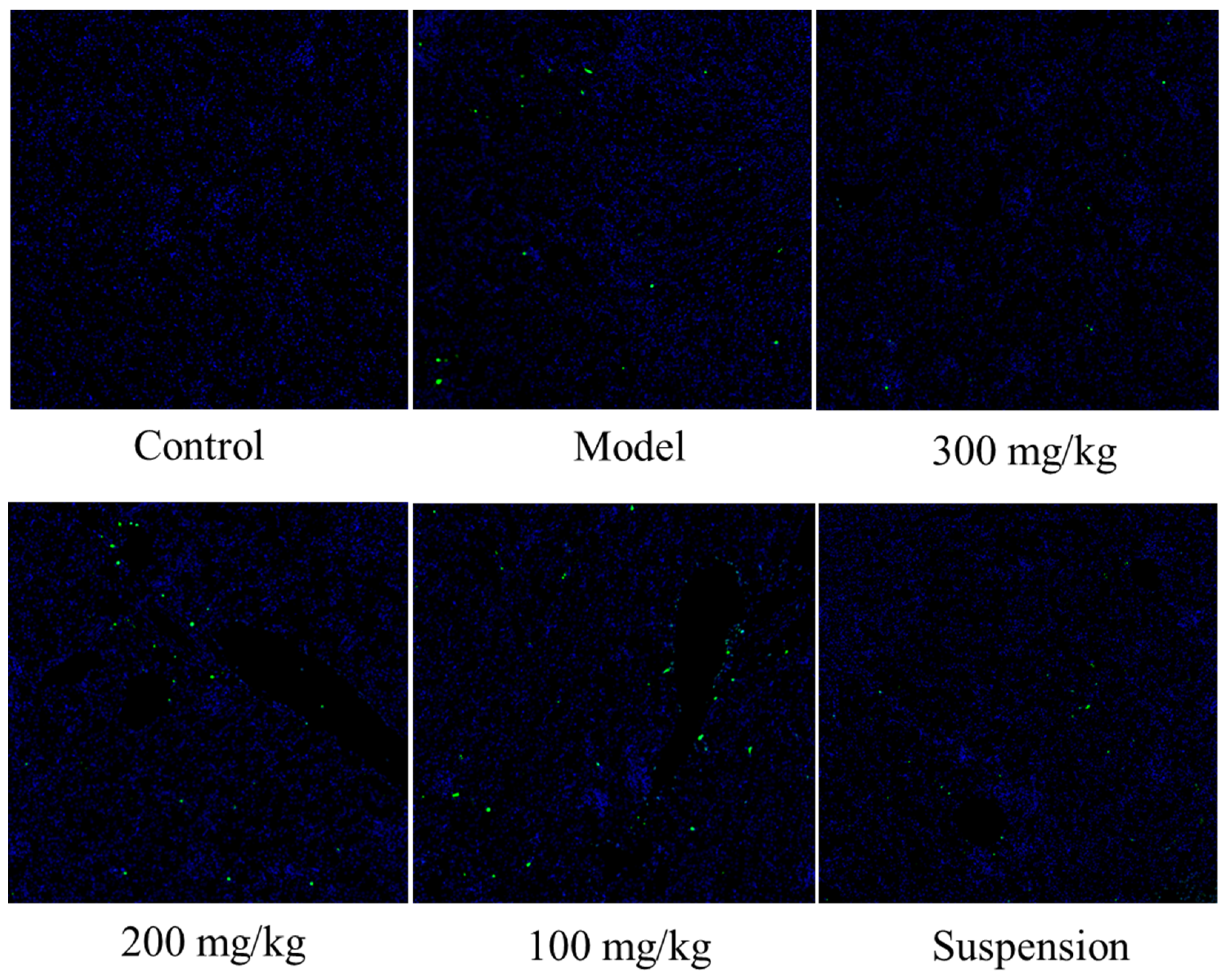
3.5. DMY Decreased the TUNEL Staining Signal
3.6. CS-DMY-NPs Reduced Cell Apoptosis in the Kidneys
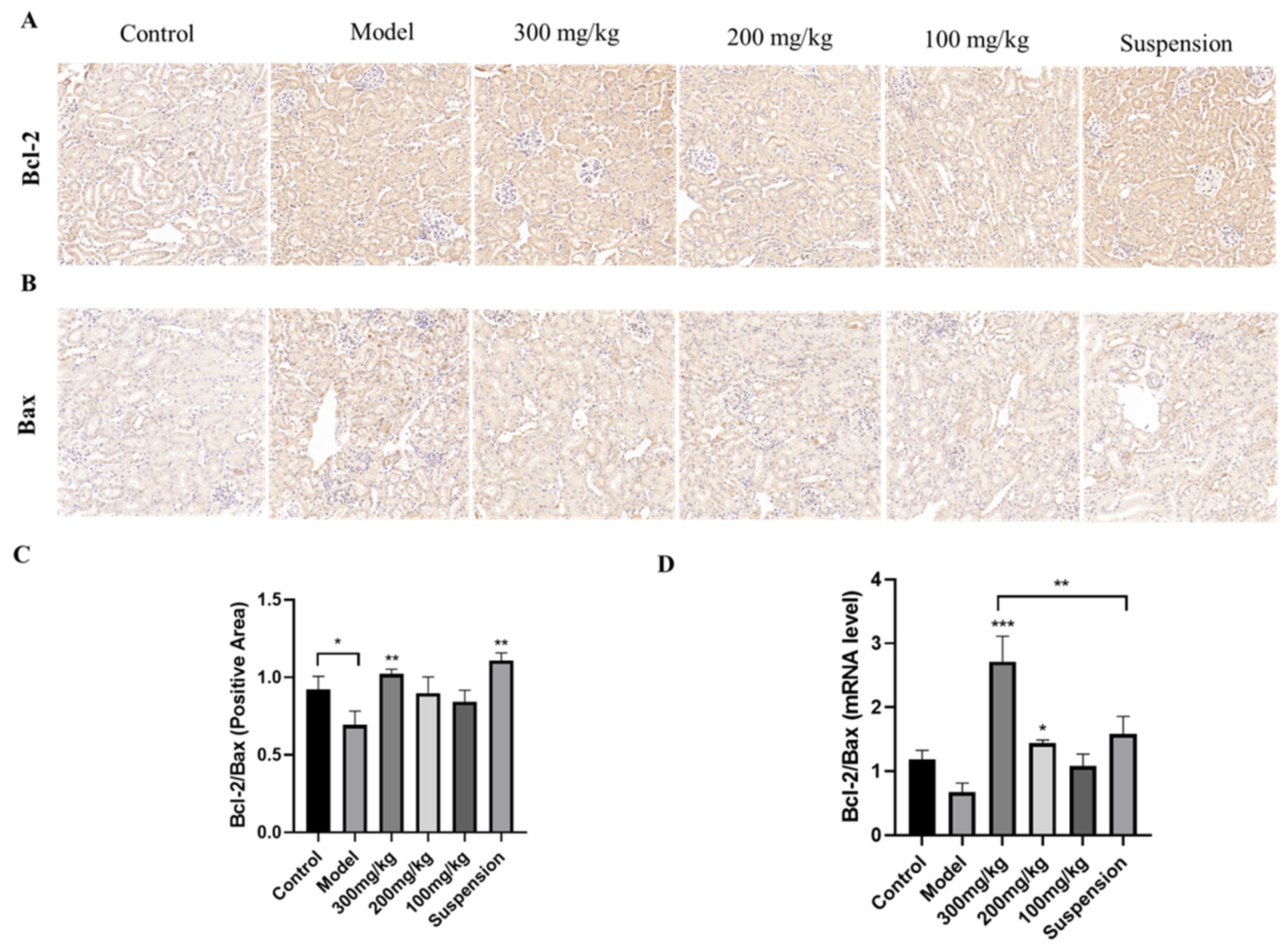
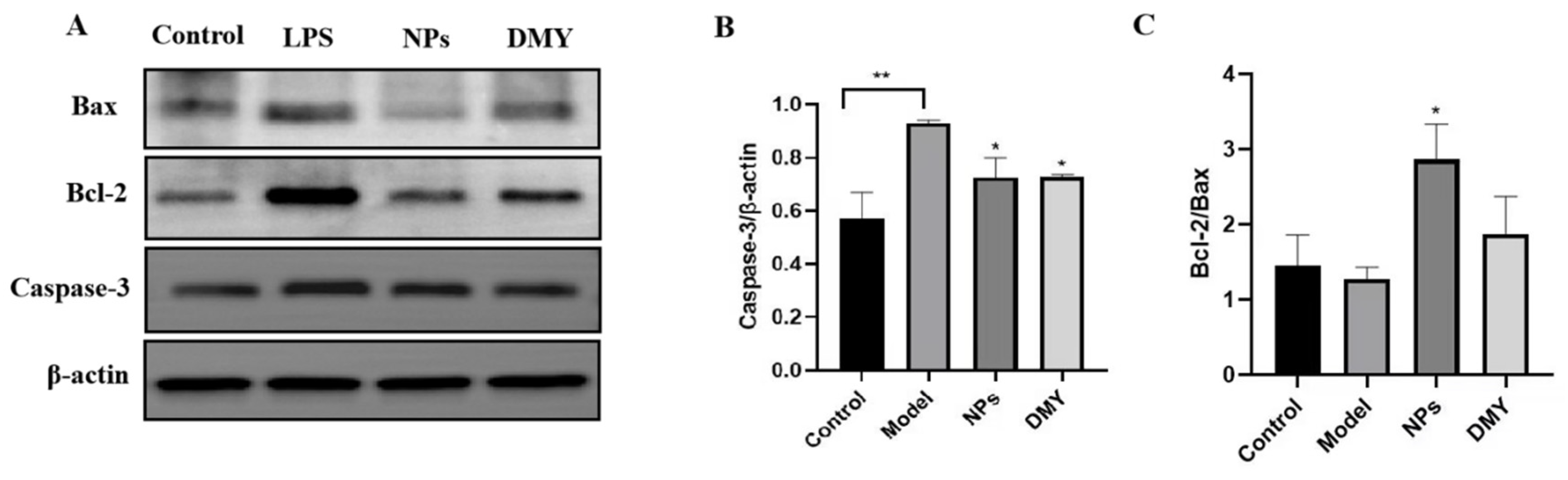
3.7. The Impact of CS-DMY-NPs on Apoptotic Signaling Pathways
3.8. The Impact of CS-DMY-NPs on the TLR4/NF-κB Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levey, A.S.; James, M.T. Acute Kidney Injury. Ann. Intern. Med. 2017, 167, ITC66–ITC80. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Shad, F.; Smith, M.C. Acute kidney injury: A guide to diagnosis and management. Am. Fam. Physician 2012, 86, 631–639. [Google Scholar] [PubMed]
- Sharawy, M.H.; Serrya, M.S. Pirfenidone attenuates gentamicin-induced acute kidney injury by inhibiting inflammasome-dependent NLRP3 pathway in rats. Life Sci. 2020, 260, 118454. [Google Scholar] [CrossRef]
- Duann, P.; Lianos, E.A.; Ma, J.; Lin, P.-H. Autophagy, Innate Immunity and Tissue Repair in Acute Kidney Injury. Int. J. Mol. Sci. 2016, 17, 662. [Google Scholar] [CrossRef]
- Tian, X.-H.; Jiang, W.-S.; Li, X.-L.; Li, M.-F.; Liu, C.-L.; Li, X.-X. Protective effect of fasudil hydrochloride against acute renal injury in septicopyemia rats. Asian Pac. J. Trop. Med. 2015, 8, 1071–1075. [Google Scholar] [CrossRef]
- Yu, C.; Wang, D.; Li, Q.; Tong, Y.; Yang, Z.; Wang, T. Trans-anethole ameliorates LPS-induced inflammation via suppression of TLR4/NF-κB pathway in IEC-6 cells. Int. Immunopharmacol. 2022, 108, 108872. [Google Scholar] [CrossRef] [PubMed]
- Zusso, M.; Lunardi, V.; Franceschini, D.; Pagetta, A.; Lo, R.; Stifani, S.; Frigo, A.C.; Giusti, P.; Moro, S. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J. Neuroinflammation 2019, 16, 148. [Google Scholar] [CrossRef]
- Huang, G.; Bao, J.; Shao, X.; Zhou, W.; Wu, B.; Ni, Z.; Wang, L. Inhibiting pannexin-1 alleviates sepsis-induced acute kidney injury via decreasing NLRP3 inflammasome activation and cell apoptosis. Life Sci. 2020, 254, 117791. [Google Scholar] [CrossRef]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 2020, 122, 109772. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Z.; Li, H.; Zhu, N.; Gu, J.; Wang, W.; Liu, C.; Wang, W.; Qin, L. Present Status, Challenges, and Prospects of Dihydromyricetin in the Battle against Cancer. Cancers 2022, 14, 3487. [Google Scholar] [CrossRef]
- Dong, S.; Zhu, M.; Wang, K.; Zhao, X.; Hu, L.; Jing, W.; Lu, H.; Wang, S. Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism. Pharmacol. Res. 2021, 171, 105767. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, S.; Yang, S.; Chen, C.; Yang, Y.; Lin, M.; Liu, C.; Wang, W.; Zhou, X.; Ai, Q. Mechanism of dihydromyricetin on inflammatory diseases. Front. Pharmacol. 2022, 12, 794563. [Google Scholar] [CrossRef]
- Di Santo, M.C.; D’Antoni, C.L.; Rubio, A.P.D.; Alaimo, A.; Pérez, O.E. Chitosan-tripolyphosphate nanoparticles designed to encapsulate polyphenolic compounds for biomedical and pharmaceutical applications—A review. Biomed. Pharmacother. 2021, 142, 111970. [Google Scholar] [CrossRef]
- Yan, Q.; Li, M.; Dong, L.; Luo, J.; Zhong, X.; Shi, F.; Ye, G.; Zhao, L.; Fu, H.; Shu, G. Preparation, characterization and protective effect of chitosan-Tripolyphosphate encapsulated dihydromyricetin nanoparticles on acute kidney injury caused by cisplatin. Int. J. Biol. Macromol. 2023, 245, 125569. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, C.; Yu, Z. Protective effect of dihydromyricetin against lipopolysaccharide-induced HK2 cells by upregulating HIF-1α. Biotechnol. Genet. Eng. Rev. 2023, 25, 1–11. [Google Scholar] [CrossRef]
- Li, N.; Xu, M.; Wu, M.; Zhao, G. Cinnamtannin A2 protects the renal injury by attenuates the altered expression of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) expression in 5/6 nephrectomized rat model. AMB Express 2020, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Wang, L.; Jiang, L.; Qin, Z.; Zhao, Y.; Su, B. Maresin 1 Attenuates Lipopolysaccharide-Induced Acute Kidney Injury via Inhibiting NOX4/ROS/NF-κB Pathway. Front. Pharmacol. 2021, 12, 782660. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.F.; Feng, J.K.; Zhao, X.C.; Liu, W.; Gu, S.A.; Li, R.; Lu, Y.L.; Mao, R.J.; Xia, L.L.; Dong, L.L.; et al. The Extract of Pinellia Ternata-Induced Apoptosis of Leukemia Cells by Regulating the Expression of Bax, Bcl-2 and Caspase-3 Protein Expression in Mice. Transplant. Proc. 2023, 55, 2232–2240. [Google Scholar] [CrossRef] [PubMed]
- Eslami, F.; Mahdavi, M.; Babaei, E.; Hussen, B.M.; Mostafavi, H.; Shahbazi, A.; Hidayat, H.J. Down-regulation of Survivin and Bcl-2 concomitant with the activation of caspase-3 as a mechanism of apoptotic death in KG1a and K562 cells upon exposure to a derivative from ciprofloxacin family. Toxicol. Appl. Pharmacol. 2020, 409, 115331. [Google Scholar] [CrossRef]
- Xu, D.; Chen, M.; Ren, X.; Ren, X.; Wu, Y. Leonurine ameliorates LPS-induced acute kidney injury via suppressing ROS-mediated NF-κB signaling pathway. Fitoterapia 2014, 97, 148–155. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, M.; Li, B.; Kan, Y.; Wang, S.; Cao, B.; Huang, Y.; Zheng, X.; Feng, W. Arbutin attenuates LPS-induced acute kidney injury by inhibiting inflammation and apoptosis via the PI3K/Akt/Nrf2 pathway. Phytomedicine 2021, 82, 153466. [Google Scholar] [CrossRef] [PubMed]
- Elks, C.M.; Reed, S.D.; Mariappan, N.; Shukitt-Hale, B.; Joseph, J.A.; Ingram, D.K.; Francis, J. A blueberry-enriched diet attenuates nephropathy in a rat model of hypertension via reduction in oxidative stress. PLoS ONE 2011, 6, e24028. [Google Scholar] [CrossRef]
- Song, J.; Fan, H.-j.; Li, H.; Ding, H.; Lv, Q.; Hou, S.-k. Zingerone ameliorates lipopolysaccharide-induced acute kidney injury by inhibiting Toll-like receptor 4 signaling pathway. Eur. J. Pharmacol. 2016, 772, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Miao, L.; Yu, H.; Han, Z.; Sun, H. Ethanol Extract of Illicium henryi Attenuates LPS-Induced Acute Kidney Injury in Mice via Regulating Inflammation and Oxidative Stress. Nutrients 2019, 11, 1412. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.-W.; Cui, Z.-G.; Jin, Y.-J.; Sun, L.; Li, M.-L.; Zakki, S.A.; Zhou, D.-J.; Inadera, H. Protective effect of dihydromyricetin on hyperthermia-induced apoptosis in human myelomonocytic lymphoma cells. Apoptosis 2019, 24, 290–300. [Google Scholar] [CrossRef]
- Hou, X.L.; Tong, Q.; Wang, W.Q.; Shi, C.Y.; Xiong, W.; Chen, J.; Liu, X.; Fang, J.G. Suppression of Inflammatory Responses by Dihydromyricetin, a Flavonoid from Ampelopsis grossedentata, via Inhibiting the Activation of NF-κB and MAPK Signaling Pathways. J. Nat. Prod. 2015, 78, 1689–1696. [Google Scholar] [CrossRef]
- Dai, Y.; Jia, P.; Fang, Y.; Liu, H.; Jiao, X.; He, J.C.; Ding, X. miR-146a is essential for lipopolysaccharide (LPS)-induced cross-tolerance against kidney ischemia/reperfusion injury in mice. Sci. Rep. 2016, 6, 27091. [Google Scholar] [CrossRef]
- Niu, X.; Yao, Q.; Li, W.; Zang, L.; Li, W.; Zhao, J.; Liu, F.; Zhi, W. Harmine mitigates LPS-induced acute kidney injury through inhibition of the TLR4-NF-κB/NLRP3 inflammasome signalling pathway in mice. Eur. J. Pharmacol. 2019, 849, 160–169. [Google Scholar] [CrossRef]
- Gui, Y.; Yang, Y.; Xu, D.; Tao, S.; Li, J. Schisantherin A attenuates sepsis-induced acute kidney injury by suppressing inflammation via regulating the NRF2 pathway. Life Sci. 2020, 258, 118161. [Google Scholar] [CrossRef]
- Shi, M.; Zeng, X.; Guo, F.; Huang, R.; Feng, Y.; Ma, L.; Zhou, L.; Fu, P. Anti-Inflammatory Pyranochalcone Derivative Attenuates LPS-Induced Acute Kidney Injury via Inhibiting TLR4/NF-κB Pathway. Molecules 2017, 22, 1683. [Google Scholar] [CrossRef]
- Tang, N.; Ma, J.; Wang, K.S.; Mi, C.; Lv, Y.; Piao, L.X.; Xu, G.H.; Li, X.; Lee, J.J.; Jin, X. Dihydromyricetin suppresses TNF-α-induced NF-κB activation and target gene expression. Mol. Cell. Biochem. 2016, 422, 11–20. [Google Scholar] [CrossRef]
- Zhang, X.; Su, C.; Zhao, S.; Li, J.; Yu, F. Combination therapy of Ulinastatin with Thrombomodulin alleviates endotoxin (LPS)—Induced liver and kidney injury via inhibiting apoptosis, oxidative stress and HMGB1/TLR4/NF-κB pathway. Bioengineered 2022, 13, 2951–2970. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, H.; Xu, Z.; Zhang, X.; Tan, X.; He, N.; Shen, J.; Dong, J. Liensinine pretreatment reduces inflammation, oxidative stress, apoptosis, and autophagy to alleviate sepsis acute kidney injury. Int. Immunopharmacol. 2023, 122, 110563. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Chen, X.; Chen, D.; Yu, B.; Zheng, P.; He, J.; Chen, H.; Yan, H.; Luo, Y.; Huang, Z. Dihydromyricetin enhances intestinal antioxidant capacity of growing-finishing pigs by activating ERK/Nrf2/HO-1 signaling pathway. Antioxidants 2022, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lu, S.; Dong, X.; Xu, L.; Sun, G.; Sun, X. Dihydromyricetin protects human umbilical vein endothelial cells from injury through ERK and Akt mediated Nrf2/HO-1 signaling pathway. Apoptosis 2017, 22, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Leem, J.; Park, K.-K. Antioxidative, Antiapoptotic, and Anti-Inflammatory Effects of Apamin in a Murine Model of Lipopolysaccharide-Induced Acute Kidney Injury. Molecules 2020, 25, 5717. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zeng, M.; Li, B.; Kan, Y.; Zhang, B.; Zheng, X.; Feng, W. Raw and salt-processed Achyranthes bidentata attenuate LPS-induced acute kidney injury by inhibiting ROS and apoptosis via an estrogen-like pathway. Biomed. Pharmacother. 2020, 129, 110403. [Google Scholar] [CrossRef]
- He, Z.; Zhang, L.; Zhuo, C.; Jin, F.; Wang, Y. Apoptosis inhibition effect of Dihydromyricetin against UVA-exposed human keratinocyte cell line. J. Photochem. Photobiol. B Biol. 2016, 161, 40–49. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Qiu, E. Protection of oxidative stress induced apoptosis in osteosarcoma cells by dihydromyricetin through down-regulation of caspase activation and up-regulation of BcL-2. Saudi J. Biol. Sci. 2017, 24, 837–842. [Google Scholar] [CrossRef]



| Genes | Type | Sequences (5→3) |
|---|---|---|
| KIM-1 | Fw | CTGGAATGGCACTGTGACATCC |
| Rev | GCAGATGCCAACATAGAAGCCC | |
| IL-1β | Fw | GCAACTGTTCCTGAACTCAACT |
| Rev | ATCTTTTGGGGTCCGTCAACT | |
| MCP-1 | Fw | CATCCACGTGTTGGCTCA |
| Rev | GATCATCTTGCTGGTGAATGAGT | |
| IL-6 | Fw | AAAGAGTTGTGCAATGGCAATTCT |
| Rev | AAGTGCATCATCGTTGTTCATACA | |
| Bcl-2 | Fw | TGTGAGGACCCAATCTGGAAA |
| Rev | TTGCAATGAATCGGGAGTTG | |
| Bax | Fw | GATCAGCTCGGGCACTTTAG |
| Rev | TTGCTGATGGCAACTTCAAC | |
| NF-κB | Fw | ATGTGGAGATCATTGAGCAGC |
| Rev | CCTGGTCCTGTGTAGCCATT | |
| GAPDH | Fw | AGGTCGGTGTGAACGGATTTG |
| Rev | TGTAGACCATGTAGTTGAGGTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.; Yan, Q.; Li, Y.; Tang, H. Dihydromyricetin Nanoparticles Alleviate Lipopolysaccharide-Induced Acute Kidney Injury by Decreasing Inflammation and Cell Apoptosis via the TLR4/NF-κB Pathway. J. Funct. Biomater. 2024, 15, 249. https://doi.org/10.3390/jfb15090249
Yin H, Yan Q, Li Y, Tang H. Dihydromyricetin Nanoparticles Alleviate Lipopolysaccharide-Induced Acute Kidney Injury by Decreasing Inflammation and Cell Apoptosis via the TLR4/NF-κB Pathway. Journal of Functional Biomaterials. 2024; 15(9):249. https://doi.org/10.3390/jfb15090249
Chicago/Turabian StyleYin, Hongmei, Qiaohua Yan, Yinglun Li, and Huaqiao Tang. 2024. "Dihydromyricetin Nanoparticles Alleviate Lipopolysaccharide-Induced Acute Kidney Injury by Decreasing Inflammation and Cell Apoptosis via the TLR4/NF-κB Pathway" Journal of Functional Biomaterials 15, no. 9: 249. https://doi.org/10.3390/jfb15090249
APA StyleYin, H., Yan, Q., Li, Y., & Tang, H. (2024). Dihydromyricetin Nanoparticles Alleviate Lipopolysaccharide-Induced Acute Kidney Injury by Decreasing Inflammation and Cell Apoptosis via the TLR4/NF-κB Pathway. Journal of Functional Biomaterials, 15(9), 249. https://doi.org/10.3390/jfb15090249






