The Impact of the Methacrylation Process on the Usefulness of Chitosan as a Biomaterial Component for 3D Printing
Abstract
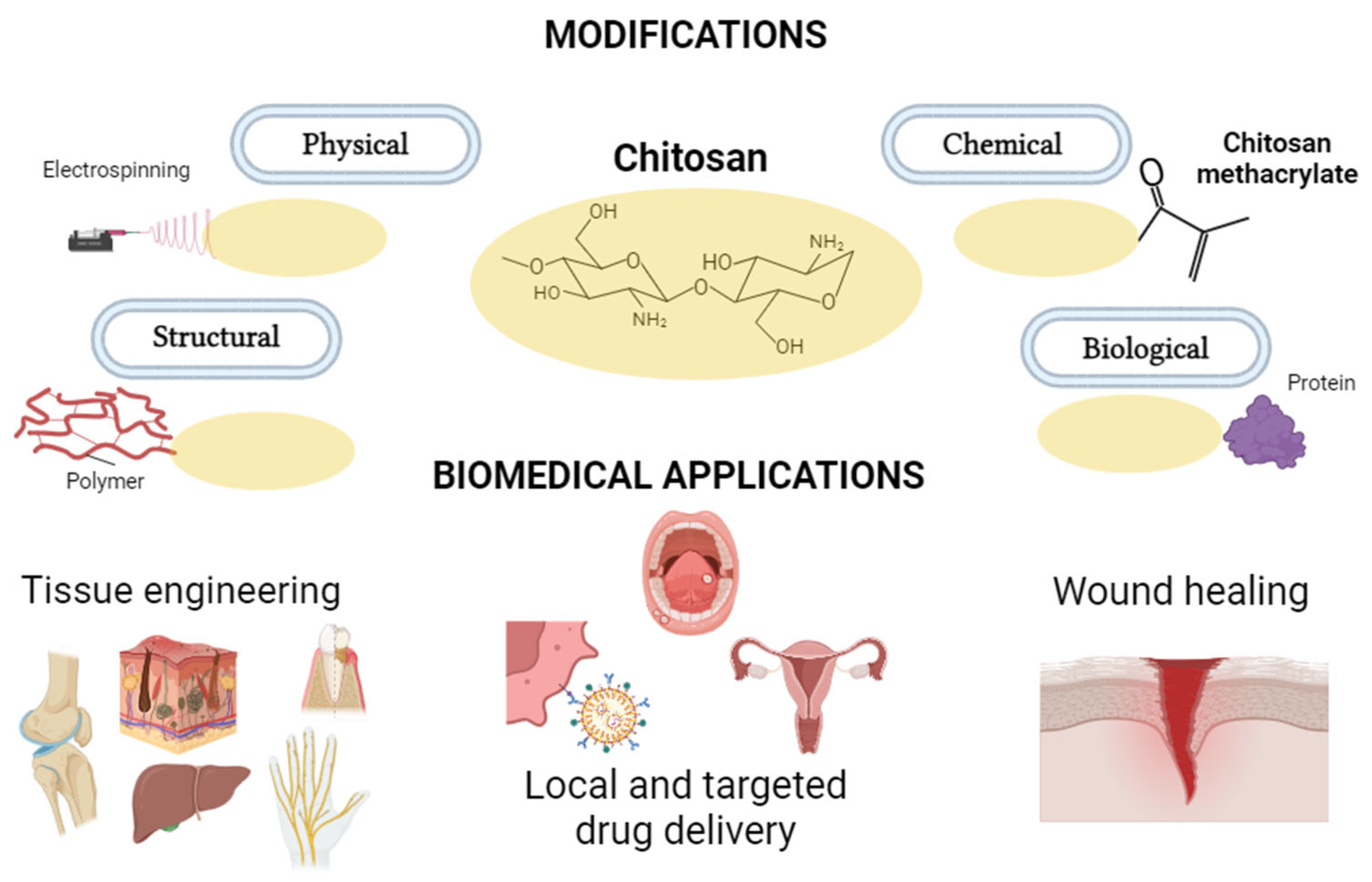
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Biomaterials Components
2.1.2. Cell Culture
2.2. Methods
2.2.1. Preparation of CHIMA
2.2.2. Degree of Substitution
2.2.3. Rheology
2.2.4. Printability
- At—theoretical pore surface area;
- Aa—the actual surface area of the pore;
- La—the actual perimeter of the pore.
- Aac—the actual area under the curve;
- Atc—the theoretical area under the curve.
2.2.5. Mechanical Testing
2.2.6. Degradation
2.2.7. Effective Swelling and Absorbability
2.2.8. The Assessment of Cell Biocompatibility Following Model Bioprinting and Cell Culture on the Surface of Biomaterials
Preparation and Bioprinting of Cell-Laden Biomaterial
Preparation and Cell Seeding on the Surface of Biomaterials
Microscopic Image Analysis
Cytotoxicity Assay
Cell Proliferation
Gene Expression
2.2.9. Statistical Analysis
3. Results
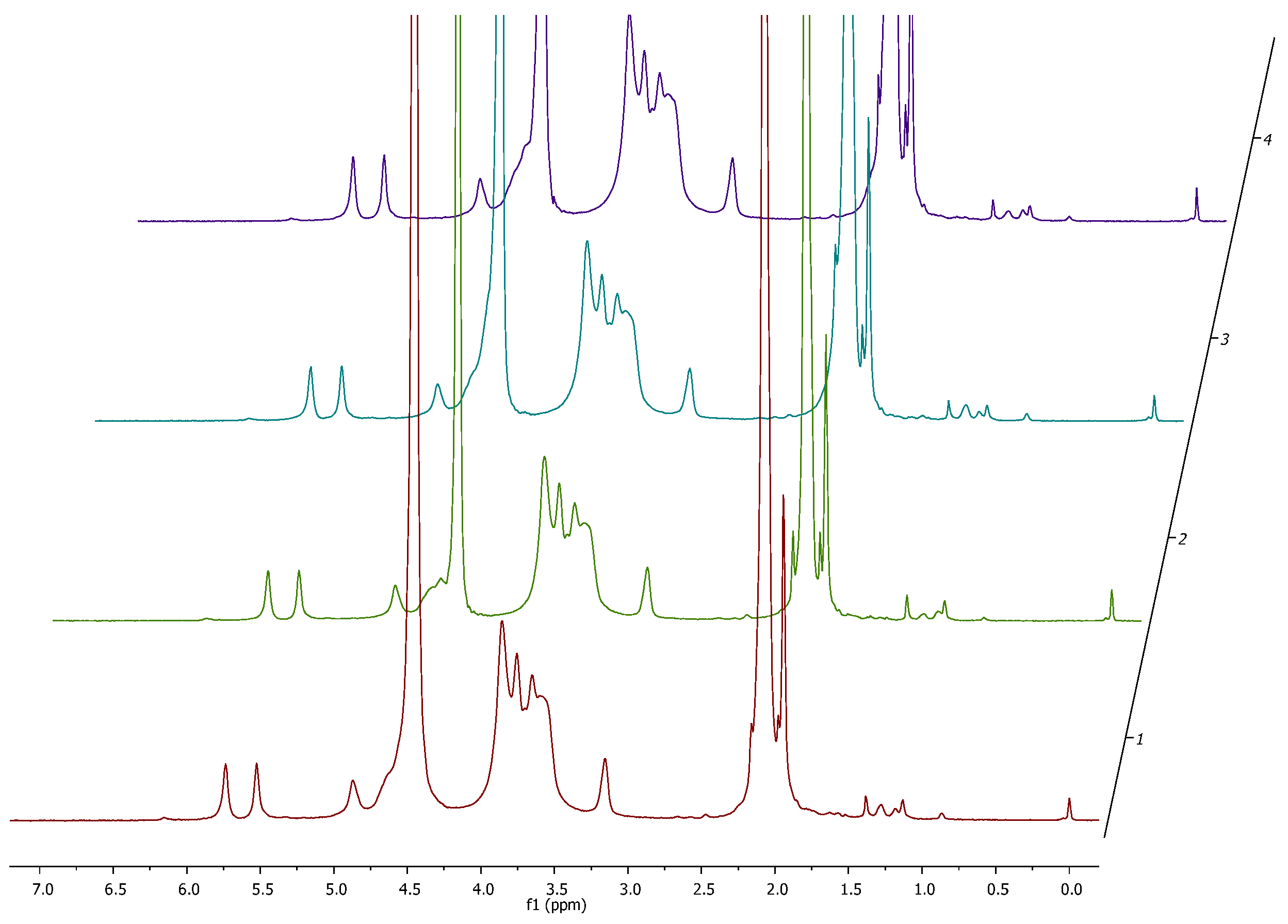
3.1. Degree of Substitution and 1H NMR Analysis
3.2. Rheology
3.3. Printability
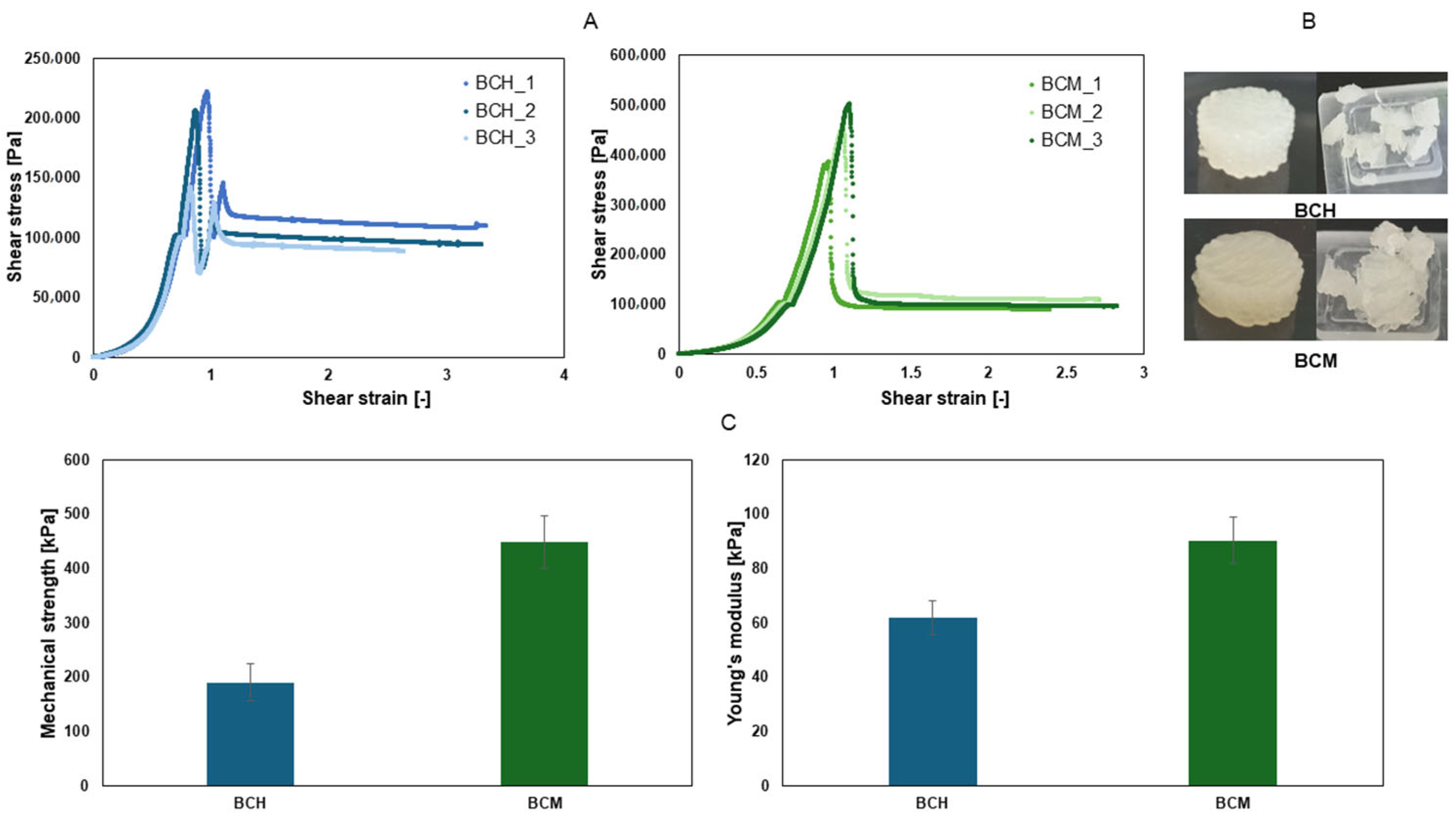
3.4. Mechanical Testing
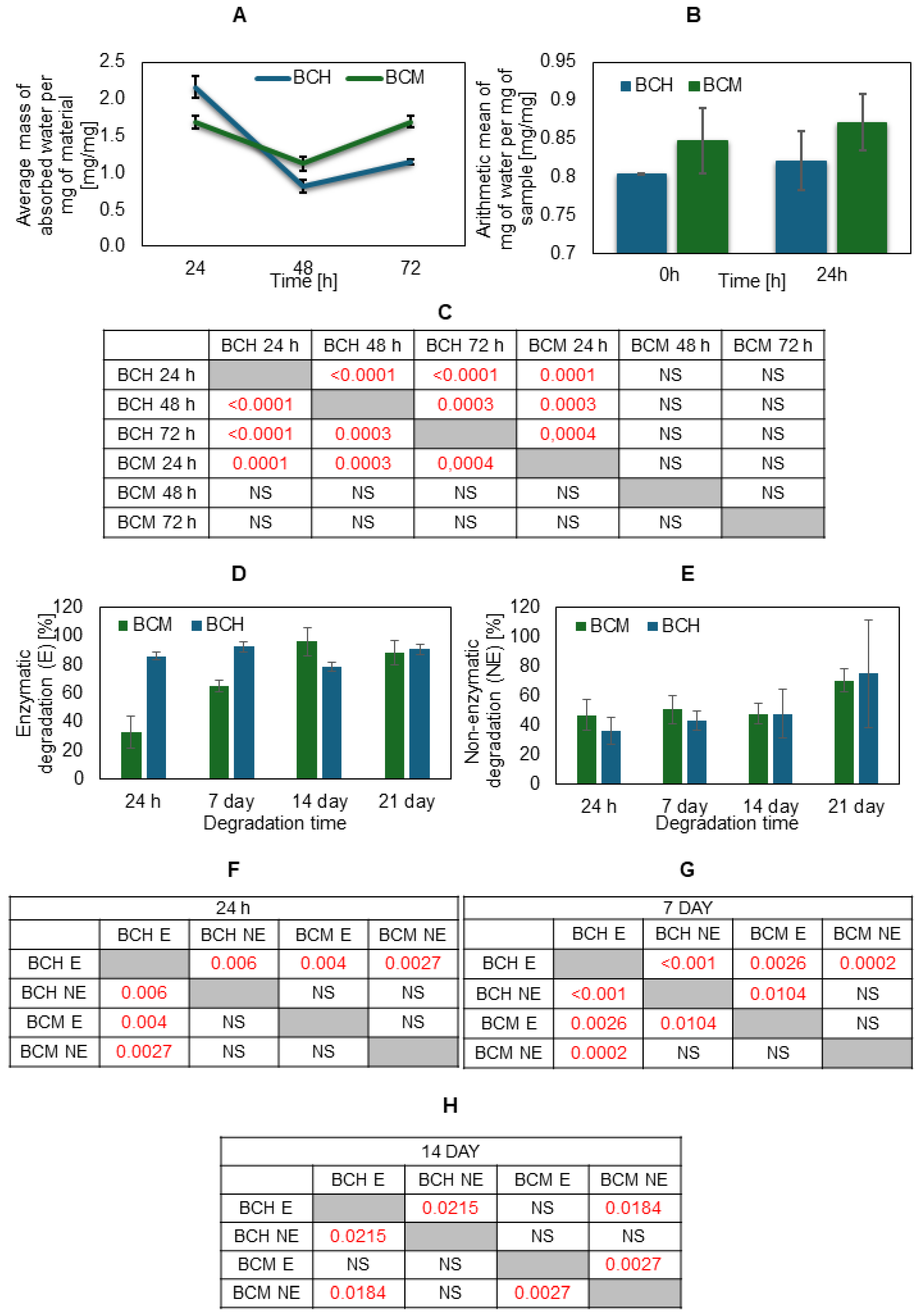
3.5. Degradation, Absorbability, and Swelling Ratio
3.6. Assessment of Biocompatibility of Cells Cultured on Biomaterial Surfaces and 3D-Printed Models
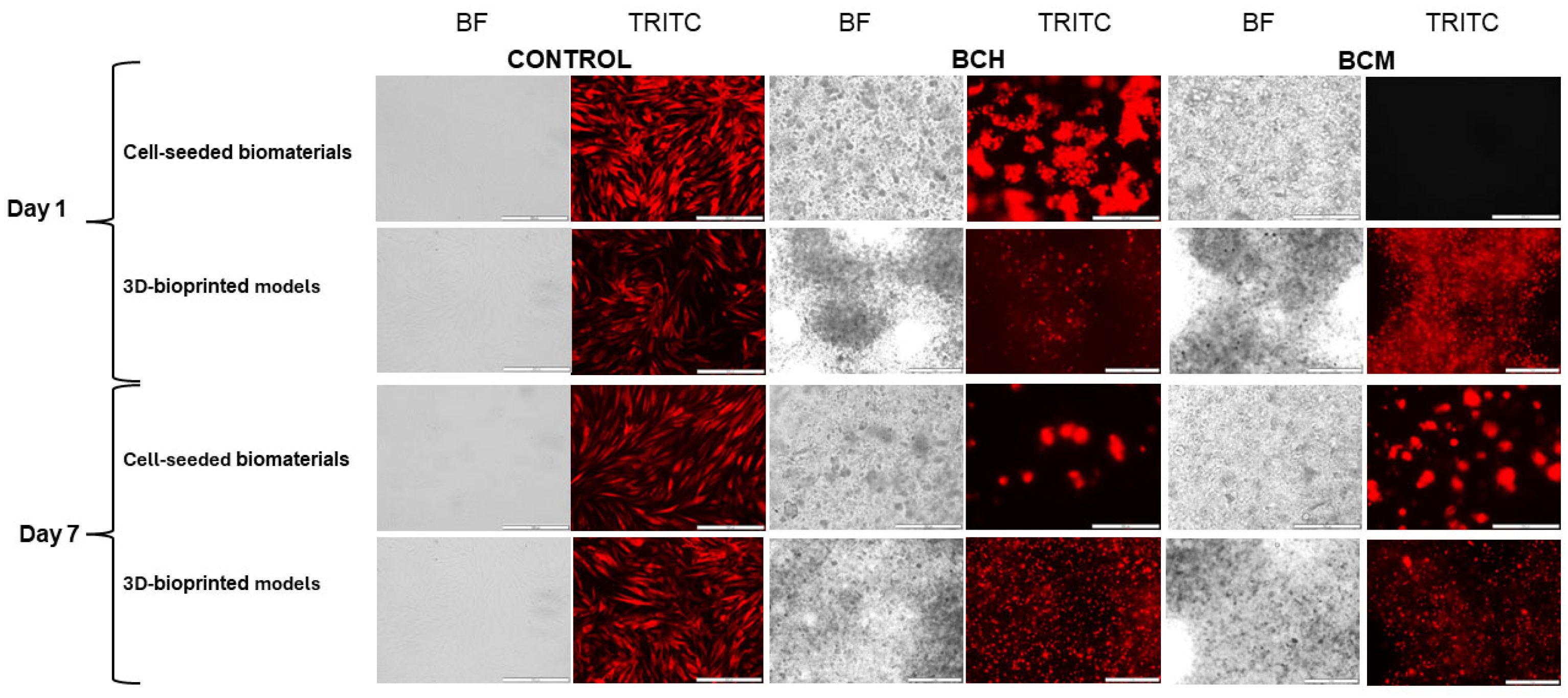
3.6.1. Microscopic Imaging Analysis
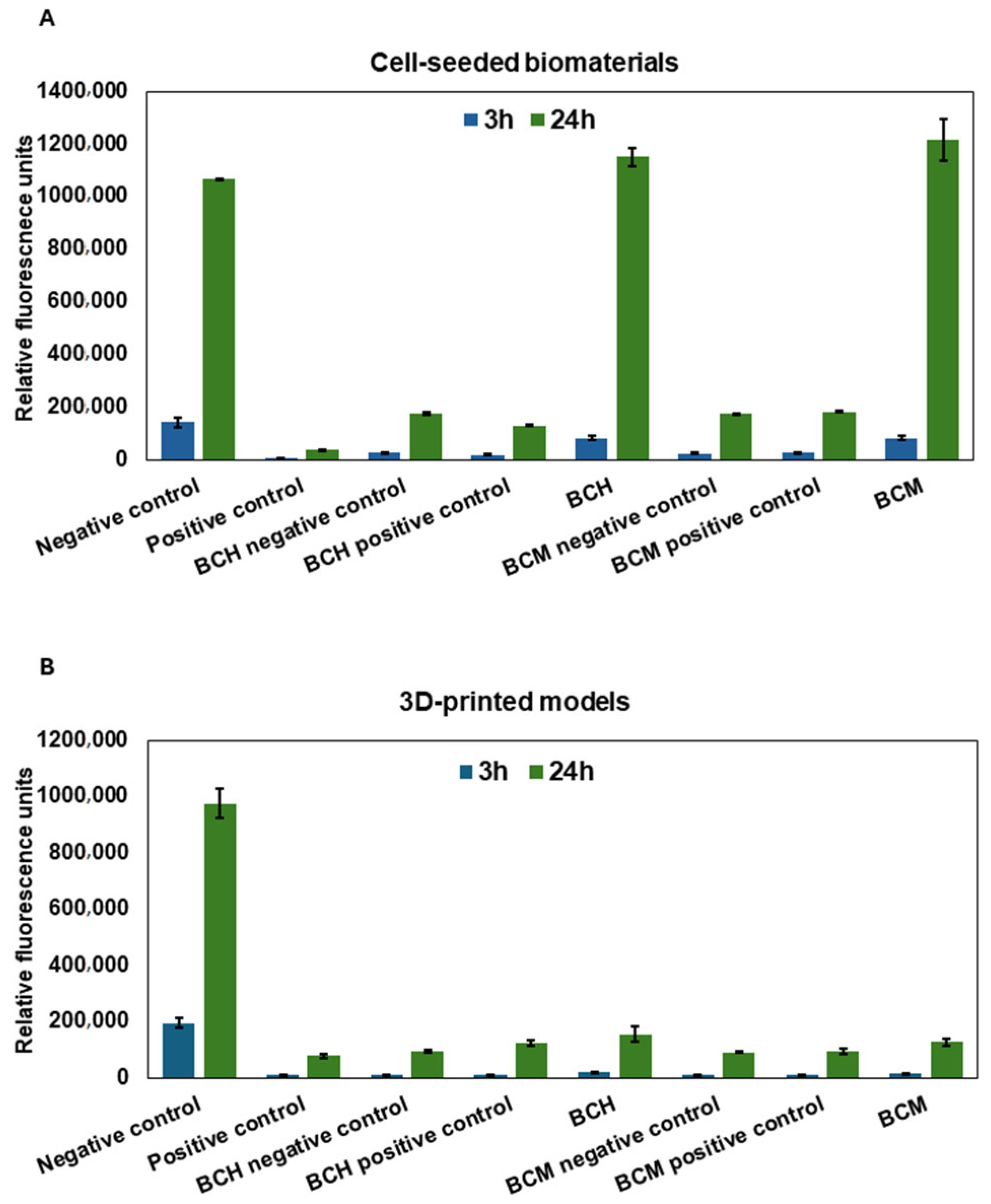
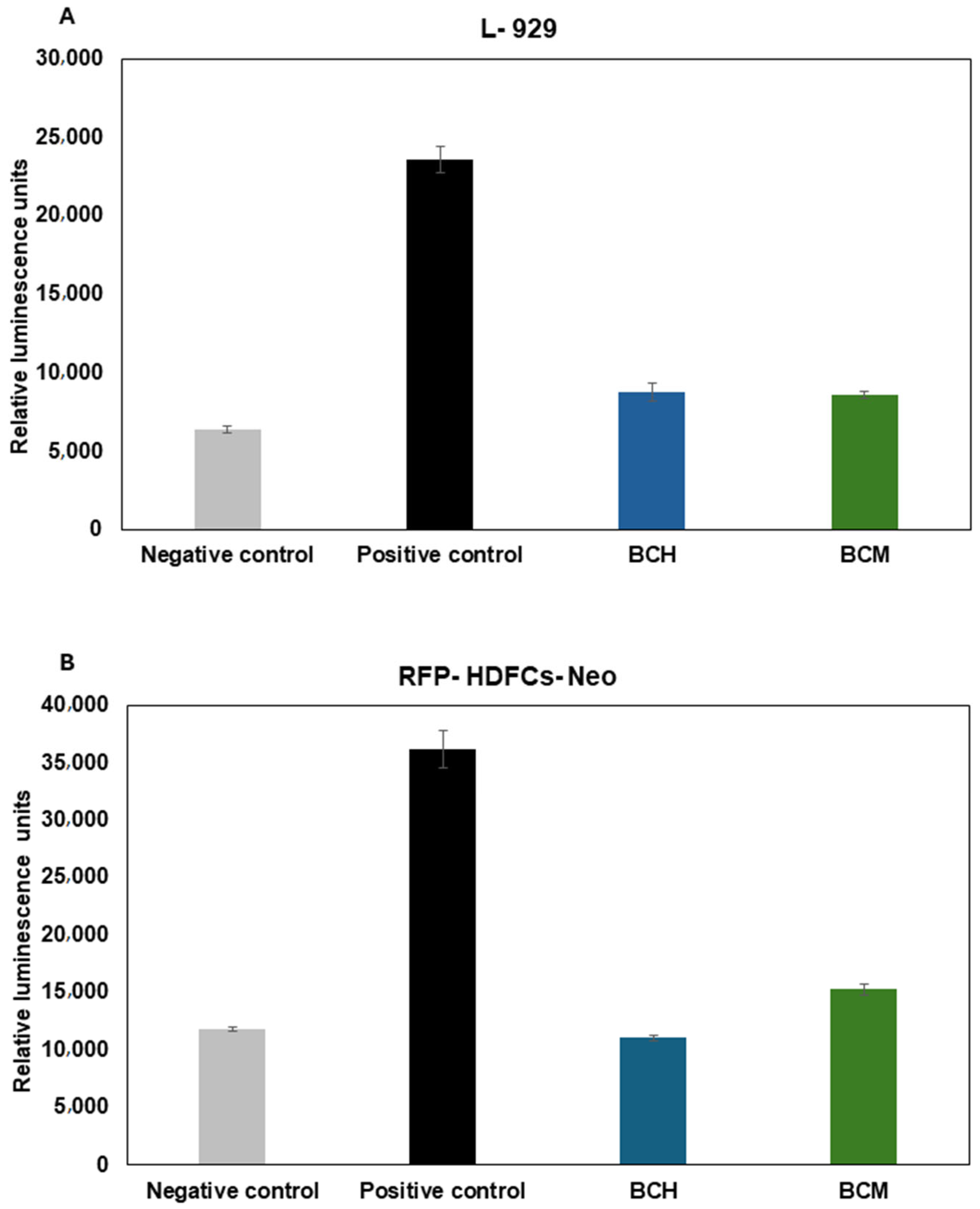
3.6.2. Cytotoxicity Assay
3.6.3. Cell Proliferation Assay
3.6.4. The Impact of Biomaterial Extracts on Cell Cytotoxicity
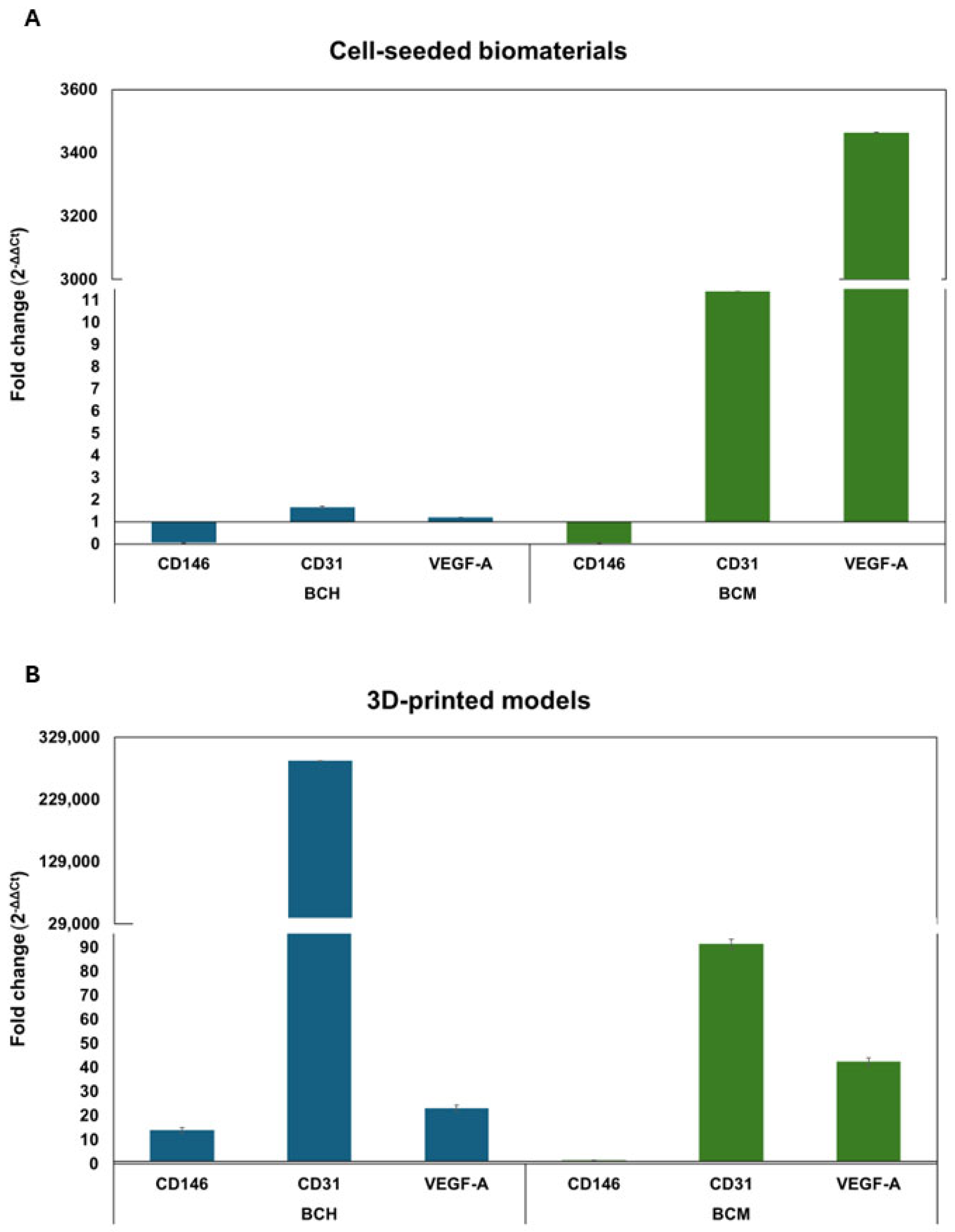
3.6.5. Gene Expression Analysis
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kołodziejska, M.; Jankowska, K.; Klak, M.; Wszoła, M. Chitosan as an Underrated Polymer in Modern Tissue Engineering. Nanomaterials 2021, 11, 3019. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, Z.; Maniruzzaman, M. Bioprinting: A Focus on Improving Bioink Printability and Cell Performance Based on Different Process Parameters. Int. J. Pharm. 2023, 640, 123020. [Google Scholar] [CrossRef] [PubMed]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D Bioprinting: An Overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Sun, W.; Starly, B.; Daly, A.C.; Burdick, J.A.; Groll, J.; Skeldon, G.; Shu, W.; Sakai, Y.; Shinohara, M.; Nishikawa, M.; et al. The Bioprinting Roadmap. Biofabrication 2020, 12, 022002. [Google Scholar] [CrossRef] [PubMed]
- Thayer, P.; Martinez, H.; Gatenholm, E. History and Trends of 3D Bioprinting. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 2140. [Google Scholar] [CrossRef]
- Kim, J. Characterization of Biocompatibility of Functional Bioinks for 3D Bioprinting. Bioengineering 2023, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef]
- Magin, C.M.; Alge, D.L.; Anseth, K.S. Bio-Inspired 3D Microenvironments: A New Dimension in Tissue Engineering. Biomed. Mater. 2016, 11, 022001. [Google Scholar] [CrossRef]
- Meiabadi, M.S.; Moradi, M.; Karamimoghadam, M.; Ardabili, S.; Bodaghi, M.; Shokri, M.; Mosavi, A.H. Modeling the Producibility of 3d Printing in Polylactic Acid Using Artificial Neural Networks and Fused Filament Fabrication. Polymers 2021, 13, 3219. [Google Scholar] [CrossRef]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface Modification of Biomaterials and Biomedical Devices Using Additive Manufacturing. Acta Biomater. 2018, 66, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Bupphathong, S.; Quiroz, C.; Huang, W.; Chung, P.F.; Tao, H.Y.; Lin, C.H. Gelatin Methacrylate Hydrogel for Tissue Engineering Applications—A Review on Material Modifications. Pharmaceuticals 2022, 15, 171. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Yang, M.; Liu, M.; Jin, Y.; Wang, Y.; Yang, R.; Wang, Y.; Zhang, K.; Fu, Q. Review on Additives in Hydrogels for 3D Bioprinting of Regenerative Medicine: From Mechanism to Methodology. Pharmaceutics 2023, 15, 1700. [Google Scholar] [CrossRef] [PubMed]
- Oleksy, M.; Dynarowicz, K.; Aebisher, D. Advances in Biodegradable Polymers and Biomaterials for Medical Applications—A Review. Molecules 2023, 28, 6213. [Google Scholar] [CrossRef] [PubMed]
- Ze, Y.; Li, Y.; Huang, L.; Shi, Y.; Li, P.; Gong, P.; Lin, J.; Yao, Y. Biodegradable Inks in Indirect Three-Dimensional Bioprinting for Tissue Vascularization. Front. Bioeng. Biotechnol. 2022, 10, 856398. [Google Scholar] [CrossRef]
- Ahmadi Soufivand, A.; Faber, J.; Hinrichsen, J.; Budday, S. Multilayer 3D Bioprinting and Complex Mechanical Properties of Alginate-Gelatin Mesostructures. Sci. Rep. 2023, 13, 11253. [Google Scholar] [CrossRef]
- Klak, M.; Bryniarski, T.; Kowalska, P.; Gomolka, M.; Tymicki, G.; Kosowska, K.; Cywoniuk, P.; Dobrzanski, T.; Turowski, P.; Wszola, M. Novel Strategies in Artificial Organ Development: What Is the Future of Medicine? Micromachines 2020, 11, 646. [Google Scholar] [CrossRef]
- Lazaridou, M.; Bikiaris, D.N.; Lamprou, D.A. 3D Bioprinted Chitosan-Based Hydrogel Scaffolds in Tissue Engineering and Localised Drug Delivery. Pharmaceutics 2022, 14, 1978. [Google Scholar] [CrossRef]
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for Tissue Repair and Organ Three-Dimensional (3D) Bioprinting. Micromachines 2019, 10, 765. [Google Scholar] [CrossRef]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioǧlu, M. A Bioprintable Form of Chitosan Hydrogel for Bone Tissue Engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef]
- Salar Amoli, M.; Anand, R.; EzEldeen, M.; Amorim, P.A.; Geris, L.; Jacobs, R.; Bloemen, V. The Development of a 3D Printable Chitosan-Based Copolymer with Tunable Properties for Dentoalveolar Regeneration. Carbohydr. Polym. 2022, 289, 119441. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Cui, Y.; Zhang, M.; Zhao, D.; Liu, G.; Ding, J. Engineered Three-Dimensional Scaffolds for Enhanced Bone Regeneration in Osteonecrosis. Bioact. Mater. 2020, 5, 584–601. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, E.; Ulag, S.; Sahin, A.; Yilmaz, B.K.; Ekren, N.; Kilic, O.; Sengor, M.; Kalaskar, D.M.; Oktar, F.N.; Gunduz, O. Fabrication of Tissue-Engineered Tympanic Membrane Patches Using 3D-Printing Technology. J. Mech. Behav. Biomed. Mater. 2021, 114, 104219. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, R.; Nakamatsu, J.; Ramírez, E.; Elgegren, M.; Ayarza, J.; Kim, S.; Pando, M.A.; Ortega-San-Martin, L. The Potential Use of Chitosan as a Biopolymer Additive for Enhanced Mechanical Properties and Water Resistance of Earthen Construction. Constr. Build. Mater. 2016, 114, 625–637. [Google Scholar] [CrossRef]
- Charoenwongpaiboon, T.; Supraditaporn, K.; Klaimon, P.; Wangpaiboon, K.; Pichyangkura, R.; Issaragrisil, S.; Lorthongpanich, C. Effect of Alternan: Versus Chitosan on the Biological Properties of Human Mesenchymal Stem Cells. RSC Adv. 2019, 9, 4370–4379. [Google Scholar] [CrossRef]
- Ravindranathan, S.; Koppolu, B.P.; Smith, S.G.; Zaharoff, D.A. Effect of Chitosan Properties on Immunoreactivity. Mar. Drugs 2016, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, F.; Islam, M.R.; Li, H. The Fabrication of the Chitosan-Based Bioink for in Vitro Tissue Repair and Regeneration: A Review. Int. J. Biol. Macromol. 2024, 257, 128504. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, Y.; Jiang, Y.; Quan, W.; Luo, H.; Wu, K.; Li, S.; Ouyang, Q. Progress in Research of Chitosan Chemical Modification Technologies and Their Applications. Mar. Drugs 2022, 20, 536. [Google Scholar] [CrossRef]
- Sharma, D.S.; Wadhwa, S.; Gulati, M.; Kumar, B.; Chitranshi, N.; Gupta, V.K.; Alrouji, M.; Alhajlah, S.; AlOmeir, O.; Vishwas, S.; et al. Chitosan Modified 5-Fluorouracil Nanostructured Lipid Carriers for Treatment of Diabetic Retinopathy in Rats: A New Di-Mension to an Anticancer Drug. Int. J. Biol. Macromol. 2023, 224, 810–830. [Google Scholar] [CrossRef] [PubMed]
- Wegrzynowska-Drzymalska, K.; Grebicka, P.; Mlynarczyk, D.T.; Chelminiak-Dudkiewicz, D.; Kaczmarek, H.; Goslinski, T.; Ziegler-Borowska, M. Crosslinking of Chitosan with Dialdehyde Chitosan as a New Approach for Biomedical Applications. Materials 2020, 13, 3413. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Ma, X.; Wang, X.; Liu, L. Structure and Properties of Chitosan Films: Effect of the Type of Solvent Acid. LWT 2021, 135, 109984. [Google Scholar] [CrossRef]
- Sabzevari, M.; Cree, D.E.; Wilson, L.D. Graphene Oxide-Chitosan Composite Material for Treatment of a Model Dye Effluent. ACS Omega 2018, 3, 13045–13054. [Google Scholar] [CrossRef]
- Hua, Y.; Ma, C.; Wei, T.; Zhang, L.; Shen, J. Collagen/Chitosan Complexes: Preparation, Antioxidant Activity, Tyrosinase Inhibition Activity, and Melanin Synthesis. Int. J. Mol. Sci. 2020, 21, 313. [Google Scholar] [CrossRef] [PubMed]
- Blachnio, M.; Zienkiewicz-Strzalka, M.; Derylo-Marczewska, A.; Nosach, L.V.; Voronin, E.F. Chitosan–Silica Composites for Adsorption Application in the Treatment of Water and Wastewater from Anionic Dyes. Int. J. Mol. Sci. 2023, 24, 11818. [Google Scholar] [CrossRef]
- Montazeri, S.; Rastegari, A.; Mohammadi, Z.; Nazari, M.; Yousefi, M.; Samadi, F.Y.; Najafzadeh, S.; Aghsami, M. Chitosan Nanoparticle Loaded by Epidermal Growth Factor as a Potential Protein Carrier for Wound Healing: In Vitro and in Vivo Studies. IET Nanobiotechnol. 2023, 17, 204–211. [Google Scholar] [CrossRef]
- Brun, P.; Zamuner, A.; Cassari, L.; D’auria, G.; Falcigno, L.; Franchi, S.; Contini, G.; Marsotto, M.; Battocchio, C.; Iucci, G.; et al. Chitosan Covalently Functionalized with Peptides Mapped on Vitronectin and Bmp-2 for Bone Tissue Engineering. Nanomaterials 2021, 11, 2784. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug Release Study of the Chitosan-Based Nanoparticles. Heliyon 2021, 8, e08674. [Google Scholar] [CrossRef]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A Review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef]
- Torkaman, S.; Rahmani, H.; Ashori, A.; Najafi, S.H.M. Modification of Chitosan Using Amino Acids for Wound Healing Purposes: A Review. Carbohydr. Polym. 2021, 258, 117675. [Google Scholar] [CrossRef] [PubMed]
- Tiboni, M.; Elmowafy, E.; El-Derany, M.O.; Benedetti, S.; Campana, R.; Verboni, M.; Potenza, L.; Palma, F.; Citterio, B.; Sisti, M.; et al. A Combination of Sugar Esters and Chitosan to Promote in Vivo Wound Care. Int. J. Pharm. 2022, 616, 121508. [Google Scholar] [CrossRef]
- Lohiya, G.; Katti, D.S. Carboxylated Chitosan-Mediated Improved Efficacy of Mesoporous Silica Nanoparticle-Based Targeted Drug Delivery System for Breast Cancer Therapy. Carbohydr. Polym. 2022, 277, 118822. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Methacrylated Chitosan as a Polymer with Enhanced Mucoadhesive Properties for Transmucosal Drug Delivery. Int. J. Pharm. 2018, 550, 123–129. [Google Scholar] [CrossRef]
- Osi, A.R.; Zhang, H.; Chen, J.; Zhou, Y.; Wang, R.; Fu, J.; Müller-Buschbaum, P.; Zhong, Q. Three-Dimensional-Printable Thermo/Photo-Cross-Linked Methacrylated Chitosan-Gelatin Hydrogel Composites for Tissue Engineering. ACS Appl. Mater. Interfaces 2021, 13, 22902–22913. [Google Scholar] [CrossRef] [PubMed]
- Hemming, E.B.; Masters, A.F.; Perosa, A.; Selva, M.; Maschmeyer, T. Single-Step Methylation of Chitosan Using Dimethyl Carbonate as a Green Methylating Agent. Molecules 2019, 24, 3986. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.J.; Lee, B.H. Gelatin Methacryloyl and Its Hydrogels with an Exceptional Degree of Controllability and Batch-to-Batch Consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef]
- Naghieh, S.; Chen, X. Printability–A Key Issue in Extrusion-Based Bioprinting. J. Pharm. Anal. 2021, 11, 564–579. [Google Scholar] [CrossRef]
- Habib, A.; Sathish, V.; Mallik, S.; Khoda, B. 3D Printability of Alginate-Carboxymethyl Cellulose Hydrogel. Materials 2018, 11, 454. [Google Scholar] [CrossRef]
- Sarker, M.; Izadifar, M.; Schreyer, D.; Chen, X. Influence of Ionic Crosslinkers (Ca2+/Ba2+/Zn2+) on the Mechanical and Biological Properties of 3D Bioplotted Hydrogel Scaffolds. J. Biomater. Sci. Polym. Ed. 2018, 29, 1126–1154. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, S.M.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Synthesis and Characterization of a Photocrosslinkable Chitosan-Gelatin Hydrogel Aimed for Tissue Regeneration. RSC Adv. 2015, 5, 63478–63488. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoo, J.J.; Lee, S.J. Three-Dimensional Cell-Based Bioprinting for Soft Tissue Regeneration. Tissue Eng. Regen. Med. 2016, 13, 647–662. [Google Scholar] [CrossRef]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan Hydrogels in 3D Printing for Biomedical Applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Taghizadeh, A.; Yazdi, M.K.; Zarrintaj, P.; Stadler, F.J.; Ramsey, J.D.; Habibzadeh, S.; Hosseini Rad, S.; Naderi, G.; Saeb, M.R.; et al. Chitosan-Based Inks for 3D Printing and Bioprinting. Green Chem. 2021, 24, 62–101. [Google Scholar] [CrossRef]
- Pei, M.; Mao, J.; Xu, W.; Zhou, Y.; Xiao, P. Photocrosslinkable Chitosan Hydrogels and Their Biomedical Applications. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1862–1871. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Wu, Z.; Cao, L. Photocrosslinked Methacrylated Natural Macromolecular Hydrogels for Tissue Engineering: A Review. Int. J. Biol. Macromol. 2023, 246, 125570. [Google Scholar] [CrossRef] [PubMed]
- Teoh, J.H.; Mozhi, A.; Sunil, V.; Tay, S.M.; Fuh, J.; Wang, C.H. 3D Printing Personalized, Photocrosslinkable Hydrogel Wound Dressings for the Treatment of Thermal Burns. Adv. Funct. Mater. 2021, 31, 2105932. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, H.; Huang, X.; Hang, R.; Zhang, X.; Wang, Y.; Yao, X. DLP Printing Photocurable Chitosan to Build Bio-Constructs for Tissue Engineering. Carbohydr. Polym. 2020, 235, 115970. [Google Scholar] [CrossRef] [PubMed]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to Assess Printability of Bioinks for Extrusion-Based Bioprinting and Evaluation of Rheological Properties Governing Bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef]
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Whitaker, I.S. ‘Printability’ of Candidate Biomaterials for Extrusion Based 3D Printing: State-of-the-Art. Adv. Healthc. Mater. 2017, 6, 1700264. [Google Scholar] [CrossRef] [PubMed]
- Rutz, A.L.; Hyland, K.E.; Jakus, A.E.; Burghardt, W.R.; Shah, R.N. A Multimaterial Bioink Method for 3D Printing Tunable, Cell-Compatible Hydrogels. Adv. Mater. 2015, 27, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fu, Q.; Yoo, J.; Chen, X.; Chandra, P.; Mo, X.; Song, L.; Atala, A.; Zhao, W. 3D Bioprinting of Urethra with PCL/PLCL Blend and Dual Autologous Cells in Fibrin Hydrogel: An in Vitro Evaluation of Biomimetic Mechanical Property and Cell Growth Environment. Acta Biomater. 2017, 50, 154–164. [Google Scholar] [CrossRef]
- Göhl, J.; Markstedt, K.; Mark, A.; Håkansson, K.; Gatenholm, P.; Edelvik, F. Simulations of 3D Bioprinting: Predicting Bioprintability of Nanofibrillar Inks. Biofabrication 2018, 10, 034105. [Google Scholar] [CrossRef] [PubMed]
- Panwar, A.; Tan, L.P. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Jakus, A.E.; Rutz, A.L.; Shah, R.N. Advancing the Field of 3D Biomaterial Printing. Biomed. Mater. 2016, 11, 014102. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Hermida, M.A.; Leslie, N.R.; Shu, W. Three-Dimensional Bioprinting of Complex Cell Laden Alginate Hydrogel Structures. Biofabrication 2015, 7, 045012. [Google Scholar] [CrossRef]
- Duty, C.; Ajinjeru, C.; Kishore, V.; Compton, B.; Hmeidat, N.; Chen, X.; Liu, P.; Hassen, A.A.; Lindahl, J.; Kunc, V. What Makes a Material Printable? A Viscoelastic Model for Extrusion-Based 3D Printing of Polymers. J. Manuf. Process. 2018, 35, 526–537. [Google Scholar] [CrossRef]
- Gao, T.; Gillispie, G.J.; Copus, J.S.; Kumar, A.P.R.; Seol, Y.J.; Atala, A.; Yoo, J.J.; Lee, S.J. Optimization of Gelatin-Alginate Composite Bioink Printability Using Rheological Parameters: A Systematic Approach. Biofabrication 2018, 10, 034106. [Google Scholar] [CrossRef]
- García-García, A.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Larrea-Sebal, A.; Martin, C.; Vilas-Vilela, J.L. Extrusion-Based 3D Printing of Photocrosslinkable Chitosan Inks. Gels 2024, 10, 126. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Feng, Y. Chitosan Hydrogel as Tissue Engineering Scaffolds for Vascular Regeneration Applications. Gels 2023, 9, 373. [Google Scholar] [CrossRef]
- Victor, R.D.S.; Santos, A.M.D.C.; de Sousa, B.V.; Neves, G.D.A.; Santana, L.N.D.L.; Menezes, R.R. A Review on Chitosan’s Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and Cancer Treatment. Materials 2020, 13, 4995. [Google Scholar] [CrossRef] [PubMed]
- Gheran, C.V.; Voicu, S.N.; Galateanu, B.; Callewaert, M.; Moreau, J.; Cadiou, C.; Chuburu, F.; Dinischiotu, A. In Vitro Studies Regarding the Safety of Chitosan and Hyaluronic Acid-Based Nanohydrogels Containing Contrast Agents for Magnetic Resonance Imaging. Int. J. Mol. Sci. 2022, 23, 3258. [Google Scholar] [CrossRef]
- Su, F.; Wang, Y.; Liu, X.; Shen, X.; Zhang, X.; Xing, Q.; Wang, L.; Chen, Y. Biocompatibility and in Vivo Degradation of Chitosan Based Hydrogels as Potential Drug Carrier. J. Biomater. Sci. Polym. Ed. 2018, 29, 1515–1528. [Google Scholar] [CrossRef] [PubMed]
- Céspedes-Valenzuela, D.N.; Sánchez-Rentería, S.; Cifuentes, J.; Gantiva-Diaz, M.; Serna, J.A.; Reyes, L.H.; Ostos, C.; Cifuentes-De la Portilla, C.; Muñoz-Camargo, C.; Cruz, J.C. Preparation and Characterization of an Injectable and Photo-Responsive Chitosan Methacrylate/Graphene Oxide Hydrogel: Potential Applications in Bone Tissue Adhesion and Repair. Polymers 2022, 14, 126. [Google Scholar] [CrossRef]
- Chen, C.C.; Wang, J.M.; Huang, Y.R.; Yu, Y.H.; Wu, T.M.; Ding, S.J. Synergistic Effect of Thermoresponsive and Photocuring Methacrylated Chitosan-Based Hybrid Hydrogels for Medical Applications. Pharmaceutics 2023, 15, 1090. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Collins, G.; Yip, D.; Cho, C.H. Functional 3-D Cardiac Co-Culture Model Using Bioactive Chitosan Nanofiber Scaffolds. Biotechnol. Bioeng. 2013, 110, 637–647. [Google Scholar] [CrossRef]
- Howling, G.I.; Dettmar, P.W.; Goddard, P.A.; Hampson, F.C.; Dornish, M.; Wood, E.J. The Effect of Chitin and Chitosan on the Proliferation of Human Skin Fibroblasts and Keratinocytes in Vitro. Biomaterials 2001, 22, 2959–2966. [Google Scholar] [CrossRef]
- He, J.; Wu, F.; Wang, D.; Yao, R.; Wu, Y.; Wu, F. Modulation of Cationicity of Chitosan for Tuning Mesenchymal Stem Cell Adhesion, Proliferation, and Differentiation. Biointerphases 2015, 10, 04A304. [Google Scholar] [CrossRef]
- Patel, B.; Manne, R.; Patel, D.B.; Gorityala, S.; Palaniappan, A.; Kurakula, M. Chitosan as Functional Biomaterial for Designing Delivery Systems in Cardiac Therapies. Gels 2021, 7, 253. [Google Scholar] [CrossRef]
- Celikkin, N.; Mastrogiacomo, S.; Jaroszewicz, J.; Walboomers, X.F.; Swieszkowski, W. Gelatin Methacrylate Scaffold for Bone Tissue Engineering: The Influence of Polymer Concentration. J. Biomed. Mater. Res.—Part A 2018, 106, 201–209. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klak, M.; Kosowska, K.; Czajka, M.; Dec, M.; Domański, S.; Zakrzewska, A.; Korycka, P.; Jankowska, K.; Romanik-Chruścielewska, A.; Wszoła, M. The Impact of the Methacrylation Process on the Usefulness of Chitosan as a Biomaterial Component for 3D Printing. J. Funct. Biomater. 2024, 15, 251. https://doi.org/10.3390/jfb15090251
Klak M, Kosowska K, Czajka M, Dec M, Domański S, Zakrzewska A, Korycka P, Jankowska K, Romanik-Chruścielewska A, Wszoła M. The Impact of the Methacrylation Process on the Usefulness of Chitosan as a Biomaterial Component for 3D Printing. Journal of Functional Biomaterials. 2024; 15(9):251. https://doi.org/10.3390/jfb15090251
Chicago/Turabian StyleKlak, Marta, Katarzyna Kosowska, Milena Czajka, Magdalena Dec, Sylwester Domański, Agnieszka Zakrzewska, Paulina Korycka, Kamila Jankowska, Agnieszka Romanik-Chruścielewska, and Michał Wszoła. 2024. "The Impact of the Methacrylation Process on the Usefulness of Chitosan as a Biomaterial Component for 3D Printing" Journal of Functional Biomaterials 15, no. 9: 251. https://doi.org/10.3390/jfb15090251






