Investigation of Mechanical and Corrosion Properties of New Mg-Zn-Ga Amorphous Alloys for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alloys Preparation for Preliminary Investigation
2.2. Alloys Preparation for Melt Spinning and Its Analysis
2.3. Mechanical Properties
2.4. Corrosion Test
3. Results
3.1. Analysis of Solidification and Microstructure of Mg-Zn-Ga Alloys
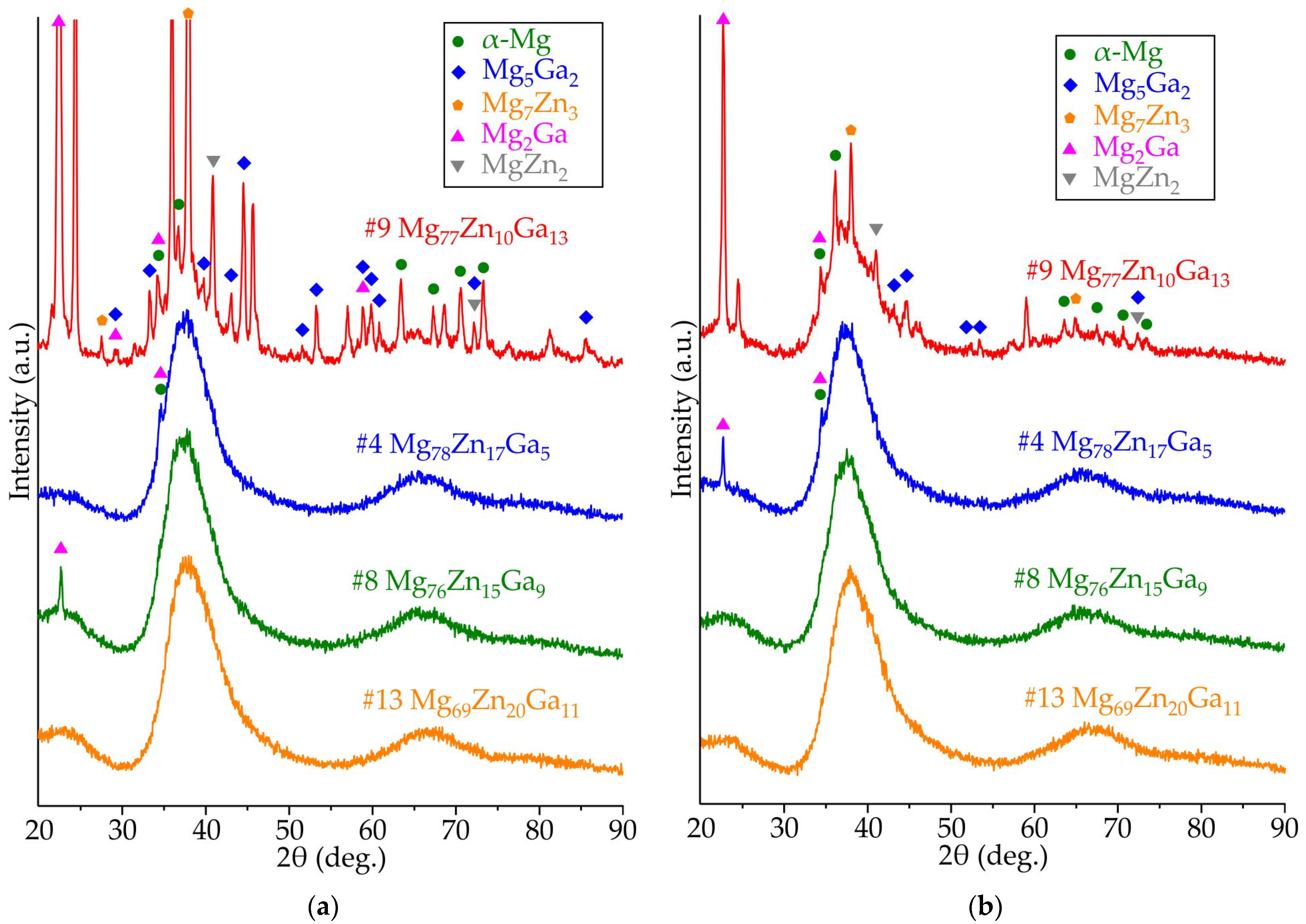
3.2. Results of XRD Analysis of Ribbons Made of #4 Mg78Zn17Ga5, #8 Mg76Zn15Ga9, #9 Mg77Zn10Ga13, and #13 Mg69Zn20Ga11 Alloys
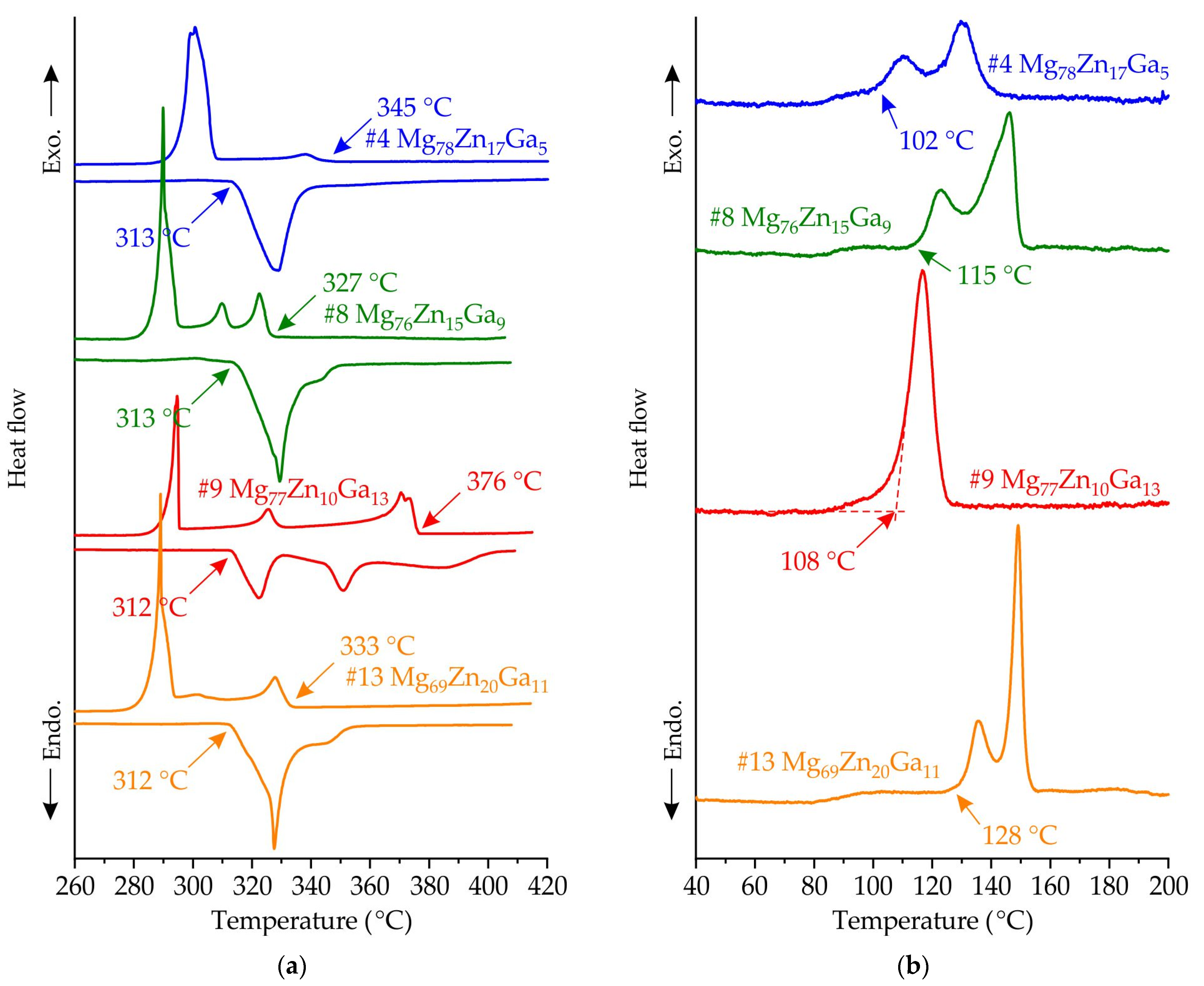
3.3. Results of DSC Analysis of Master Alloys and Ribbons Made of #4 Mg78Zn17Ga5, #8 Mg76Zn15Ga9, #9 Mg77Zn10Ga13 and #13 Mg69Zn20Ga11 Alloys
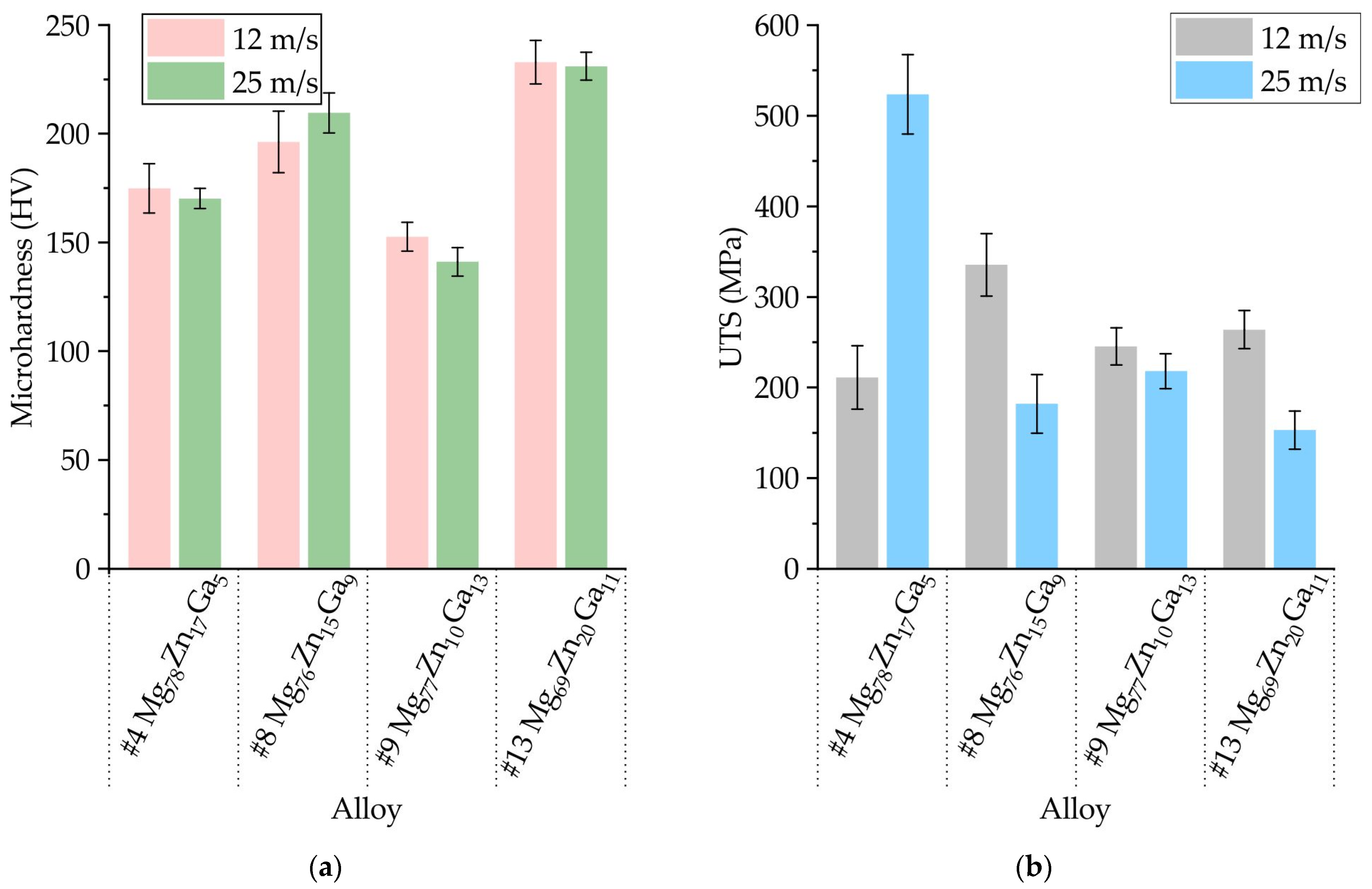
3.4. Mechanical Properties of Ribbons Made of #4 Mg78Zn17Ga5, #8 Mg76Zn15Ga9, #9 Mg77Zn10Ga13, and #13 Mg69Zn20Ga11 Alloys
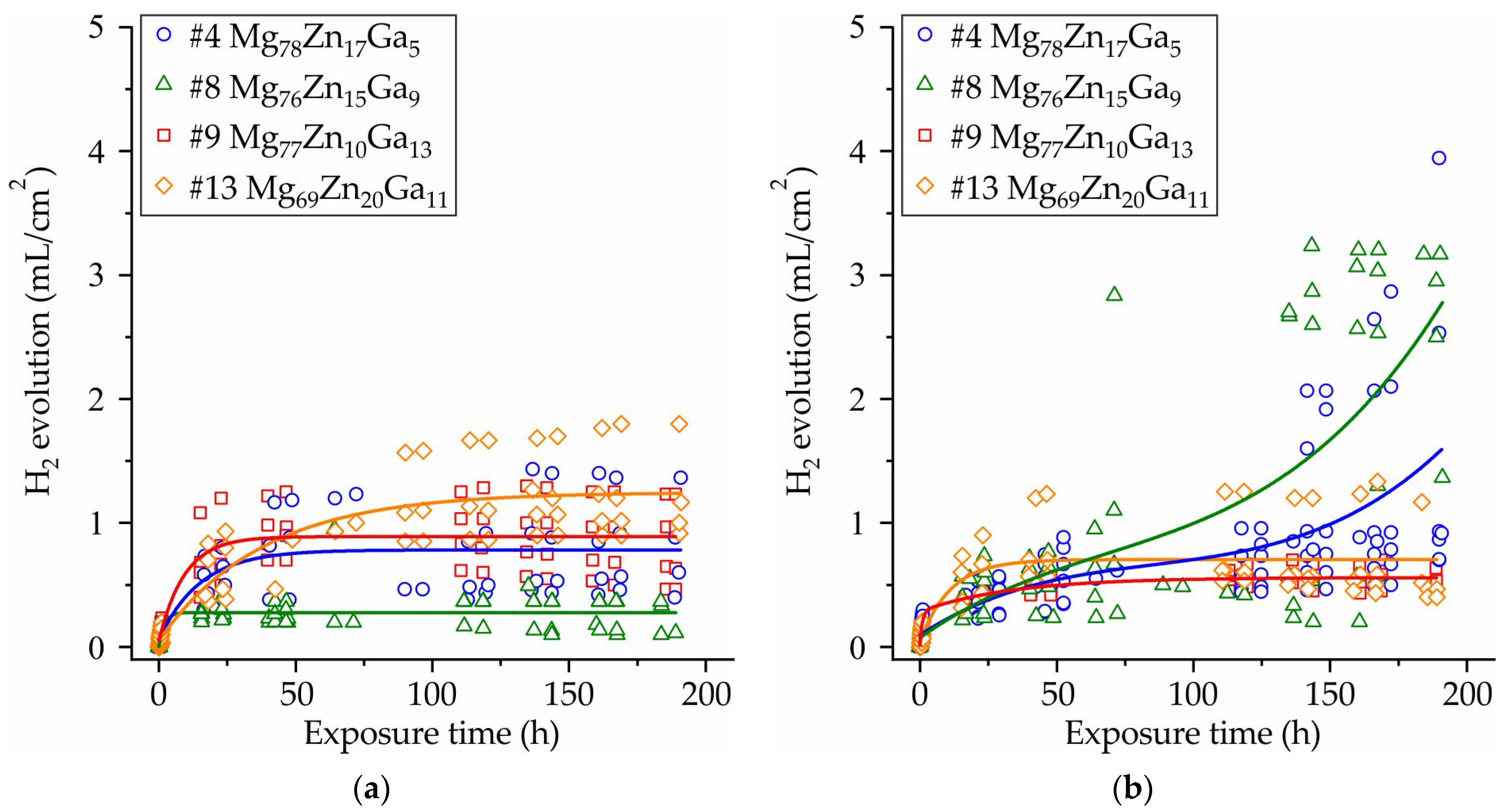
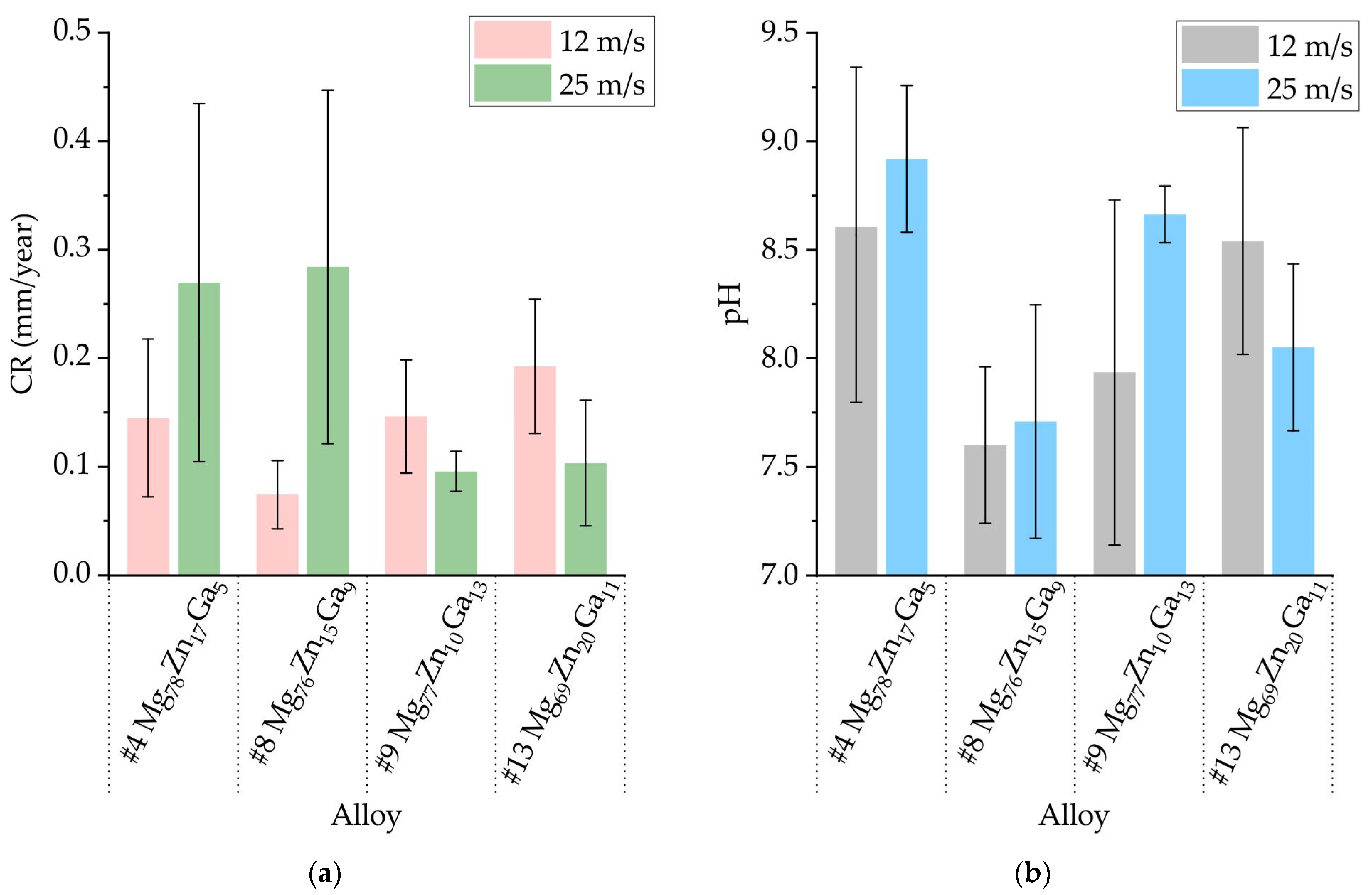
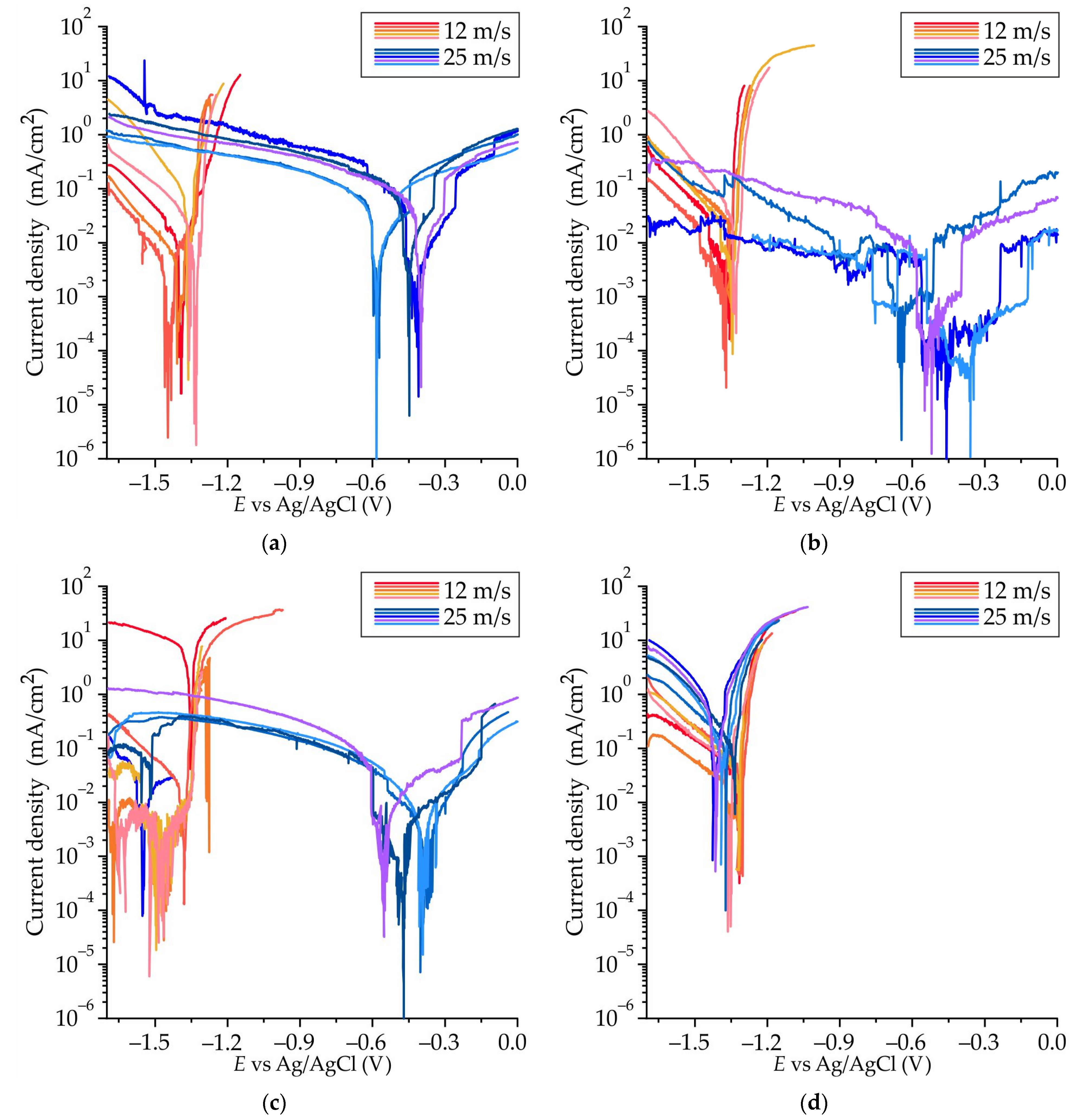
3.5. Corrosion Properties of Ribbons Made of #4 Mg78Zn17Ga5, #8 Mg76Zn15Ga9, #9 Mg77Zn10Ga13, and #13 Mg69Zn20Ga11 Alloys
4. Discussion
5. Conclusions
- (i)
- The XRD and DSC analysis confirmed that the ribbon of the #13 Mg69Zn20Ga11 alloy has a fully amorphous structure, and the #4 Mg78Zn17Ga5, #8 Mg76Zn15Ga9 and #9 Mg77Zn10Ga13 ribbons are partially crystalline. The onset of the crystallization temperature of the first exothermic peak (Tx) is in the range of 102–128 °C, and increasing the Zn and Ga content in the ribbons may result in a higher Tx.
- (ii)
- The ribbons’ microhardness was 141 and 231 HV and increased with the increase in the total content of Zn and Ga in alloys, but the rotational speed of the melt spinner wheel had no effect on the microhardness. The tensile properties of the ribbons have a large influence on the ribbon quality, and the maximal UTS of 524 MPa was observed for #4 Mg78Zn17Ga5 ribbon.
- (iii)
- The results of an immersion corrosion test show that the CR of the investigated ribbons is in the range of 0.1–0.3 mm/year. This is in accordance with requirements to biodegradable materials, making the Mg-Zn-Ga ribbons a candidate material for biodegradable applications. The potentiodynamic corrosion test shows that ribbon #9 Mg77Zn10Ga13 has both amorphous and crystalline parts because there were two corrosion potentials, −0.5 and −1.5 V, observed in the polarization curve.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gareb, B.; Van Bakelen, N.B.; Vissink, A.; Bos, R.R.M.; Van Minnen, B. Titanium or Biodegradable Osteosynthesis in Maxillofacial Surgery? In Vitro and In Vivo Performances. Polymers 2022, 14, 2782. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.; Fischerauer, S.F.; Hänzi, A.C.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Kačarević, Ž.P.; Elad, A.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.; Molnar, B.; Hesse, B.; et al. Biodegradation of a Magnesium Alloy Fixation Screw Used in a Guided Bone Regeneration Model in Beagle Dogs. Materials 2022, 15, 4111. [Google Scholar] [CrossRef] [PubMed]
- Delsmann, M.M.; Stürznickel, J.; Kertai, M.; Stücker, R.; Rolvien, T.; Rupprecht, M. Radiolucent Zones of Biodegradable Magnesium-Based Screws in Children and Adolescents—A Radiographic Analysis. Arch. Orthop. Trauma. Surg. 2023, 143, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Vujović, S.; Desnica, J.; Stanišić, D.; Ognjanović, I.; Stevanovic, M.; Rosic, G. Applications of Biodegradable Magnesium-Based Materials in Reconstructive Oral and Maxillofacial Surgery: A Review. Molecules 2022, 27, 5529. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2010, 27, 1728–1734. [Google Scholar] [CrossRef]
- Gu, X.-N.; Li, S.-S.; Li, X.-M.; Fan, Y.-B. Magnesium based degradable biomaterials: A review. Front. Mater. Sci. 2014, 8, 200–218. [Google Scholar] [CrossRef]
- Tran, N.T.; Kim, Y.-K.; Kim, S.-Y.; Lee, M.-H.; Lee, K.-B. Comparative Osteogenesis and Degradation Behavior of Magnesium Implant in Epiphysis and Diaphysis of the Long Bone in the Rat Model. Materials 2022, 15, 5630. [Google Scholar] [CrossRef]
- Gu, X.-N.; Zheng, Y.-F. A review on magnesium alloys as biodegradable materials. Front. Mater. Sci. China 2010, 4, 111–115. [Google Scholar] [CrossRef]
- Nguyen, A.; Kunert, M.; Hort, N.; Schrader, C.; Weisser, J.; Schmidt, J. Cytotoxicity of the Ga-containing coatings on biodegradable magnesium alloys. Surf. Innovat. 2015, 3, 10–19. [Google Scholar] [CrossRef]
- Shan, Z.; Xie, X.; Wu, X.; Zhuang, S.; Zhang, C. Development of degradable magnesium-based metal implants and their function in promoting bone metabolism (A review). J. Orthop. Transl. 2022, 36, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Seal, C.K.; Vince, K.; Hodgson, M.A. Biodegradable surgical implants based on magnesium alloys—A review of current research. IOP Conf. Ser.Mat. Sci. Eng. 2009, 4, 012011. [Google Scholar] [CrossRef]
- Marco, I.; Feyerabend, F.; Willumeit-Römer, R.; Van der Biest, O. Influence of Testing Environment on the Degradation Behavior of Magnesium Alloys for Bioabsorbable Implants. In Proceedings of the Annual Meeting & Exhibition (TMS2015), Hoboken, Orlando, FL, USA, 15–19 March 2015. [Google Scholar] [CrossRef]
- Meagher, P.; O’Cearbhaill, E.D.; Byrne, J.H.; Browne, D.J. Bulk Metallic Glasses for Implantable Medical Devices and Surgical Tools. Adv. Mater. 2016, 28, 5755–5762. [Google Scholar] [CrossRef]
- Wong, C.-C.; Wong, P.-C.; Tsai, P.-H.; Jang, J.S.-C.; Cheng, C.-K.; Chen, H.-H.; Chen, C.-H. Biocompatibility and Osteogenic Capacity of Mg-Zn-Ca Bulk Metallic Glass for Rabbit Tendon-Bone Interference Fixation. Int. J. Mol. Sci. 2019, 20, 2191. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Liu, Z.; Yu, W.; Qin, C.; Yu, H.; Wang, Z. Biodegradable Mg–Zn–Ca-Based Metallic Glasses. Materials 2022, 15, 2172. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zheng, Y.; Zhong, S.; Xi, T.; Wang, J.; Wang, W. Corrosion of, and cellular responses to Mg–Zn–Ca bulk metallic glasses. Biomaterials 2010, 31, 1093–1103. [Google Scholar] [CrossRef]
- Wong, P.-C.; Tsai, P.-H.; Li, T.-H.; Cheng, C.-K.; Jang, J.S.C.; Huang, J.C. Degradation behavior and mechanical strength of Mg-Zn-Ca bulk metallic glass composites with Ti particles as biodegradable materials. J. Alloys Compd. 2017, 699, 914–920. [Google Scholar] [CrossRef]
- Fijołek, A.; Lelito, J.; Krawiec, H.; Ryba, J.; Rogal, Ł. Corrosion Resistance of Mg72Zn24Ca4 and Zn87Mg9Ca4 Alloys for Application in Medicine. Materials 2020, 13, 3515. [Google Scholar] [CrossRef]
- Bin, S.J.B.; Fong, K.S.; Chua, B.W.; Gupta, M. Development of Biocompatible Bulk MgZnCa Metallic Glass with Very High Corrosion Resistance in Simulated Body Fluid. Materials 2022, 15, 8989. [Google Scholar] [CrossRef]
- Li, K.; Li, B.; Du, P.; Xiang, T.; Yang, X.; Xie, G. Effect of powder size on strength and corrosion behavior of Mg66Zn30Ca4 bulk metallic glass. J. Alloys Compd. 2022, 897, 163219. [Google Scholar] [CrossRef]
- Wang, J.; Meng, L.; Xie, W.; Ji, C.; Wang, R.; Zhang, P.; Jin, L.; Sheng, L.; Zheng, Y. Corrosion and in vitro cytocompatibility investigation on the designed Mg-Zn-Ag metallic glasses for biomedical application. J. Magnes. Alloy 2022, 12, 1566–1580. [Google Scholar] [CrossRef]
- Li, H.; Pang, S.; Liu, Y.; Liaw, P.K.; Zhang, T. In vitro investigation of Mg–Zn–Ca–Ag bulk metallic glasses for biomedical applications. J. Non-Cryst. Solids 2015, 427, 134–138. [Google Scholar] [CrossRef]
- Li, H.; Pang, S.; Liu, Y.; Sun, L.; Liaw, P.K.; Zhang, T. Biodegradable Mg–Zn–Ca–Sr bulk metallic glasses with enhanced corrosion performance for biomedical applications. Mater. Des. 2015, 67, 9–19. [Google Scholar] [CrossRef]
- Li, H.; He, W.; Pang, S.; Liaw, P.K.; Zhang, T. In vitro responses of bone-forming MC3T3-E1 pre-osteoblasts to biodegradable Mg-based bulk metallic glasses. Mater. Sci. Eng. C 2016, 68, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Wan, Y.; Ma, Z.-J.; Guo, Y.-C.; Yang, Z.; Wang, P.; Li, J.-P. Glass-forming ability and corrosion performance of Mn-doped Mg–Zn–Ca amorphous alloys for biomedical applications. Rare Met. 2018, 37, 579–586. [Google Scholar] [CrossRef]
- Meifeng, H.; Hao, W.; Kunguang, Z.; Deng, P.; Fang, L. Effects of Li addition on the corrosion behaviour and biocompatibility of Mg(Li)–Zn–Ca metallic glasses. J. Mater. Sci. 2018, 53, 9928–9942. [Google Scholar] [CrossRef]
- Zai, W.; Man, H.C.; Su, Y.; Li, G.; Lian, J. Impact of microalloying element Ga on the glass-forming ability (GFA), mechanical properties and corrosion behavior of Mg-Zn-Ca bulk metallic glass. Mater. Chem. Phys. 2020, 255, 123555. [Google Scholar] [CrossRef]
- Roche, V.; Koga, G.Y.; Matias, T.B.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J.; Nogueira, R.P.; Jorge Junior, A.M. Degradation of biodegradable implants: The influence of microstructure and composition of Mg-Zn-Ca alloys. J. Alloys Compd. 2019, 774, 168–181. [Google Scholar] [CrossRef]
- Zhang, X.L.; Chen, G.; Bauer, T. Mg-based bulk metallic glass composite with high bio-corrosion resistance and excellent mechanical properties. Intermetallics 2012, 29, 56–60. [Google Scholar] [CrossRef]
- Bernstein, L.R. Mechanisms of Therapeutic Activity for Gallium. Pharmacol. Rev. 1998, 50, 665–682. [Google Scholar]
- Warrell, R.P., Jr.; Bockman, R.S.; Coonley, C.J.; Isaacs, M.; Staszewski, H. Gallium nitrate inhibits calcium resorption from bone and is effective treatment for cancer-related hypercalcemia. J. Clin. Investig. 1984, 73, 1487–1490. [Google Scholar] [CrossRef] [PubMed]
- Warrell, R.P., Jr.; Skelos, A.; Alcock, N.W.; Bockman, R.S. Gallium Nitrate for Acute Treatment of Cancer-related Hypercalcemia: Clinicopharmacological and Dose Response Analysis. Cancer Res. 1986, 46, 4208–4212. [Google Scholar] [PubMed]
- Warrell, R.P., Jr.; Israel, R.; Frisone, M.; Snyder, T.; Gaynor, J.J.; Bockman, R.S. Gallium Nitrate for Acute Treatment of Cancer-Related Hypercalcemia: A Randomized, Double-Blind Comparison to Calcitonin. Ann. Intern. Med. 1988, 108, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Warrell, R.P., Jr.; Bosco, B.; Weinerman, S.; Levine, B.; Lane, J.; Bockman, R.S. Gallium Nitrate for Advanced Paget Disease of Bone: Effectiveness and Dose-Response Analysis. Ann. Intern. Med. 1990, 113, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Matkovic, V.; Apseloff, G.; Shepard, D.R.; Gerber, N. Use of gallium to treat Paget’s disease of bone: A pilot study. Lancet 1990, 335, 72–75. [Google Scholar] [CrossRef]
- Li, H.F.; Zheng, Y.F. Recent advances in bulk metallic glasses for biomedical applications. Acta Biomater. 2016, 36, 1–20. [Google Scholar] [CrossRef]
- Kaur, G.; Pandey, O.P.; Singh, K.; Homa, D.; Scott, B.; Pickrel, G. A review of bioactive glasses: Their structure, properties, fabrication, and apatite formation. J. Biomed. Mater. Res. Part. A 2014, 102, 254–274. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Zberg, B.; Uggowitzer, P.J.; Löffler, J.F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef]
- Melnikov, P.; Teixeira, A.R.; Malzac, A.; Coelho, M. de B. Gallium-containing hydroxyapatite for potential use in orthopedics. Mater. Chem. Phys. 2009, 117, 86–90. [Google Scholar] [CrossRef]
- Ma, Z.; Fu, Q. Therapeutic Effect of Organic Gallium on Ovariectomized Osteopenic Rats by Decreased Serum Minerals and Increased Bone Mineral Content. Biol. Trace Elem. Res. 2010, 133, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Kasinath, R.; Ernsberg, C.; Vass, S.; Ginn, S.N.; Qu, H.; Tong, W. Orthopedic Implant Having a Crystalline Gallium-Containing Hydroxyapatite Coating and Methods for Making the Same. U.S. Patent 11,141,505, 14 September 2021. [Google Scholar]
- Bazhenov, V.; Koltygin, A.; Komissarov, A.; Anishchenko, A.; Khasenova, R.; Komissarova, J.; Bautin, V.; Seferyan, A.; Fozilov, B. Microstructure, Mechanical and Corrosion Properties of Biodegradable Mg-Ga-Zn-X (X = Ca, Y, Nd) Alloys, Metal 2018. In Proceedings of the 27th Anniversary International Conference on Metallurgy and Materials, Brno, Czech Republic; 23–25 May 2018; TANGER Ltd.: Ostrava, Czech Republic, 2018; pp. 1375–1380. [Google Scholar]
- Bazhenov, V.; Koltygin, A.; Komissarov, A.; Li, A.; Bautin, V.; Khasenova, R.; Anishchenko, A.; Seferyan, A.; Komissarova, J.; Estrin, Y. Gallium-containing magnesium alloy for potential use as temporary implants in osteosynthesis. J. Magnes. Alloys 2020, 8, 352–363. [Google Scholar] [CrossRef]
- Bazhenov, V.; Li, A.; Tavolzhanskii, S.; Bazlov, A.; Tabachkova, N.; Koltygin, A.; Komissarov, A.; Shin, K.S. Microstructure and Mechanical Properties of Hot-Extruded Mg–Zn–Ga–(Y) Biodegradable Alloys. Materials 2022, 15, 6849. [Google Scholar] [CrossRef] [PubMed]
- Bazhenov, V.; Li, A.; Iliasov, A.; Bautin, V.; Plegunova, S.; Koltygin, A.; Komissarov, A.; Abakumov, M.; Redko, N.; Shin, K.S. Corrosion Behavior and Biocompatibility of Hot-Extruded Mg–Zn–Ga–(Y) Biodegradable Alloys. J. Funct. Biomater. 2022, 13, 294. [Google Scholar] [CrossRef] [PubMed]
- Rogachev, S.O.; Bazhenov, V.E.; Komissarov, A.A.; Ten, D.V.; Li, A.V.; Andreev, V.A.; Statnik, E.S.; Sadykova, I.A.; Plegunova, S.V.; Yushchuk, V.V.; et al. High strength and ductility in a new Mg–Zn–Ga biocompatible alloy by drawing and rotary forging. Results Mater. 2024, 21, 100524. [Google Scholar] [CrossRef]
- Rogachev, S.O.; Bazhenov, V.E.; Komissarov, A.A.; Li, A.V.; Munzaferova, N.E.; Plegunova, S.V.; Ten, D.V. Microstructure, Mechanical and Corrosion Properties of Mg–Zn–Ga Alloy after Hot Rolling. J. Mater. Eng. Perform. 2024. [Google Scholar] [CrossRef]
- Takeichi, N.; Fukunaga, T.; Mizutani, U. Development in the short- and medium-range structure in amorphous alloys. J. Phys. Condens. Matter 1998, 10, 10179–10192. [Google Scholar] [CrossRef]
- Ranganathan, S.; Biswas, T.; Subramaniam, A. Discovering and designing bulk metallic glasses. In Book Series on Complex Metallic Alloys; World Scientific: Singapore, 2009; pp. 413–449. [Google Scholar] [CrossRef]
- Mizutani, U.; Matsuda, T. Electronic properties of Mg-based simple metallic glasses. J. Phys. F Met. Phys. 1984, 14, 2995–3006. [Google Scholar] [CrossRef]
- Uhlenhaut, D.I.; Furrer, A.; Uggowitzer, P.J.; Löffler, J.F. Corrosion properties of glassy Mg70Al15Ga15 in 0.1M NaCl solution. Intermetallics 2009, 17, 811–817. [Google Scholar] [CrossRef]
- Predel, B. Thermodynamic investigations on the formation and decomposition of metallic glasses. Phys. B+C 1981, 103, 113–122. [Google Scholar] [CrossRef]
- Ramya, M.; Sarwat, S.G.; Udhayabanu, V.; Raj, B.; Ravi, K.R. Exploring Mg-Zn-Ca-Based Bulk Metallic Glasses for Biomedical Applications Based on Thermodynamic Approach. Metall. Mater. Trans. A 2015, 46, 5962–5971. [Google Scholar] [CrossRef]
- Okamoto, H. Ga-Mg (Gallium-Magnesium). J. Phase Equilibria Diffus. 2013, 34, 148. [Google Scholar] [CrossRef]
- Predel, B. Mg-Zn (Magnesium-Zinc). In Li-Mg–Nd-Zr. Landolt-Börnstein—Group IV Physical Chemistry (Numerical Data and Functional Relationships in Science and Technology); Madelung, O., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; Volume 5H. [Google Scholar] [CrossRef]
- Matsuda, T.; Shiotani, N.; Mizutani, U. Electronic properties of Mg0.7Zn0.3−xGax simple metallic glasses. J. Phys. F Met. Phys. 1984, 14, 1193–1204. [Google Scholar] [CrossRef]
- ASTM Standard G1–03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2011. [CrossRef]
- Kubásek, J.; Vojtěch, D.; Lipov, J.; Ruml, T. Structure, mechanical properties, corrosion behavior and cytotoxicity of biodegradable Mg–X (X = Sn, Ga, In) alloys. Mater. Sci. Eng. C 2013, 33, 2421–2432. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef] [PubMed]
- ASTM Standard G102–89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Greer, A.L. Physical Metallurgy, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 305–385. [Google Scholar] [CrossRef]
- Zhang, P.; Li, S.X.; Zhang, Z.F. General relationship between strength and hardness. Mater. Sci. Eng. A 2011, 529, 62–73. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, E. Biocorrosion behavior of magnesium alloy in different simulated fluids for biomedical application. Mater. Sci. Eng. C 2009, 29, 1691–1696. [Google Scholar] [CrossRef]
- Salahshoor, M.; Guo, Y. Biodegradable Orthopedic Magnesium-Calcium (MgCa) Alloys, Processing, and Corrosion Performance. Materials 2012, 5, 135–155. [Google Scholar] [CrossRef]
- Ibriş, N.; Rosca, J.C.M. EIS study of Ti and its alloys in biological media. J. Electochem. Chem. 2002, 526, 53–62. [Google Scholar] [CrossRef]
- Sazou, D. Current oscillations and mass-transport control during electrodissolution of iron in phosphoric acid solutions. Electochem. Acta 1997, 42, 627–637. [Google Scholar] [CrossRef]
- Sazou, D.; Pagitsas, M. Bifurcation to limit cycle oscillations induced by the ohmic potential drop during the anodic polarisation of iron in 14.8 M H3PO4. J. Electroanal. Chem. 1998, 451, 77–87. [Google Scholar] [CrossRef]
- Bojinov, M.; Betova, I.; Fabricius, G.; Laitinen, T.; Raicheff, R. Passivation mechanism of iron in concentrated phosphoric acid. J. Electroanal. Chem. 1999, 475, 58–65. [Google Scholar] [CrossRef]
- Opitek, B.; Lelito, J.; Szucki, M.; Piwowarski, G.; Gondek, Ł.; Rogal, Ł. Analysis of the Crystallization Kinetics and Thermal Stability of the Amorphous Mg72Zn24Ca4 Alloy. Materials 2021, 14, 3583. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Xie, G.; Dan, Z.; Zhu, S.; Seki, I. Corrosion behavior and mechanical properties of Mg–Zn–Ca amorphous alloys. Intermetallics 2013, 42, 9–13. [Google Scholar] [CrossRef]
- Dambatta, M.S.; Izman, S.; Yahaya, B.; Lim, J.Y.; Kurniawan, D. Mg-based bulk metallic glasses for biodegradable implant materials: A review on glass forming ability, mechanical properties, and biocompatibility. J. Non-Cryst. Solids 2015, 426, 110–115. [Google Scholar] [CrossRef]
- Li, Q.F.; Weng, H.R.; Suo, Z.Y.; Ren, Y.L.; Yuan, X.G.; Qiu, K.Q. Microstructure and mechanical properties of bulk Mg–Zn–Ca amorphous alloys and amorphous matrix composites. Mater. Sci. Eng. A 2008, 487, 301–308. [Google Scholar] [CrossRef]
- Nowosielski, R.; Cesarz-Andraczke, K. Impact of Zn and Ca on dissolution rate, mechanical properties and GFA of resorbable Mg–Zn–Ca metallic glasses. Arch. Civ. Mech. Eng. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Lu, W.; He, M.; Yu, D.; Xie, X.; Wang, H.; Wang, S.; Yuan, C.; Chen, A. Ductile behavior and excellent corrosion resistance of Mg-Zn-Yb-Ag metallic glasses. Mater. Des. 2021, 210, 110027. [Google Scholar] [CrossRef]
- Greer, A.L.; Cheng, Y.Q.; Ma, E. Shear bands in metallic glasses. Mater. Sci. Eng. R 2013, 74, 71–132. [Google Scholar] [CrossRef]
- Ganeshan, S.; Shang, S.L.; Wang, Y.; Liu, Z.-K. Effect of alloying elements on the elastic properties of Mg from first-principles calculations. Acta Mater. 2009, 57, 3876–3884. [Google Scholar] [CrossRef]
- Yang, F.; Fan, T.-W.; Wu, J.; Tang, B.-Y.; Peng, L.-M.; Ding, W.-J. Effects of Y and Zn atoms on the elastic properties of Mg solid solution from first-principles calculations. Phys. Status Solidi B 2011, 248, 2809–2815. [Google Scholar] [CrossRef]
- Sumitomo, T.; Cáceres, C.H.; Veidt, M. The elastic modulus of cast Mg–Al–Zn alloys. J. Light. Metal. 2002, 2, 49–56. [Google Scholar] [CrossRef]
- Kiani, F.; Wen, C.; Li, Y. Prospects and strategies for magnesium alloys as biodegradable implants from crystalline to bulk metallic glasses and composites—A review. Acta Biomater. 2020, 103, 1–23. [Google Scholar] [CrossRef]
- Li, K.; Zhou, S.; Bao, W.; Chen, J.; Li, J.; Xie, G. New biodegradable Mg–Zn–Ca bulk metallic glass composite with large plasticity reinforced by SnZn alloy. Mater. Sci. Eng. A 2023, 873, 145045. [Google Scholar] [CrossRef]
- Nordlien, H.; Ono, S.; Masuko, N.; Nisancioclu, K. A TEM investigation of naturally formed oxide films on pure magnesium. Corr. Sci. 1997, 39, 1397–1414. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef]
- Chen, S.; Tu, J.; Hu, Q.; Xiong, X.; Wu, J.; Zou, J.; Zeng, X. Corrosion resistance and in vitro bioactivity of Si-containing coating prepared on a biodegradable Mg-Zn-Ca bulk metallic glass by micro-arc oxidation. J. Non-Cryst. Solids 2017, 456, 125–131. [Google Scholar] [CrossRef]
- Ramya, M.; Sarwat, S.G.; Udhayabanu, V.; Subramanian, S.; Raj, B.; Ravi, K.R. Role of partially amorphous structure and alloying elements on the corrosion behavior of Mg–Zn–Ca bulk metallic glass for biomedical applications. Mater. Des. 2015, 86, 829–835. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, M.J.; Pang, J.; Wang, Z.; Jarfors, A.W.E. In vitro corrosion behaviors of Mg67Zn28Ca5 alloy: From amorphous to crystalline. Mater. Chem. Phys. 2012, 134, 1079–1087. [Google Scholar] [CrossRef]
- Zhou, J.; Li, K.; Wang, B.; Ai, F. Nano-hydroxyapatite/ZnO coating prepared on a biodegradable Mg–Zn–Ca bulk metallic glass by one-step hydrothermal method in acid situation. Ceram. Int. 2020, 46, 6958–6964. [Google Scholar] [CrossRef]
- Wang, C.; Shuai, Y.; Yang, Y.; Zeng, D.; Liang, X.; Peng, S.; Shuai, C. Amorphous magnesium alloy with high corrosion resistance fabricated by laser powder bed fusion. J. Alloys Compd. 2022, 897, 163247. [Google Scholar] [CrossRef]
- Miskovic, D.M.; Pohl, K.; Birbilis, N.; Laws, K.J.; Ferry, M. Examining the elemental contribution towards the biodegradation of Mg–Zn–Ca ternary metallic glasses. J. Mater. Chem. B 2016, 4, 2679–2690. [Google Scholar] [CrossRef] [PubMed]
- Matias, T.B.; Roche, V.; Nogueira, R.P.; Asato, G.H.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J.; Jorge, A.M. Mg-Zn-Ca amorphous alloys for application as temporary implant: Effect of Zn content on the mechanical and corrosion properties. Mater. Des. 2016, 110, 188–195. [Google Scholar] [CrossRef]
- Bin, S.J.B.; Fong, K.S.; Chua, B.W.; Gupta, M. Mg-based bulk metallic glasses: A review of recent developments. J. Magnes. Alloys 2022, 10, 899–914. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
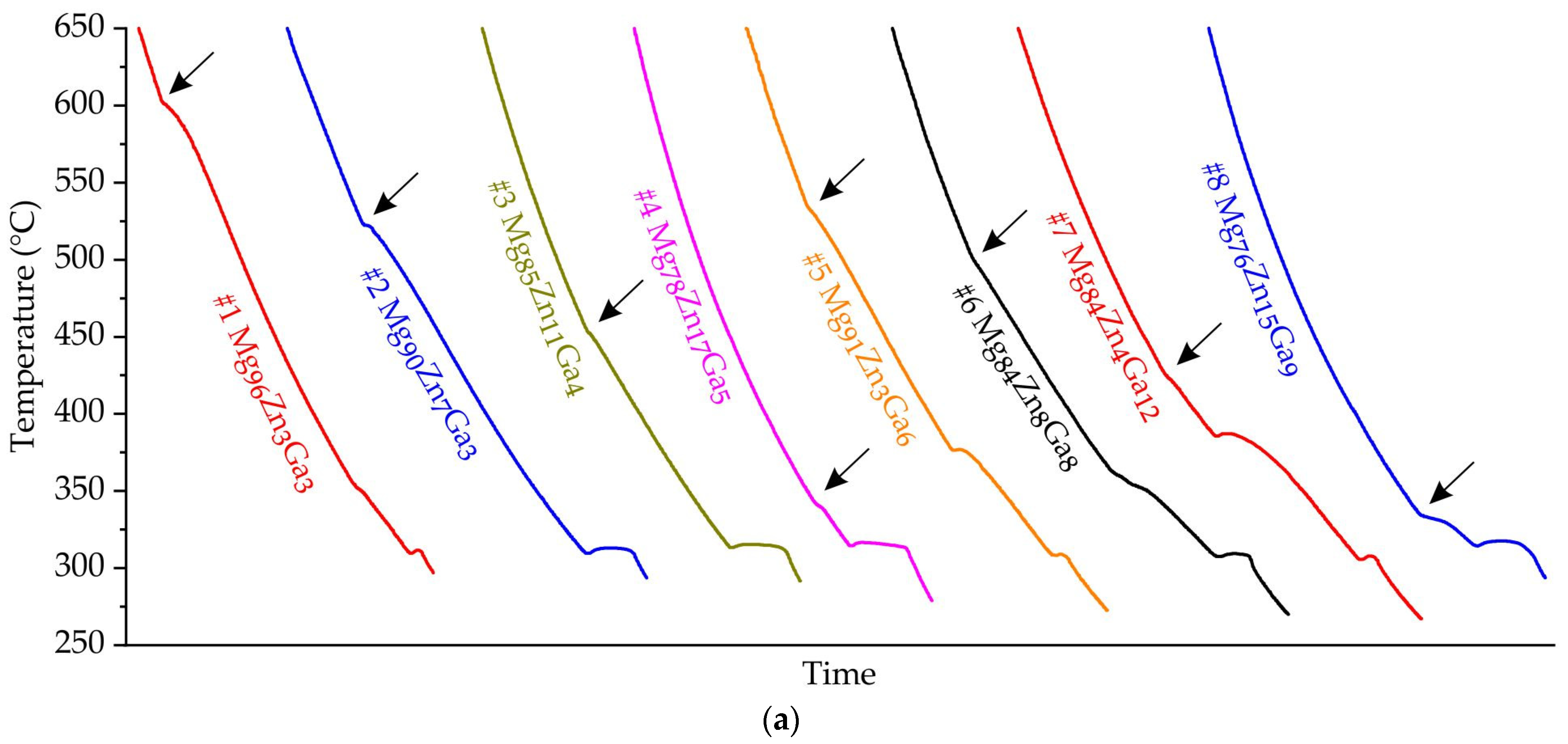

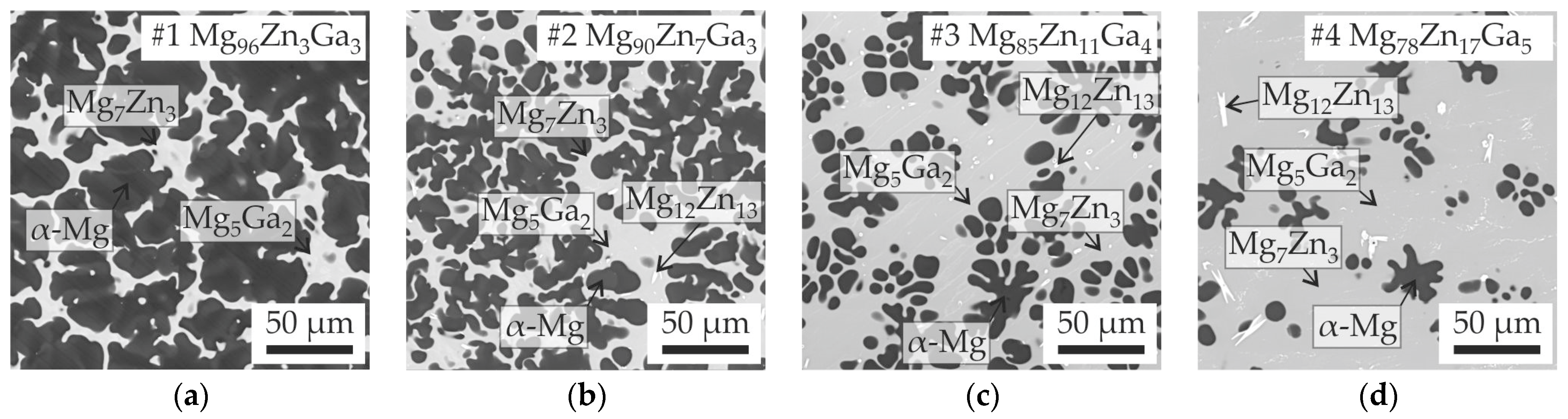
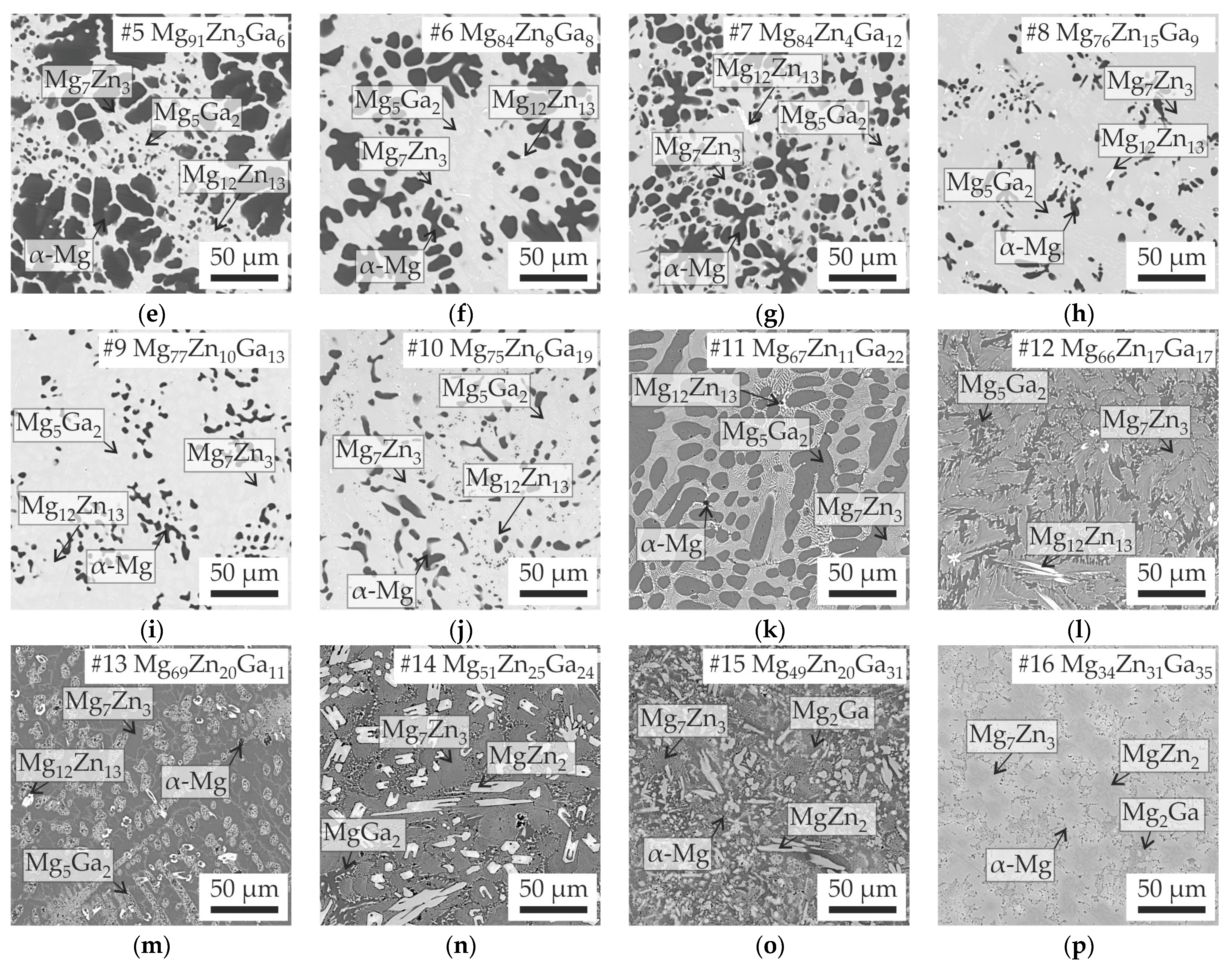
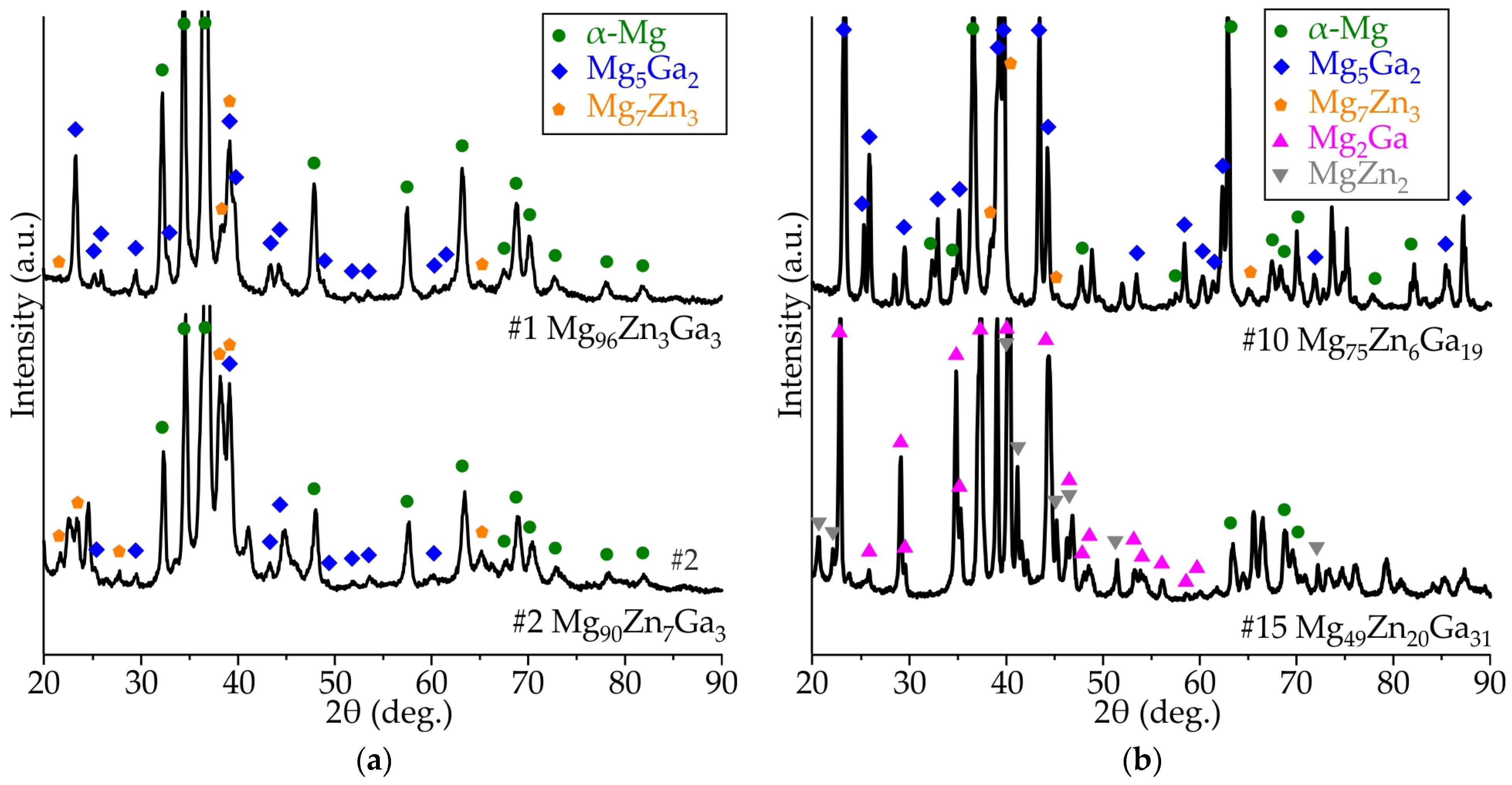


| Alloy | Element Content (at.%) | Liquidus Temperature (°C) | Calculated Solidus Temperature (°C) | Equilibrium Solidification Pathway | ||||
|---|---|---|---|---|---|---|---|---|
| Mg | Zn | Ga | Experimental | Calculated | Error | |||
| #1 Mg96Zn3Ga3 | Bal. | 3.2 | 2.9 | 602.8 | 597.5 | +5.3 | 295 | L→α-Mg; L→ α-Mg + Mg12Zn13; L→α-Mg + Mg12Zn13 + Mg5Ga2 |
| #2 Mg90Zn7Ga3 | Bal. | 6.7 | 3.0 | 522.4 | 562.7 | –40.3 | 295 | |
| #3 Mg85Zn11Ga4 | Bal. | 11.1 | 3.8 | 452.4 | 505.4 | –48.0 | 295 | |
| #4 Mg78Zn17Ga5 | Bal. | 17.4 | 5.0 | 341.5 | 406.8 | –65.3 | 295 | |
| #5 Mg91Zn3Ga6 | Bal. | 3.4 | 6.1 | 535.3 | 560.6 | –25.2 | 295 | L→α-Mg; L→α-Mg + Mg5Ga2; L→α-Mg + Mg12Zn13 + Mg5Ga2 |
| #6 Mg84Zn8Ga8 | Bal. | 8.2 | 8.0 | 502.6 | 479.4 | +23.2 | 295 | |
| #7 Mg84Zn4Ga12 | Bal. | 4.2 | 11.5 | 424.8 | 476.9 | –52.1 | 295 | |
| #8 Mg76Zn15Ga9 | Bal. | 14.8 | 9.3 | 328.1 | 361.8 | –33.7 | 295 | |
| #9 Mg77Zn10Ga13 | Bal. | 10.2 | 13.3 | 383.9 | 356.1 | +27.8 | 295 | L→Mg5Ga2; L→α-Mg + Mg5Ga2; L→α-Mg + Mg12Zn13 + Mg5Ga2 |
| #10 Mg75Zn6Ga19 | Bal. | 5.5 | 18.9 | 425.3 | 413.5 | +11.8 | 295 | |
| #11 Mg67Zn11Ga22 | Bal. | 11.3 | 22.4 | 401.8 | 402.5 | –0.7 | 298 | L→Mg5Ga2; L→Mg5Ga2 + MgZn2; L + MgZn2→ Mg5Ga2 + Mg2Zn3; L→Mg5Ga2 + Mg2Zn3 + Mg12Zn13 |
| #12 Mg66Zn17Ga17 | Bal. | 16.5 | 16.7 | 368.2 | 368.7 | –0.5 | 295 | L→Mg5Ga2; L→Mg5Ga2 + MgZn2; L + MgZn2→Mg5Ga2 + Mg2Zn3; L + Mg2Zn3→Mg5Ga2 + Mg12Zn13; L→α-Mg + Mg12Zn13 + Mg5Ga2 |
| #13 Mg69Zn20Ga11 | Bal. | 19.9 | 10.6 | 333 | 318.5 | –14.8 | 295 | L→Mg2Zn3; L→Mg2Zn3 + Mg5Ga2; L + Mg2Zn3→Mg5Ga2 + Mg12Zn13; L→α-Mg + Mg12Zn13 + Mg5Ga2 |
| #14 Mg51Zn25Ga24 | Bal. | 25.0 | 24.2 | 428.2 | 436.4 | –8.2 | 317 | L→MgZn2; L→MgZn2 + Mg2Ga; L→MgZn2 + Mg2Ga + MgGa |
| #15 Mg49Zn20Ga31 | Bal. | 19.8 | 31.4 | 425.0 | 407.5 | +17.5 | 317 | |
| #16 Mg34Zn31Ga35 | Bal. | 31.1 | 35.0 | 421.4 | 412.9 | +8.5 | 259 | L→MgZn2; L→MgZn2 + MgGa2; L→MgZn2 + MgGa + MgGa2 |
| Symbol | Temperature (°C) | Transition |
|---|---|---|
| E1 | 359 | L→MgZn2 + Mg2Ga + Mg5Ga2 |
| P1 | 339 | L + MgZn2→Mg2Zn3 + Mg5Ga2 |
| P2 | 298 | L + Mg2Zn3→Mg12Zn13 + Mg5Ga2 |
| E2 | 295 | L→α-Mg + Mg12Zn13 + Mg5Ga2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazhenov, V.E.; Gorobinskiy, M.V.; Bazlov, A.I.; Bautin, V.A.; Koltygin, A.V.; Komissarov, A.A.; Ten, D.V.; Li, A.V.; Drobyshev, A.Y.; Kang, Y.; et al. Investigation of Mechanical and Corrosion Properties of New Mg-Zn-Ga Amorphous Alloys for Biomedical Applications. J. Funct. Biomater. 2024, 15, 275. https://doi.org/10.3390/jfb15090275
Bazhenov VE, Gorobinskiy MV, Bazlov AI, Bautin VA, Koltygin AV, Komissarov AA, Ten DV, Li AV, Drobyshev AY, Kang Y, et al. Investigation of Mechanical and Corrosion Properties of New Mg-Zn-Ga Amorphous Alloys for Biomedical Applications. Journal of Functional Biomaterials. 2024; 15(9):275. https://doi.org/10.3390/jfb15090275
Chicago/Turabian StyleBazhenov, Viacheslav E., Mikhail V. Gorobinskiy, Andrey I. Bazlov, Vasiliy A. Bautin, Andrey V. Koltygin, Alexander A. Komissarov, Denis V. Ten, Anna V. Li, Alexey Yu. Drobyshev, Yoongu Kang, and et al. 2024. "Investigation of Mechanical and Corrosion Properties of New Mg-Zn-Ga Amorphous Alloys for Biomedical Applications" Journal of Functional Biomaterials 15, no. 9: 275. https://doi.org/10.3390/jfb15090275
APA StyleBazhenov, V. E., Gorobinskiy, M. V., Bazlov, A. I., Bautin, V. A., Koltygin, A. V., Komissarov, A. A., Ten, D. V., Li, A. V., Drobyshev, A. Y., Kang, Y., Jung, I.-H., & Shin, K. S. (2024). Investigation of Mechanical and Corrosion Properties of New Mg-Zn-Ga Amorphous Alloys for Biomedical Applications. Journal of Functional Biomaterials, 15(9), 275. https://doi.org/10.3390/jfb15090275







