Analysis of the Feasibility of the OrthoNail Hybrid Intramedullary Implant in the Human Body with Respect to Material Durability
Abstract
:1. Introduction
- 13.6 kg (30 lbs) for an implant with a diameter of 8.5 mm;
- 22.7 kg (50 lbs) for an implant with a diameter of 10.7 mm;
- 22.7 kg (50 lbs) for an implant with a diameter of 12.5 mm.
2. Materials and Methods
- Material testing (structure, hardness, and strength of the material);
- Testing on a proprietary strength testing setup, including the following:
- –
- Compression testing;
- –
- Tensile testing;
- –
- 3-point bending in frontal and sagittal planes;
- –
- Maximum load testing in compression, tension, and 3-point bending;
- Fatigue testing in 3-point bending;
- Static 4-point bending test;
- Static torsion test;
- Dynamic 4-point bending test;
- Finite element method (FEM) analysis of stress distribution in bone tissue at the site of bone screw insertion.
2.1. Implant Material Testing
2.2. Implant Strength Testing
2.2.1. Testing on a Custom Strength Testing Setup
- Compression in the range ;
- Tension in the range ;
- 3-point bending with a bending moment range (in both frontal and sagittal planes).
2.2.2. Compression Test
2.2.3. Tensile Test
2.2.4. Bending Test
2.2.5. Fatigue Testing in 3-Point Bending
- Mean bending force (The mean value was calculated for cycles in the range of 100 to 500,000, after stabilization of the recorded results): ;
- Mean bending moment: ;
- Mean deflection: .
2.2.6. Static 4-Point Bending Test
2.2.7. Static Torsion Test
2.2.8. Dynamic 4-Point Bending Test
2.3. Numerical Calculations
3. Results
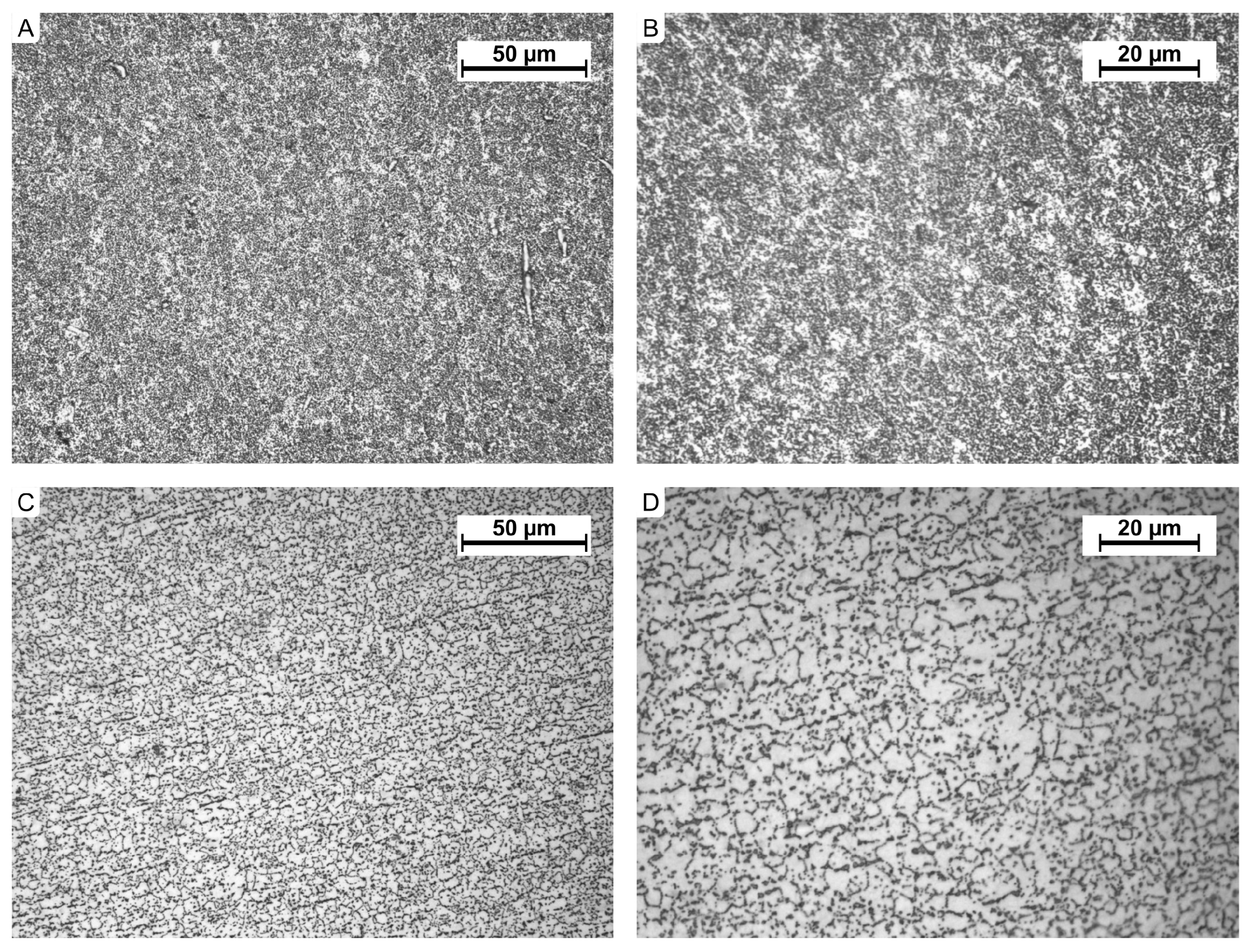
3.1. Implant Material Analysis
3.2. Mechanical Strength Testing of the Implant
3.2.1. Testing on a Custom Strength Testing Rig
- Axial compression: 500 N;
- Axial tension: 100 N;
- Bending: 20 Nm (in both frontal and sagittal planes).
- Axial compression: 1400 N;
- Axial tension: 1000 N;
- Bending: 112–115 Nm (in both frontal and sagittal planes).
3.2.2. Fatigue Testing in 3-Point Bending
3.2.3. Static 4-Point Bending Test
3.2.4. Static Torsion Test
3.2.5. Dynamic 4-Point Bending Test
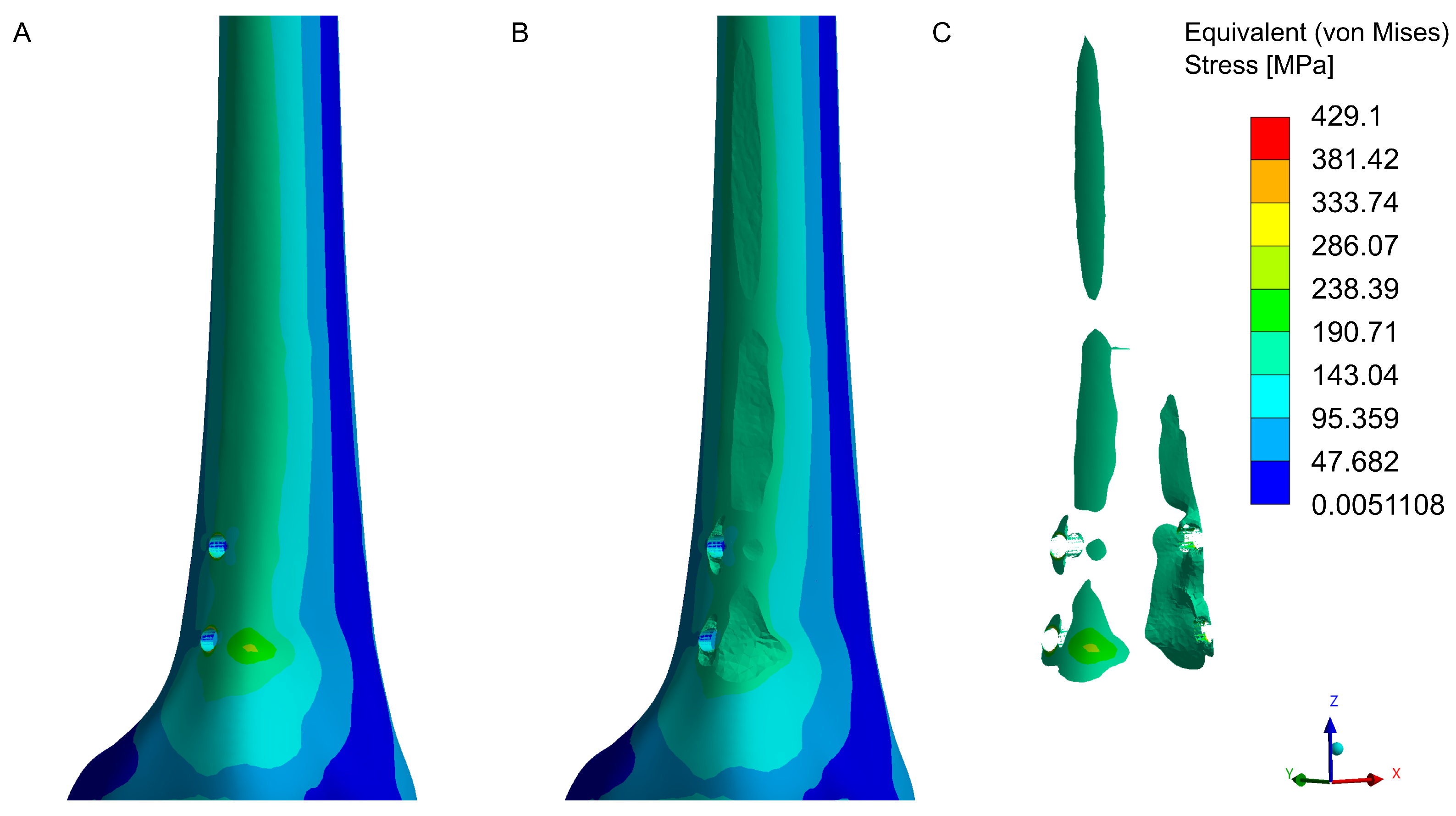
3.3. Numerical Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, H.R.; Roh, T.S.; Shim, K.W.; Kim, Y.O.; Lew, D.H.; Yun, I.S. Skull Reconstruction with Custom Made Three-Dimensional Titanium Implant. Arch. Craniofacial Surg. 2015, 16, 11. [Google Scholar] [CrossRef]
- Park, E.K.; Lim, J.Y.; Yun, I.S.; Kim, J.S.; Woo, S.H.; Kim, D.S.; Shim, K.W. Cranioplasty enhanced by three-dimensional printing: Custom-made three-dimensional-printed titanium implants for skull defects. J. Craniofacial Surg. 2016, 27, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, W.; Chen, J.; Yu, J.; Zhang, J.; Chen, J. The application of polyetheretherketone (PEEK) implants in cranioplasty. Brain Res. Bull. 2019, 153, 143–149. [Google Scholar] [CrossRef]
- Xu, L.; Qin, H.; Tan, J.; Cheng, Z.; Luo, X.; Tan, H.; Huang, W. Clinical study of 3D printed personalized prosthesis in the treatment of bone defect after pelvic tumor resection. J. Orthop. Transl. 2021, 29, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Hollensteiner, M.; Sandriesser, S.; Bliven, E.; von Rüden, C.; Augat, P. Biomechanics of Osteoporotic Fracture Fixation. Curr. Osteoporos. Rep. 2019, 17, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.; Michel, M.C.; Genge, M.; Zuber, K.; Ganz, R.; Perren, S.M. Loads acting in an intramedullary nail during fracture healing in the human femur. J. Biomech. 2001, 34, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Malkova, T.A.; Borzunov, D.Y. International recognition of the Ilizarov bone reconstruction techniques: Current practice and research (dedicated to 100th birthday of G. A. Ilizarov). World J. Orthop. 2021, 12, 515–533. [Google Scholar] [CrossRef]
- Brånemark, R.; Brånemark, P.I.; Rydevik, B.; Myers, R.R. Osseointegration in skeletal reconstruction and rehabilitation: A review. J. Rehabil. Res. Dev. 2001, 38, 175–181. [Google Scholar]
- Carlsson, L.; Röstlund, T.; Albrektsson, B.; Albrektsson, T.; Brånemark, P.I. Osseointegration of titanium implants. Acta Orthopaedica Scandinavica 1986, 57, 285–289. [Google Scholar] [CrossRef]
- Dailey, H.L.; Daly, C.J.; Galbraith, J.G.; Cronin, M.; Harty, J.A. A novel intramedullary nail for micromotion stimulation of tibial fractures. Clin. Biomech. 2012, 27, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Dailey, H.L.; Schwarzenberg, P.; Webb, E.B., III; Boran, S.A.M.; Guerin, S.; Harty, J.A. Pilot study of micromotion nailing for mechanical stimulation of tibial fracture healing. Bone Jt. Open 2021, 2, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Triana, L.; Huatuco, R.M.P.; Campilgio, G.; Liscano, E. Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery—ISAPS. Aesthetic Plast. Surg. 2024, 48, 4217–4227. [Google Scholar] [CrossRef] [PubMed]
- Valina, G.; Sessa, A. Current Trends of Cosmetic Surgical Procedures With the General Cosmetic Surgery Fellowships by the American Academy of Cosmetic Surgery. Am. Cosmet. Surg. 2020, 37, 5–13. [Google Scholar] [CrossRef]
- Sabharwal, S.; Mittal, A.; Allahabadi, S.; Jayaram, R.; Nalluri, A.; Callahan, M. Trends and Practices in Limb Lengthening: An 11-year US Database Study. Strateg. Trauma Limb Reconstr. 2023, 18, 21–31. [Google Scholar] [CrossRef]
- Sarwer, D.B.; Crerand, C.E. Body image and cosmetic medical treatments. Body Image 2004, 1, 99–111. [Google Scholar] [CrossRef]
- Dang, H.S.; Nguyen, T.M.T.; Wang, C.N.; Day, J.D.; Dang, T.M.H. Grey System Theory in the Study of Medical Tourism Industry and Its Economic Impact. Int. Environ. Res. Public Health 2020, 17, 961. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.R.; Batra, R.; Banerji, A. Low Cost, Quality Treatment and Excellent Hospitality Makes India the Best Destination for Medical Tourism. Int. J. Innov. Res. Med. Sci. 2020, 5, 10–15. [Google Scholar] [CrossRef]
- Liew, S.; Wu, W.T.L.; Chan, H.H.; Ho, W.W.S.; Kim, H.J.; Goodman, G.J.; Peng, P.H.L.; Rogers, J.D. Consensus on Changing Trends, Attitudes, and Concepts of Asian Beauty. Aesthetic Plast. Surg. 2016, 40, 193–201. [Google Scholar] [CrossRef]
- Panfiluk, E. Aesthetic medicine tourism—Nature and scope of the services. Ekon. Zarz. 2016, 8, 71–79. [Google Scholar] [CrossRef]
- Guerreschi, F.; Tsibidakis, H. Cosmetic lengthening: What are the limits? J. Child. Orthop. 2016, 10, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Catagni, M.A.; Lovisetti, L.; Guerreschi, F.; Combi, A.; Ottaviani, G.; Ottaviani, G. Cosmetic bilateral leg lengthening experience of 54 cases. J. Bone Jt. Surg. 2005, 87, 1402–1405. [Google Scholar] [CrossRef] [PubMed]
- Origo, C.; Lazzotti, C. Current trends on limb length discrepancy treatment: Results of a survey among 11 dedicated Italian centres. Musculoskelet. Surg. 2023, 107, 165–170. [Google Scholar] [CrossRef]
- Marwan, Y.; Cohen, D.; Alotaibi, M.; Addar, A.; Bernstein, M.; Hamdy, R. Cosmetic stature lengthening. Bone Jt. Res. 2020, 9, 341–350. [Google Scholar] [CrossRef]
- Standard, S.C.; Herzenberg, J.E.; Green, S.A. Antegrade and Retrograde Femur Operative Technique; NuVasive: San Diego, CA, USA, 2019. [Google Scholar]
- Horn, J.; Hvid, I.; Huhnstock, S.; Breen, A.B.; Steen, H. Limb lengthening and deformity correction with externally controlled motorized intramedullary nails: Evaluation of 50 consecutive lengthenings. Acta Orthop. 2019, 90, 81–87. [Google Scholar] [CrossRef]
- Frommer, A.; Roedl, R.; Gosheger, G.; Niemann, M.; Turkowski, D.; Toporowski, G.; Theil, C.; Laufer, A.; Vogt, B. What Are the Potential Benefits and Risks of Using Magnetically Driven Antegrade Intramedullary Lengthening Nails for Femoral Lengthening to Treat Leg Length Discrepancy? Clin. Orthop. Relat. Res. 2022, 480, 790–803. [Google Scholar] [CrossRef]
- Paley, D. PRECICE intramedullary limb lengthening system. Expert Rev. Med. Devices 2015, 12, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Garg, V.; Goyal, A.; Aggarwal, N. Removal of a broken distal cannulated intramedullary femoral nail with solid reamer with closed methods and without using C-arm: A case report. J. Arthrosc. Jt. Surg. 2015, 2, 9–11. [Google Scholar] [CrossRef]
- Rölfing, J.D.; Kold, S.; Nygaard, T.; Mikuzis, M.; Brix, M.; Faergemann, C.; Gottliebsen, M.; Davidsen, M.; Petruskevicius, J.; Olesen, U.K. Pain, osteolysis, and periosteal reaction are associated with the STRYDE limb lengthening nail: A nationwide cross-sectional study. Acta Orthop. 2021, 92, 479–484. [Google Scholar] [CrossRef]
- Hidden, K.A.; Dahl, M.T.; Ly, T.V. Management of a Broken PRECICE Femoral Nail at an Ununited Distraction Osteogenesis Site: A Case Report. JBJS Case Connect. 2020, 10, e0267. [Google Scholar] [CrossRef] [PubMed]
- Eltayeby, H.H.; Alrabai, H.M.; Jauregui, J.J.; Shabtai, L.Y.; Herzenberg, J.E. Post-retrieval functionality testing of PRECICE lengthening nails: The “Sleeper” nail concept. J. Clin. Orthop. Trauma 2021, 14, 151–155. [Google Scholar] [CrossRef]
- Alrabai, H.M. Breakage of a re-activated PRECICE® nail: A case report. Int. J. Surg. Case Rep. 2023, 106, 108182. [Google Scholar] [CrossRef]
- ISO 5832-3:2021; Implants for Surgery—Metallic Materials Part 3: Wrought Titanium 6-Aluminium 4-Vanadium Alloy. ISO: Geneva, Switzerland, 2021.
- PN-EN ISO 6507-1:2018; Metallic Materials: Vickers Hardness Test. Part 1, Test Method. ISO: Geneva, Switzerland, 2018.
- ISO 6892-1:2019; Metallic Materials—Tensile Testing. ISO: Geneva, Switzerland, 2019.
- Croccolo, D.; Agostinis, M.D.; Fini, S.; Funaioli, S.; Olmi, G.; Robusto, F. Structural validation of intramedullary nails: From experimentation to virtual testing. In Proceedings of the 6th International Conference on Integrity-Reliability-Failure, Lisbon, Portugal, 22–26 July 2018; Silva Gomes, J., Meguid, S., Eds.; Universidade do Porto: Porto, Portugal, 2018; pp. 1011–1016. [Google Scholar]
- Karuppiah, S.V.; Johnstone, A.J. Distal locking in femoral intramedullary nailing system: Is one cross screw sufficient? J. Biomed. Sci. Eng. 2012, 5, 593–596. [Google Scholar] [CrossRef]
- PN-EN 13018:2016-04; Badania Nieniszczące—Badania Wizualne—Zasady Ogólne. Polish Committee for Standardization: Warsaw, Poland, 2016.
- PN-EN ISO 7438:2021-04; Metallic Materiale—Bend Test (ISO 7438:2020). ISO: Geneva, Switzerland, 2021.
- ASTM F1264-16e1; Standard Specification and Test Methods for Intramedullary Fixation Devices. ASTM: West Conshohocken, PA, USA, 2024.
- Little, R.E. Manual on Statistical Planning and Analysis for Fatigue Experiments; ASTM International: West Conshohocken, PA, USA, 1975; Volume 588. [Google Scholar]
- Baleani, M.; Erani, P.; Blaise, M.; Fognani, R.; Palmas, M.; Manfrini, M. Intercalary reconstruction of long bones by massive allograft: Comparison of construct stability ensured by three different host-graft junctions and two types of fixations in a synthetic femur model. Front. Pediatr. 2022, 10, 868299. [Google Scholar] [CrossRef]
- Edwards, W.B.; Gillette, J.C.; Thomas, J.M.; Derrick, T.R. Internal femoral forces and moments during running: Implications for stress fracture development. Clin. Biomech. 2008, 23, 1269–1278. [Google Scholar] [CrossRef]
- Becerra, L.H.C.; Rodríguez, M.A.L.H.; Arroyo, R.L.; Solís, H.E.; Castro, A.T. Effect of sterilization on 3-point dynamic response to in vitro bending of an Mg implant. Biomater. Res. 2021, 25, 9. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, K.; Choi, Y.S. Comparison of mechanical rigidity between plate augmentation leaving the nail in situ and interlocking nail using cadaveric fracture model of the femur. Int. Orthop. 2011, 35, 581–585. [Google Scholar] [CrossRef]
- Untaroiu, C.; Genovese, D.; Ivarsson, J.; Crandall, J. A Finite Element Analysis of Mid-Shaft Femoral Tolerance under Combined Axial-Bending Loading. In Proceedings of the 10th International LS-DYNA Conference, Detroit, MI, USA, 8–10 June 2008. [Google Scholar]
- Zhang, Z.K.; Guo, X.; Lao, J.; Qin, Y.X. Effect of capsaicin-sensitive sensory neurons on bone architecture and mechanical properties in the rat hindlimb suspension model. J. Orthop. Transl. 2017, 10, 12–17. [Google Scholar] [CrossRef] [PubMed]
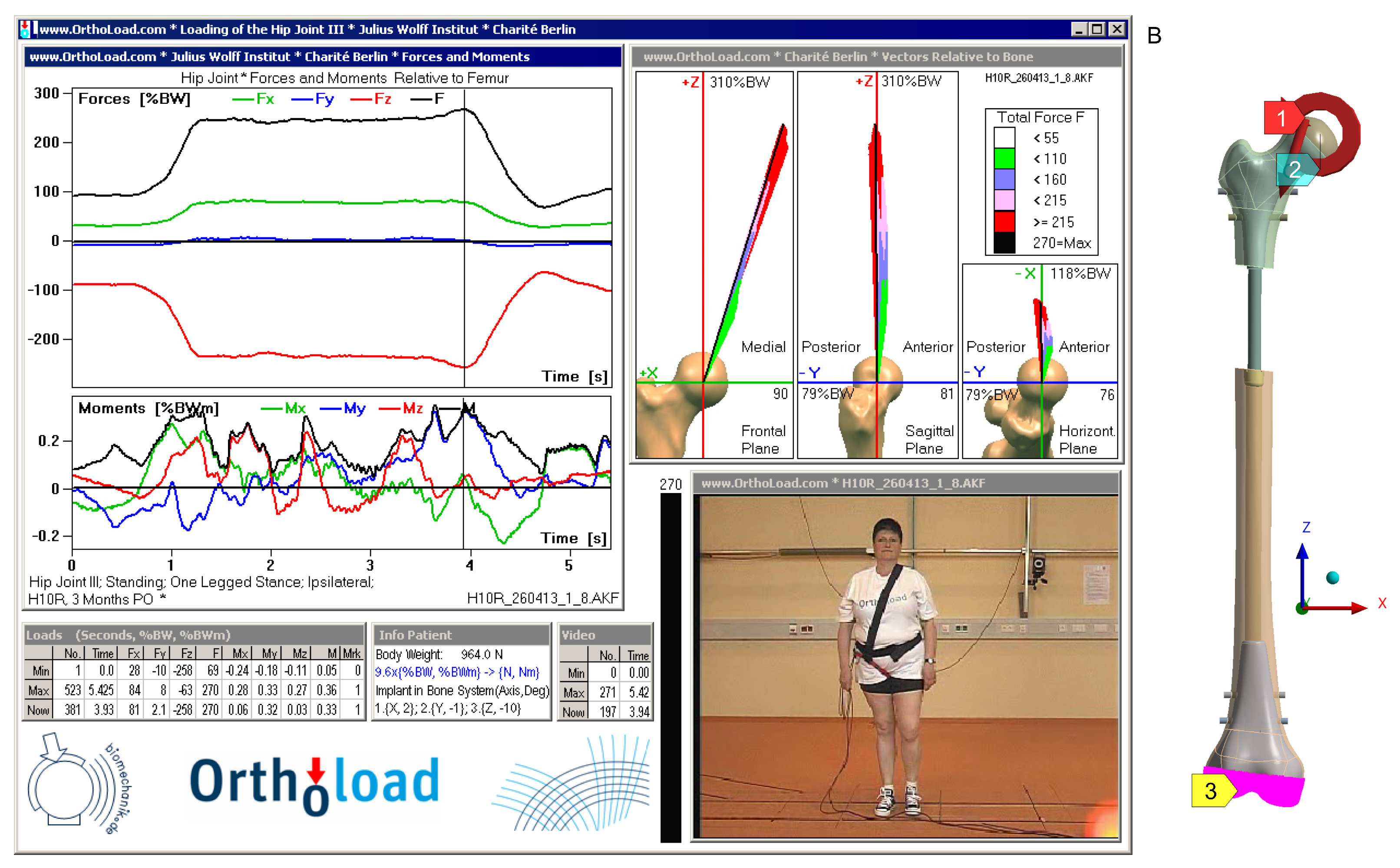
- Bergmann, G.; Damm, P. Julius Wolff Institute, Berlin Institute of Health at Charité Universitätsmedizin Berlin, 2008. Available online: https://orthoload.com/database/?implantId=2355&activityId=2541&activityIndentationLevel=1¶meterId=1¶meterIndentationLevel=-1&patientId=h10r&fileId=h10r_260413_1_8&fileType=t&selectBox=file (accessed on 1 December 2024).
- Aprisunadi, N.N.; Mustikasari, M.; Ifadah, E.; Hapsari, E.D. Effect of Early Mobilization on Hip and Lower Extremity Postoperative: A Literature Review. SAGE Open Nurs. 2023, 9, 23779608231167825. [Google Scholar] [CrossRef] [PubMed]
- Kenyon-Smith, T.; Nguyen, E.; Oberai, T.; Jarsma, R. Early Mobilization Post–Hip Fracture Surgery. Geriatr. Orthop. Surg. Rehabil. 2019, 10, 2151459319826431. [Google Scholar] [CrossRef]
- Hlukha, L.P.; Alrabai, H.M.; Sax, O.C.; Hammouda, A.I.; McClure, P.K.; Herzenberg, J.E. Mechanical Failures in Magnetic Intramedullary Lengthening Nails. J. Bone Jt. Surg. 2023, 105, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Ramlawi, A.A.; Assayag, M.; McClure, P. PRECICE nail bending in femur lengthening. J. Orthop. 2024, 56, 127–132. [Google Scholar] [CrossRef] [PubMed]
- ASTM F136-13(2021)e1; Standard Specification for Wrought Titanium-6Aluminum-4Vanadium ELI (Extra Low Interstitial) Alloy for Surgical Implant Applications. ASTM: West Conshohocken, PA, USA, 2021.
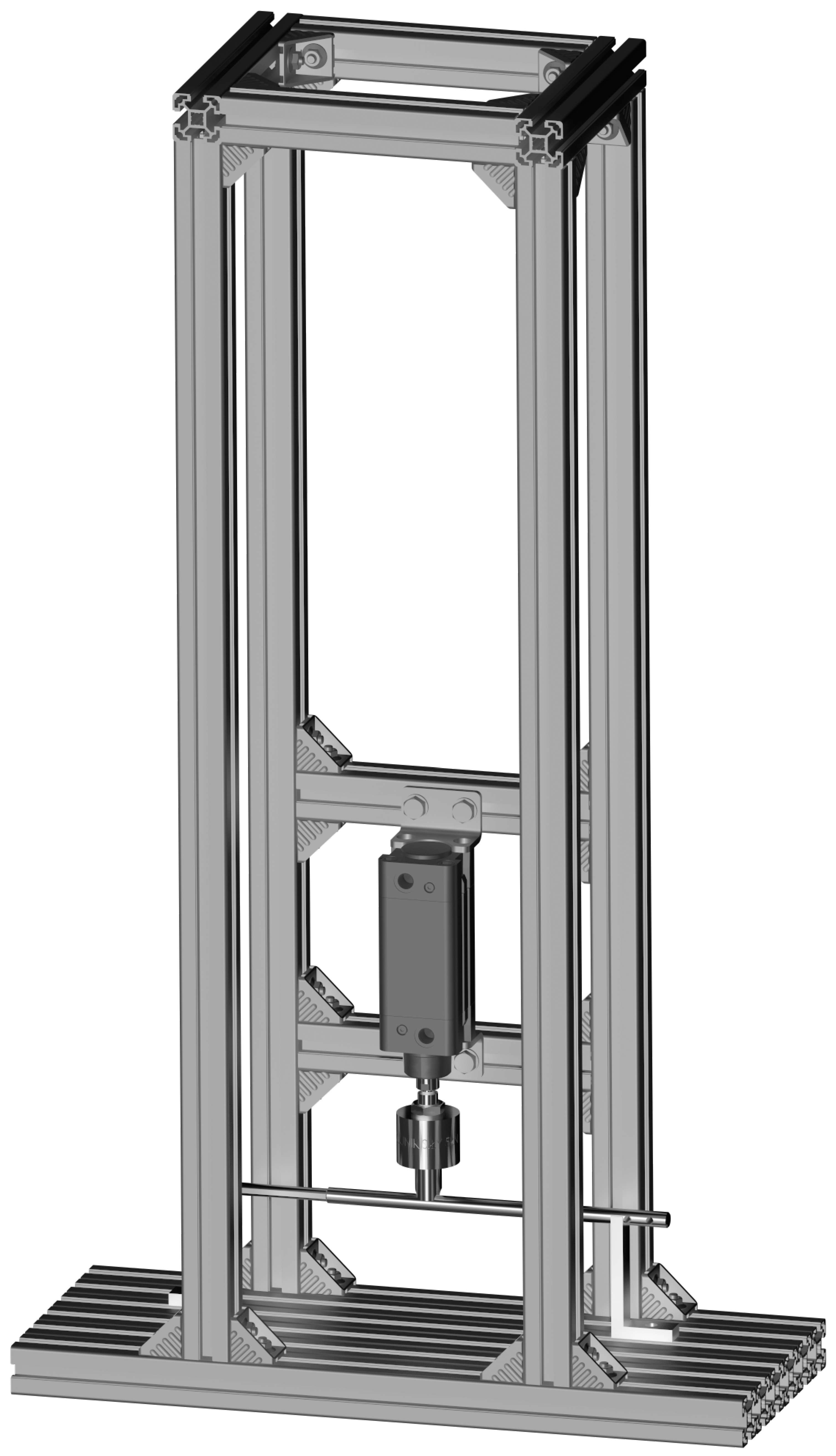
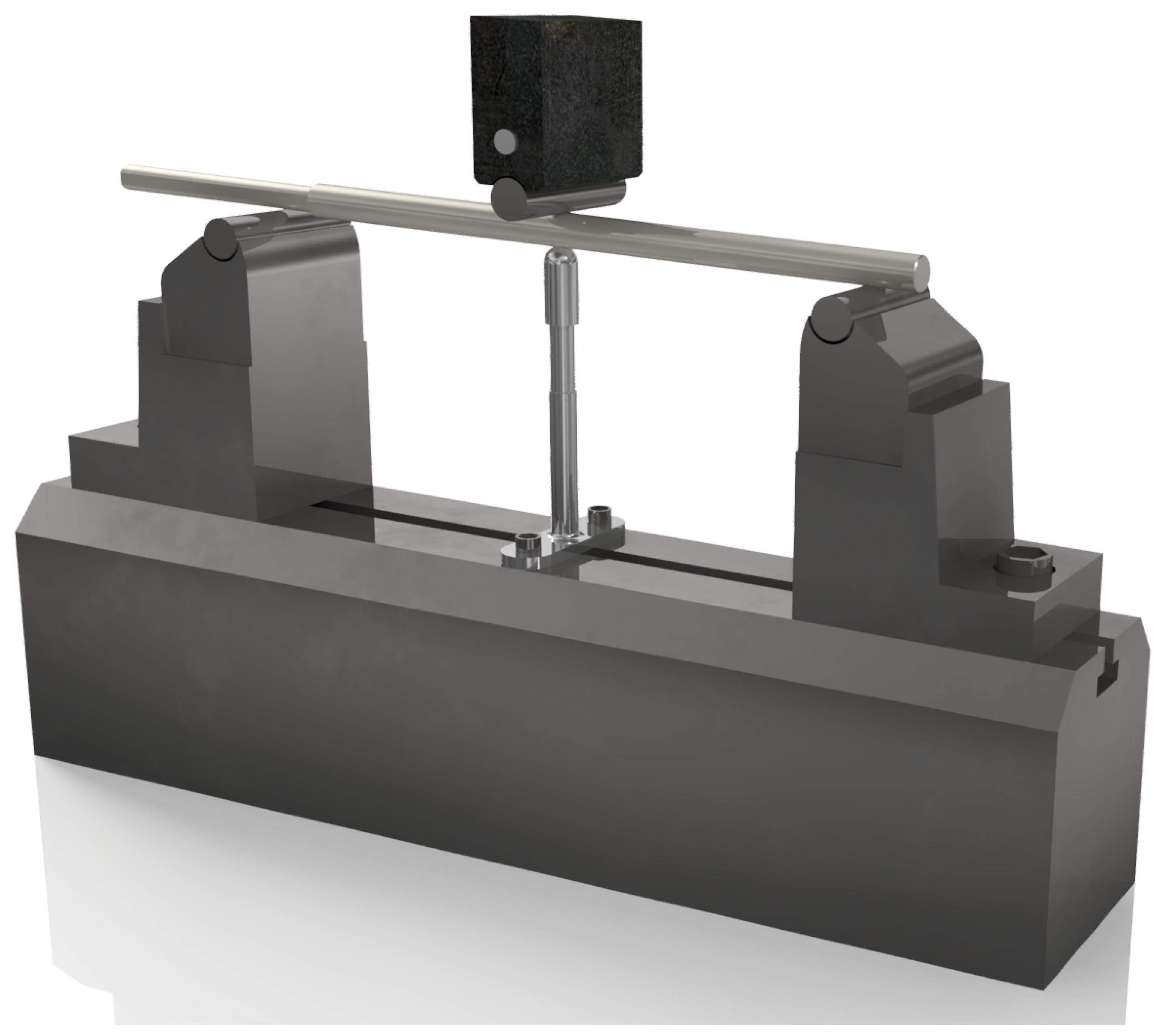



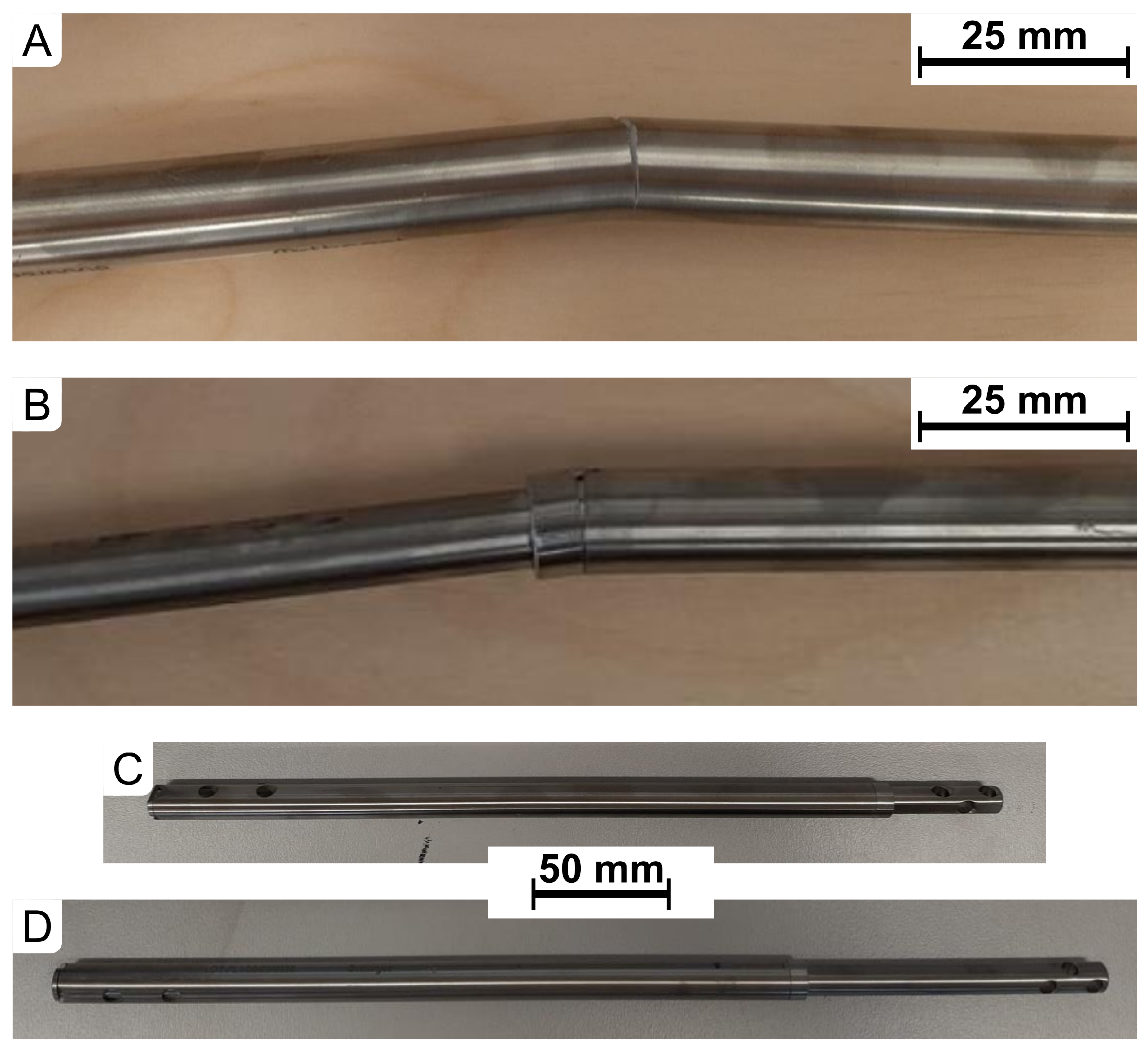

| Axial Compression of the Implant | ||||
|---|---|---|---|---|
| IL* = 0 mm | 6 repetitions for N for each implant lenght | |||
| IL = 40 mm | ||||
| IL = 80 mm | ||||
| Axial Tension of the Implant | ||||
| IL = 0 mm | 6 repetitions for N for each implant lenght | |||
| IL = 40 mm | ||||
| IL = 80 mm | ||||
| 3-Point Bending of the Implant | ||||
| Support Span | ||||
| 250 mm | 290 mm | 315 mm | ||
| IL = 0 mm | Frontal Plane | 6 repetitions for | ||
| Sagittal Plane | ||||
| IL = 40 mm | Frontal Plane | 6 repetitions for | ||
| Sagittal Plane | ||||
| IL = 80 mm | Frontal Plane | 6 repetitions for | ||
| Sagittal Plane | ||||
| Cycles | Data Recording Frequency |
|---|---|
| 1–10 | Every cycle |
| 11–100 | Every 10 cycles |
| 101–1000 | Every 100 cycles |
| 1001–500,000 | Every 1000 cycles |
| Material | E [GPa] | v [-] |
|---|---|---|
| Cortical bone tissue | 18 | 0.3 |
| Cancellous bone tissue | 0.480 | 0.42 |
| Titanium alloy | 114 | 0.34 |
| No. | Hardness HV1 (9.807 N) | Mean Hardness HV1 |
|---|---|---|
| 1 | 296.5 | |
| 2 | 300.3 | |
| 3 | 301.6 | |
| 4 | 299.3 | |
| 5 | 307.3 |
| No. | Young’s Modulus | Elastic Limit | Yield Strength | Tensile Strength | Cross-Section Reduction | Elongation at Break |
|---|---|---|---|---|---|---|
| [ MPa] | [MPa] | [MPa] | [MPa] | [%] | [%] | |
| 1 | 1.10 | 772 | 792 | 1046 | 31 | 7.3 |
| 2 | 1.13 | 947 | 964 | 1051 | 34 | 9.3 |
| 3 | 1.13 | 944 | 959 | 1087 | 25 | 8.0 |
| Average | 1.12 | 888 | 905 | 1061 | 30 | 8.2 |
| Axial Compression of the Implant | ||||
|---|---|---|---|---|
| Results (Load ± Displacement) | ||||
| IL * = 0 mm | 508.12 ± 0.91 N 0.24 ± 0.02 mm | |||
| IL = 40 mm | 511.53 ± 8.68 N 0.24 ± 0.03 mm | |||
| IL = 80 mm | 507.89 ± 2.09 N 0.49 ± 0.04 mm | |||
| Axial Tension of the Implant | ||||
| IL = 0 mm | 105.18 ± 0.76 N 0.05 ± 0.00 mm | |||
| IL = 40 mm | 104.27 ± 0.29 N 0.10 ± 0.01 mm | |||
| IL = 80 mm | 103.55 ± 0.82 N 0.07 ± 0.01 mm | |||
| 3-Point Bending of the Implant | ||||
| Support Span | ||||
| 250 mm | 290 mm | 315 mm | ||
| IL = 0 mm | Frontal Plane | 25.06 ± 0.03 Nm 1.55 ± 0.01 mm | ||
| Sagittal Plane | 25.28 ± 0.29 Nm 1.56 ± 0.07 mm | |||
| IL = 40 mm | Frontal Plane | 25.35 ± 0.24 Nm 2.42 ± 0.04 mm | ||
| Sagittal Plane | 25.18 ± 0.09 Nm 2.42 ± 0.03 mm | |||
| IL = 80 mm | Frontal Plane | 25.17 ± 0.04 Nm 3.03 ± 0.00 mm | ||
| Sagittal Plane | 25.25 ± 0.03 Nm 3.02 ± 0.03 mm | |||
| Parameter | Value | Unit |
|---|---|---|
| Maximum deflection for cycle 100 | mm | |
| Maximum deflection for cycle 500,000 | 500,000) | mm |
| Deflection change | mm | |
| Deflection change * | 1.43% | |
| Visual evaluation of implant post-test | no signs of damage | |
| Implant | q | ||||||
|---|---|---|---|---|---|---|---|
| 5.101 | 148.2 | 1.6 | 3.9 | 4.54 | 11.2 | 74 | |
| 4.890 | 110.2 | 1.6 | 2.9 | 4.10 | 12.9 | 58 |
| Implant Length | Torsional Moment [Nm] | Rotation Angle [°] |
|---|---|---|
| 17.16 | 18.45 | |
| 19.36 | 19.37 |
| Sample | Extension | Recorded Cycles | Failure Mode | ||
|---|---|---|---|---|---|
| a | 390.0 | 3900.0 | 7404 | Fracture | |
| b | 290.0 | 2900.0 | 6734 | Fracture | |
| c | 100.0 | 1000.0 | 1,000,000 | No failure | |
| d | 100.0 | 1000.0 | 1,000,000 | No failure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grygier, D.; Kowalewski, P.; Opałka, M.; Słowiński, J.J.; Dziubek, M.; Pyka, D. Analysis of the Feasibility of the OrthoNail Hybrid Intramedullary Implant in the Human Body with Respect to Material Durability. J. Funct. Biomater. 2025, 16, 27. https://doi.org/10.3390/jfb16010027
Grygier D, Kowalewski P, Opałka M, Słowiński JJ, Dziubek M, Pyka D. Analysis of the Feasibility of the OrthoNail Hybrid Intramedullary Implant in the Human Body with Respect to Material Durability. Journal of Functional Biomaterials. 2025; 16(1):27. https://doi.org/10.3390/jfb16010027
Chicago/Turabian StyleGrygier, Dominika, Piotr Kowalewski, Mariusz Opałka, Jakub J. Słowiński, Mateusz Dziubek, and Dariusz Pyka. 2025. "Analysis of the Feasibility of the OrthoNail Hybrid Intramedullary Implant in the Human Body with Respect to Material Durability" Journal of Functional Biomaterials 16, no. 1: 27. https://doi.org/10.3390/jfb16010027
APA StyleGrygier, D., Kowalewski, P., Opałka, M., Słowiński, J. J., Dziubek, M., & Pyka, D. (2025). Analysis of the Feasibility of the OrthoNail Hybrid Intramedullary Implant in the Human Body with Respect to Material Durability. Journal of Functional Biomaterials, 16(1), 27. https://doi.org/10.3390/jfb16010027










