Eco-Friendly Synthesis of Chitosan–Fatty Acid Nano Micelles and Their Differential Antibacterial Activity Against Escherichia coli and Bacillus subtilis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
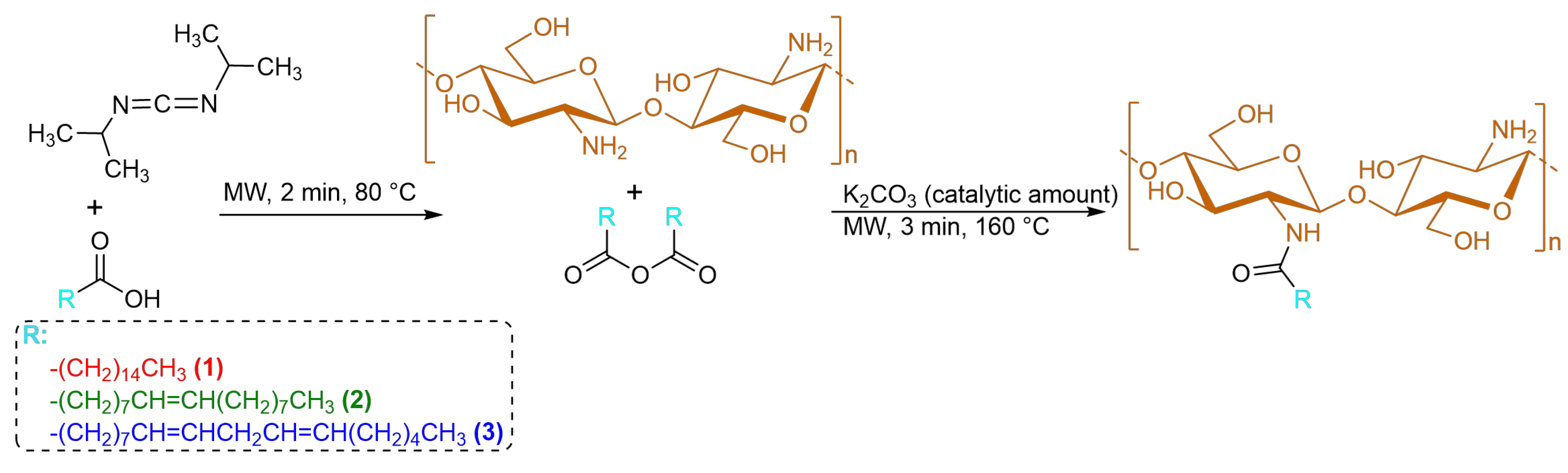
2.2. Synthesis of CS–FA Conjugates
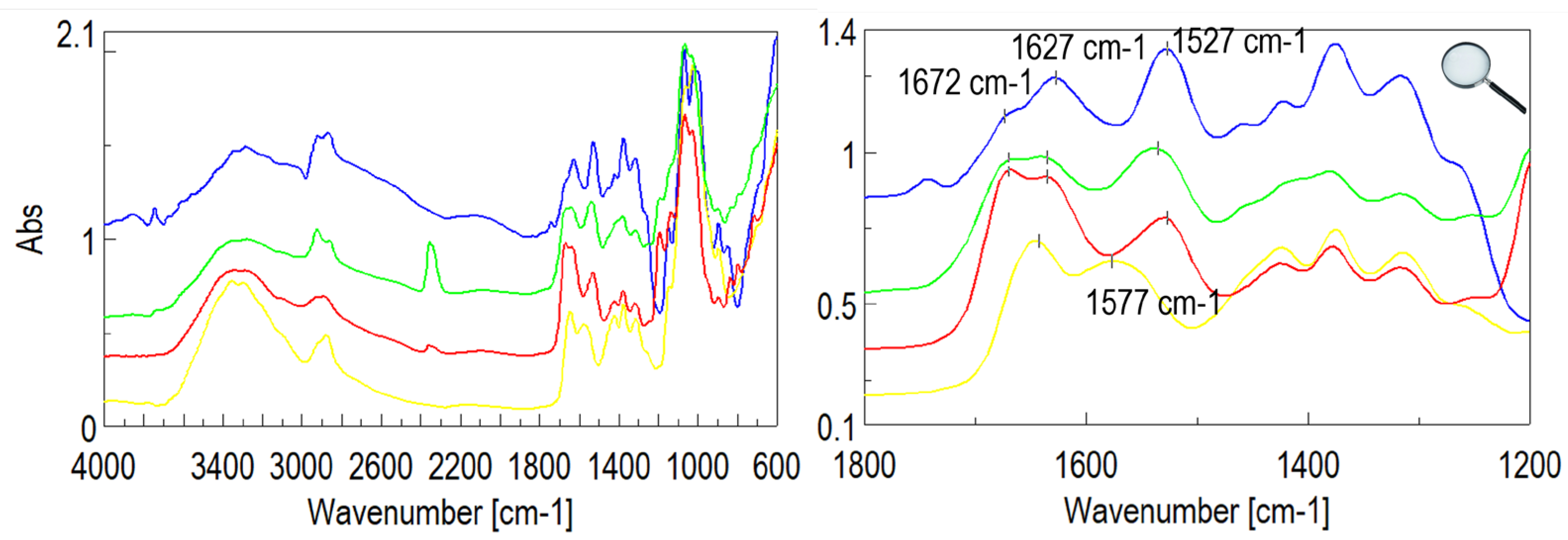
2.3. FT-IR Spectroscopy Characterization
2.4. Preparation and Size Distribution Characterization of CS-FA Micelles (Dynamic Light Scattering)
2.5. Determination of Critical Micellar Concentration (CMC)
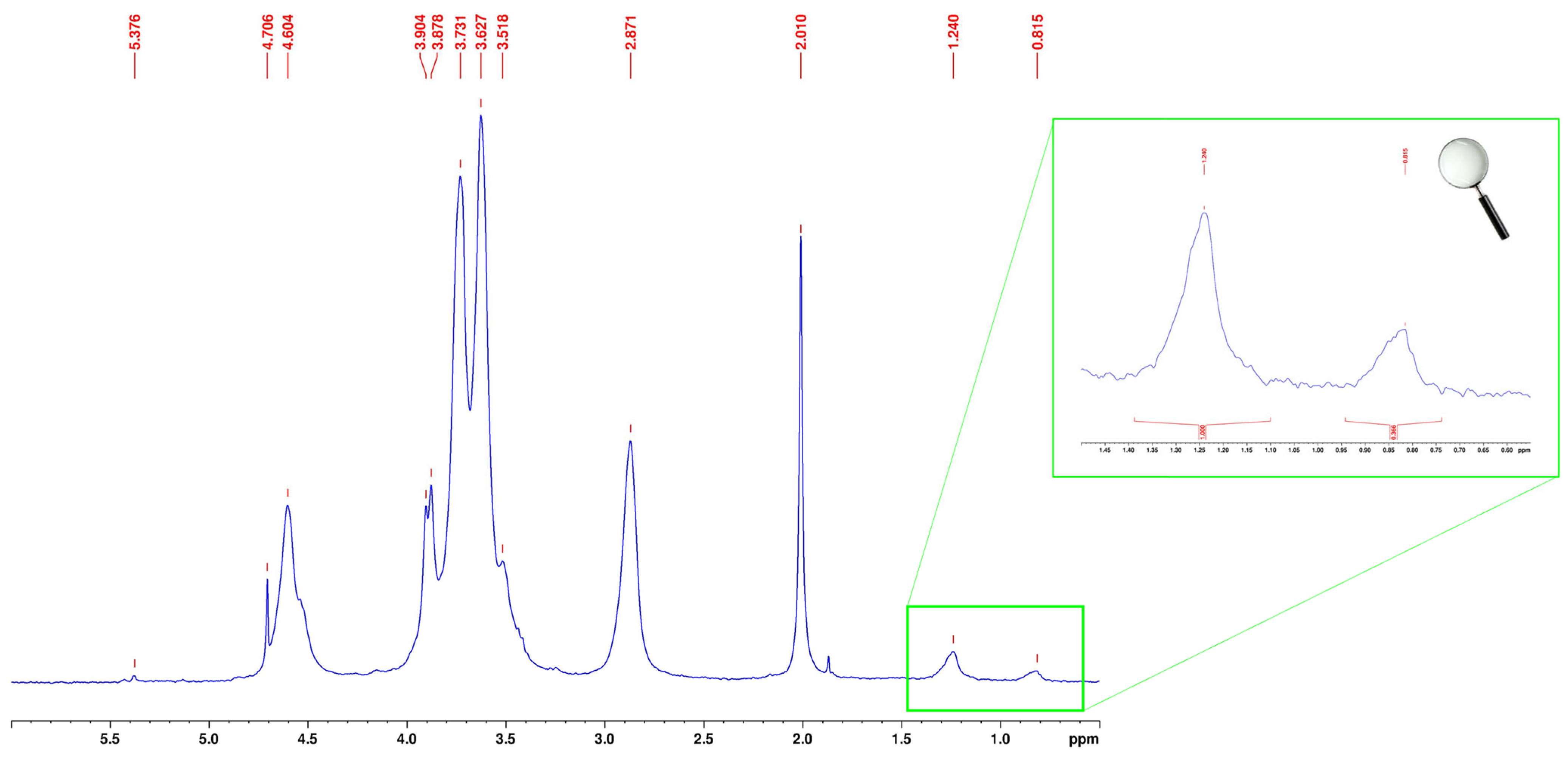
2.6. Nuclear Magnetic Resonance (1H NMR) Characterization
2.7. Antibacterial Assay
3. Results and Discussion
3.1. Synthesis and Structural Characterization of CS–FA Conjugates
3.2. Evaluation of CMC and Investigation of CS-FA Micelles Size via Dynamic Light Scattering Technique
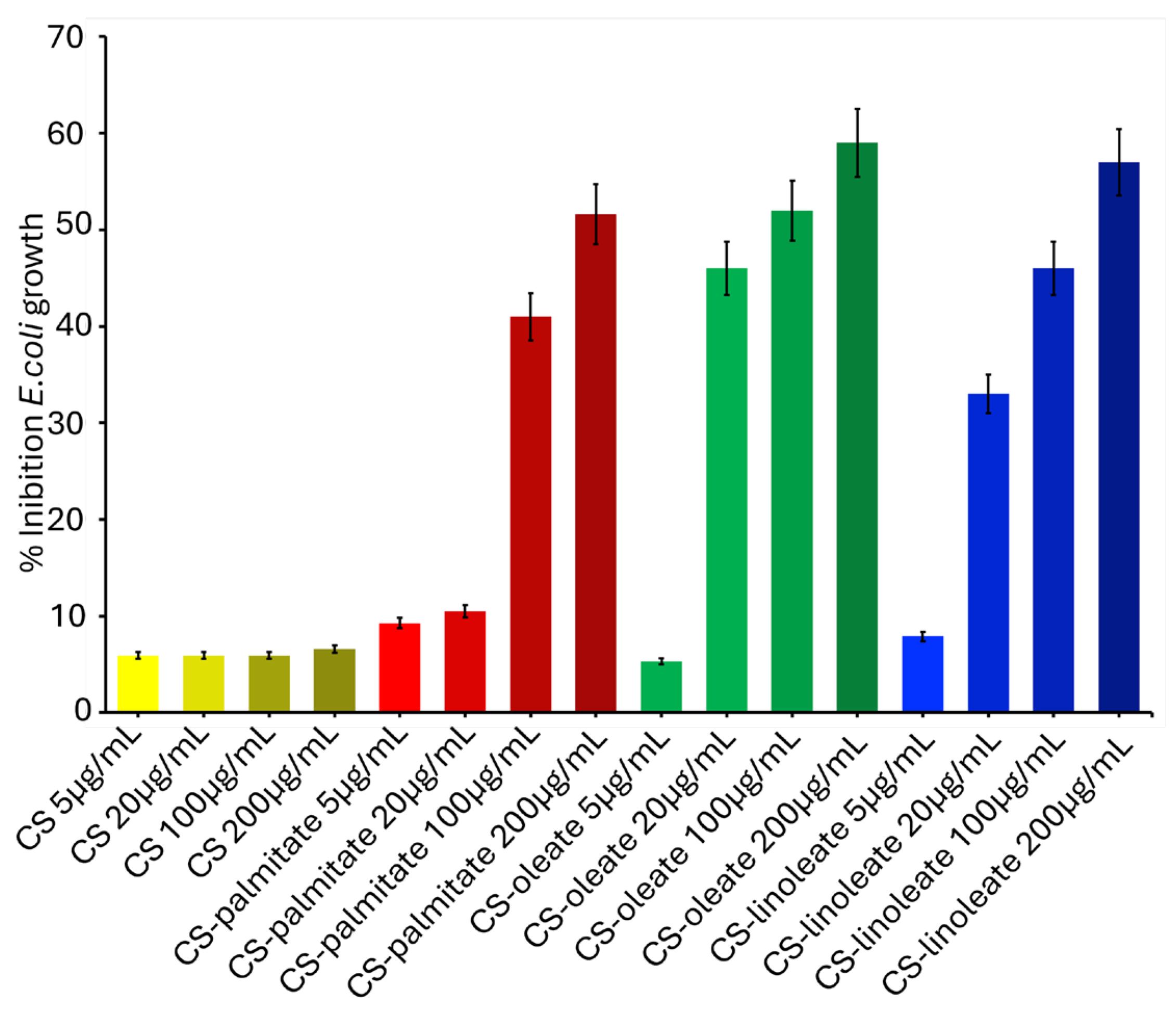
3.3. Antibacterial Assay Using CS-FA Micelles Against E. coli and B. subtilis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1D-DOSY | One-Dimensional Diffusion-Ordered Spectroscopy |
| ATCC | American Type Culture Collection |
| B. subtilis | Bacillus subtilis |
| CFU | Colony Forming Units |
| CMC | Critical Micellar Concentration |
| CS | Chitosan |
| CS-FA | Chitosan–Fatty Acid |
| D2O | Deuterium Oxide |
| DIC | N,N′-Diisopropylcarbodiimide |
| DLS | Dynamic Light Scattering |
| E. coli | Escherichia coli |
| FA | Fatty Acid |
| 1H NMR | Proton Nuclear Magnetic Resonance Spectroscopy |
| HCl | Hydrochloric acid |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| K2CO3 | Potassium Carbonate |
| LPS | Lipopolysaccharides |
| MH | Mueller–Hinton |
| MW | Microwave |
| MWCO | Molecular Weight Cut-Off |
| NMR | Nuclear Magnetic Resonance |
| OD600 | Optical Density at 600 nm |
| PDI | Polydispersity Index |
| S. aureus | Staphylococcus aureus |
| TECAN | Tecan Group Ltd. |
References
- Arellano, H.; Nardello-Rataj, V.; Szunerits, S.; Boukherroub, R.; Fameau, A.L. Saturated Long Chain Fatty Acids as Possible Natural Alternative Antibacterial Agents: Opportunities and Challenges. Adv. Colloid. Interface Sci. 2023, 318, 102952. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P. Potential Applications of Antimicrobial Fatty Acids in Medicine, Agriculture and Other Industries. Recent. Pat. Antiinfect. Drug Discov. 2012, 7, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Travkova, O.G.; Moehwald, H.; Brezesinski, G. The Interaction of Antimicrobial Peptides with Membranes. Adv. Colloid. Interface Sci. 2017, 247, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, Y. Structure–Activity Relationship of Cationic Surfactants as Antimicrobial Agents. Curr. Opin. Colloid. Interface Sci. 2020, 45, 28–43. [Google Scholar] [CrossRef]
- Kumar, P.; Lee, J.H.; Beyenal, H.; Lee, J. Fatty Acids as Antibiofilm and Antivirulence Agents. Trends Microbiol. 2020, 28, 753–768. [Google Scholar] [CrossRef]
- Jackman, J.A.; Yoon, B.K.; Li, D.; Cho, N.J. Nanotechnology Formulations for Antibacterial Free Fatty Acids and Monoglycerides. Molecules 2016, 21, 305. [Google Scholar] [CrossRef]
- Jung, S.W.; Thamphiwatana, S.; Zhang, L.; Obonyo, M. Mechanism of Antibacterial Activity of Liposomal Linolenic Acid against Helicobacter Pylori. PLoS ONE 2015, 10, e0116519. [Google Scholar] [CrossRef]
- Tran, T.Q.M.; Hsieh, M.F.; Chang, K.L.; Pho, Q.H.; Nguyen, V.C.; Cheng, C.Y.; Huang, C.M. Bactericidal Effect of Lauric Acid-Loaded PCL-PEG-PCL Nano-Sized Micelles on Skin Commensal Propionibacterium Acnes. Polymers 2016, 8, 321. [Google Scholar] [CrossRef]
- Calce, E.; Mignogna, E.; Bugatti, V.; Galdiero, M.; Vittoria, V.; De Luca, S. Pectin Functionalized with Natural Fatty Acids as Antimicrobial Agent. Int. J. Biol. Macromol. 2014, 68, 28–32. [Google Scholar] [CrossRef]
- Verdoliva, V.; Muzio, G.; Autelli, R.; Saviano, M.; Bedini, E.; De Luca, S. Microwave-Assisted, Solid-State Procedure to Covalently Conjugate Hyaluronic Acid to Curcumin: Validation of a Green Synthetic Protocol. ACS Polym. Au 2024, 4, 214–221. [Google Scholar] [CrossRef]
- Verdoliva, V.; Muzio, G.; Autelli, R.; De Luca, S. Solid State Synthesis of Hyaluronic Acid–Quercetin Conjugate: Sustainable Protocol to Improve the Biological Activity of Quercetin. Chem. Biodivers. 2024, 22, e202402495. [Google Scholar] [CrossRef]
- Verdoliva, V.; Bedini, E.; De Luca, S. Sustainable Chemical Modification of Natural Polysaccharides: Mechanochemical, Solvent-Free Conjugation of Pectins and Hyaluronic Acid Promoted by Microwave Radiations. Biomacromolecules 2024, 25, 6217–6228. [Google Scholar] [CrossRef] [PubMed]
- Traboni, S.; Esposito, F.; Ziaco, M.; Bedini, E.; Iadonisi, A. A Comprehensive Solvent-Free Approach for the Esterification and Amidation of Carboxylic Acids Mediated by Carbodiimides. Tetrahedron 2023, 133, 133291. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A Review on Properties, Biological Activities and Recent Progress in Biomedical Applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Manigandan, V.; Karthik, R.; Ramachandran, S.; Rajagopal, S. Chitosan Applications in Food Industry. In Biopolymers for Food Design; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 469–491. ISBN 9780128115015. [Google Scholar]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Zhao, L. Antibacterial Activity and Mechanism of Chitosan with Ultra High Molecular Weight. Carbohydr. Polym. 2016, 148, 200–205. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, J. Synthesis and Characterization of Fatty Acid Grafted Chitosan Polymer and Their Nanomicelles for Nonviral Gene Delivery Applications. Bioconjug Chem. 2017, 28, 2772–2783. [Google Scholar] [CrossRef]
- Lee, C.M.; Jang, D.; Kim, J.; Cheong, S.J.; Kim, E.M.; Jeong, M.H.; Kim, S.H.; Kim, D.W.; Lim, S.T.; Sohn, M.H.; et al. Oleyl-Chitosan Nanoparticles Based on a Dual Probe for Optical/MR Imaging in Vivo. Bioconjug Chem. 2011, 22, 186–192. [Google Scholar] [CrossRef]
- Mubeen, I.; Abbas, G.; Shah, S.; Assiri, A.A. Conjugated Linoleic Acid–Carboxymethyl Chitosan Polymeric Micelles to Improve the Solubility and Oral Bioavailability of Paclitaxel. Pharmaceutics 2024, 16, 342. [Google Scholar] [CrossRef]
- Abdulsalam, R.A.; Ijabadeniyi, O.A.; Sabiu, S. Fatty Acid-Modified Chitosan and Nanoencapsulation of Essential Oils: A Snapshot of Applications. Carbohydr. Res. 2024, 542, 109196. [Google Scholar] [CrossRef]
- Saranya, T.S.; Rajan, V.K.; Biswas, R.; Jayakumar, R.; Sathianarayanan, S. Synthesis, Characterisation and Biomedical Applications of Curcumin Conjugated Chitosan Microspheres. Int. J. Biol. Macromol. 2018, 110, 227–233. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Li, D.; Lin, S.; Chen, H.; Wu, W.; Zhang, W. Linolenic Acid Conjugated Chitosan Micelles for Improving the Oral Absorption of Doxorubicin via Fatty Acid Transporter. Carbohydr. Polym. 2023, 300, 120233. [Google Scholar] [CrossRef] [PubMed]
- Le-Vinh, B.; Le, N.M.N.; Nazir, I.; Matuszczak, B.; Bernkop-Schnürch, A. Chitosan Based Micelle with Zeta Potential Changing Property for Effective Mucosal Drug Delivery. Int. J. Biol. Macromol. 2019, 133, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Farion, I.A.; Burdukovskii, V.F.; Kholkhoev, B.C.; Timashev, P.S.; Chailakhyan, R.K. Functionalization of Chitosan with Carboxylic Acids and Derivatives of Them: Synthesis Issues and Prospects of Practical Use: A Review. Express Polym. Lett. 2018, 12, 1081–1105. [Google Scholar] [CrossRef]
- Métro, T.X.; Bantreil, X.; Martinez, J.; Lamaty, F. Solvent-Free Chemistry. In Biphasic Chemistry and the Solvent Case; Wiley: Hoboken, NJ, USA, 2020; pp. 174–220. ISBN 9781119695080. [Google Scholar]
- Calce, E.; Mercurio, F.A.; Leone, M.; Saviano, M.; De Luca, S. Eco-Friendly Microwave-Assisted Protocol to Prepare Hyaluronan-Fatty Acid Conjugates and to Induce Their Self-Assembly Process. Carbohydr. Polym. 2016, 143, 84–89. [Google Scholar] [CrossRef]
- Méndez, P.A.; Vásquez, G.M.; Gartner, C.; López, B.L. Chitosan/OA Nanoparticle as Delivery System for Celecoxib: Parameters Affecting the Particle Size, Encapsulation, and Release. J. Appl. Polym. Sci. 2017, 134, 44472. [Google Scholar] [CrossRef]
- Niemczyk, A.; Goszczyńska, A.; Gołda-Cępa, M.; Kotarba, A.; Sobolewski, P.; El Fray, M. Biofunctional Catheter Coatings Based on Chitosan-Fatty Acids Derivatives. Carbohydr. Polym. 2019, 225, 115263. [Google Scholar] [CrossRef]
- Calce, E.; Ringhieri, P.; Mercurio, F.A.; Leone, M.; Bugatti, V.; Saviano, M.; Vittoria, V.; De Luca, S. A Biocompatible Process to Prepare Hyaluronan-Based Material Able to Self-Assemble into Stable Nano-Particles. RSC Adv. 2015, 5, 29573–29576. [Google Scholar] [CrossRef]
- Suryani, S.; Chaerunisaa, A.Y.; Joni, I.M.; Ruslin, R.; Anton, A.; Sartinah, A.; Ramadhan, L.O.A.N.; Aspadiah, V. The Chemical Modification to Improve Solubility of Chitosan and Its Derivatives Application, Preparation Method, Toxicity as a Nanoparticles. Nanotechnol. Sci. Appl. 2024, 17, 41–57. [Google Scholar] [CrossRef]
- Wang, L.; Pang, Y.; Xin, M.; Li, M.; Shi, L.; Mao, Y. Evaluating the Antibacterial and Antibiofilm Activities of Chitosan Derivatives Containing Six-Membered Heterocyclics against E. Coli and S. Aureus. Colloids Surf. B Biointerfaces 2024, 242, 114084. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-G.; Lee, C.M.; Park, H.-J. O/W Emulsification for the Self-Aggregation and Nanoparticle Formation of Linoleic AcidsModified Chitosan in the Aqueous System. J. Agric. Food Chem. 2003, 51, 3135–3139. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, F.C.; Baddiley, J. A Continuum of Anionic Charge: Structures and Functions of d -Alanyl-Teichoic Acids in Gram-Positive Bacteria. Microbiol. Mol. Biol. Rev. 2003, 67, 686–723. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Otto, M.; Jack, R.W.; Kalbacher, H.; Jung, G.; Götz, F. Inactivation of the Dlt Operon in Staphylococcus Aureus Confers Sensitivity to Defensins, Protegrins, and Other Antimicrobial Peptides. J. Biol. Chem. 1999, 274, 8405–8410. [Google Scholar] [CrossRef]
- Atrih, A.; Zöllner, P.; Allmaierr, G.; Foster, S.J. Structural Analysis of Bacillus Subtilis 168 Endospore Peptidoglycan and Its Role during Differentiation. J. Bacteriol. 1996, 178, 6173–6183. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, Y.; Li, J.; Zhang, Z.; Han, R.J.; Zuo, J.; Bai, Y.; Zhang, J. Application of Chitosan-Based Drug Delivery Systems in the Treatment of Bacterial Diseases: A Review. Drug Deliv. 2025, 32, 2514140. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus Lipopeptides: Versatile Weapons for Plant Disease Biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Hecker, M.; Pané-Farré, J.; Völker, U. SigB-Dependent General Stress Response in Bacillus Subtilis and Related Gram-Positive Bacteria. Annu. Rev. Microbiol. 2007, 61, 215–236. [Google Scholar] [CrossRef]
- Krajewska, B.; Wydro, P.; Jańczyk, A.J. Probing the Modes of Antibacterial Activity of Chitosan. Effects of PH and Molecular Weight on Chitosan Interactions with Membrane Lipids in Langmuir Films. Biomacromolecules 2011, 12, 4144–4152. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, Q.; Cai, X.; Liu, L.; Jiang, Y.; Zhu, X.; Huang, Z.; Wu, K.; Luo, H.; Ouyang, Q. Self-Assembled Amphiphilic Chitosan Nanomicelles: Synthesis, Characterization and Antibacterial Activity. Biomolecules 2023, 13, 1595. [Google Scholar] [CrossRef]

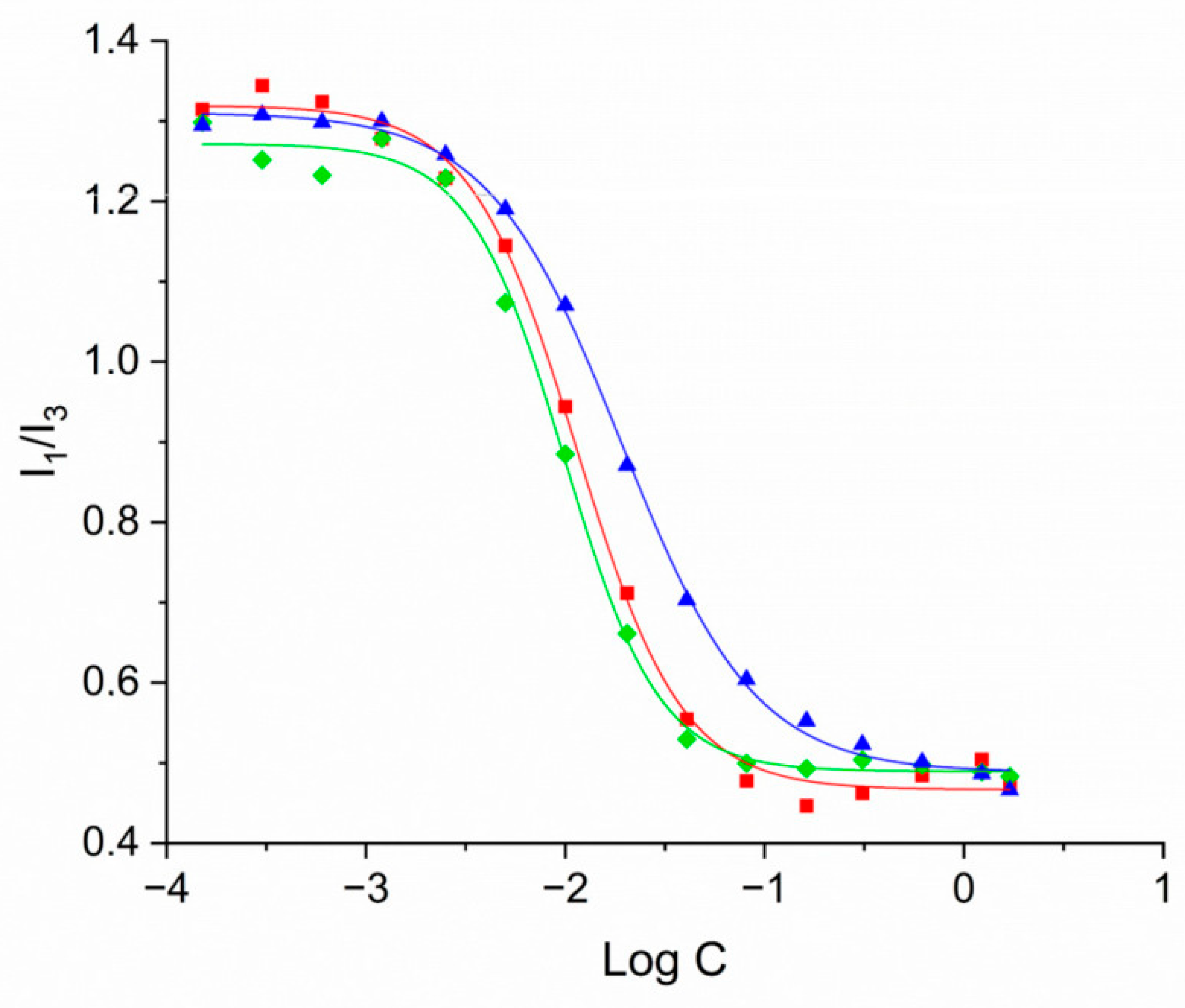
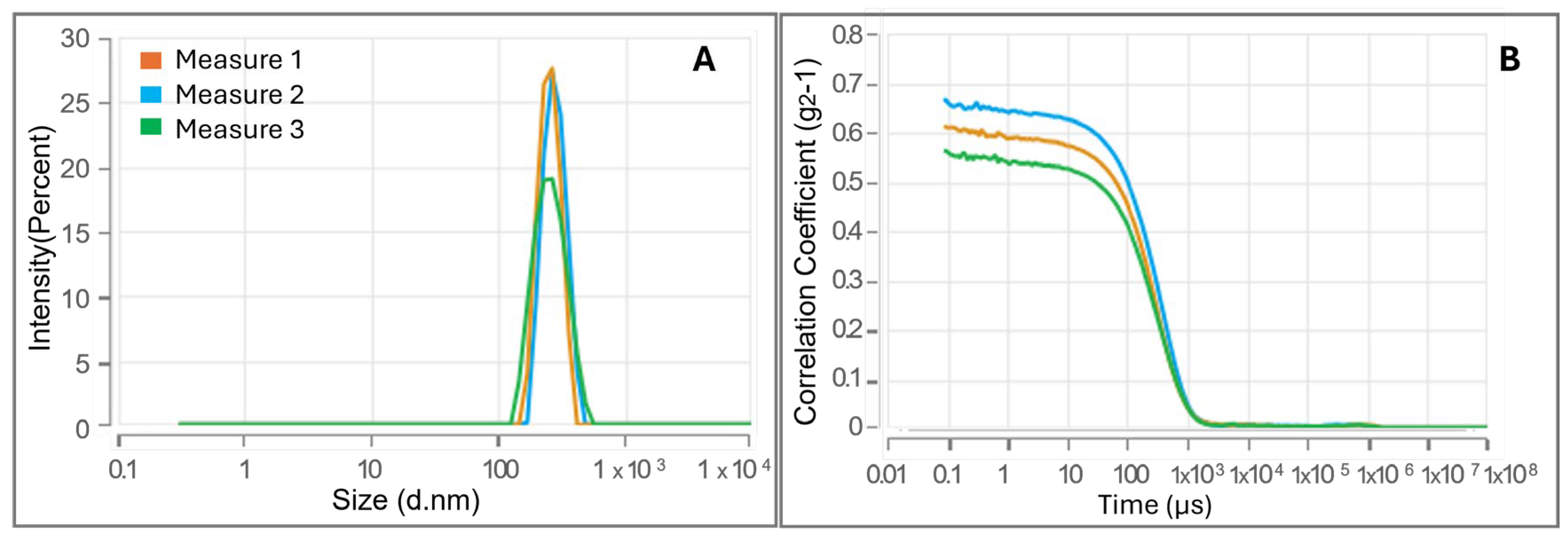
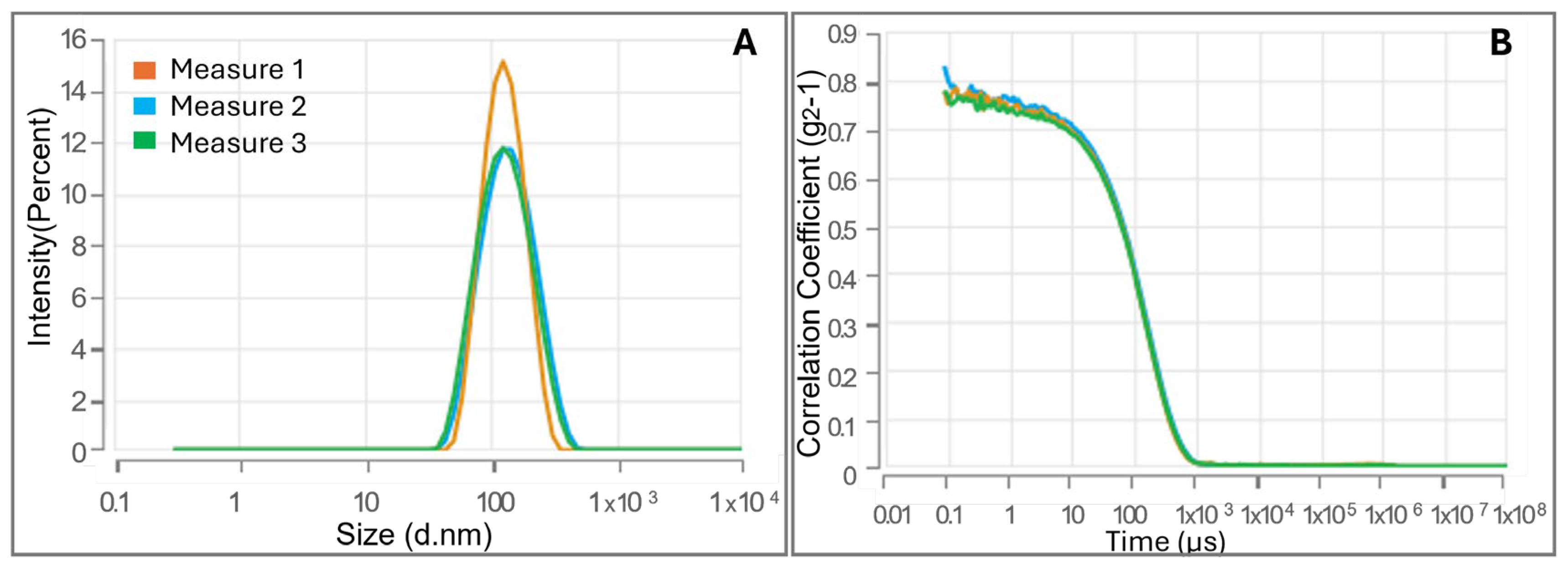
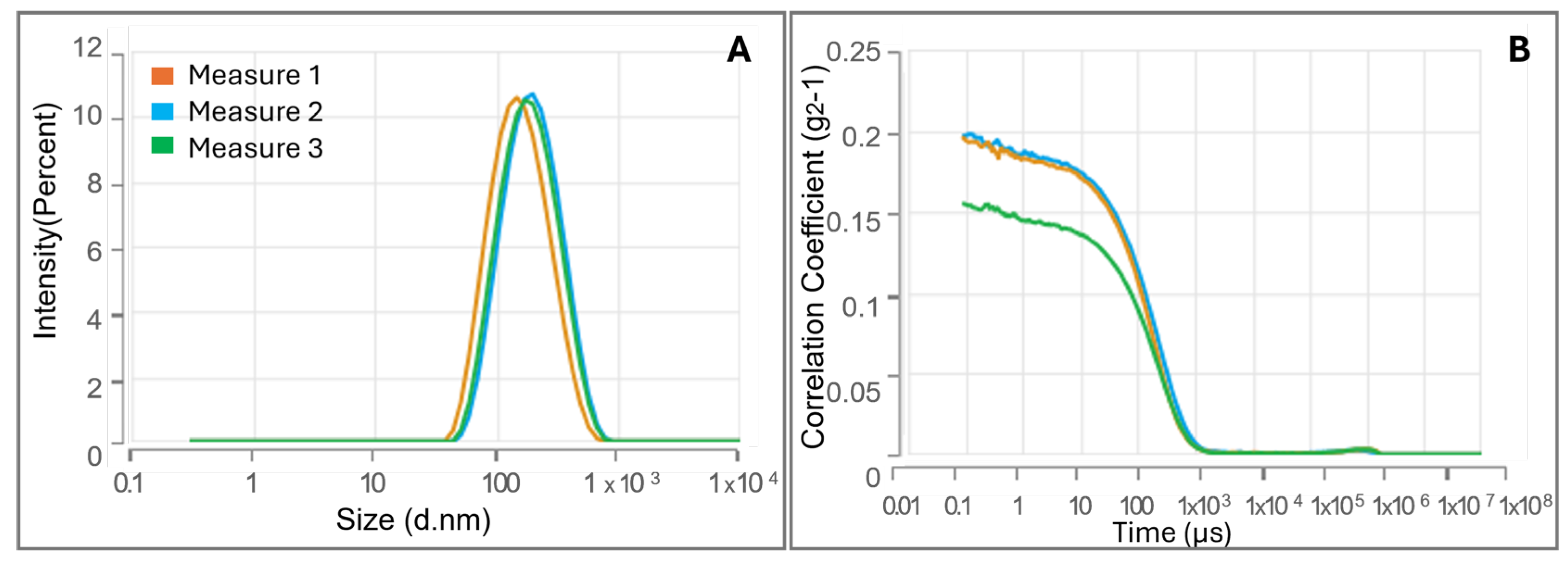

| Mean Diameter (nm) | PDI | Δ (mV) | CMC (LogC) | CMC (mg/mL) | |
|---|---|---|---|---|---|
| CS-PALMITATE | 252.3 ± 20.25 | 0.101 ± 0.055 | 17.53 ± 1.62 | –1.945 ± 0.022 | 0.0114 |
| CS-OLEATE | 109.8 ± 1.972 | 0.239 ± 0.025 | 16.81 ± 1.53 | –2.005 ± 0.020 | 0.0099 |
| CS-LINOLEATE | 136.2 ± 9.415 | 0.319 ± 0.045 | 17.03 ± 1.21 | –1.719 ± 0.016 | 0.0191 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulvirenti, A.; Verdoliva, V.; De Luca, V.; Traboni, S.; Capasso, C.; De Luca, S. Eco-Friendly Synthesis of Chitosan–Fatty Acid Nano Micelles and Their Differential Antibacterial Activity Against Escherichia coli and Bacillus subtilis. J. Funct. Biomater. 2025, 16, 373. https://doi.org/10.3390/jfb16100373
Pulvirenti A, Verdoliva V, De Luca V, Traboni S, Capasso C, De Luca S. Eco-Friendly Synthesis of Chitosan–Fatty Acid Nano Micelles and Their Differential Antibacterial Activity Against Escherichia coli and Bacillus subtilis. Journal of Functional Biomaterials. 2025; 16(10):373. https://doi.org/10.3390/jfb16100373
Chicago/Turabian StylePulvirenti, Alfio, Valentina Verdoliva, Viviana De Luca, Serena Traboni, Clemente Capasso, and Stefania De Luca. 2025. "Eco-Friendly Synthesis of Chitosan–Fatty Acid Nano Micelles and Their Differential Antibacterial Activity Against Escherichia coli and Bacillus subtilis" Journal of Functional Biomaterials 16, no. 10: 373. https://doi.org/10.3390/jfb16100373
APA StylePulvirenti, A., Verdoliva, V., De Luca, V., Traboni, S., Capasso, C., & De Luca, S. (2025). Eco-Friendly Synthesis of Chitosan–Fatty Acid Nano Micelles and Their Differential Antibacterial Activity Against Escherichia coli and Bacillus subtilis. Journal of Functional Biomaterials, 16(10), 373. https://doi.org/10.3390/jfb16100373










