Porous Hydrogels Prepared by Two-Step Gelation Method for Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
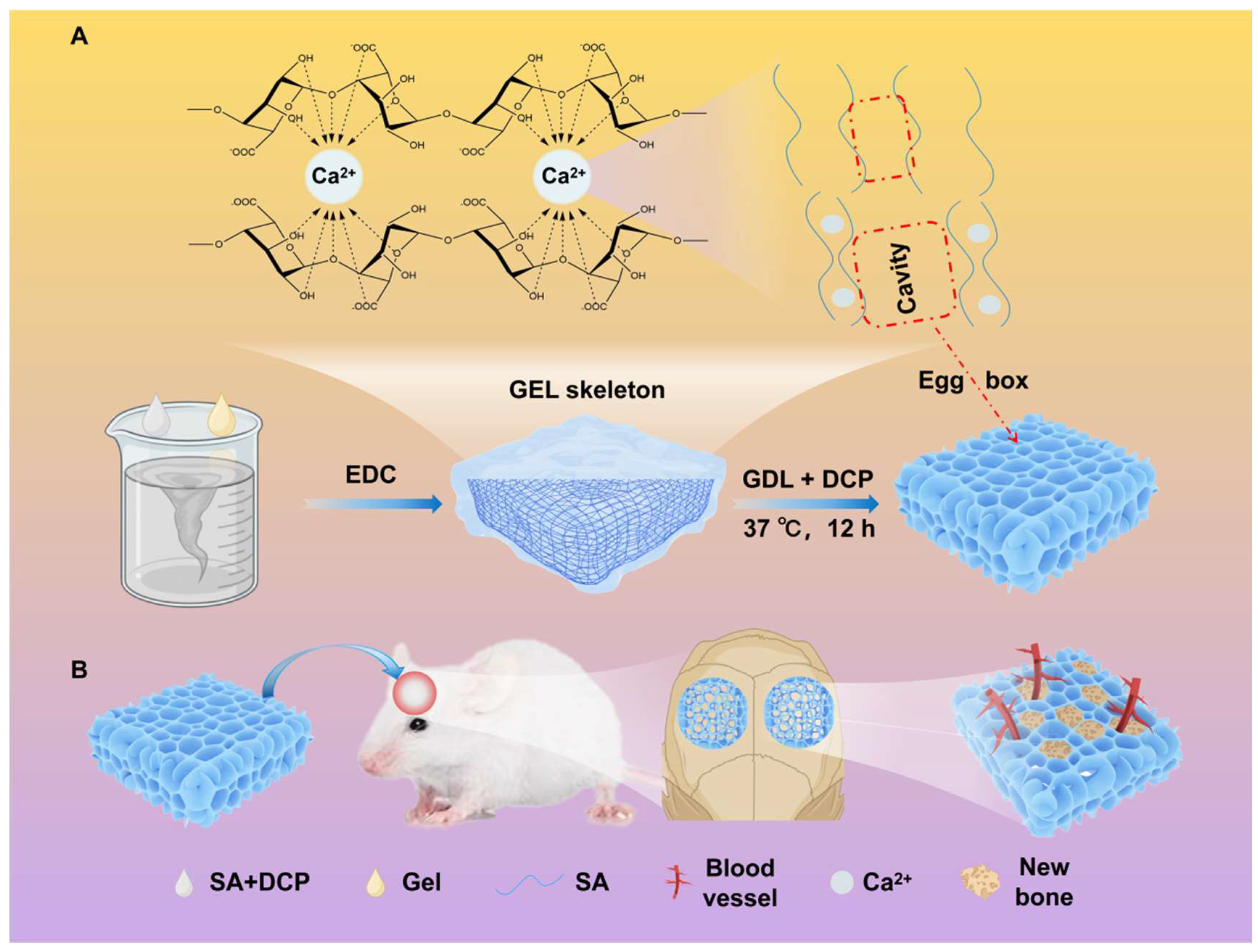
2.2. Preparation of Porous Hydrogel
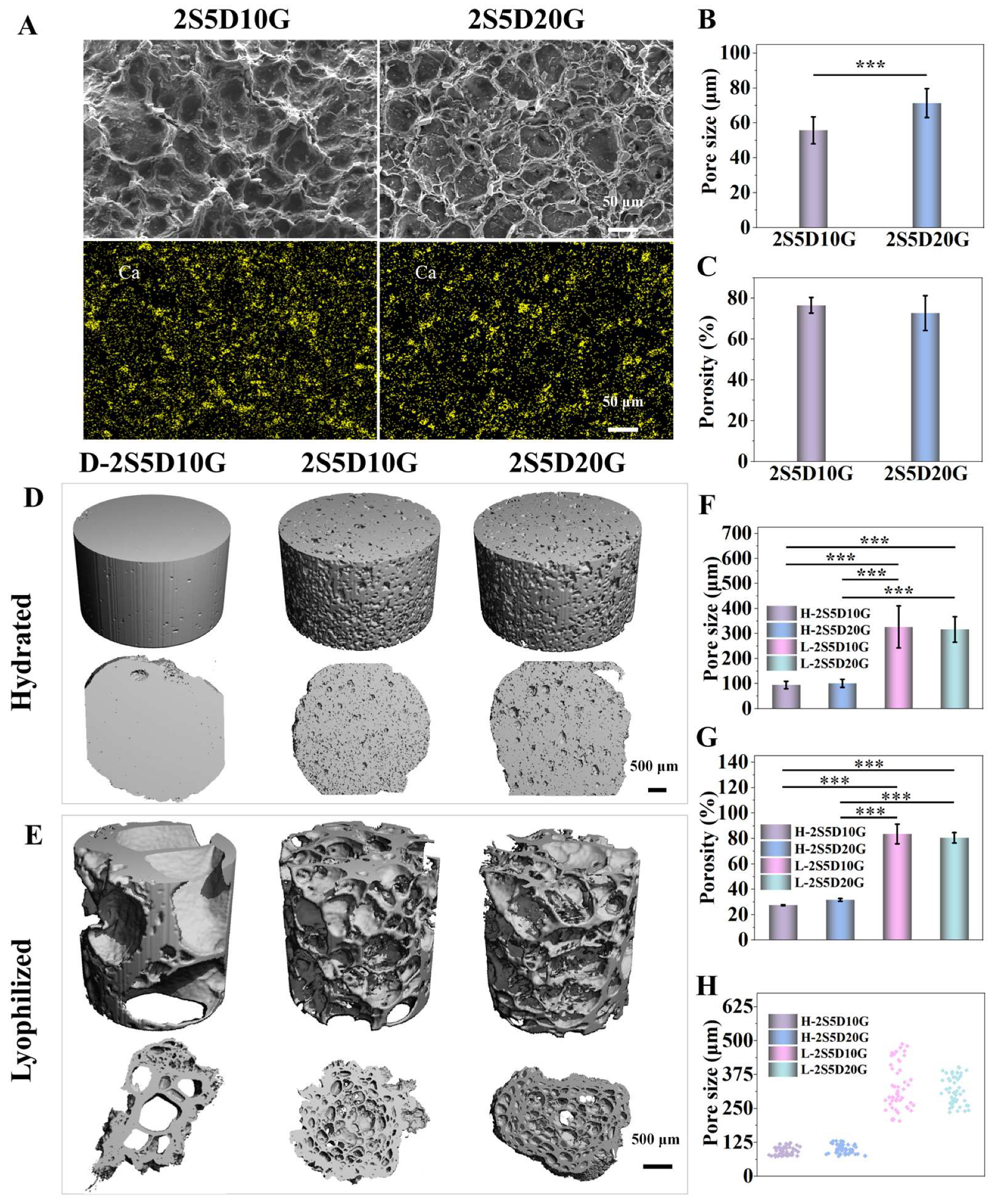
2.3. Morphological Characterization of the Hydrogels
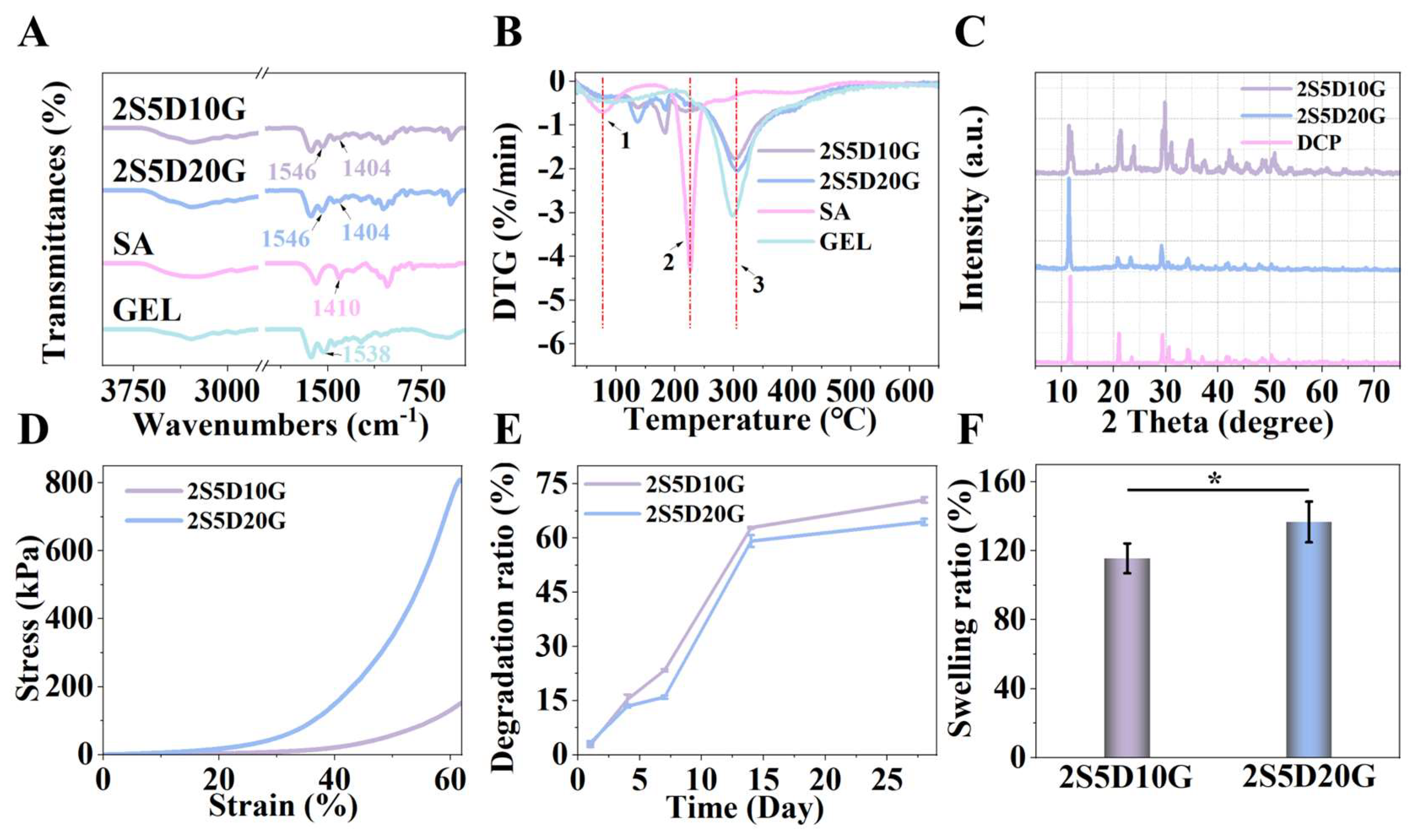
2.4. Compositional Characterization of the Hydrogel
2.5. Swelling Test
2.6. Mechanical Measurement
2.7. Degradation Test
2.8. Effect of the Scaffolds on BMSC Behaviors
2.8.1. BMSC Culture
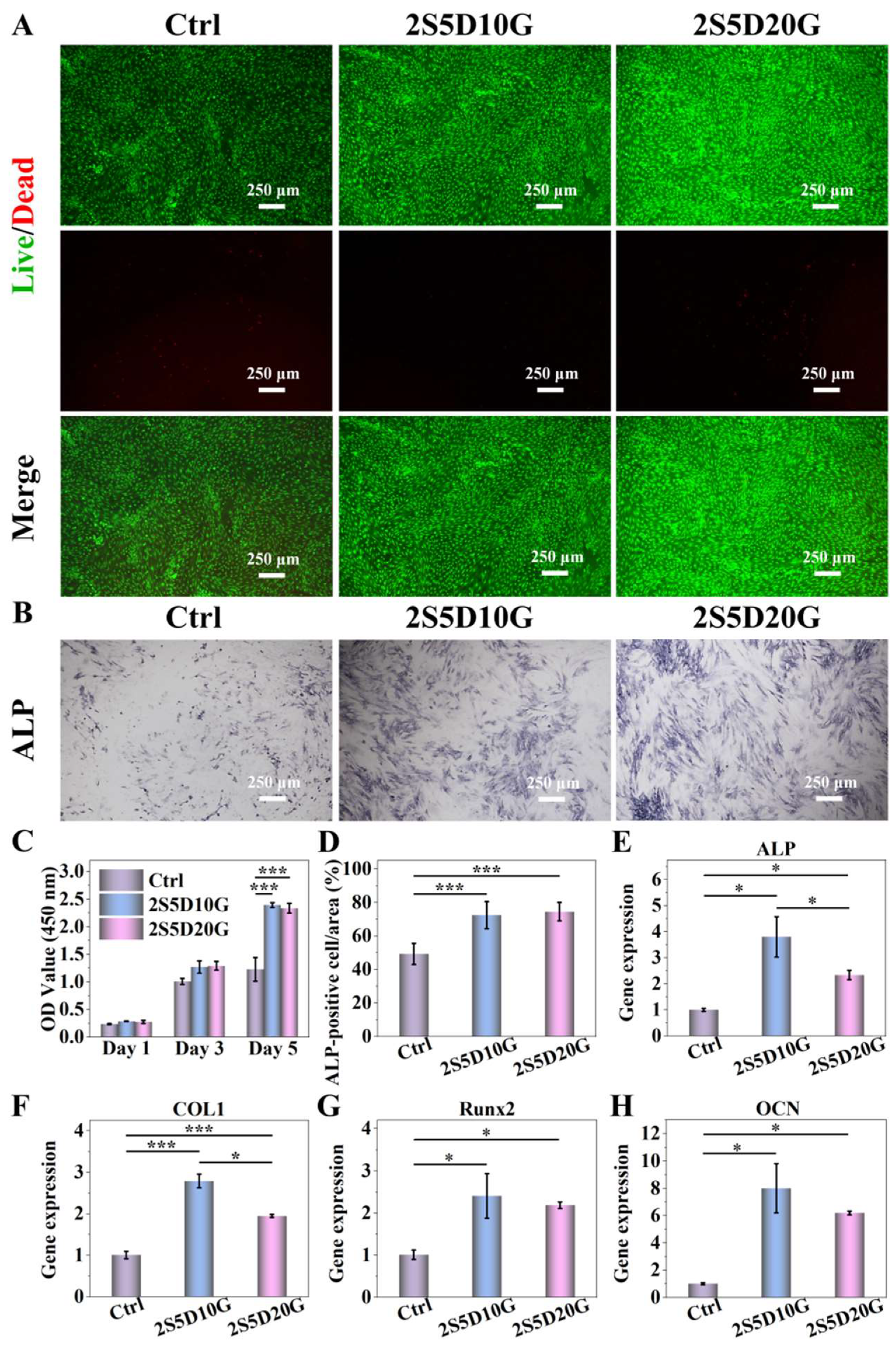
2.8.2. Cell Proliferation
2.8.3. Effect of Cell Behavior on Hydrogels
2.8.4. Osteogenic Differentiation
2.8.5. Gene Expression
2.9. Subcutaneous Implantation of Hydrogels in Rats
2.10. Osteogenesis of Hydrogels
2.10.1. Surgical Procedure
2.10.2. Micro-CT Analysis
2.10.3. Histological Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Morphology and Three-Dimensional Structure of Porous Hydrogels
3.2. Physicochemical Properties of Porous Hydrogels
3.3. Cytocompatibility of Porous Hydrogels
3.4. The Degradation of Porous Hydrogels in the Cellular Environment
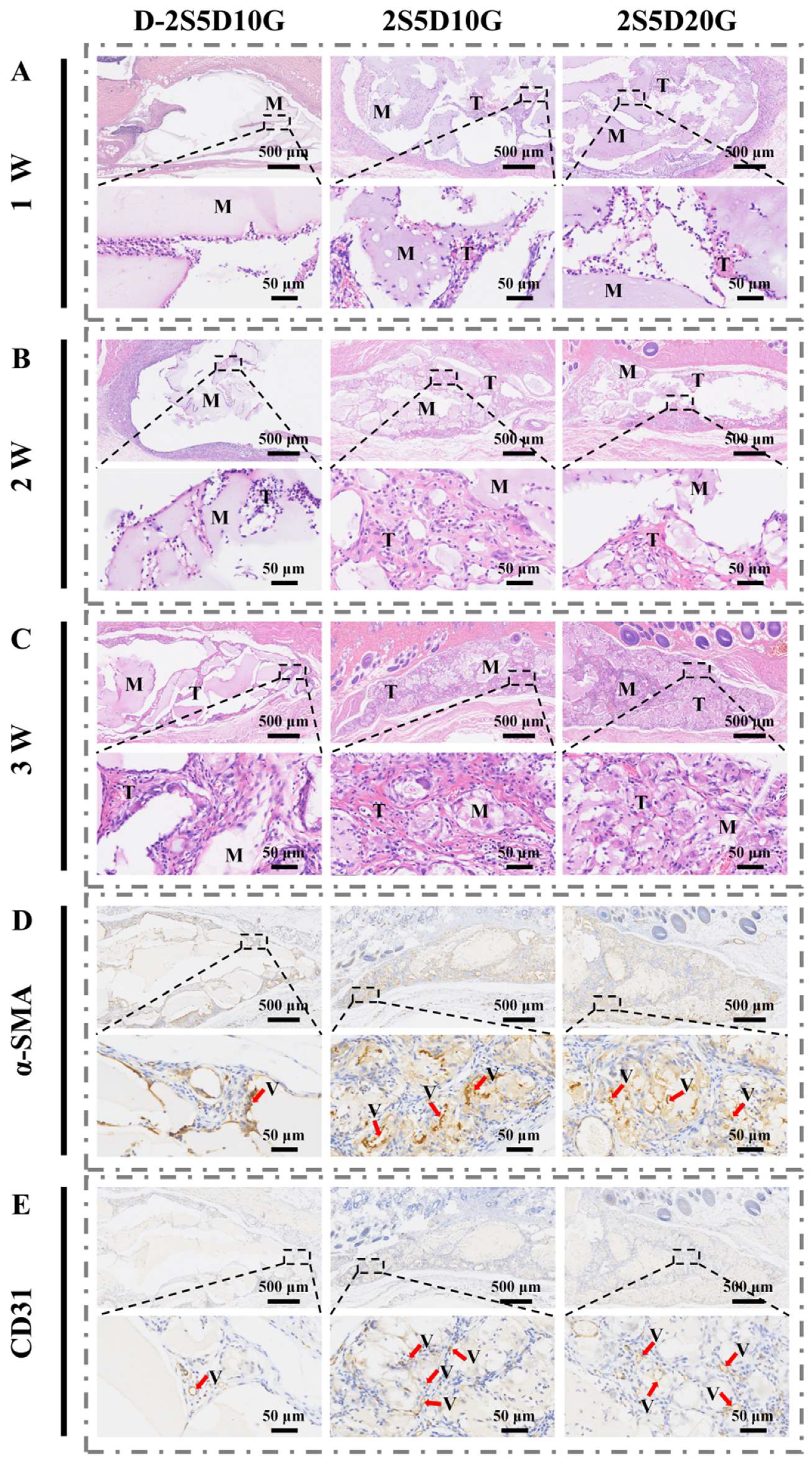
3.5. Subcutaneous Implantation of Porous Hydrogels in Rats
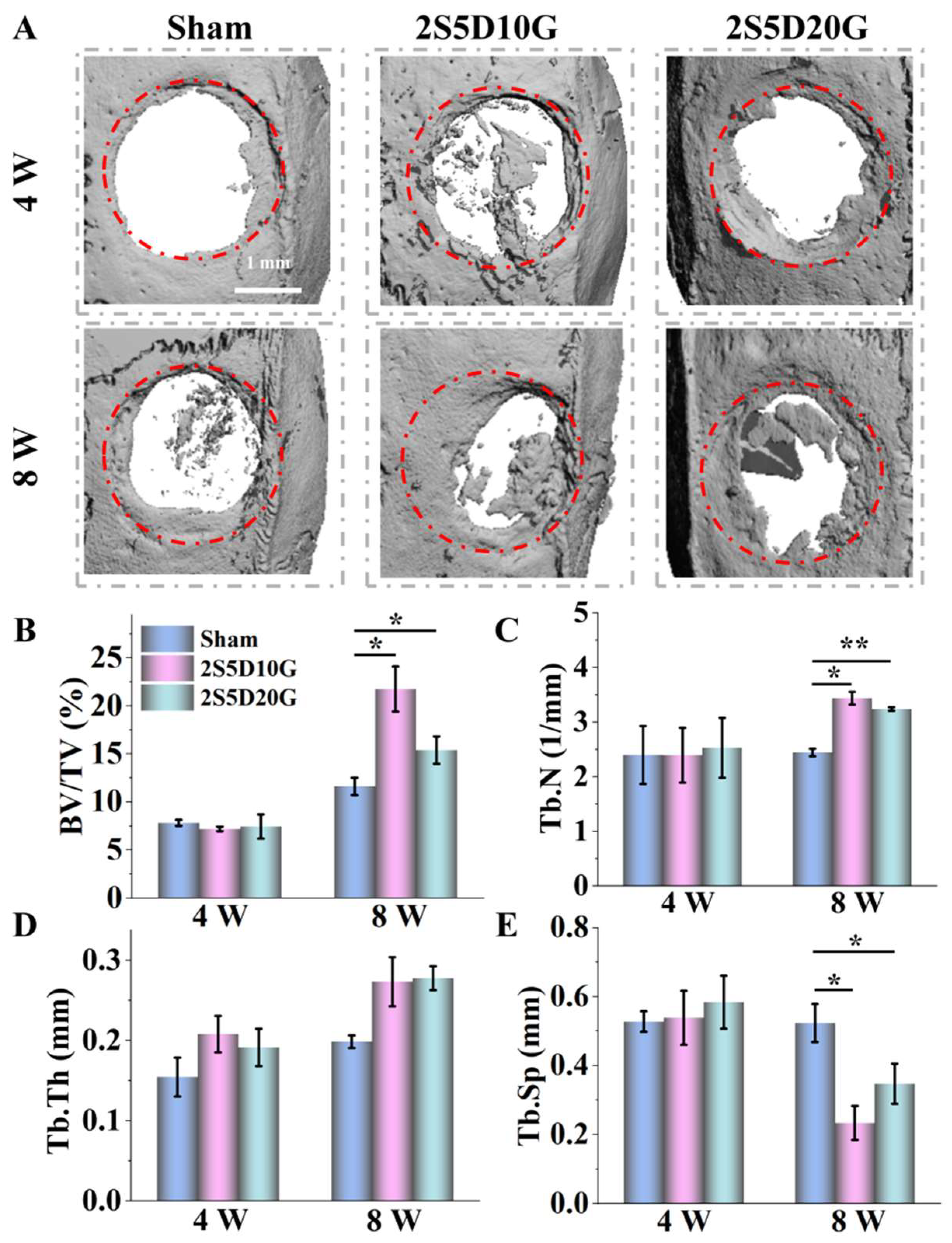
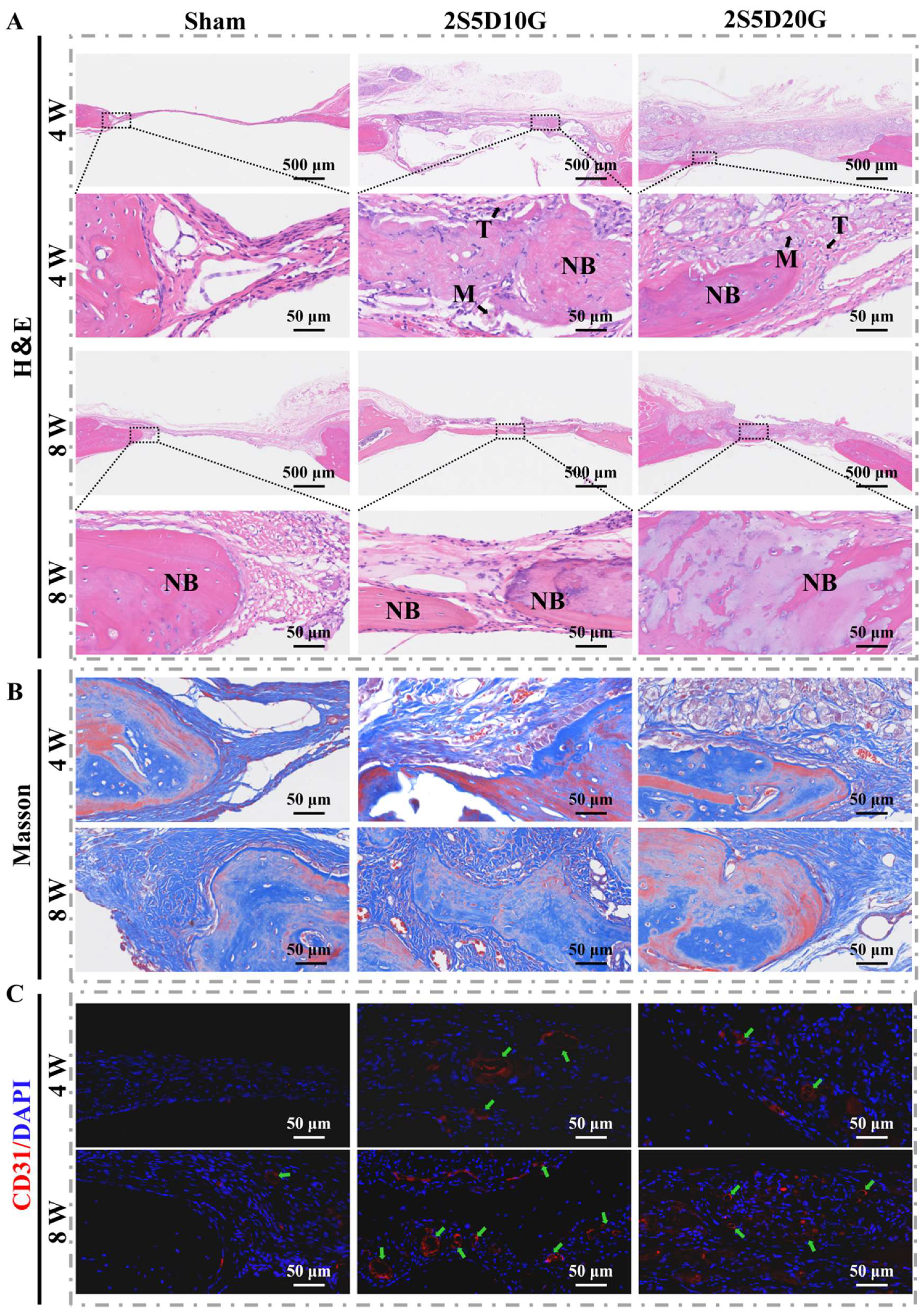
3.6. Repair of Rat Cranial Defects with Porous Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Y.; de Vasconcelos, L.S.; Manohar, N.; Geng, J.; Johnston, K.P.; Yu, G. Highly Elastic Interconnected Porous Hydrogels through Self-Assembled Templating for Solar Water Purification. Angew. Chem. Int. Ed. 2022, 61, e202114074. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, D.; Liu, B.; Nian, G.; Li, X.; Yin, J.; Qu, S.; Yang, W. 3D Printing of Multifunctional Hydrogels. Adv. Funct. Mater. 2019, 29, 1900971. [Google Scholar] [CrossRef]
- Liu, G.W.; Pickett, M.J.; Kuosmanen, J.L.P.; Ishida, K.; Madani, W.A.M.; White, G.N.; Jenkins, J.; Park, S.; Feig, V.R.; Jimenez, M.; et al. Drinkable in situ-forming tough hydrogels for gastrointestinal therapeutics. Nat. Mater. 2024, 23, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, C.S.; Mooney, D.J. Injectable, Pore-Forming Hydrogels for In Vivo Enrichment of Immature Dendritic Cells. Adv. Healthc. Mater. 2015, 4, 2677–2687. [Google Scholar] [CrossRef]
- Li, G.; Zhou, D.; Sheng, S.; Lin, Q.; Jing, Y.; Ren, X.; Su, J. Hydrogel for bone microenvironment: Strategy and application. Chem. Eng. J. 2024, 499, 156554. [Google Scholar] [CrossRef]
- Taheri, S.; Bao, G.; He, Z.; Mohammadi, S.; Ravanbakhsh, H.; Lessard, L.; Li, J.; Mongeau, L. Injectable, Pore-Forming, Perfusable Double-Network Hydrogels Resilient to Extreme Biomechanical Stimulations. Adv. Sci. 2022, 9, 2102627. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Li, M.; Nie, L.; Wei, Q.; Okoro, O.V.; Jafari, H.; Wang, S.; Deng, J.; Chen, J.; et al. Bioactive wound dressing based on decellularized tendon and GelMA with incorporation of PDA-loaded asiaticoside nanoparticles for scarless wound healing. Chem. Eng. J. 2023, 466, 143016. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, W.; Wu, J.; Liu, L.; Yue, L.; Zhang, X.; Li, J.; She, P.; Yang, J.; Sun, C.; et al. 3D-Printed Dendritic Cell Vaccines for Post-Surgery Cancer Immunotherapy. Adv. Funct. Mater. 2024, 34, 2400507. [Google Scholar] [CrossRef]
- Najberg, M.; Haji Mansor, M.; Taillé, T.; Bouré, C.; Molina-Peña, R.; Boury, F.; Cenis, J.L.; Garcion, E.; Alvarez-Lorenzo, C. Aerogel sponges of silk fibroin, hyaluronic acid and heparin for soft tissue engineering: Composition-properties relationship. Carbohydr. Polym. 2020, 237, 116107. [Google Scholar] [CrossRef]
- Li, Z.; Xing, X.; Zhao, C.; Wu, Q.; Liu, J.; Qiu, X.; Wang, L. A rapid interactive chitosan-based medium with antioxidant and pro-vascularization properties for infected burn wound healing. Carbohydr. Polym. 2024, 333, 121991. [Google Scholar] [CrossRef]
- Walthers, C.M.; Nazemi, A.K.; Patel, S.L.; Wu, B.M.; Dunn, J.C.Y. The effect of scaffold macroporosity on angiogenesis and cell survival in tissue-engineered smooth muscle. Biomaterials 2014, 35, 5129–5137. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jin, M.; Wang, D.-A. In vitro expansion of hematopoietic stem cells in a porous hydrogel-based 3D culture system. Acta Biomater. 2023, 161, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.H.G.; Lemos, A.F.; Agathopoulos, S.; Kannan, S.; Valério, P.; Ferreira, J.M.F. Hydrothermal growth of hydroxyapatite scaffolds from aragonitic cuttlefish bones. J. Biomed. Mater. Res. Part A 2006, 77A, 160–168. [Google Scholar] [CrossRef]
- Zeng, L.; Yao, Y.; Wang, D.-A.; Chen, X. Effect of microcavitary alginate hydrogel with different pore sizes on chondrocyte culture for cartilage tissue engineering. Mater. Sci. Eng. C 2014, 34, 168–175. [Google Scholar] [CrossRef]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Wagoner Johnson, A.J. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, S.; Yin, S.; Jiang, F.; Zhou, M.; Yang, G.; Sun, N.; Zhang, W.; Jiang, X. In situ gas foaming based on magnesium particle degradation: A novel approach to fabricate injectable macroporous hydrogels. Biomaterials 2020, 232, 119727. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, M.; Zhai, D.; Qin, C.; Wang, Y.; Ma, J.; Zhuang, H.; Shi, Z.; Wang, L.; Wu, C. Polyhedron-like Biomaterials for Innervated and Vascularized Bone Regeneration. Adv. Mater. 2023, 35, 2302716. [Google Scholar] [CrossRef]
- Marenzana, M.; Arnett, T.R. The Key Role of the Blood Supply to Bone. Bone Res. 2013, 1, 203–215. [Google Scholar] [CrossRef]
- Jiang, S.; Shang, L.; Liang, H.; Li, B.; Li, J. Preparation of konjac glucomannan/xanthan gum/sodium alginate composite gel by freezing combining moisture regulation. Food Hydrocoll. 2022, 127, 107499. [Google Scholar] [CrossRef]
- Ying, G.-L.; Jiang, N.; Maharjan, S.; Yin, Y.-X.; Chai, R.-R.; Cao, X.; Yang, J.-Z.; Miri, A.K.; Hassan, S.; Zhang, Y.S. Aqueous Two-Phase Emulsion Bioink-Enabled 3D Bioprinting of Porous Hydrogels. Adv. Mater. 2018, 30, 1805460. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. A green approach for alginate extraction from Sargassum muticum brown seaweed using ultrasound-assisted technique. Int. J. Biol. Macromol. 2019, 124, 451–459. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Chen, K.; Cai, L.; Huang, S.; Zhang, Y. Super gastro-resistant microcapsules based on CaCO3 nanocrystal buffered alginate/pectin composites for colon-targeted probiotic delivery: In vitro and in vivo evaluation. Adv. Compos. Hybrid Mater. 2024, 7, 207. [Google Scholar] [CrossRef]
- Porter, G.C.; Schwass, D.R.; Tompkins, G.R.; Bobbala, S.K.R.; Medlicott, N.J.; Meledandri, C.J. AgNP/Alginate Nanocomposite hydrogel for antimicrobial and antibiofilm applications. Carbohydr. Polym. 2021, 251, 117017. [Google Scholar] [CrossRef]
- Guo, Y.; Han, Y.; Cao, Y.; Chen, Y.; Xie, J.; Ding, H.; Liang, S.; Liu, X.; Sun, W.; Tang, J.; et al. Facile Fabrication of Tough Super Macroporous Hydrogel via Enhanced Phase Separation. Adv. Funct. Mater. 2025, 35, 2412412. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, L.; Ye, D.; Dong, Y.; Zhan, Y.; Jiang, X. Facile preparation of sodium alginate/poly(vinyl alcohol)/graphite hybrid porous hydrogel for efficient solar desalination. Chem. Eng. J. 2024, 480, 148226. [Google Scholar] [CrossRef]
- Donati, I.; Christensen, B.E. Alginate-metal cation interactions: Macromolecular approach. Carbohydr. Polym. 2023, 321, 121280. [Google Scholar] [CrossRef]
- Skjåk-Bræk, G.; Grasdalen, H.; Smidsrød, O. Inhomogeneous polysaccharide ionic gels. Carbohydr. Polym. 1989, 10, 31–54. [Google Scholar] [CrossRef]
- Kroneková, Z.; Pelach, M.; Mazancová, P.; Uhelská, L.; Treľová, D.; Rázga, F.; Némethová, V.; Szalai, S.; Chorvát, D.; McGarrigle, J.J.; et al. Structural changes in alginate-based microspheres exposed to in vivo environment as revealed by confocal Raman microscopy. Sci. Rep. 2018, 8, 1637. [Google Scholar] [CrossRef]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yuan, K.; Zhang, H.; Luo, S.; Wang, S.; Yang, X.; Guo, Y. Regulation on gel textures of Nicandra physalodes (Linn.) Gaertn. pectin by its synergistic interaction with sodium alginate. Food Hydrocoll. 2023, 134, 108047. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Wang, W.; Xiang, Y.; Liu, J.; An, Y.; Shi, J.; Qi, H.; Huang, Z. Sodium alginate/sodium lignosulfonate hydrogel based on inert Ca2+ activation for water conservation and growth promotion. Environ. Res. 2024, 246, 118144. [Google Scholar] [CrossRef]
- Huang, K.; Wu, J.; Gu, Z. Black Phosphorus Hydrogel Scaffolds Enhance Bone Regeneration via a Sustained Supply of Calcium-Free Phosphorus. ACS Appl. Mater. Interfaces 2019, 11, 2908–2916. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, J.; Liu, W.; Huang, X.; Wu, X.; Wei, W.; Zhu, H.; Zhang, J.; Xiao, J.; Dai, H. Bioadaptable bioactive glass-β-tricalcium phosphate scaffolds with TPMS-gyroid structure by stereolithography for bone regeneration. J. Mater. Sci. Technol. 2023, 155, 54–65. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef]
- Julien, M.; Khoshniat, S.; Lacreusette, A.; Gatius, M.; Bozec, A.; Wagner, E.F.; Wittrant, Y.; Masson, M.; Weiss, P.; Beck, L.; et al. Phosphate-Dependent Regulation of MGP in Osteoblasts: Role of ERK1/2 and Fra-11. J. Bone Miner. Res. 2009, 24, 1856–1868. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, T.; Qu, X.; Liu, Z.; Guo, Y.; Li, G.; Zhang, Z.; Lian, J.; Ren, L. Preparation of anticorrosion, biocompatible and antibacterial dicalcium phosphate dihydrate/polycaprolactone-titania composite coating on Mg alloy. Prog. Org. Coat. 2022, 172, 107133. [Google Scholar] [CrossRef]
- Bej, R.; Stevens, C.A.; Nie, C.; Ludwig, K.; Degen, G.D.; Kerkhoff, Y.; Pigaleva, M.; Adler, J.M.; Bustos, N.A.; Page, T.M.; et al. Mucus-Inspired Self-Healing Hydrogels: A Protective Barrier for Cells against Viral Infection. Adv. Mater. 2024, 36, 2401745. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Ma, Z.; Chen, H.; Chen, F.; Wang, K.; Zhang, C.; Li, H. Fabrication of novel Hydrogel/Epoxy resin composites with Soft-Hard combined Bi-Continuous aqueous load-bearing electrolytes and application in structural energy storage devices. Chem. Eng. J. 2024, 486, 150295. [Google Scholar] [CrossRef]
- Thilagashanthi, T.; Gunasekaran, K.; Satyanarayanan, K.S. Microstructural pore analysis using SEM and ImageJ on the absorption of treated coconut shell aggregate. J. Clean. Prod. 2021, 324, 129217. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Seifi, S.; Shamloo, A.; Barzoki, A.K.; Bakhtiari, M.A.; Zare, S.; Cheraghi, F.; Peyrovan, A. Engineering biomimetic scaffolds for bone regeneration: Chitosan/alginate/polyvinyl alcohol-based double-network hydrogels with carbon nanomaterials. Carbohydr. Polym. 2024, 339, 122232. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.N.; Lopez Silva, T.L.; Carrejo, N.C.; Origel Marmolejo, C.A.; Li, I.C.; Hartgerink, J.D. Nanofibrous peptide hydrogel elicits angiogenesis and neurogenesis without drugs, proteins, or cells. Biomaterials 2018, 161, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Mehrotra, S.; van Bochove, B.; Teotia, A.K.; Singh, P.; Laurén, I.; Lindfors, N.C.; Seppälä, J.; Kumar, A. Strontium-Substituted Nanohydroxyapatite Containing Biodegradable 3D Printed Composite Scaffolds for Bone Regeneration. ACS Appl. Mater. Interfaces 2024, 16, 65378–65393. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Wang, Y.; Xu, J.; Huang, T.; Luo, X. Construction of biomimetic cell-sheet-engineered periosteum with a double cell sheet to repair calvarial defects of rats. J. Orthop. Transl. 2023, 38, 1–11. [Google Scholar] [CrossRef]
- Thu, B.; Bruheim, P.; Espevik, T.; Smidsrød, O.; Soon-Shiong, P.; Skjåk-Bræk, G. Alginate polycation microcapsules: II. Some functional properties. Biomaterials 1996, 17, 1069–1079. [Google Scholar] [CrossRef]
- Tang, S.; Yang, J.; Lin, L.; Peng, K.; Chen, Y.; Jin, S.; Yao, W. Construction of physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem. Eng. J. 2020, 393, 124728. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Sztuka, K.; Wolska, J.; Wojtasz-Pająk, A.; Kołodziejska, I. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC films: FT-IR study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 707–712. [Google Scholar] [CrossRef]
- Gao, K.; Yang, Y.; Yan, Q.; Li, P.; Zhang, Y.; Wang, G. Preparation and study of a sodium alginate film for preventing spontaneous combustion of water-soaked coal in goaf. Energy 2024, 289, 130082. [Google Scholar] [CrossRef]
- Du, W.; Yang, Y.; Liu, J.; Zhu, Y.; Shen, T.; Chen, Q.; Miyazaki, T. In Situ Synthesis and Characterizations of a Strontium-Substituted Dicalcium Phosphate Anhydrous/Hydroxyapatite Biphasic Whisker and Its Properties Evaluation. ACS Biomater. Sci. Eng. 2024, 10, 6874–6886. [Google Scholar] [CrossRef]
- Tamimi, F.; Torres, J.; Bassett, D.; Barralet, J.; Cabarcos, E.L. Resorption of monetite granules in alveolar bone defects in human patients. Biomaterials 2010, 31, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, L.; Gbureck, U.; Bhaduri, S.B.; Sikder, P. Monetite, an important calcium phosphate compound–Its synthesis, properties and applications in orthopedics. Acta Biomater. 2021, 127, 41–55. [Google Scholar] [CrossRef]
- Septiadi, D.; Crippa, F.; Moore, T.L.; Rothen-Rutishauser, B.; Petri-Fink, A. Nanoparticle–Cell Interaction: A Cell Mechanics Perspective. Adv. Mater. 2018, 30, 1704463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Ma, Z.; He, D.; Li, H. Modulating degradation of sodium alginate/bioglass hydrogel for improving tissue infiltration and promoting wound healing. Bioact. Mater. 2021, 6, 3692–3704. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, N.; Huang, J.; Pei, Y.; Wang, F.; Tang, K. A facile fabrication of core–shell sodium alginate/gelatin beads for drug delivery systems. Polym. Bull. 2019, 76, 87–102. [Google Scholar] [CrossRef]
- Williams, D.F. Biocompatibility pathways and mechanisms for bioactive materials: The bioactivity zone. Bioact. Mater. 2022, 10, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Zivari-Ghader, T.; Shokouhi, B.; Kosari-Nasab, M.; Davaran, S.; Hamishehkar, H.; Farahpour, M.R.; Rashidi, M.-R.; Mehrali, M. Hypericum Perforatum Callus Extract-Loaded Composite Hydrogel with Diverse Bioactivities for Enhanced Wound Healing and Fibrosis Prevention. Small 2024, 20, 2407112. [Google Scholar] [CrossRef]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef]
- Hoemann, C.D.; El-Gabalawy, H.; McKee, M.D. In vitro osteogenesis assays: Influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol. Biol. 2009, 57, 318–323. [Google Scholar] [CrossRef]
- Zauchner, D.; Müller, M.Z.; Horrer, M.; Bissig, L.; Zhao, F.; Fisch, P.; Lee, S.S.; Zenobi-Wong, M.; Müller, R.; Qin, X.-H. Synthetic biodegradable microporous hydrogels for in vitro 3D culture of functional human bone cell networks. Nat. Commun. 2024, 15, 5027. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qiang, T.; Chen, X.; Ren, W.; Zhang, H.J. Fabrication and evaluation of biodegradable multi-cross-linked mulch film based on waste gelatin. Chem. Eng. J. 2021, 419, 129639. [Google Scholar] [CrossRef]
- Dai, W.; Li, S.; Jia, H.; Zhao, X.; Liu, C.; Zhou, C.; Xiao, Y.; Guo, L.; Fan, Y.; Zhang, X. Indirect 3D printing CDHA scaffolds with hierarchical porous structure to promote osteoinductivity and bone regeneration. J. Mater. Sci. Technol. 2025, 207, 295–307. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Gao, X.; Wang, H.; Luan, S.; Zhou, G. Low-Temperature Printed Hierarchically Porous Induced-Biomineralization Polyaryletherketone Scaffold for Bone Tissue Engineering. Adv. Healthc. Mater. 2022, 11, 2200977. [Google Scholar] [CrossRef]
- Wu, J.; Lei, J.H.; Li, M.; Zhang, A.; Li, Y.; Liang, X.; de Souza, S.C.; Yuan, Z.; Wang, C.; Chen, G.; et al. Carbon Dots Crosslinked Egg White Hydrogel for Tissue Engineering. Adv. Sci. 2024, 11, 2404702. [Google Scholar] [CrossRef]
- Jaberi, A.; Kedzierski, A.; Kheirabadi, S.; Tagay, Y.; Ataie, Z.; Zavari, S.; Naghashnejad, M.; Waldron, O.; Adhikari, D.; Lester, G.; et al. Engineering Microgel Packing to Tailor the Physical and Biological Properties of Gelatin Methacryloyl Granular Hydrogel Scaffolds. Adv. Healthc. Mater. 2024, 13, 2402489. [Google Scholar] [CrossRef]
- Fonseca-Silva, T.; Santos, C.C.O.; Alves, L.R.; Dias, L.C.; Brito-Júnior, M.; De Paula, A.M.B.; Guimarães, A.L.S. Detection and quantification of mast cell, vascular endothelial growth factor, and microvessel density in human inflammatory periapical cysts and granulomas. Int. Endod. J. 2012, 45, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.F.; Palgrave, R.G.; Seifalian, A.M.; Butler, P.E.; Kalaskar, D.M. Enhancing tissue integration and angiogenesis of a novel nanocomposite polymer using plasma surface polymerisation, an in vitro and in vivo study. Biomater. Sci. 2016, 4, 145–158. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, Q.; Luo, H.; Han, X.; Feng, Q.; Cao, X. Nano-vibration exciter: Hypoxia-inducible factor 1 signaling pathway-mediated extracellular vesicles as bioactive glass substitutes for bone regeneration. Bioact. Mater. 2024, 40, 460–473. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, J.; Jing, W. Motivating role of type H vessels in bone regeneration. Cell Prolif. 2020, 53, e12874. [Google Scholar] [CrossRef] [PubMed]

| Content | SA (% w/v) | DCP (% w/v) | GEL (% w/v) | GDL (% w/v) | EDC (% w/v) | |
|---|---|---|---|---|---|---|
| Name | ||||||
| 2S5D10G | 2 | 5 | 10 | 1 | 1 | |
| 2S5D20G | 2 | 5 | 20 | 1 | 1 | |
| 2S10D10G | 2 | 10 | 10 | 1 | 1 | |
| 2S10D20G | 2 | 10 | 20 | 1 | 1 | |
| D-2S5D10G | 2 | 5 | 10 | 0 | 0 | |
| 2S5D | 2 | 5 | 0 | 1 | 0 | |
| 2S5D10G-0GDL | 2 | 5 | 10 | 0 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, J.; Wei, J.; Yuan, L.; Hu, J.; Dai, S.; Li, Y.; Li, J. Porous Hydrogels Prepared by Two-Step Gelation Method for Bone Regeneration. J. Funct. Biomater. 2025, 16, 100. https://doi.org/10.3390/jfb16030100
Li Y, Liu J, Wei J, Yuan L, Hu J, Dai S, Li Y, Li J. Porous Hydrogels Prepared by Two-Step Gelation Method for Bone Regeneration. Journal of Functional Biomaterials. 2025; 16(3):100. https://doi.org/10.3390/jfb16030100
Chicago/Turabian StyleLi, Yongzhi, Jiangshan Liu, Jiawei Wei, Li Yuan, Jiaxin Hu, Siluo Dai, Yubao Li, and Jidong Li. 2025. "Porous Hydrogels Prepared by Two-Step Gelation Method for Bone Regeneration" Journal of Functional Biomaterials 16, no. 3: 100. https://doi.org/10.3390/jfb16030100
APA StyleLi, Y., Liu, J., Wei, J., Yuan, L., Hu, J., Dai, S., Li, Y., & Li, J. (2025). Porous Hydrogels Prepared by Two-Step Gelation Method for Bone Regeneration. Journal of Functional Biomaterials, 16(3), 100. https://doi.org/10.3390/jfb16030100







