Three-Dimensional Bioprinting for Intervertebral Disc Regeneration
Abstract
:1. Introduction
2. Three-Dimensional Bioprinting
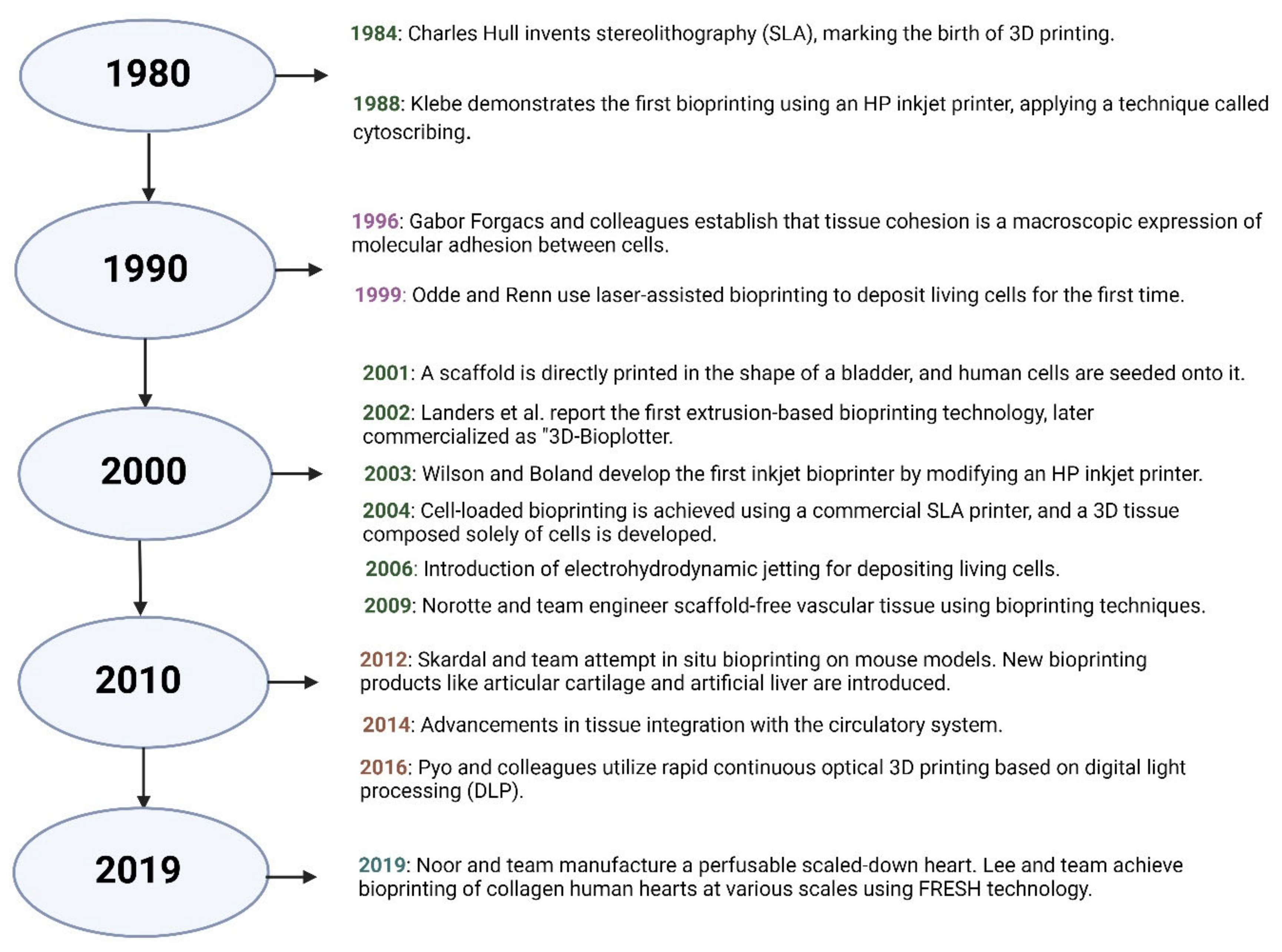
2.1. Three-Dimensional Bioprinting for Tissue Engineering Purposes
2.2. Strategies in 3D Bioprinting
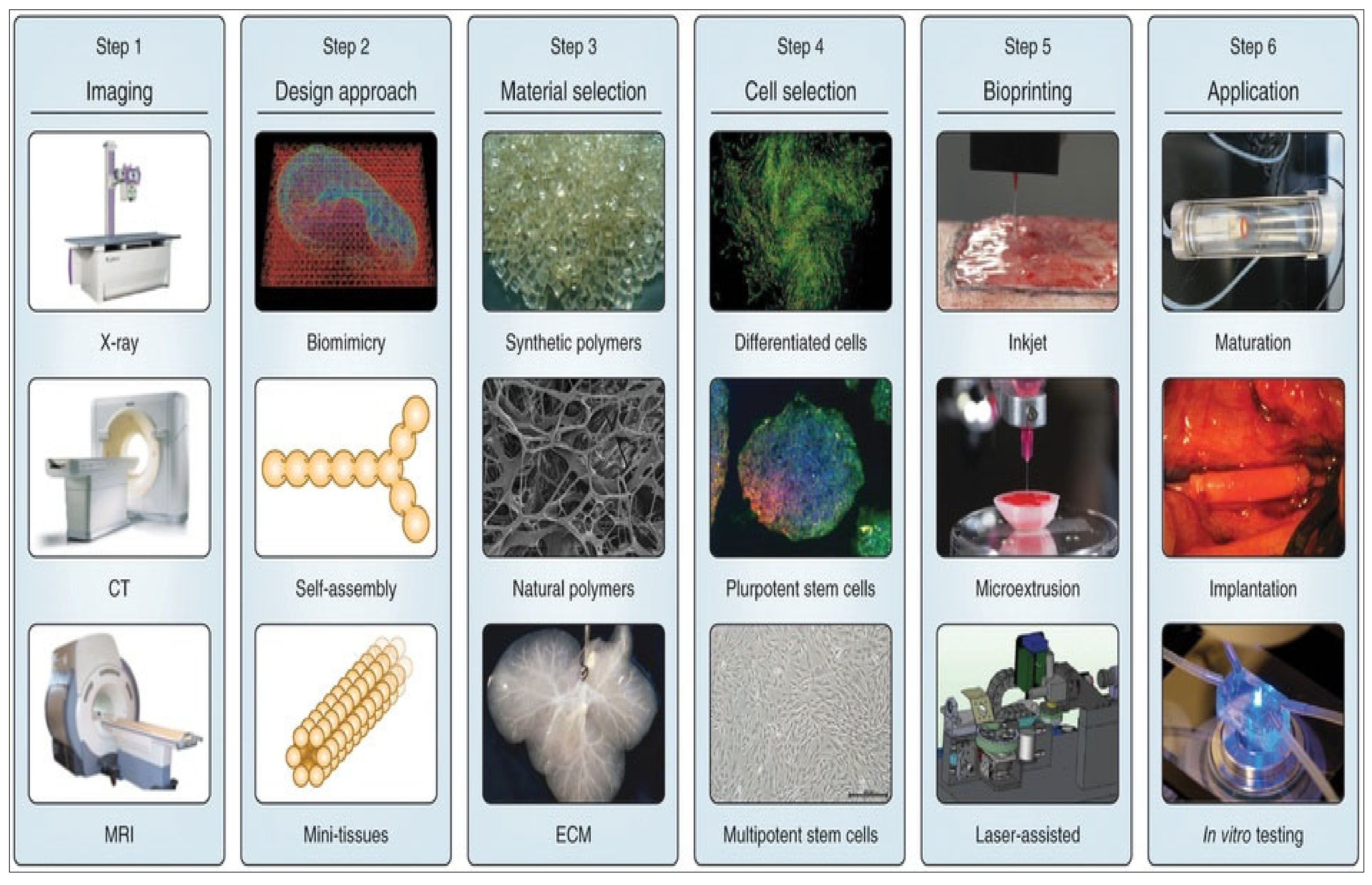
2.3. Design of 3D Bioprinting Model
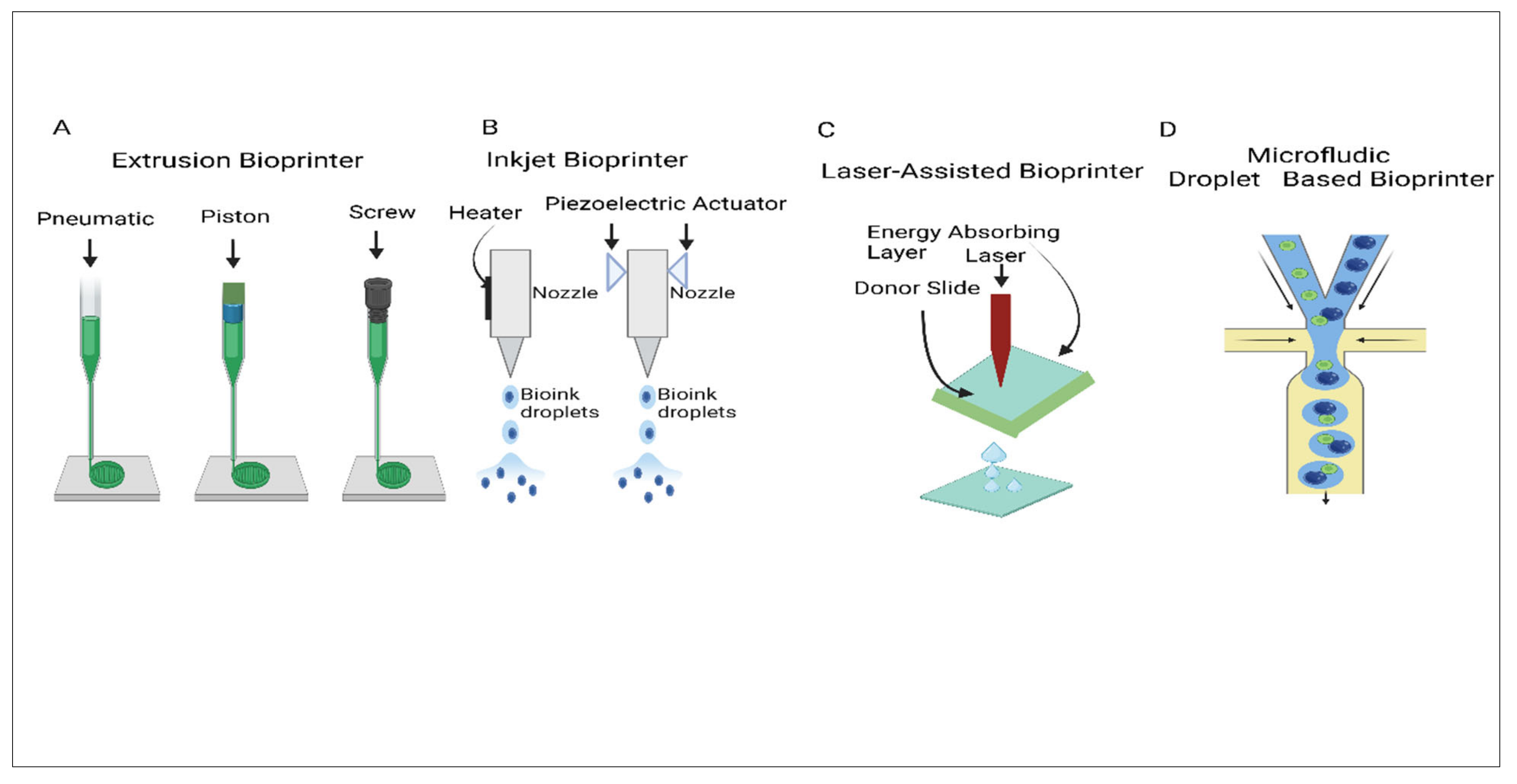
2.4. Extrusion Bioprinting
2.4.1. Pneumatic-Driven Extrusion
2.4.2. Piston-Driven Extrusion
2.5. Screw-Driven Extrusion
2.6. Inkjet Bioprinting
2.7. Light-Assisted Bioprinting
2.8. Microfluidics Bioprinting
2.9. Comparison of Different 3D Bioprinting Techniques
3. Pathophysiology of Degenerative Intervertebral Disc
4. Mechanical Properties of Biomaterials
5. Natural Biomaterials
5.1. Chitosan
5.2. Alginate
5.3. Hyaluronan
5.4. Collagen and Gelatin
5.5. Agarose
6. Synthetic Biomaterials
6.1. Polyethylene Glycol-Based Hydrogels
6.2. Polyurethane
6.3. Poly (-Caprolactone)
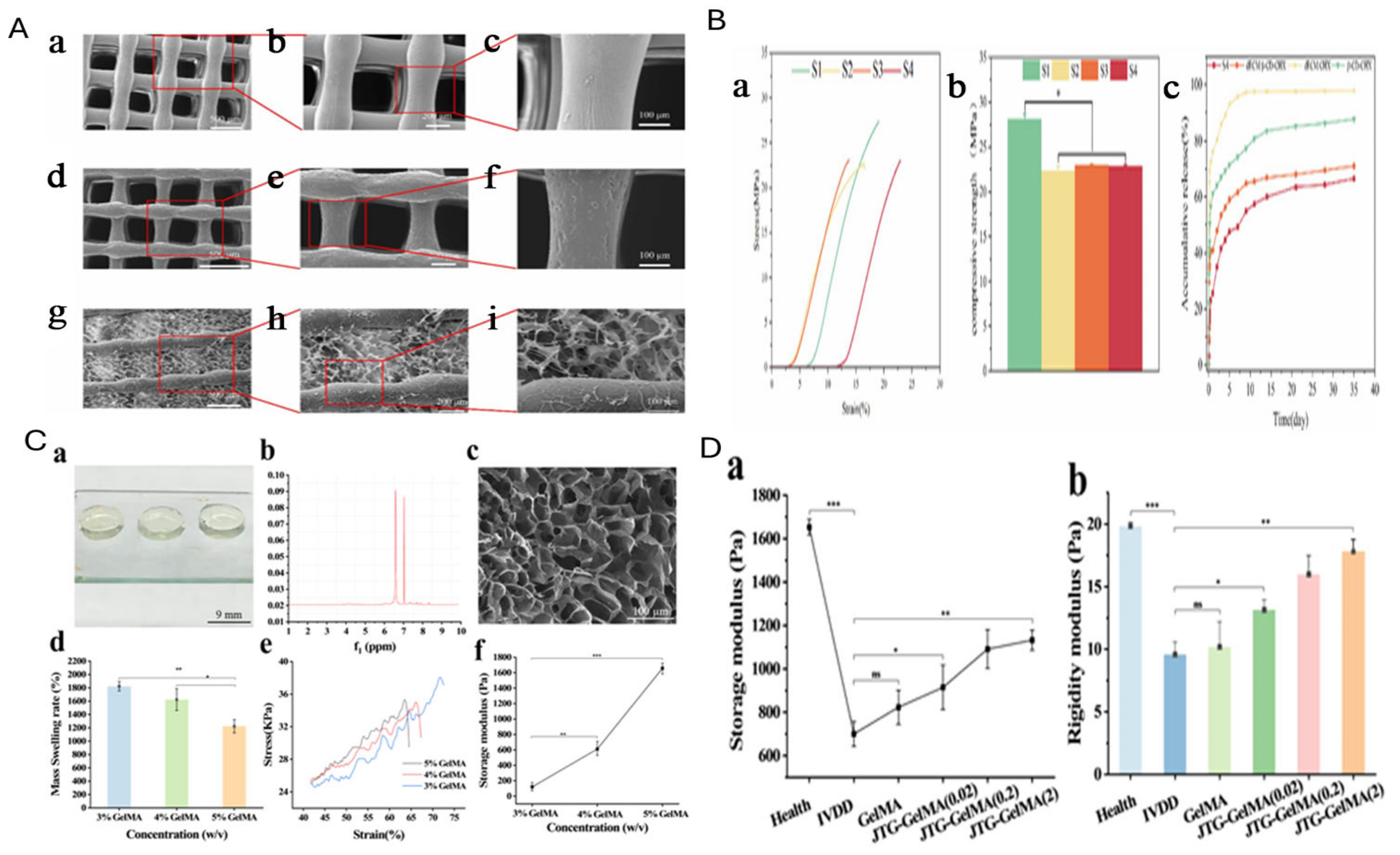
7. GelMA for IVD Regeneration
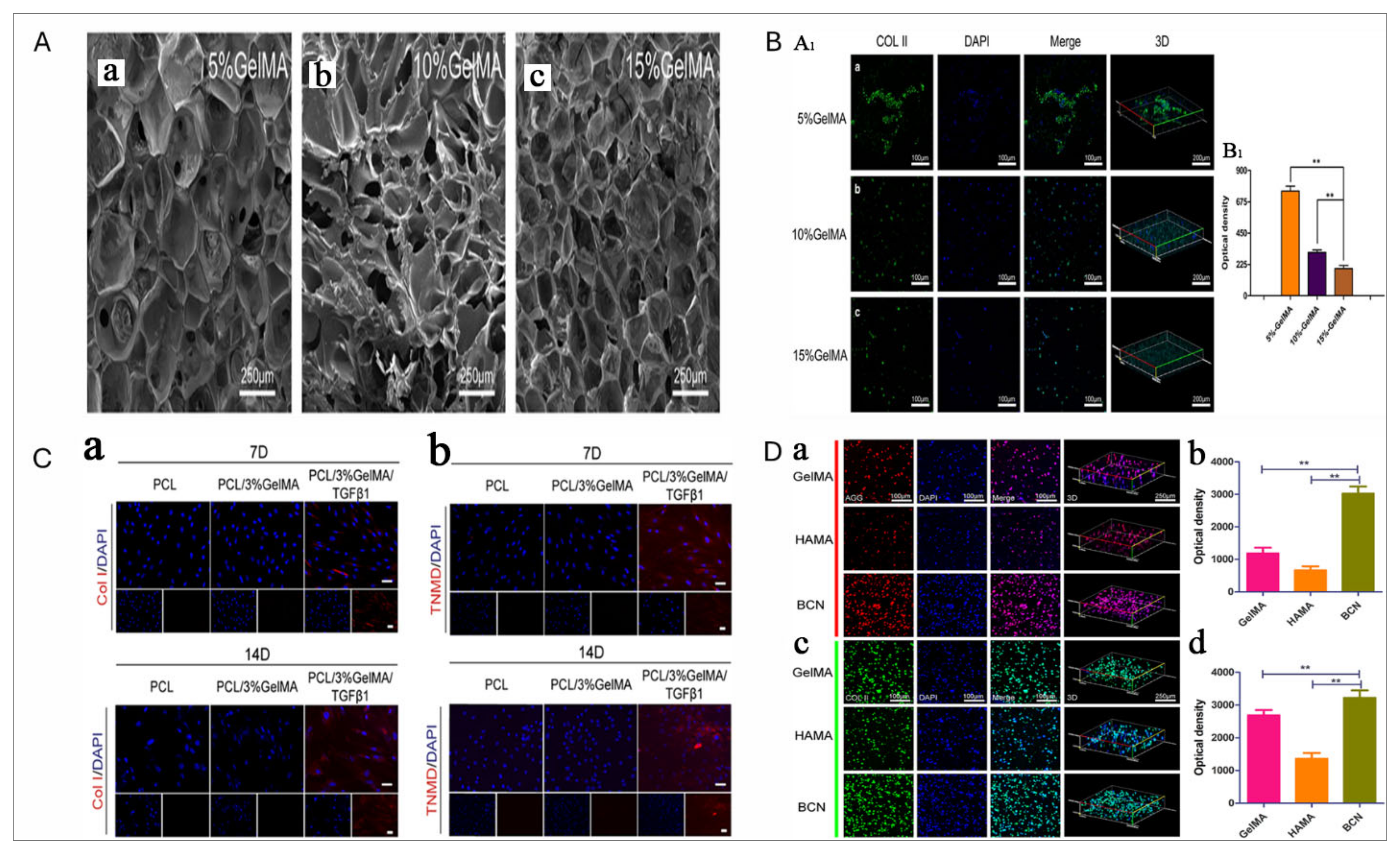
8. Cell Sources for IVD Regeneration
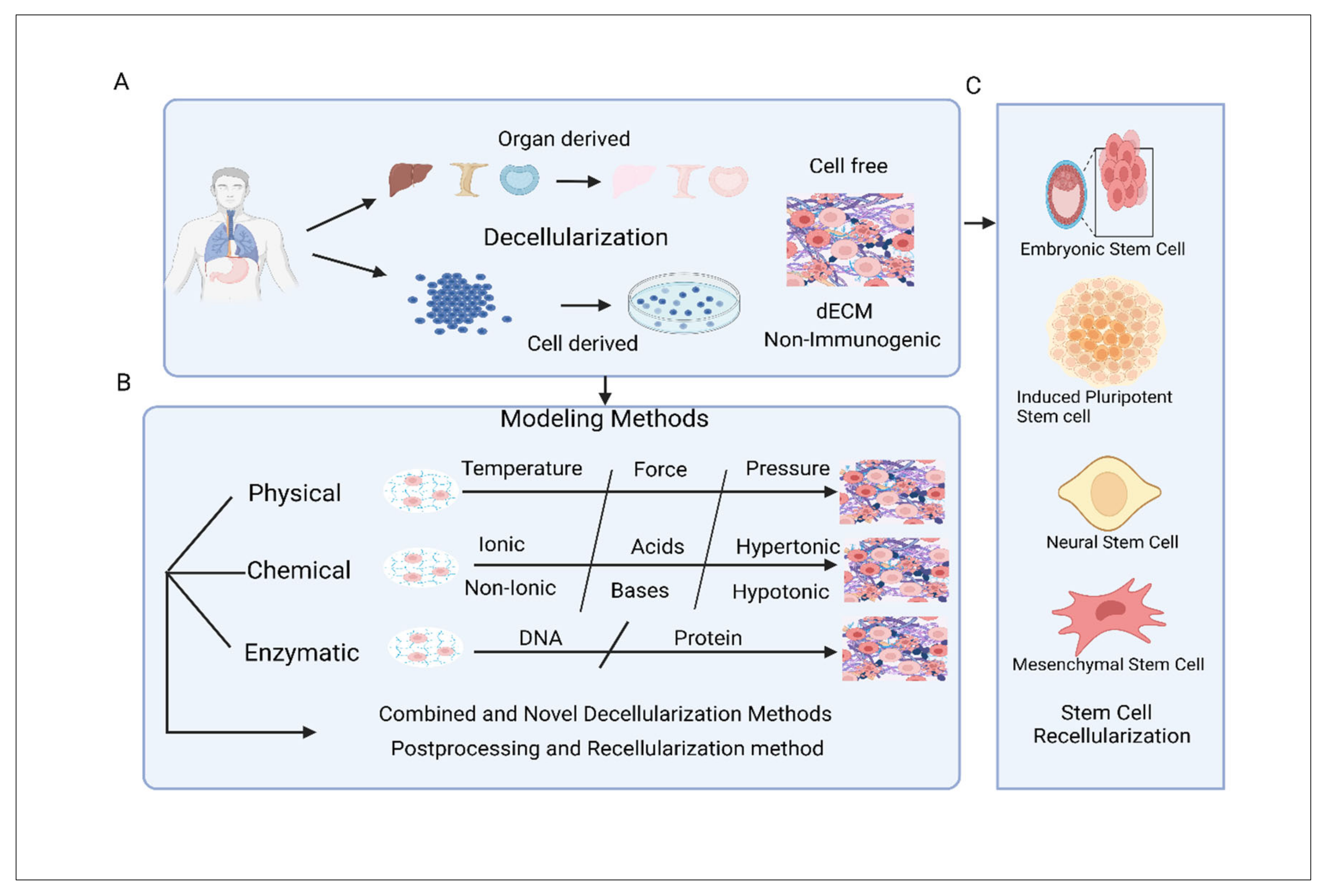
9. Categorization and Modelling of Decellularized Extracellular Matrix Scaffold
9.1. dECM Scaffolds Constructed from Organs and Tissues
9.2. dECM Scaffold Constructed from Cell
9.3. Modelling of dECM Scaffold
9.4. Combined and Novel Decellularization Methods
9.5. Postprocessing and Recellularization Method
10. Limitation and Future Perspective
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melrose, J. Strategies in regenerative medicine for intervertebral disc repair using mesenchymal stem cells and bioscaffolds. Regen. Med. 2016, 11, 705–724. [Google Scholar] [CrossRef] [PubMed]
- Khaleque, M.A.; Kim, J.-H.; Hwang, B.-J.; Kang, J.-K.; Quan, M.; Kim, Y.-Y. Role of necroptosis in intervertebral disc degeneration. Int. J. Mol. Sci. 2023, 24, 15292. [Google Scholar] [CrossRef]
- Seo, J.-Y.; Kim, J.; Kim, Y.-Y.; Ha, K.-Y.; Kim, Y.-H.; Kim, S.-I.; Lim, J.-H.; Seo, K.B.; Kang, H.; Choi, S. Autophagy in an extruded disc compared to the remaining disc after lumbar disc herniation in the same patient. Eur. Spine J. 2024, 33, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Stolworthy, D.K.; Fullwood, R.A.; Merrell, T.M.; Bridgewater, L.C.; Bowden, A.E. Biomechanical analysis of the camelid cervical intervertebral disc. J. Orthop. Transl. 2015, 3, 34–43. [Google Scholar]
- Khaleque, M.A.; Kim, J.-H.; Lee, H.-H.; Kim, G.-H.; You, W.-Y.; Lee, W.-J.; Kim, Y.-Y. Comparative Analysis of Autophagy and Apoptosis in Disc Degeneration: Understanding the Dynamics of Temporary-Compression-Induced Early Autophagy and Sustained-Compression-Triggered Apoptosis. Int. J. Mol. Sci. 2024, 25, 2352. [Google Scholar] [CrossRef] [PubMed]
- Makhni, M.C.; Caldwell, J.-M.E.; Saifi, C.; Fischer, C.R.; Lehman, R.A.; Lenke, L.G.; Lee, F.Y. Tissue engineering advances in spine surgery. Regen. Med. 2016, 11, 211–222. [Google Scholar]
- Kang, K.-T.; Koh, Y.-G.; Son, J.; Yeom, J.S.; Park, J.-H.; Kim, H.-J. Biomechanical evaluation of pedicle screw fixation system in spinal adjacent levels using polyetheretherketone, carbon-fiber-reinforced polyetheretherketone, and traditional titanium as rod materials. Compos. Part B Eng. 2017, 130, 248–256. [Google Scholar] [CrossRef]
- Fiordalisi, M.; Silva, A.J.; Barbosa, M.; Gonçalves, R.; Caldeira, J. Decellularized scaffolds for intervertebral disc regeneration. Trends Biotechnol. 2020, 38, 947–951. [Google Scholar] [CrossRef]
- James, A.R.; Bowles, R.D.; Gebhard, H.H.; Bonassar, L.J.; Härtl, R. Tissue-engineered total disc replacement: Final outcomes of a murine caudal disc in vivo study. Evid.-Based Spine-Care J. 2011, 2, 55. [Google Scholar]
- Oner, T.; Cengiz, I.; Pitikakis, M.; Cesario, L.; Parascandolo, P.; Vosilla, L.; Viano, G.; Oliveira, J.; Reis, R.; Silva-Correia, J. 3D segmentation of intervertebral discs: From concept to the fabrication of patient-specific scaffolds. J. 3D Print. Med. 2017, 1, 91–101. [Google Scholar]
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D bioprinting for organ regeneration. Adv. Healthc. Mater. 2017, 6, 1601118. [Google Scholar]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar]
- Zhu, C.; Li, J.; Liu, C.; Zhou, P.; Yang, H.; Li, B. Modulation of the gene expression of annulus fibrosus-derived stem cells using poly(ether carbonate urethane)urea scaffolds of tunable elasticity. Acta Biomater. 2016, 29, 228–238. [Google Scholar] [CrossRef] [PubMed]
- van Uden, S.; Silva-Correia, J.; Correlo, V.M.; Oliveira, J.M.; Reis, R.L. Custom-tailored tissue engineered polycaprolactone scaffolds for total disc replacement. Biofabrication 2015, 7, 015008. [Google Scholar] [CrossRef]
- Rosenzweig, D.H.; Carelli, E.; Steffen, T.; Jarzem, P.; Haglund, L. 3D-Printed ABS and PLA Scaffolds for Cartilage and Nucleus Pulposus Tissue Regeneration. Int. J. Mol. Sci. 2015, 16, 15118–15135. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Miot, S.; Gorecka, A.; Singha, K.; Loparic, M.; Dickinson, S.; Das, A.; Bhavesh, N.S.; Ray, A.R.; Martin, I.; et al. Oriented lamellar silk fibrous scaffolds to drive cartilage matrix orientation: Towards annulus fibrosus tissue engineering. Acta Biomater. 2012, 8, 3313–3325. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, M.A.H.; Khaleque, M.A.; Kim, G.-H.; Yoo, W.-Y.; Kim, Y.-Y. The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives. Biomimetics 2024, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, T.G.; Kim, B.S.; Kim, S.W.; Kwon, S.M.; Cho, D.W. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. 2016, 33, 88–95. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef]
- Illien-Junger, S.; Sedaghatpour, D.D.; Laudier, D.M.; Hecht, A.C.; Qureshi, S.A.; Iatridis, J.C. Development of a bovine decellularized extracellular matrix-biomaterial for nucleus pulposus regeneration. J. Orthop. Res. 2016, 34, 876–888. [Google Scholar] [CrossRef]
- Fan, D.; Li, Y.; Wang, X.; Zhu, T.; Wang, Q.; Cai, H.; Li, W.; Tian, Y.; Liu, Z. Progressive 3D Printing Technology and Its Application in Medical Materials. Front. Pharmacol. 2020, 11, 122. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.; Zhang, M.; Ma, Y.; Yang, Y.; Han, X.; Wang, X. Long-term delivery of alendronate through an injectable tetra-PEG hydrogel to promote osteoporosis therapy. Biomater. Sci. 2020, 8, 3138–3146. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Cui, X.; Wang, X.; Zhang, L.; Tang, P. Recent Advances on Magnetic Sensitive Hydrogels in Tissue Engineering. Front. Chem. 2020, 8, 124. [Google Scholar] [CrossRef]
- Yu, T.; Wang, H.; Zhang, Y.; Wang, X.; Han, B. The Delivery of RNA-Interference Therapies Based on Engineered Hydrogels for Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 445. [Google Scholar] [CrossRef]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Zhou, B.; Li, F.; Wang, W.; Liu, Y.; Wang, X.; Liu, C.; Ye, X. Advances of naturally derived and synthetic hydrogels for intervertebral disk regeneration. Front. Bioeng. Biotechnol. 2020, 8, 745. [Google Scholar]
- Xiao, W.; Li, J.; Qu, X.; Wang, L.; Tan, Y.; Li, K.; Li, H.; Yue, X.; Li, B.; Liao, X. Cell-laden interpenetrating network hydrogels formed from methacrylated gelatin and silk fibroin via a combination of sonication and photocrosslinking approaches. Mater. Sci. Eng. C 2019, 99, 57–67. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Gan, W.; Yin, F.; Zhao, J.; Xu, B.; Yu, Q.; Yang, L. Multi-physical field coupling for vibration feed electrochemical machining of diamond-shaped hole in titanium alloy. Int. J. Adv. Manuf. Technol. 2019, 106, 1409–1420. [Google Scholar] [CrossRef]
- Frazier, W.E. Metal Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Halloran, J.W. Ceramic Stereolithography: Additive Manufacturing for Ceramics by Photopolymerization. Annu. Rev. Mater. Res. 2016, 46, 19–40. [Google Scholar] [CrossRef]
- Kotz, F.; Arnold, K.; Bauer, W.; Schild, D.; Keller, N.; Sachsenheimer, K.; Nargang, T.M.; Richter, C.; Helmer, D.; Rapp, B.E. Three-dimensional printing of transparent fused silica glass. Nature 2017, 544, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Kotz, F.; Risch, P.; Arnold, K.; Sevim, S.; Puigmarti-Luis, J.; Quick, A.; Thiel, M.; Hrynevich, A.; Dalton, P.D.; Helmer, D.; et al. Fabrication of arbitrary three-dimensional suspended hollow microstructures in transparent fused silica glass. Nat. Commun. 2019, 10, 1439. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mulhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Heer, B. Additive manufacturing of multi-material structures. Mater. Sci. Eng. R Rep. 2018, 129, 1–16. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar]
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Erratum: Organ printing, computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2004, 22, 265. [Google Scholar] [CrossRef]
- Boland, T.; Mironov, V.; Gutowska, A.; Roth, E.A.; Markwald, R.R. Cell and organ printing 2: Fusion of cell aggregates in three-dimensional gels. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003, 272, 497–502. [Google Scholar] [CrossRef]
- Klebe, R.J. Cytoscribing: A method for micropositioning cells and the construction of two-and three-dimensional synthetic tissues. Exp. Cell Res. 1988, 179, 362–373. [Google Scholar] [CrossRef]
- Foty, R.A.; Pfleger, C.M.; Forgacs, G.; Steinberg, M.S. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development 1996, 122, 1611–1620. [Google Scholar] [CrossRef]
- Odde, D.J.; Renn, M.J. Laser-guided direct writing for applications in biotechnology. Trends Biotechnol. 1999, 17, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Karzyński, K.; Kosowska, K.; Ambrożkiewicz, F.; Berman, A.; Cichoń, J.; Klak, M.; Serwańska-Świętek, M.; Wszoła, M. Use of 3D bioprinting in biomedical engineering for clinical application. Med. Stud. 2018, 34, 93–97. [Google Scholar] [CrossRef]
- Landers, R.; Hübner, U.; Schmelzeisen, R.; Mülhaupt, R. Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering. Biomaterials 2002, 23, 4437–4447. [Google Scholar] [CrossRef]
- Wilson, W.C., Jr.; Boland, T. Cell and organ printing 1: Protein and cell printers. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003, 272, 491–496. [Google Scholar] [CrossRef]
- Dhariwala, B.; Hunt, E.; Boland, T. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng. 2004, 10, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.N.; Qureshi, A.N.; Eagles, P.A. Electrohydrodynamic jet processing: An advanced electric-field-driven jetting phenomenon for processing living cells. Small 2006, 2, 216–219. [Google Scholar] [CrossRef]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef]
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.; Soker, S. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl. Med. 2012, 1, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Duan, B. State-of-the-Art Review of 3D Bioprinting for Cardiovascular Tissue Engineering. Ann. Biomed. Eng. 2017, 45, 195–209. [Google Scholar] [CrossRef]
- Pyo, S.-H.; Wang, P.; Hwang, H.H.; Zhu, W.; Warner, J.; Chen, S. Continuous optical 3D printing of green aliphatic polyurethanes. ACS Appl. Mater. Interfaces 2017, 9, 836–844. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.; Shiwarski, D.; Tashman, J.; Hinton, T.; Yerneni, S.; Bliley, J.; Campbell, P.; Feinberg, A. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Ahn, S.H.; Lee, H.J.; Lee, J.-S.; Yoon, H.; Chun, W.; Kim, G.H. A novel cell-printing method and its application to hepatogenic differentiation of human adipose stem cell-embedded mesh structures. Sci. Rep. 2015, 5, 13427. [Google Scholar]
- Elomaa, L.; Pan, C.-C.; Shanjani, Y.; Malkovskiy, A.; Seppälä, J.V.; Yang, Y. Three-dimensional fabrication of cell-laden biodegradable poly (ethylene glycol-co-depsipeptide) hydrogels by visible light stereolithography. J. Mater. Chem. B 2015, 3, 8348–8358. [Google Scholar] [CrossRef] [PubMed]
- Elomaa, L.; Teixeira, S.; Hakala, R.; Korhonen, H.; Grijpma, D.W.; Seppälä, J.V. Preparation of poly (ε-caprolactone)-based tissue engineering scaffolds by stereolithography. Acta Biomater. 2011, 7, 3850–3856. [Google Scholar] [CrossRef]
- Chan, V.; Zorlutuna, P.; Jeong, J.H.; Kong, H.; Bashir, R. Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapsulation. Lab A Chip 2010, 10, 2062–2070. [Google Scholar]
- Seck, T.M.; Melchels, F.P.; Feijen, J.; Grijpma, D.W. Designed biodegradable hydrogel structures prepared by stereolithography using poly (ethylene glycol)/poly (d, l-lactide)-based resins. J. Control. Release 2010, 148, 34–41. [Google Scholar]
- Lee, K.; Jin, G.; Jang, C.H.; Jung, W.-K.; Kim, G. Preparation and characterization of multi-layered poly (ε-caprolactone)/chitosan scaffolds fabricated with a combination of melt-plotting/in situ plasma treatment and a coating method for hard tissue regeneration. J. Mater. Chem. B 2013, 1, 5831–5841. [Google Scholar] [CrossRef]
- Hong, J.M.; Kim, B.J.; Shim, J.-H.; Kang, K.S.; Kim, K.-J.; Rhie, J.W.; Cha, H.J.; Cho, D.-W. Enhancement of bone regeneration through facile surface functionalization of solid freeform fabrication-based three-dimensional scaffolds using mussel adhesive proteins. Acta Biomater. 2012, 8, 2578–2586. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H.; Shuang, J.; Wang, J.; Ma, J.; Zhang, S. Microsphere-based selective laser sintering for building macroporous bone scaffolds with controlled microstructure and excellent biocompatibility. Colloids Surf. B Biointerfaces 2015, 135, 81–89. [Google Scholar] [CrossRef]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Lee, M.-Y.; Shyu, V.B.-H.; Chen, Y.-C.; Chen, C.-T.; Chen, J.-P. Surface modification of polycaprolactone scaffolds fabricated via selective laser sintering for cartilage tissue engineering. Mater. Sci. Eng. C 2014, 40, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Reed, E.J.; Klumb, L.; Koobatian, M.; Viney, C. Biomimicry as a route to new materials: What kinds of lessons are useful? Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 1571–1585. [Google Scholar] [CrossRef]
- Huh, D.; Torisawa, Y.S.; Hamilton, G.A.; Kim, H.J.; Ingber, D.E. Microengineered physiological biomimicry: Organs-on-chips. Lab A Chip 2012, 12, 2156–2164. [Google Scholar] [CrossRef]
- Ingber, D.E.; Mow, V.C.; Butler, D.; Niklason, L.; Huard, J.; Mao, J.; Yannas, I.; Kaplan, D.; Vunjak-Novakovic, G. Tissue engineering and developmental biology: Going biomimetic. Tissue Eng. 2006, 12, 3265–3283. [Google Scholar] [CrossRef]
- Müller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Nanostructured Pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication 2015, 7, 035006. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yan, M.; Wang, Y.; Fu, J.; Suo, H. 3D bioprinting of low-concentration cell-laden gelatin methacrylate (GelMA) bioinks with a two-step cross-linking strategy. ACS Appl. Mater. Interfaces 2018, 10, 6849–6857. [Google Scholar] [CrossRef] [PubMed]
- Roehm, K.D.; Madihally, S.V. Bioprinted chitosan-gelatin thermosensitive hydrogels using an inexpensive 3D printer. Biofabrication 2017, 10, 015002. [Google Scholar] [CrossRef]
- Ng, W.L.; Yeong, W.Y.; Naing, M.W. Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int. J. Bioprint 2016, 2, 53–62. [Google Scholar] [CrossRef]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef]
- Tripathi, S.; Mandal, S.S.; Bauri, S.; Maiti, P. 3D bioprinting and its innovative approach for biomedical applications. MedComm 2023, 4, e194. [Google Scholar]
- Marga, F.; Neagu, A.; Kosztin, I.; Forgacs, G. Developmental biology and tissue engineering. Birth Defects Res. Part C Embryo Today Rev. 2007, 81, 320–328. [Google Scholar] [CrossRef]
- Jakab, K.; Norotte, C.; Marga, F.; Murphy, K.; Vunjak-Novakovic, G.; Forgacs, G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2010, 2, 022001. [Google Scholar]
- Yamato, M.; Utsumi, M.; Kushida, A.; Konno, C.; Kikuchi, A.; Okano, T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2001, 7, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Kushida, A.; Yamato, M.; Isoi, Y.; Kikuchi, A.; Okano, T. A noninvasive transfer system for polarized renal tubule epithelial cell sheets using temperature-responsive culture dishes. Eur. Cell Mater. 2005, 10, 23–30. [Google Scholar] [PubMed]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Kelm, J.M.; Lorber, V.; Snedeker, J.G.; Schmidt, D.; Broggini-Tenzer, A.; Weisstanner, M.; Odermatt, B.; Mol, A.; Zünd, G.; Hoerstrup, S.P. A novel concept for scaffold-free vessel tissue engineering: Self-assembly of microtissue building blocks. J. Biotechnol. 2010, 148, 46–55. [Google Scholar]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef]
- Kamei, M.; Saunders, W.B.; Bayless, K.J.; Dye, L.; Davis, G.E.; Weinstein, B.M. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 2006, 442, 453–456. [Google Scholar] [CrossRef]
- Sonntag, F.; Schilling, N.; Mader, K.; Gruchow, M.; Klotzbach, U.; Lindner, G.; Horland, R.; Wagner, I.; Lauster, R.; Howitz, S.; et al. Design and prototyping of a chip-based multi-micro-organoid culture system for substance testing, predictive to human (substance) exposure. J. Biotechnol. 2010, 148, 70–75. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv. Healthc. Mater. 2016, 5, 1429–1438. [Google Scholar] [PubMed]
- Günther, A.; Yasotharan, S.; Vagaon, A.; Lochovsky, C.; Pinto, S.; Yang, J.; Lau, C.; Voigtlaender-Bolz, J.; Bolz, S.-S. A microfluidic platform for probing small artery structure and function. Lab A Chip 2010, 10, 2341–2349. [Google Scholar]
- Sodupe-Ortega, E.; Sanz-Garcia, A.; Pernia-Espinoza, A.; Escobedo-Lucea, C. Accurate Calibration in Multi-Material 3D Bioprinting for Tissue Engineering. Materials 2018, 11, 1402. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef]
- Trachtenberg, J.E.; Placone, J.K.; Smith, B.T.; Piard, C.M.; Santoro, M.; Scott, D.W.; Fisher, J.P.; Mikos, A.G. Extrusion-Based 3D Printing of Poly(propylene fumarate) in a Full-Factorial Design. ACS Biomater. Sci. Eng. 2016, 2, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Blaeser, A.; Weber, M.; Jakel, J.; Neuss, S.; Jahnen-Dechent, W.; Fischer, H. Three-dimensional printing of stem cell-laden hydrogels submerged in a hydrophobic high-density fluid. Biofabrication 2013, 5, 015003. [Google Scholar] [CrossRef]
- Visser, J.; Peters, B.; Burger, T.J.; Boomstra, J.; Dhert, W.J.; Melchels, F.P.; Malda, J. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 2013, 5, 035007. [Google Scholar] [CrossRef]
- Pantani, R.; Turng, L.S. Manufacturing of advanced biodegradable polymeric components. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Saunders, R.E.; Derby, B. Inkjet printing biomaterials for tissue engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar]
- Lorber, B.; Hsiao, W.-K.; Hutchings, I.M.; Martin, K.R. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication 2013, 6, 015001. [Google Scholar]
- Colina, M.; Serra, P.; Fernandez-Pradas, J.M.; Sevilla, L.; Morenza, J.L. DNA deposition through laser induced forward transfer. Biosens. Bioelectron. 2005, 20, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Dinca, V.; Kasotakis, E.; Catherine, J.; Mourka, A.; Ranella, A.; Ovsianikov, A.; Chichkov, B.N.; Farsari, M.; Mitraki, A.; Fotakis, C. Directed three-dimensional patterning of self-assembled peptide fibrils. Nano Lett. 2008, 8, 538–543. [Google Scholar]
- Ringeisen, B.R.; Kim, H.; Barron, J.A.; Krizman, D.B.; Chrisey, D.B.; Jackman, S.; Auyeung, R.; Spargo, B.J. Laser printing of pluripotent embryonal carcinoma cells. Tissue Eng. 2004, 10, 483–491. [Google Scholar]
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B. Laser-assisted cell printing: Principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomedicine 2010, 5, 507–515. [Google Scholar]
- Gruene, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Hofmann, N.; Bernemann, I.; Glasmacher, B.; Chichkov, B. Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng. Part C Methods 2011, 17, 79–87. [Google Scholar] [CrossRef]
- Hopp, B.; Smausz, T.; Kresz, N.; Barna, N.; Bor, Z.; Kolozsvári, L.; Chrisey, D.B.; Szabó, A.; Nógrádi, A. Survival and proliferative ability of various living cell types after laser-induced forward transfer. Tissue Eng. 2005, 11, 1817–1823. [Google Scholar]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [PubMed]
- Barata, D.; van Blitterswijk, C.; Habibovic, P. High-throughput screening approaches and combinatorial development of biomaterials using microfluidics. Acta Biomater. 2016, 34, 1–20. [Google Scholar] [CrossRef]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv. Mater. 2016, 28, 677–684. [Google Scholar] [CrossRef]
- Costantini, M.; Testa, S.; Mozetic, P.; Barbetta, A.; Fuoco, C.; Fornetti, E.; Tamiro, F.; Bernardini, S.; Jaroszewicz, J.; Swieszkowski, W.; et al. Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials 2017, 131, 98–110. [Google Scholar] [CrossRef]
- De la Vega, L.; Rosas Gómez, D.A.; Abelseth, E.; Abelseth, L.; Allisson da Silva, V.; Willerth, S.M. 3D bioprinting human induced pluripotent stem cell-derived neural tissues using a novel lab-on-a-printer technology. Appl. Sci. 2018, 8, 2414. [Google Scholar] [CrossRef]
- Smith, C.M.; Stone, A.L.; Parkhill, R.L.; Stewart, R.L.; Simpkins, M.W.; Kachurin, A.M.; Warren, W.L.; Williams, S.K. Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue Eng. 2004, 10, 1566–1576. [Google Scholar] [PubMed]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Remy, M.; Bordenave, L.; Amedee, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef]
- Cui, X.; Dean, D.; Ruggeri, Z.M.; Boland, T. Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol. Bioeng. 2010, 106, 963–969. [Google Scholar] [CrossRef]
- Koch, L.; Kuhn, S.; Sorg, H.; Gruene, M.; Schlie, S.; Gaebel, R.; Polchow, B.; Reimers, K.; Stoelting, S.; Ma, N. Laser printing of skin cells and human stem cells. Tissue Eng. Part C Methods 2010, 16, 847–854. [Google Scholar]
- Souza, G.R.; Molina, J.R.; Raphael, R.M.; Ozawa, M.G.; Stark, D.J.; Levin, C.S.; Bronk, L.F.; Ananta, J.S.; Mandelin, J.; Georgescu, M.M.; et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat. Nanotechnol. 2010, 5, 291–296. [Google Scholar] [CrossRef]
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Shrike Zhang, Y.; Shin, S.R.; Calzone, G.; et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101. [Google Scholar] [CrossRef]
- Menon, R.G.; Zibetti, M.V.; Pendola, M.; Regatte, R.R. Measurement of three-dimensional internal dynamic strains in the intervertebral disc of the lumbar spine with mechanical loading and golden-angle radial sparse parallel-magnetic resonance imaging. J. Magn. Reson. Imaging 2021, 54, 486–496. [Google Scholar]
- Van der Veen, A.; Van Dieen, J.; Nadort, A.; Stam, B.; Smit, T. Intervertebral disc recovery after dynamic or static loading in vitro: Is there a role for the endplate? J. Biomech. 2007, 40, 2230–2235. [Google Scholar]
- Dudek, M.; Yang, N.; Ruckshanthi, J.P.; Williams, J.; Borysiewicz, E.; Wang, P.; Adamson, A.; Li, J.; Bateman, J.F.; White, M.R. The intervertebral disc contains intrinsic circadian clocks that are regulated by age and cytokines and linked to degeneration. Ann. Rheum. Dis. 2017, 76, 576–584. [Google Scholar]
- Hristova, G.I.; Jarzem, P.; Ouellet, J.A.; Roughley, P.J.; Epure, L.M.; Antoniou, J.; Mwale, F. Calcification in human intervertebral disc degeneration and scoliosis. J. Orthop. Res. 2011, 29, 1888–1895. [Google Scholar] [PubMed]
- Zhao, C.-Q.; Wang, L.-M.; Jiang, L.-S.; Dai, L.-Y. The cell biology of intervertebral disc aging and degeneration. Ageing Res. Rev. 2007, 6, 247–261. [Google Scholar]
- McCann, M.R.; Tamplin, O.J.; Rossant, J.; Séguin, C.A. Tracing notochord-derived cells using a Noto-cre mouse: Implications for intervertebral disc development. Dis. Models Mech. 2012, 5, 73–82. [Google Scholar]
- Sheyn, D.; Ben-David, S.; Tawackoli, W.; Zhou, Z.; Salehi, K.; Bez, M.; De Mel, S.; Chan, V.; Roth, J.; Avalos, P. Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model. Theranostics 2019, 9, 7506. [Google Scholar]
- Liu, C.; Xiao, L.; Zhang, Y.; Zhao, Q.; Xu, H. Regeneration of annulus fibrosus tissue using a DAFM/PECUU-blended electrospun scaffold. J. Biomater. Sci. Polym. Ed. 2020, 31, 2347–2361. [Google Scholar]
- He, R.; Wang, Z.; Cui, M.; Liu, S.; Wu, W.; Chen, M.; Wu, Y.; Qu, Y.; Lin, H.; Chen, S. HIF1A Alleviates compression-induced apoptosis of nucleus pulposus derived stem cells via upregulating autophagy. Autophagy 2021, 17, 3338–3360. [Google Scholar]
- Cui, Z.-Y.; Shen, L.; Gao, X.-S.; Dong, X.-B.; Fu, H.-Y. S-phase kinase-associated protein-2 (Skp2) promotes nucleus pulposus cell proliferation by inhibition of p27 in attenuating intervertebral disc degeneration. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2802–2810. [Google Scholar] [PubMed]
- Zhang, Y.; Yang, B.; Wang, J.; Cheng, F.; Shi, K.; Ying, L.; Wang, C.; Xia, K.; Huang, X.; Gong, Z. Cell senescence: A nonnegligible cell state under survival stress in pathology of intervertebral disc degeneration. Oxidative Med. Cell. Longev. 2020, 2020, 9503562. [Google Scholar]
- Cheung, K.M.; Karppinen, J.; Chan, D.; Ho, D.W.; Song, Y.-Q.; Sham, P.; Cheah, K.S.; Leong, J.C.; Luk, K.D. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 2009, 34, 934–940. [Google Scholar] [PubMed]
- Lyu, F.-J.; Cui, H.; Pan, H.; Mc Cheung, K.; Cao, X.; Iatridis, J.C.; Zheng, Z. Painful intervertebral disc degeneration and inflammation: From laboratory evidence to clinical interventions. Bone Res. 2021, 9, 7. [Google Scholar]
- Lan, T.; Shen, Z.; Yan, B.; Chen, J. New insights into the interplay between miRNAs and autophagy in the aging of intervertebral discs. Ageing Res. Rev. 2021, 65, 101227. [Google Scholar]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar]
- Chen, J.; Xie, J.-J.; Jin, M.-Y.; Gu, Y.-T.; Wu, C.-C.; Guo, W.-J.; Yan, Y.-Z.; Zhang, Z.-J.; Wang, J.-L.; Zhang, X.-L. Sirt6 overexpression suppresses senescence and apoptosis of nucleus pulposus cells by inducing autophagy in a model of intervertebral disc degeneration. Cell Death Dis. 2018, 9, 56. [Google Scholar]
- Hu, S.; Chen, L.; Al Mamun, A.; Ni, L.; Gao, W.; Lin, Y.; Jin, H.; Zhang, X.; Wang, X. The therapeutic effect of TBK1 in intervertebral disc degeneration via coordinating selective autophagy and autophagic functions. J. Adv. Res. 2021, 30, 1–13. [Google Scholar]
- Li, S.; Liu, Z.; Gao, X.; Cheng, L.; Xu, Z.; Li, L.; Diao, Y.; Chen, L.; Liu, Y.; Sun, J. Preparation and properties of a 3D printed nHA/PLA bone tissue engineering scaffold loaded with a β-CD–CHX combined dECM hydrogel. RSC Adv. 2024, 14, 9848–9859. [Google Scholar]
- Zhang, Y.; Gao, R.; Xie, X.; Zhang, J.; Liang, Z.; Wei, Z.; Xu, F.; Ding, T. Research on the dual mode of JTG-GelMA hydrogel system to promote the regeneration of degenerated intervertebral disc. Eur. Polym. J. 2024, 220, 113475. [Google Scholar]
- Groll, J.; Burdick, J.A.; Cho, D.W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jungst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef] [PubMed]
- Sasson, A.; Patchornik, S.; Eliasy, R.; Robinson, D.; Haj-Ali, R. Hyperelastic mechanical behavior of chitosan hydrogels for nucleus pulposus replacement-experimental testing and constitutive modeling. J. Mech. Behav. Biomed. Mater. 2012, 8, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Yang, S.-H.; Su, W.-Y.; Chen, Y.-C.; Yang, K.-C.; Cheng, W.T.-K.; Wu, S.-C.; Lin, F.-H. Thermosensitive chitosan–gelatin–glycerol phosphate hydrogels as a cell carrier for nucleus pulposus regeneration: An in vitro study. Tissue Eng. Part A 2010, 16, 695–703. [Google Scholar] [CrossRef]
- Smith, L.J.; Gorth, D.J.; Showalter, B.L.; Chiaro, J.A.; Beattie, E.E.; Elliott, D.M.; Mauck, R.L.; Chen, W.; Malhotra, N.R. In vitro characterization of a stem-cell-seeded triple-interpenetrating-network hydrogel for functional regeneration of the nucleus pulposus. Tissue Eng. Part A 2014, 20, 1841–1849. [Google Scholar] [CrossRef]
- Li, Z.; Shim, H.; Cho, M.O.; Cho, I.S.; Lee, J.H.; Kang, S.W.; Kwon, B.; Huh, K.M. Thermo-sensitive injectable glycol chitosan-based hydrogel for treatment of degenerative disc disease. Carbohydr. Polym. 2018, 184, 342–353. [Google Scholar] [CrossRef]
- Li, P.; Fu, L.; Liao, Z.; Peng, Y.; Ning, C.; Gao, C.; Zhang, D.; Sui, X.; Lin, Y.; Liu, S.; et al. Chitosan hydrogel/3D-printed poly(epsilon-caprolactone) hybrid scaffold containing synovial mesenchymal stem cells for cartilage regeneration based on tetrahedral framework nucleic acid recruitment. Biomaterials 2021, 278, 121131. [Google Scholar] [CrossRef]
- Foss, B.L.; Maxwell, T.W.; Deng, Y. Chondroprotective supplementation promotes the mechanical properties of injectable scaffold for human nucleus pulposus tissue engineering. J. Mech. Behav. Biomed. Mater. 2014, 29, 56–67. [Google Scholar] [CrossRef]
- Ukeba, D.; Sudo, H.; Tsujimoto, T.; Ura, K.; Yamada, K.; Iwasaki, N. Bone marrow mesenchymal stem cells combined with ultra-purified alginate gel as a regenerative therapeutic strategy after discectomy for degenerated intervertebral discs. eBioMedicine 2020, 53, 102698. [Google Scholar] [CrossRef] [PubMed]
- Jarrah, R.M.; Potes, M.D.A.; Vitija, X.; Durrani, S.; Ghaith, A.K.; Mualem, W.; Zamanian, C.; Bhandarkar, A.R.; Bydon, M. Alginate hydrogels: A potential tissue engineering intervention for intervertebral disc degeneration. J. Clin. Neurosci. 2023, 113, 32–37. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Huang, B.; Liu, L.T.; Liu, M.H.; Wang, J.; Li, C.Q.; Zhang, Z.F.; Chu, T.W.; Xiong, C.J. Utilization of stem cells in alginate for nucleus pulposus tissue engineering. Tissue Eng. Part A 2014, 20, 908–920. [Google Scholar] [CrossRef]
- Kenne, L.; Gohil, S.; Nilsson, E.M.; Karlsson, A.; Ericsson, D.; Helander Kenne, A.; Nord, L.I. Modification and cross-linking parameters in hyaluronic acid hydrogels--definitions and analytical methods. Carbohydr. Polym. 2013, 91, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Beecham, M.; Mauck, R.L.; Burdick, J.A. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials 2009, 30, 4287–4296. [Google Scholar] [CrossRef] [PubMed]
- Kazezian, Z.; Li, Z.; Alini, M.; Grad, S.; Pandit, A. Injectable hyaluronic acid down-regulates interferon signaling molecules, IGFBP3 and IFIT3 in the bovine intervertebral disc. Acta Biomater. 2017, 52, 118–129. [Google Scholar] [CrossRef]
- Sloan, S.R., Jr.; Galesso, D.; Secchieri, C.; Berlin, C.; Hartl, R.; Bonassar, L.J. Initial investigation of individual and combined annulus fibrosus and nucleus pulposus repair ex vivo. Acta Biomater. 2017, 59, 192–199. [Google Scholar] [CrossRef]
- Endres, M.; Abbushi, A.; Thomale, U.W.; Cabraja, M.; Kroppenstedt, S.N.; Morawietz, L.; Casalis, P.A.; Zenclussen, M.L.; Lemke, A.J.; Horn, P.; et al. Intervertebral disc regeneration after implantation of a cell-free bioresorbable implant in a rabbit disc degeneration model. Biomaterials 2010, 31, 5836–5841. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.E.; Cameron, A.R.; Menzies, D.J.; Ghosh, P.; Whitehead, D.L.; Gronthos, S.; Zannettino, A.C.; Cooper-White, J.J. An injectable hydrogel incorporating mesenchymal precursor cells and pentosan polysulphate for intervertebral disc regeneration. Biomaterials 2013, 34, 9430–9440. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfeghian, A.; Madihally, S.V. In vitro characterization of chitosan-gelatin scaffolds for tissue engineering. Biomaterials 2005, 26, 7616–7627. [Google Scholar] [CrossRef]
- Bron, J.L.; Mulder, H.W.; Vonk, L.A.; Doulabi, B.Z.; Oudhoff, M.J.; Smit, T.H. Migration of intervertebral disc cells into dense collagen scaffolds intended for functional replacement. J. Mater. Sci. Mater. Med. 2012, 23, 813–821. [Google Scholar] [CrossRef]
- Wang, S.J.; Jiang, D.; Zhang, Z.Z.; Chen, Y.R.; Yang, Z.D.; Zhang, J.Y.; Shi, J.; Wang, X.; Yu, J.K. Biomimetic Nanosilica-Collagen Scaffolds for In Situ Bone Regeneration: Toward a Cell-Free, One-Step Surgery. Adv. Mater. 2019, 31, e1904341. [Google Scholar] [CrossRef]
- Tsaryk, R.; Gloria, A.; Russo, T.; Anspach, L.; De Santis, R.; Ghanaati, S.; Unger, R.E.; Ambrosio, L.; Kirkpatrick, C.J. Collagen-low molecular weight hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres for cell and growth factor delivery for nucleus pulposus regeneration. Acta Biomater. 2015, 20, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gunn, J.; Chen, M.H.; Cooper, A.; Zhang, M. On-site alginate gelation for enhanced cell proliferation and uniform distribution in porous scaffolds. J. Biomed. Mater. Res. A 2008, 86, 552–559. [Google Scholar] [CrossRef]
- Chen, Y.C.; Su, W.Y.; Yang, S.H.; Gefen, A.; Lin, F.H. In situ forming hydrogels composed of oxidized high molecular weight hyaluronic acid and gelatin for nucleus pulposus regeneration. Acta Biomater. 2013, 9, 5181–5193. [Google Scholar] [CrossRef]
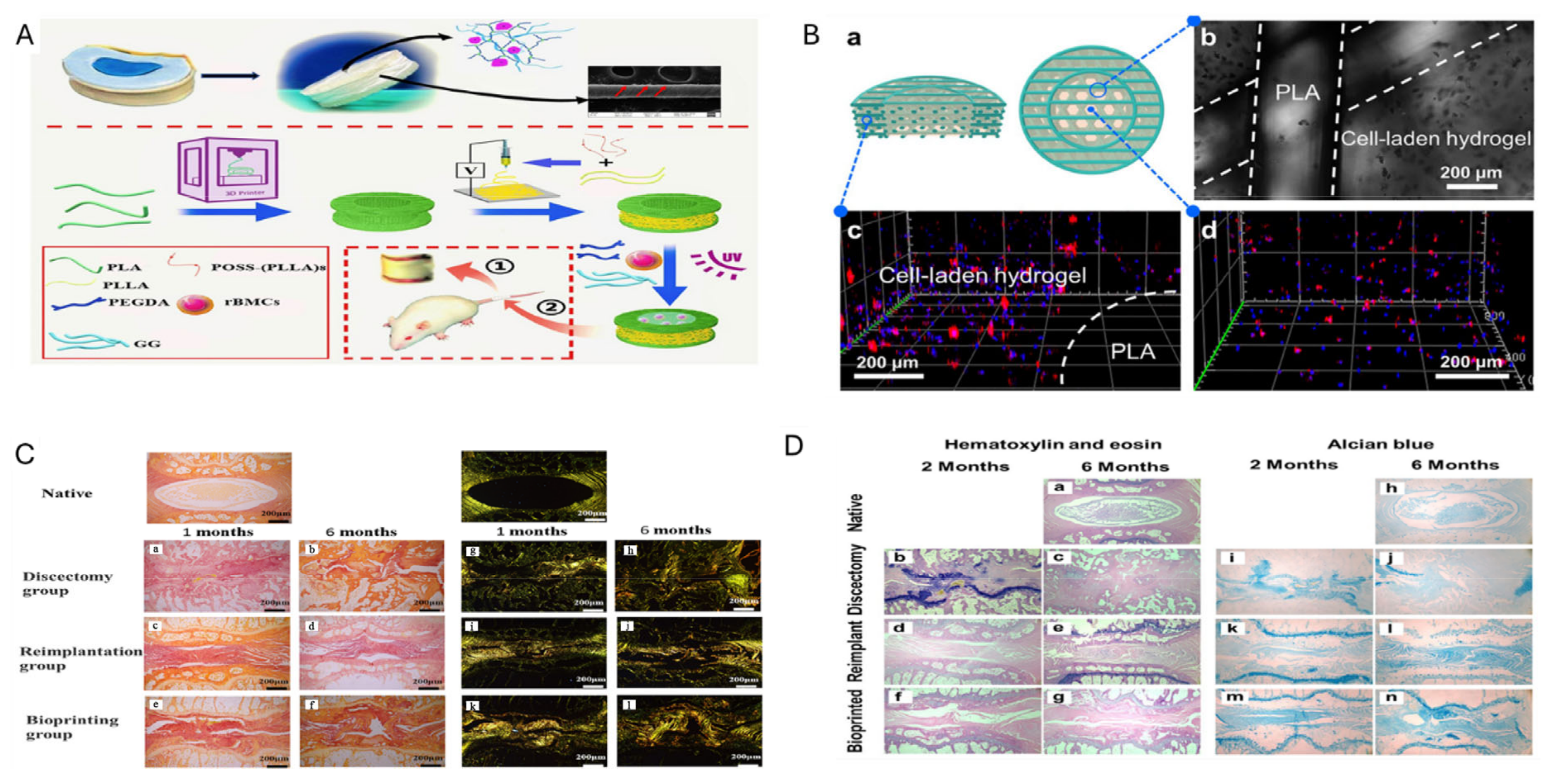
- Wu, D.; Tan, J.; Yao, L.; Tian, J.; Luo, B.; Li, L.; Zhou, C.; Lu, L. Customized composite intervertebral disc scaffolds by integrated 3D bioprinting for therapeutic implantation. Compos. Part A Appl. Sci. Manuf. 2021, 147, 106468. [Google Scholar] [CrossRef]
- Zhu, M.; Tan, J.; Liu, L.; Tian, J.; Li, L.; Luo, B.; Zhou, C.; Lu, L. Construction of biomimetic artificial intervertebral disc scaffold via 3D printing and electrospinning. Mater. Sci. Eng. C 2021, 128, 112310. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.C.; Grover, L.M. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol. Lett. 2010, 32, 733–742. [Google Scholar] [CrossRef]
- Tilwani, R.K.; Bader, D.L.; Chowdhury, T.T. Biomechanical Conditioning Enhanced Matrix Synthesis in Nucleus Pulposus Cells Cultured in Agarose Constructs with TGFbeta. J. Funct. Biomater. 2012, 3, 23–36. [Google Scholar] [CrossRef]
- Benoit, D.S.; Anseth, K.S. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials 2005, 26, 5209–5220. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Anseth, K.S. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef]
- Francisco, A.T.; Hwang, P.Y.; Jeong, C.G.; Jing, L.; Chen, J.; Setton, L.A. Photocrosslinkable laminin-functionalized polyethylene glycol hydrogel for intervertebral disc regeneration. Acta Biomater. 2014, 10, 1102–1111. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, Z.; Dang, M.; Zhang, X.; Doleyres, Y.; Song, Y.; Chen, D.; Ma, P.X. Injectable nanofibrous spongy microspheres for NR4A1 plasmid DNA transfection to reverse fibrotic degeneration and support disc regeneration. Biomaterials 2017, 131, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.G.; Francisco, A.T.; Niu, Z.; Mancino, R.L.; Craig, S.L.; Setton, L.A. Screening of hyaluronic acid-poly(ethylene glycol) composite hydrogels to support intervertebral disc cell biosynthesis using artificial neural network analysis. Acta Biomater. 2014, 10, 3421–3430. [Google Scholar] [CrossRef]
- Hung, K.C.; Tseng, C.S.; Hsu, S.H. Synthesis and 3D printing of biodegradable polyurethane elastomer by a water-based process for cartilage tissue engineering applications. Adv. Healthc. Mater. 2014, 3, 1578–1587. [Google Scholar] [CrossRef]
- Hu, J.; Chen, B.; Guo, F.; Du, J.; Gu, P.; Lin, X.; Yang, W.; Zhang, H.; Lu, M.; Huang, Y.; et al. Injectable silk fibroin/polyurethane composite hydrogel for nucleus pulposus replacement. J. Mater. Sci. Mater. Med. 2012, 23, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Gong, M.S.; Park, J.H.; Moon, S.I.; Wall, I.B.; Kim, H.W.; Lee, J.H.; Knowles, J.C. Silk fibroin-polyurethane blends: Physical properties and effect of silk fibroin content on viscoelasticity, biocompatibility and myoblast differentiation. Acta Biomater. 2013, 9, 8962–8971. [Google Scholar] [CrossRef]
- Li, Z.; Lang, G.; Chen, X.; Sacks, H.; Mantzur, C.; Tropp, U.; Mader, K.T.; Smallwood, T.C.; Sammon, C.; Richards, R.G.; et al. Polyurethane scaffold with in situ swelling capacity for nucleus pulposus replacement. Biomaterials 2016, 84, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Dash, T.K.; Konkimalla, V.B. Poly-small je, Ukrainian-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Guarino, V.; Lewandowska, M.; Bil, M.; Polak, B.; Ambrosio, L. Morphology and degradation properties of PCL/HYAFF11® composite scaffolds with multi-scale degradation rate. Compos. Sci. Technol. 2010, 70, 1826–1837. [Google Scholar] [CrossRef]
- Nerurkar, N.L.; Baker, B.M.; Sen, S.; Wible, E.E.; Elliott, D.M.; Mauck, R.L. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat. Mater. 2009, 8, 986–992. [Google Scholar] [CrossRef]
- Lopez, A.; Persson, C.; Hilborn, J.; Engqvist, H. Synthesis and characterization of injectable composites of poly[D,L-lactide-co-(epsilon-caprolactone)] reinforced with beta-TCP and CaCO3 for intervertebral disk augmentation. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95, 75–83. [Google Scholar] [CrossRef]
- Martin, J.T.; Milby, A.H.; Chiaro, J.A.; Kim, D.H.; Hebela, N.M.; Smith, L.J.; Elliott, D.M.; Mauck, R.L. Translation of an engineered nanofibrous disc-like angle-ply structure for intervertebral disc replacement in a small animal model. Acta Biomater. 2014, 10, 2473–2481. [Google Scholar] [CrossRef] [PubMed]
- Gloria, A.; Russo, T.; D’Amora, U.; Santin, M.; De Santis, R.; Ambrosio, L. Customised multiphasic nucleus/annulus scaffold for intervertebral disc repair/regeneration. Connect. Tissue Res. 2020, 61, 152–162. [Google Scholar] [CrossRef]
- Sun, B.; Lian, M.; Han, Y.; Mo, X.; Jiang, W.; Qiao, Z.; Dai, K. A 3D-Bioprinted dual growth factor-releasing intervertebral disc scaffold induces nucleus pulposus and annulus fibrosus reconstruction. Bioact. Mater. 2021, 6, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Schmocker, A.; Khoushabi, A.; Frauchiger, D.A.; Gantenbein, B.; Schizas, C.; Moser, C.; Bourban, P.E.; Pioletti, D.P. A photopolymerized composite hydrogel and surgical implanting tool for a nucleus pulposus replacement. Biomaterials 2016, 88, 110–119. [Google Scholar] [CrossRef]
- Yan, C.; Wang, X.; Xiang, C.; Wang, Y.; Pu, C.; Chen, L.; Jiang, K.; Li, Y. Applications of Functionalized Hydrogels in the Regeneration of the Intervertebral Disc. Biomed. Res. Int. 2021, 2021, 2818624. [Google Scholar] [CrossRef]
- Xu, H.; Sun, M.; Wang, C.; Xia, K.; Xiao, S.; Wang, Y.; Ying, L.; Yu, C.; Yang, Q.; He, Y. GDF5-GelMA injectable microspheres laden with adipose-derived stem cells for disc degeneration repair. Biofabrication 2020, 1758–5090. [Google Scholar]
- Xu, P.; Guan, J.; Chen, Y.; Xiao, H.; Yang, T.; Sun, H.; Wu, N.; Zhang, C.; Mao, Y. Stiffness of photocrosslinkable gelatin hydrogel influences nucleus pulposus cell propertiesin vitro. J. Cell. Mol. Med. 2021, 25, 880–891. [Google Scholar] [CrossRef]
- Li, Z.; Jia, Y.; Zhang, T.; Du, L.; Li, W.; Li, J.; Zhang, K.; Jia, Y.; Zhu, M.; Xu, B. Combined nucleus pulposus and annulus fibrosus bioactive scaffolds enhance intervertebral disc defect regeneration. Chem. Eng. J. 2024, 487, 150548. [Google Scholar] [CrossRef]
- Krouwels, A.; Melchels, F.P.; Van Rijen, M.H.; Ten Brink, C.B.; Dhert, W.J.; Öner, F.C.; Tryfonidou, M.A.; Creemers, L.B. Focal adhesion signaling affects regeneration by human nucleus pulposus cells in collagen-but not carbohydrate-based hydrogels. Acta Biomater. 2018, 66, 238–247. [Google Scholar] [CrossRef]
- Silva, A.J.; Ferreira, J.R.; Cunha, C.; Corte-Real, J.V.; Bessa-Goncalves, M.; Barbosa, M.A.; Santos, S.G.; Goncalves, R.M. Macrophages Down-Regulate Gene Expression of Intervertebral Disc Degenerative Markers Under a Pro-inflammatory Microenvironment. Front. Immunol. 2019, 10, 1508. [Google Scholar] [CrossRef]
- Liu, Y.; Du, J.; Peng, P.; Cheng, R.; Lin, J.; Xu, C.; Yang, H.; Cui, W.; Mao, H.; Li, Y.; et al. Regulation of the inflammatory cycle by a controllable release hydrogel for eliminating postoperative inflammation after discectomy. Bioact. Mater. 2021, 6, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid. Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Xu, P.; Li, C.; Jin, S.; Hu, J.; Fang, Y.; Zhu, K.; Xu, G.; Han, Z.; Zhang, Z. Bioactive hydrogel encapsulated dual-gene engineered nucleus pulposus stem cells towards intervertebral disc tissue repair. Chem. Eng. J. 2023, 453, 139717. [Google Scholar] [CrossRef]
- Ambesi-Impiombato, F.S.; Parks, L.; Coon, H. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc. Natl. Acad. Sci. USA 1980, 77, 3455–3459. [Google Scholar] [CrossRef]
- Hamm, A.; Krott, N.; Breibach, I.; Blindt, R.; Bosserhoff, A.K. Efficient transfection method for primary cells. Tissue Eng. 2002, 8, 235–245. [Google Scholar] [CrossRef]
- Okumura, N.; Ueno, M.; Koizumi, N.; Sakamoto, Y.; Hirata, K.; Hamuro, J.; Kinoshita, S. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Invest. Ophthalmol. Vis. Sci. 2009, 50, 3680–3687. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, M.; Fu, P.; Xie, M.; Wang, W.; Luo, X. ROCK inhibition with Y27632 promotes the proliferation and cell cycle progression of cultured astrocyte from spinal cord. Neurochem. Int. 2012, 61, 1114–1120. [Google Scholar] [CrossRef]
- Abbott, R.D.; Purmessur, D.; Monsey, R.D.; Iatridis, J.C. Regenerative potential of TGFbeta3 + Dex and notochordal cell conditioned media on degenerated human intervertebral disc cells. J. Orthop. Res. 2012, 30, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Colombier, P.; Clouet, J.; Boyer, C.; Ruel, M.; Bonin, G.; Lesoeur, J.; Moreau, A.; Fellah, B.H.; Weiss, P.; Lescaudron, L.; et al. TGF-beta1 and GDF5 Act Synergistically to Drive the Differentiation of Human Adipose Stromal Cells toward Nucleus Pulposus-like Cells. Stem Cells 2016, 34, 653–667. [Google Scholar] [CrossRef]
- Xu, P.; Lou, L.; Zhan, W.; Wang, C.; Wu, S.; Liu, Z.; Wang, Y. Bicomponent hydrogel laden with TGF-β3-nucleus pulposus stem cells for disc degeneration repair. Chem. Eng. J. 2024, 479, 147788. [Google Scholar] [CrossRef]
- Farhang, N.; Brunger, J.M.; Stover, J.D.; Thakore, P.I.; Lawrence, B.; Guilak, F.; Gersbach, C.A.; Setton, L.A.; Bowles, R.D. (*) CRISPR-Based Epigenome Editing of Cytokine Receptors for the Promotion of Cell Survival and Tissue Deposition in Inflammatory Environments. Tissue Eng. Part A 2017, 23, 738–749. [Google Scholar] [CrossRef]
- Krupkova, O.; Cambria, E.; Besse, L.; Besse, A.; Bowles, R.; Wuertz-Kozak, K. The potential of CRISPR/Cas9 genome editing for the study and treatment of intervertebral disc pathologies. JOR Spine 2018, 1, e1003. [Google Scholar] [CrossRef] [PubMed]
- Piazza, N.; Dehghani, M.; Gaborski, T.R.; Wuertz-Kozak, K. Therapeutic Potential of Extracellular Vesicles in Degenerative Diseases of the Intervertebral Disc. Front. Bioeng. Biotechnol. 2020, 8, 311. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Poldervaart, M.T.; Goversen, B.; de Ruijter, M.; Abbadessa, A.; Melchels, F.P.W.; Oner, F.C.; Dhert, W.J.A.; Vermonden, T.; Alblas, J. 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS ONE 2017, 12, e0177628. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; White, L.J.; Shakesheff, K.M.; Tatullo, M. Odontogenic Differentiation of Human Dental Pulp Stem Cells on Hydrogel Scaffolds Derived from Decellularized Bone Extracellular Matrix and Collagen Type I. PLoS ONE 2016, 11, e0148225. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965. [Google Scholar] [CrossRef]
- Ullah, I.; Busch, J.F.; Rabien, A.; Ergun, B.; Stamm, C.; Knosalla, C.; Hippenstiel, S.; Reinke, P.; Kurtz, A. Adult Tissue Extracellular Matrix Determines Tissue Specification of Human iPSC-Derived Embryonic Stage Mesodermal Precursor Cells. Adv. Sci. 2020, 7, 1901198. [Google Scholar] [CrossRef] [PubMed]
- Safari, F.; Fani, N.; Eglin, D.; Alini, M.; Stoddart, M.J.; Baghaban Eslaminejad, M. Human umbilical cord-derived scaffolds for cartilage tissue engineering. J. Biomed. Mater. Res. A 2019, 107, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Hoshiba, T. Cultured cell-derived decellularized matrices: A review towards the next decade. J. Mater. Chem. B 2017, 5, 4322–4331. [Google Scholar] [CrossRef]
- Kumar, A.; Nune, K.C.; Misra, R.D. Biological functionality and mechanistic contribution of extracellular matrix-ornamented three dimensional Ti-6Al-4V mesh scaffolds. J. Biomed. Mater. Res. A 2016, 104, 2751–2763. [Google Scholar] [CrossRef]
- Cai, D.; Weng, W. Development potential of extracellular matrix hydrogels as hemostatic materials. Front. Bioeng. Biotechnol. 2023, 11, 1187474. [Google Scholar] [CrossRef]
- Gupta, S.K.; Mishra, N.C.; Dhasmana, A. Decellularization Methods for Scaffold Fabrication. Methods Mol. Biol. 2018, 1577, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cai, Y.; Xia, C.; Wu, H.; Li, Q.; Xu, Z.; Lu, F. An innovative method to obtain porous porcine aorta scaffolds for tissue engineering. Artif. Organs 2019, 43, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Yang, S.; Rye, S.M.; Choi, S.W. Effect of pulsatile flow perfusion on decellularization. Biomed. Eng. Online 2018, 17, 15. [Google Scholar] [CrossRef]
- Guimaraes, A.B.; Correia, A.T.; Alves, B.P.; Da Silva, R.S.; Martins, J.K.; Pego-Fernandes, P.M.; Xavier, N.S.; Dolhnikoff, M.; Cardoso, P.F.G. Evaluation of a Physical-Chemical Protocol for Porcine Tracheal Decellularization. Transpl. Proc. 2019, 51, 1611–1613. [Google Scholar] [CrossRef]
- Poornejad, N.; Schaumann, L.B.; Buckmiller, E.M.; Momtahan, N.; Gassman, J.R.; Ma, H.H.; Roeder, B.L.; Reynolds, P.R.; Cook, A.D. The impact of decellularization agents on renal tissue extracellular matrix. J. Biomater. Appl. 2016, 31, 521–533. [Google Scholar] [CrossRef]
- Cornelison, R.C.; Wellman, S.M.; Park, J.H.; Porvasnik, S.L.; Song, Y.H.; Wachs, R.A.; Schmidt, C.E. Development of an apoptosis-assisted decellularization method for maximal preservation of nerve tissue structure. Acta Biomater. 2018, 77, 116–126. [Google Scholar] [CrossRef]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed. Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef] [PubMed]
- Willemse, J.; Verstegen, M.M.A.; Vermeulen, A.; Schurink, I.J.; Roest, H.P.; van der Laan, L.J.W.; de Jonge, J. Fast, robust and effective decellularization of whole human livers using mild detergents and pressure controlled perfusion. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110200. [Google Scholar] [CrossRef] [PubMed]
- Burk, J.; Erbe, I.; Berner, D.; Kacza, J.; Kasper, C.; Pfeiffer, B.; Winter, K.; Brehm, W. Freeze-thaw cycles enhance decellularization of large tendons. Tissue Eng. Part C Methods 2014, 20, 276–284. [Google Scholar] [CrossRef]
- Goecke, T.; Theodoridis, K.; Tudorache, I.; Ciubotaru, A.; Cebotari, S.; Ramm, R.; Hoffler, K.; Sarikouch, S.; Vasquez Rivera, A.; Haverich, A.; et al. In vivo performance of freeze-dried decellularized pulmonary heart valve allo- and xenografts orthotopically implanted into juvenile sheep. Acta Biomater. 2018, 68, 41–52. [Google Scholar] [CrossRef]
- Rowland, C.R.; Lennon, D.P.; Caplan, A.I.; Guilak, F. The effects of crosslinking of scaffolds engineered from cartilage ECM on the chondrogenic differentiation of MSCs. Biomaterials 2013, 34, 5802–5812. [Google Scholar] [CrossRef] [PubMed]
- Hillebrandt, K.H.; Everwien, H.; Haep, N.; Keshi, E.; Pratschke, J.; Sauer, I.M. Strategies based on organ decellularization and recellularization. Transpl. Int. 2019, 32, 571–585. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Kenry; Lee, W.C.; Loh, K.P.; Lim, C.T. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials 2018, 155, 236–250. [Google Scholar] [CrossRef]
- Sart, S.; Yan, Y.; Li, Y.; Lochner, E.; Zeng, C.; Ma, T.; Li, Y. Crosslinking of extracellular matrix scaffolds derived from pluripotent stem cell aggregates modulates neural differentiation. Acta Biomater. 2016, 30, 222–232. [Google Scholar] [CrossRef]
- Hong, X.; Yuan, Y.; Sun, X.; Zhou, M.; Guo, G.; Zhang, Q.; Hescheler, J.; Xi, J. Skeletal Extracellular Matrix Supports Cardiac Differentiation of Embryonic Stem Cells: A Potential Scaffold for Engineered Cardiac Tissue. Cell Physiol. Biochem. 2018, 45, 319–331. [Google Scholar] [CrossRef]
- Sambi, M.; Chow, T.; Whiteley, J.; Li, M.; Chua, S.; Raileanu, V.; Rogers, I.M. Acellular Mouse Kidney ECM can be Used as a Three-Dimensional Substrate to Test the Differentiation Potential of Embryonic Stem Cell Derived Renal Progenitors. Stem Cell Rev. Rep. 2017, 13, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, M.; Zhou, H.; Nadif Kasri, N. Choices for Induction of Pluripotency: Recent Developments in Human Induced Pluripotent Stem Cell Reprogramming Strategies. Stem Cell Rev. Rep. 2016, 12, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jakus, A.E.; Baptista, P.M.; Soker, S.; Soto-Gutierrez, A.; Abecassis, M.M.; Shah, R.N.; Wertheim, J.A. Functional Maturation of Induced Pluripotent Stem Cell Hepatocytes in Extracellular Matrix-A Comparative Analysis of Bioartificial Liver Microenvironments. Stem Cells Transl. Med. 2016, 5, 1257–1267. [Google Scholar] [CrossRef]
- Wan, J.; Huang, Y.; Zhou, P.; Guo, Y.; Wu, C.; Zhu, S.; Wang, Y.; Wang, L.; Lu, Y.; Wang, Z. Culture of iPSCs Derived Pancreatic beta-Like Cells In Vitro Using Decellularized Pancreatic Scaffolds: A Preliminary Trial. Biomed. Res. Int. 2017, 2017, 4276928. [Google Scholar] [CrossRef]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 013001. [Google Scholar] [CrossRef]
- Elomaa, L.; Almalla, A.; Keshi, E.; Hillebrandt, K.; Sauer, I.M.; Weinhart, M. Rise of tissue-and species-specific 3D bioprinting based on decellularized extracellular matrix-derived bioinks and bioresins. Biomater. Biosyst. 2023, 12, 100084. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Barceló, X.; Von Euw, S.; Kelly, D.J. 3D printing of mechanically functional meniscal tissue equivalents using high concentration extracellular matrix inks. Mater. Today Bio 2023, 20, 100624. [Google Scholar] [CrossRef] [PubMed]
- Garreta, E.; Oria, R.; Tarantino, C.; Pla-Roca, M.; Prado, P.; Fernández-Avilés, F.; Campistol, J.M.; Samitier, J.; Montserrat, N. Tissue engineering by decellularization and 3D bioprinting. Mater. Today 2017, 20, 166–178. [Google Scholar] [CrossRef]
- Philips, C.; Terrie, L.; Thorrez, L. Decellularized skeletal muscle: A versatile biomaterial in tissue engineering and regenerative medicine. Biomaterials 2022, 283, 121436. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Zheng, Z.; Wei, X.; Chen, L.; Wu, Y.; Huang, W.; Yang, L. Strategies for improving the 3D printability of decellularized extracellular matrix bioink. Theranostics 2023, 13, 2562–2587. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Huang, X.; Fang, W.; Tao, Y.; Zhao, T.; Liang, C.; Hua, J.; Chen, Q.; Li, F. Injectable decellularized nucleus pulposus-based cell delivery system for differentiation of adipose-derived stem cells and nucleus pulposus regeneration. Acta Biomater. 2018, 81, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Kuang, W.; Liu, C.; Xu, H. The application of decellularized nucleus pulposus matrix/chitosan with transforming growth factor β3 for nucleus pulposus tissue engineering. Cytotechnology 2021, 73, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, H.; Lee, J.; Atala, A.; Yoo, J.J.; Lee, S.J.; Kim, G.H. Efficient myotube formation in 3D bioprinted tissue construct by biochemical and topographical cues. Biomaterials 2020, 230, 119632. [Google Scholar] [CrossRef] [PubMed]
- Hoshiba, T.; Chen, G.; Endo, C.; Maruyama, H.; Wakui, M.; Nemoto, E.; Kawazoe, N.; Tanaka, M. Decellularized Extracellular Matrix as an In Vitro Model to Study the Comprehensive Roles of the ECM in Stem Cell Differentiation. Stem Cells Int. 2016, 2016, 6397820. [Google Scholar] [CrossRef]
- Jang, J.; Park, H.J.; Kim, S.W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Mironov, V.; Kasyanov, V.; Markwald, R.R. Organ printing: From bioprinter to organ biofabrication line. Curr. Opin. Biotechnol. 2011, 22, 667–673. [Google Scholar] [CrossRef]


| Methods | Advantages | Disadvantages | Materials | Ref. |
|---|---|---|---|---|
| SLA, DLP | Fabricated in both basic and complex designs; High speed with excellent resolution. Does not require support materials. | Costly machinery and materials; Limited to photopolymer materials; Potential cytotoxicity of residual photoinitiators. | PEG, PCL, PEG-co-PDP, PEGDA. | [54,55,56,57] |
| FFF | Convenient and easy to operate Exhibits strong mechanical properties; No need for solvents. | Restricted to thermoplastic materials; Filament required; Incompatible with living cells. | PCL/PLGA/β-TCP, PCL/PLGA | [58,59] |
| SLS | No need for support materials; Various biomaterials. | Irregular surface texture; Costly and bulky equipment. | PCL/HA, PCL, HA/PEEK, Titanium | [60,61,62] |
| Bioprinting Method | Advantages | Limitations | Applications | References |
|---|---|---|---|---|
| Extrusion-Based Bioprinting | Wide range of biocompatible materials (cell aggregates, hydrogels); Handles viscosities from 30 to 6 × 107 mPa/s; Simple and cost-effective setup. | Lower printing accuracy (~100 μm); Potential for cell damage from shear forces during extrusion. | Creating structures with both high and low cell densities; Research and customized services. | [12,106,107] |
| Inkjet Bioprinting | Low cost, high precision, and speed; Supports multiple nozzles for simultaneous printing of various cells and materials. | Limited to low-viscosity bioinks, compromising structural integrity; Struggles with high cell density printing, affecting viability and practical application. | Printing different cells and materials simultaneously. | [108]. |
| Laser-Assisted Bioprinting | Nozzle-free, non-contact technique avoiding nozzle clogging and mechanical damage to cells. | High cost and time-consuming. | Printing high-viscosity materials with high cell density and precision. | [99,107,109]. |
| Microfluidic Bioprinting | High precision, and ability to create complex gradients and patterns. | Complexity of microfluidic systems. | Ideal for replicating intricate tissue structures. | [110,111]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanvir, M.A.H.; Khaleque, M.A.; Lee, J.; Park, J.-B.; Kim, G.-H.; Lee, H.-H.; Kim, Y.-Y. Three-Dimensional Bioprinting for Intervertebral Disc Regeneration. J. Funct. Biomater. 2025, 16, 105. https://doi.org/10.3390/jfb16030105
Tanvir MAH, Khaleque MA, Lee J, Park J-B, Kim G-H, Lee H-H, Kim Y-Y. Three-Dimensional Bioprinting for Intervertebral Disc Regeneration. Journal of Functional Biomaterials. 2025; 16(3):105. https://doi.org/10.3390/jfb16030105
Chicago/Turabian StyleTanvir, Md Amit Hasan, Md Abdul Khaleque, Junhee Lee, Jong-Beom Park, Ga-Hyun Kim, Hwan-Hee Lee, and Young-Yul Kim. 2025. "Three-Dimensional Bioprinting for Intervertebral Disc Regeneration" Journal of Functional Biomaterials 16, no. 3: 105. https://doi.org/10.3390/jfb16030105
APA StyleTanvir, M. A. H., Khaleque, M. A., Lee, J., Park, J.-B., Kim, G.-H., Lee, H.-H., & Kim, Y.-Y. (2025). Three-Dimensional Bioprinting for Intervertebral Disc Regeneration. Journal of Functional Biomaterials, 16(3), 105. https://doi.org/10.3390/jfb16030105









