Biomechanical Evaluation of a Novel Non-Engaging Abutment and Screw in Internal Implant Systems: Comparative Fatigue and Load Testing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Implant Groups
2.2. Prosthesis Fabrication and Assembly
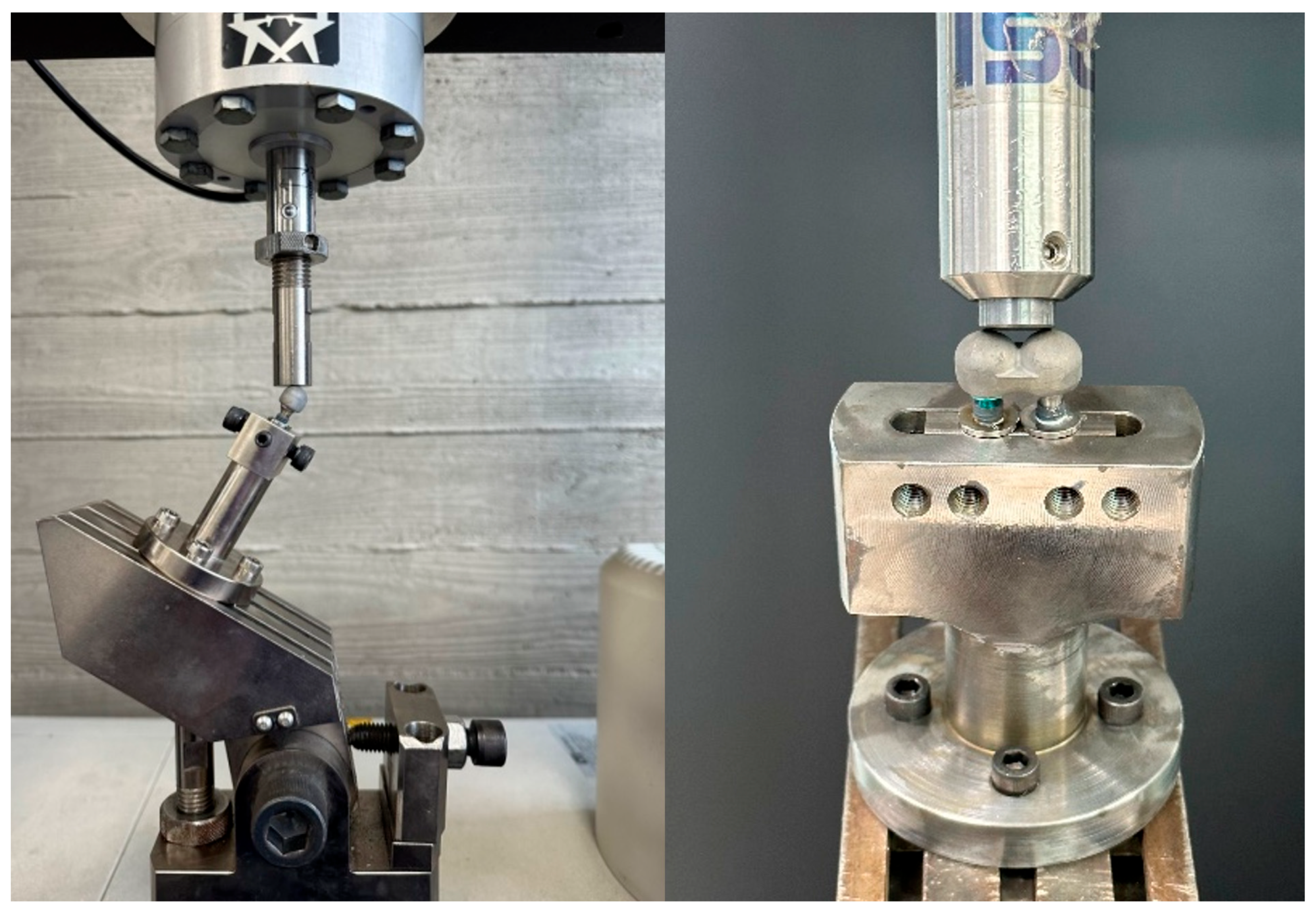
2.3. Mechanical Testing Protocol
2.3.1. Compressive Load Test
2.3.2. Fatigue Test
2.3.3. Precision Fit and Surface Analysis
2.4. Statistical Analysis
3. Results
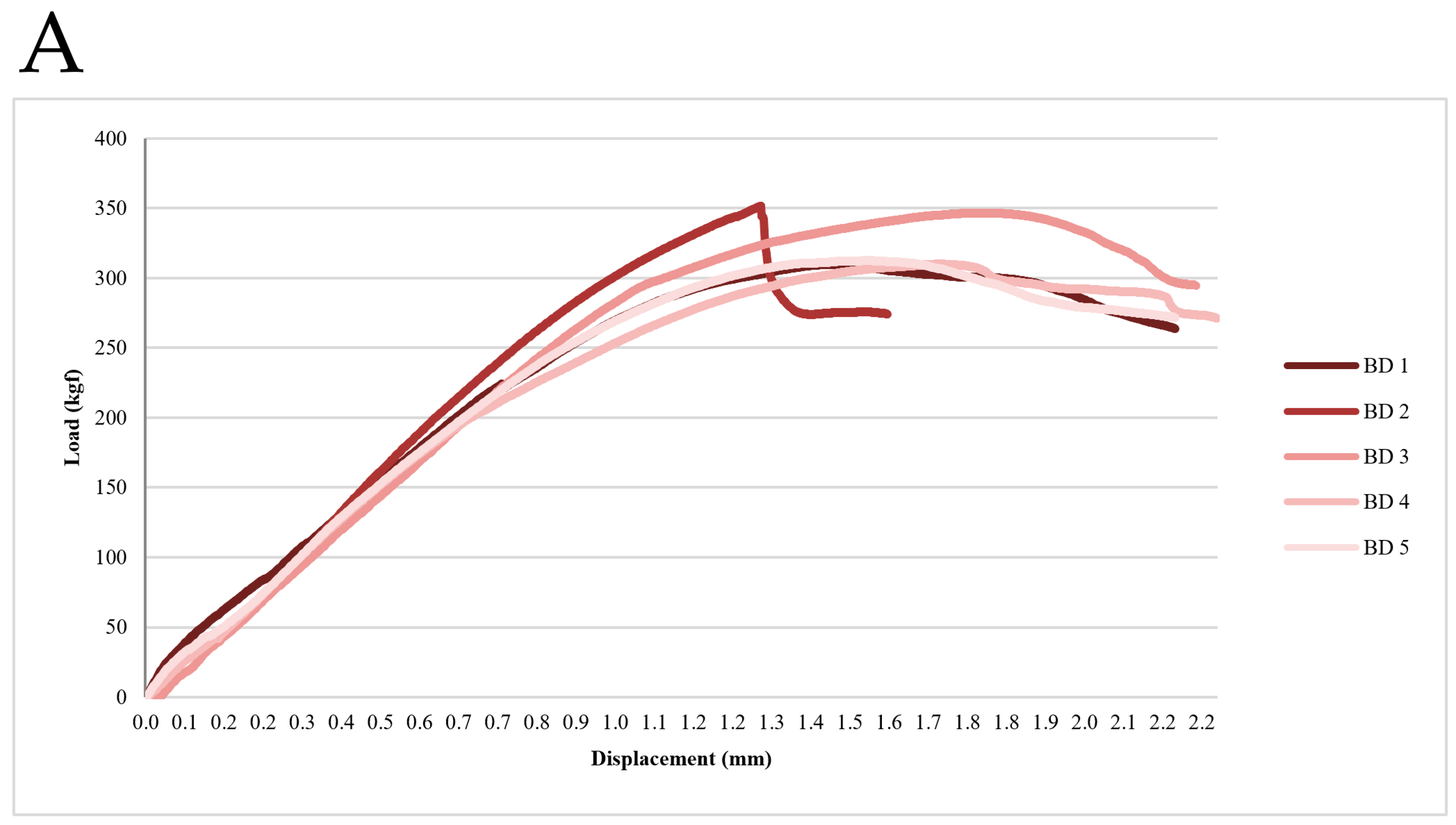
3.1. Static Compression-Strength Test Results
3.2. Fatigue Test Results
3.3. Precision Fit and Structural Integrity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cochran, D.L.; Buser, D.; Ten Bruggenkate, C.M.; Weingart, D.; Taylor, T.M.; Bernard, J.P.; Peters, F.; Simpson, J.P. The use of reduced healing times on ITI® implants with a sandblasted and acid-etched (SLA) surface: Early results from clinical trials on ITI® SLA implants. Clin. Oral Implant. Res. 2002, 13, 144–153. [Google Scholar] [CrossRef]
- Meredith, N. Assessment of implant stability as a prognostic determinant. Int. J. Prosthodont. 1998, 11, 491–501. [Google Scholar]
- Albrektsson, T.; Dahlin, C.; Jemt, T.; Sennerby, L.; Turri, A.; Wennerberg, A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin. Implant. Dent. Relat. Res. 2014, 16, 155–165. [Google Scholar] [CrossRef]
- Şahin, S.; Cehreli, M.C.; Yalçın, E. The influence of functional forces on the biomechanics of implant-supported prostheses—A review. J. Dent. 2002, 30, 271–282. [Google Scholar] [CrossRef]
- Chee, Y. Implant Surgery for Fixed Implant-supported Prostheses in the Edentulous Mandible: A Case Report. J. Implantol. Appl. Sci. 2021, 25, 84–94. [Google Scholar] [CrossRef]
- Wang, T.-M.; Leu, L.-J.; Wang, J.-S.; Lin, L.-D. Effects of prosthesis materials and prosthesis splinting on peri-implant bone stress around implants in poor-quality bone: A numeric analysis. Int. J. Oral Maxillofac. Implant. 2002, 17, 231–237. [Google Scholar]
- Ahumada-DeGirolamo, D.; Azocar, A.; Delpiano-Mesina, C.; Maldonado-Cortés, P.; Muñoz, M.A.; Luque-Martínez, I.; Bravo-Gallardo, F. Splinting or non-splinting of fixed prostheses on adjacent implants: A critical review. J. Prosthodont. Res. 2023, 68, 206–214. [Google Scholar] [CrossRef]
- Manchikalapudi, G. 11. Accuracy of abutment level impressions compared to implant level impressions-a systematic review. J. Indian. Prosthodont. Soc. 2018, 18, S78. [Google Scholar] [CrossRef]
- Toia, M.; Parpaiola, A.; Stevanello, N.; Tattan, M.; Saleh, M.H.; Ravidà, A. Clinical outcomes of implant-versus abutment-level connection in screw-retained fixed dental prostheses: A 5-year randomized controlled trial. Clin. Oral Implant. Res. 2024, 35, 230–241. [Google Scholar] [CrossRef]
- Behnaz, E.; Ramin, M.; Abbasi, S.; Pouya, M.A.; Mahmood, F. The effect of implant angulation and splinting on stress distribution in implant body and supporting bone: A finite element analysis. Eur. J. Dent. 2015, 9, 311–318. [Google Scholar] [CrossRef]
- Sullivan, D.; Siddiqui, A. Wide-diameter implants: Overcoming problems. Dent. Today 1994, 13, 50, 52–57. [Google Scholar]
- Goodacre, C.J.; Bernal, G.; Rungcharassaeng, K.; Kan, J.Y. Clinical complications with implants and implant prostheses. J. Prosthet. Dent. 2003, 90, 121–132. [Google Scholar] [CrossRef]
- Balshi, T.J.; Hernandez, R.E.; Pryszlak, M.C.; Rangert, B. A comparative study of one implant versus two replacing a single molar. Int. J. Oral Maxillofac. Implant. 1996, 11, 372–378. [Google Scholar] [CrossRef]
- Kwon, M.-J.; Yeo, I.-S.; Kim, Y.-K.; Yi, Y.-J.; Yang, J.-H. Use of separate single-tooth implant restorations to replace two or more consecutive posterior teeth: A prospective cohort study for up to 1 year. J. Adv. Prosthodont. 2010, 2, 54–57. [Google Scholar] [CrossRef]
- Wittneben, J.-G.; Millen, C.; Brägger, U. Clinical Performance of Screw-Versus Cement-Retained Fixed Implant-Supported Reconstructions—A Systematic Review. Int. J. Oral Maxillofac. Implant. 2014, 29, 84–98. [Google Scholar] [CrossRef]
- Di Fiore, A.; Vigolo, P.; Graiff, L.; Stellini, E. Digital vs Conventional Workflow for Screw-Retained Single-Implant Crowns: A Comparison of Key Considerations. Int. J. Prosthodont. 2018, 31, 577–579. [Google Scholar] [CrossRef]
- Rajan, M.; Gunaseelan, R. Fabrication of a cement-and screw-retained implant prosthesis. J. Prosthet. Dent. 2004, 92, 578–580. [Google Scholar] [CrossRef]
- Katsoulis, J.; Takeichi, T.; Sol Gaviria, A.; Peter, L.; Katsoulis, K. Misfit of implant prostheses and its impact on clinical outcomes. Definition, assessment and a systematic review of the literature. Eur. J. Oral Implant. 2017, 10, 121–138. [Google Scholar]
- Proussaefs, P.; AlHelal, A. The combination prosthesis: A digitally designed retrievable cement-and screw-retained implant-supported prosthesis. J. Prosthet. Dent. 2018, 119, 535–539. [Google Scholar] [CrossRef]
- Heo, Y.-K.; Lim, Y.-J. A Newly Designed Screw-and Cement-Retained Prosthesis and Its Abutments. Int. J. Prosthodont. 2015, 28, 612–614. [Google Scholar] [CrossRef]
- Al-Omari, W.M.; Shadid, R.; Abu-Naba’a, L.; Masoud, B.E. Porcelain fracture resistance of screw-retained, cement-retained, and screw-cement-retained implant-supported metal ceramic posterior crowns. J. Prosthodont. Implant. Esthet. Reconstr. Dent. 2010, 19, 263–273. [Google Scholar] [CrossRef]
- Jemt, T. In vivo measurements of precision of fit involving implant-supported prostheses in the edentulous jaw. Int. J. Oral Maxillofac. Implant. 1996, 11, 151–158. [Google Scholar]
- Seong, D.-J.; Hong, S.-J.; Ha, S.-R. External vs. internal connection implant system. J. Korean Dent. Assoc. 2016, 54, 184–190. [Google Scholar] [CrossRef]
- Esposito, M.; Maghaireh, H.; Pistilli, R.; Grusovin, M.G.; Lee, S.T.; Gualini, F.; Yoo, J.; Buti, J. Dental implants with internal versus external connections: 1-year post-loading results from a pragmatic multicenter randomised controlled trial. Eur. J. Oral Implantol. 2015, 8, 331–344. [Google Scholar] [CrossRef]
- Michalakis, K.X.; Hirayama, H.; Garefis, P.D. Cement-retained versus screw-retained implant restorations: A critical review. Int. J. Oral Maxillofac. Implant. 2003, 18, 719–728. [Google Scholar]
- Karl, M.; Graef, F.; Taylor, T.D.; Heckmann, S.M. In vitro effect of load cycling on metal-ceramic cement-and screw-retained implant restorations. J. Prosthet. Dent. 2007, 97, 137–140. [Google Scholar] [CrossRef]
- Naguib, G.H.; Hashem, A.B.H.; Natto, Z.S.; Abougazia, A.O.; Mously, H.A.; Hamed, M.T. The effect of implant length and diameter on stress distribution of tooth-implant and implant supported fixed prostheses: An in vitro finite element analysis study. J. Oral Implantol. 2023, 49, 46–54. [Google Scholar] [CrossRef]
- Grant, B.-T.N.; Pancko, F.X.; Kraut, R.A. Outcomes of placing short dental implants in the posterior mandible: A retrospective study of 124 cases. J. Oral Maxillofac. Surg. 2009, 67, 713–717. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Ku, J.-K.; Kim, H.-S.; Yun, P.-Y.; Kim, Y.-K. A retrospective clinical study of single short implants (less than 8 mm) in posterior edentulous areas. J. Adv. Prosthodont. 2018, 10, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Song, E.S.; Ju, K.W.; Lim, D.; Han, D.-W.; Jung, T.-G.; Jeong, Y.-H.; Lee, J.-H.; Kim, B. Mechanical assessment of fatigue characteristics between single-and multi-directional cyclic loading modes on a dental implant system. Materials 2020, 13, 1545. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-M.; Tsai, C.-M.; Chang, C.-C.; Lin, C.-T.; Lee, S.-Y. Evaluation of loading conditions on fatigue-failed implants by fracture surface analysis. Int. J. Oral Maxillofac. Implant. 2005, 20, 854–859. [Google Scholar]
- Alves, D.C.C.; de Carvalho, P.S.P.; Elias, C.N.; Vedovatto, E.; Martinez, E.F. In vitro analysis of the microbiological sealing of tapered implants after mechanical cycling. Clin. Oral Investig. 2016, 20, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Wiskott, H.W.; Nicholls, J.I.; Belser, U.C.; Wiskott, H.; Nicholls, J.; Belser, U. Stress fatigue: Basic principles and prosthodontic implications. Int. J. Prosthodont. 1995, 8, 105–116. [Google Scholar]
- García-González, M.; Blasón-González, S.; García-García, I.; Lamela-Rey, M.J.; Fernández-Canteli, A.; Álvarez-Arenal, Á. Optimized planning and evaluation of dental implant fatigue testing: A specific software application. Biology 2020, 9, 372. [Google Scholar] [CrossRef]
- Choi, N.-H.; Yoon, H.-I.; Kim, T.-H.; Park, E.-J. Improvement in fatigue behavior of dental implant fixtures by changing internal connection design: An in vitro pilot study. Materials 2019, 12, 3264. [Google Scholar] [CrossRef]
- Agarwal, S.; Mistry, L.; Mistry, S.; Kadam, I.; Bhiwapurkar, S.; Talekar, S.; Kondkari, S. The Per-Ingvar Brånemark Era (1929–2014): Evolution of a No Compromise Prosthetic Dental Replacement. Cureus 2024, 16, e71708. [Google Scholar] [CrossRef]
- Sakka, S.; Baroudi, K.; Nassani, M.Z. Factors associated with early and late failure of dental implants. J. Investig. Clin. Dent. 2012, 3, 258–261. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yun, B.-H.; Jang, J.-E.; Huh, J.-B.; Jeong, C.-M. Retrievable SCP (screw-cement prosthesis) implant-supported fixed partial dentures in a fully edentulous patient: A case report. J. Korean Acad. Prosthodont. 2012, 50, 318–323. [Google Scholar] [CrossRef]
- Chung, C.; Son, M. The classification and comparison of implant prosthesis according to types of retention. Part I: Screw retained prosthesis vs cement retained prosthesis. Implantology 2010, 14, 138–151. [Google Scholar]
- Lee, A.; Okayasu, K.; Wang, H.-L. Screw-versus cement-retained implant restorations: Current concepts. Implant. Dent. 2010, 19, 8–15. [Google Scholar] [CrossRef]
- Shadid, R.; Sadaqa, N. A comparison between screw-and cement-retained implant prostheses. A literature review. J. Oral Implantol. 2012, 38, 298–307. [Google Scholar] [CrossRef]
- Aparicio, C. A new method for achieving passive fit of an interim restoration supported by Brånemark implants: A technical note. Int. J. Oral Maxillofac. Implant. 1995, 10, 614–618. [Google Scholar]
- Arcuri, L.; Pozzi, A.; Lio, F.; Rompen, E.; Zechner, W.; Nardi, A. Influence of implant scanbody material, position and operator on the accuracy of digital impression for complete-arch: A randomized in vitro trial. J. Prosthodont. Res. 2020, 64, 128–136. [Google Scholar] [CrossRef]
- Abduo, J.; Judge, R.B. Implications of implant framework misfit: A systematic review of biomechanical sequelae. Int. J. Oral Maxillofac. Implant. 2014, 29, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.C.; Cornacchia, T.M.; de Magalhães, C.S.; Bueno, A.C.; Moreira, A.N. Biomechanical evaluation of screw-and cement-retained implant-supported prostheses: A nonlinear finite element analysis. J. Prosthet. Dent. 2014, 112, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Malpartida-Carrillo, V.; Tinedo-López, P.L.; Ortiz-Culca, F.; Guerrero, M.E.; Amaya-Pajares, S.P.; Özcan, M. Fracture resistance of cement-retained, screw-retained, and combined cement-and screw-retained metalceramic implant-supported molar restorations. J. Contemp. Dent. Pr. 2020, 21, 868–873. [Google Scholar]
- Park, D.-U.; Kim, J.-Y.; Lee, J.-R.; Kim, H.-S.; Sim, H.-Y.; Lee, H.; Han, Y.-S. Screw-and-cement–retained prosthesis versus cement-retained prosthesis: Which is more appropriate for the upper premolar area? J. Dent. Sci. 2022, 17, 1553–1558. [Google Scholar] [CrossRef]
- Rella, E.; De Angelis, P.; Damis, G.; D’Addona, A.; Manicone, P.F. The application of angulated screw-channels in metal-free, implant-supported restorations: A retrospective survival analysis. Materials 2021, 14, 7006. [Google Scholar] [CrossRef]
- Fiorillo, L.; D’Amico, C.; Ronsivalle, V.; Cicciù, M.; Cervino, G. Single Dental Implant Restoration: Cemented or Screw-Retained? A Systematic Review of Multi-Factor Randomized Clinical Trials. Prosthesis 2024, 6, 871–886. [Google Scholar] [CrossRef]
- Hansson, S. Implant-abutment interface: Biomechanical study of flat top versus conical. Clin. Implant. Dent. Relat. Res. 2000, 2, 33–41. [Google Scholar]
- Byun, S.-H.; Seo, J.-H.; Cho, R.-Y.; Yi, S.-M.; Kim, L.-K.; Han, H.-S.; On, S.-W.; Kim, W.-H.; An, H.-W.; Yang, B.-E. Finite Element Analysis of a New Non-Engaging Abutment System for Three-Unit Implant-Supported Fixed Dental Prostheses. Bioengineering 2022, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- Sailer, I.; Mühlemann, S.; Zwahlen, M.; Hämmerle, C.H.; Schneider, D. Cemented and screw-retained implant reconstructions: A systematic review of the survival and complication rates. Clin. Oral Implant. Res. 2012, 23, 163–201. [Google Scholar] [CrossRef] [PubMed]
- Joda, T.; Brägger, U. Time-efficiency analysis of the treatment with monolithic implant crowns in a digital workflow: A randomized controlled trial. Clin. Oral Implant. Res. 2016, 27, 1401–1406. [Google Scholar] [CrossRef]
- Wilson, T.G., Jr. The positive relationship between excess cement and peri-implant disease: A prospective clinical endoscopic study. J. Periodontol. 2009, 80, 1388–1392. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Gallucci, G.O.; Chen, C.J.; Hanssen, S.; Naert, I.; Vandenberghe, B. Digital versus conventional implant impressions for edentulous patients: Accuracy outcomes. Clin. Oral Implant. Res. 2016, 27, 465–472. [Google Scholar] [CrossRef]
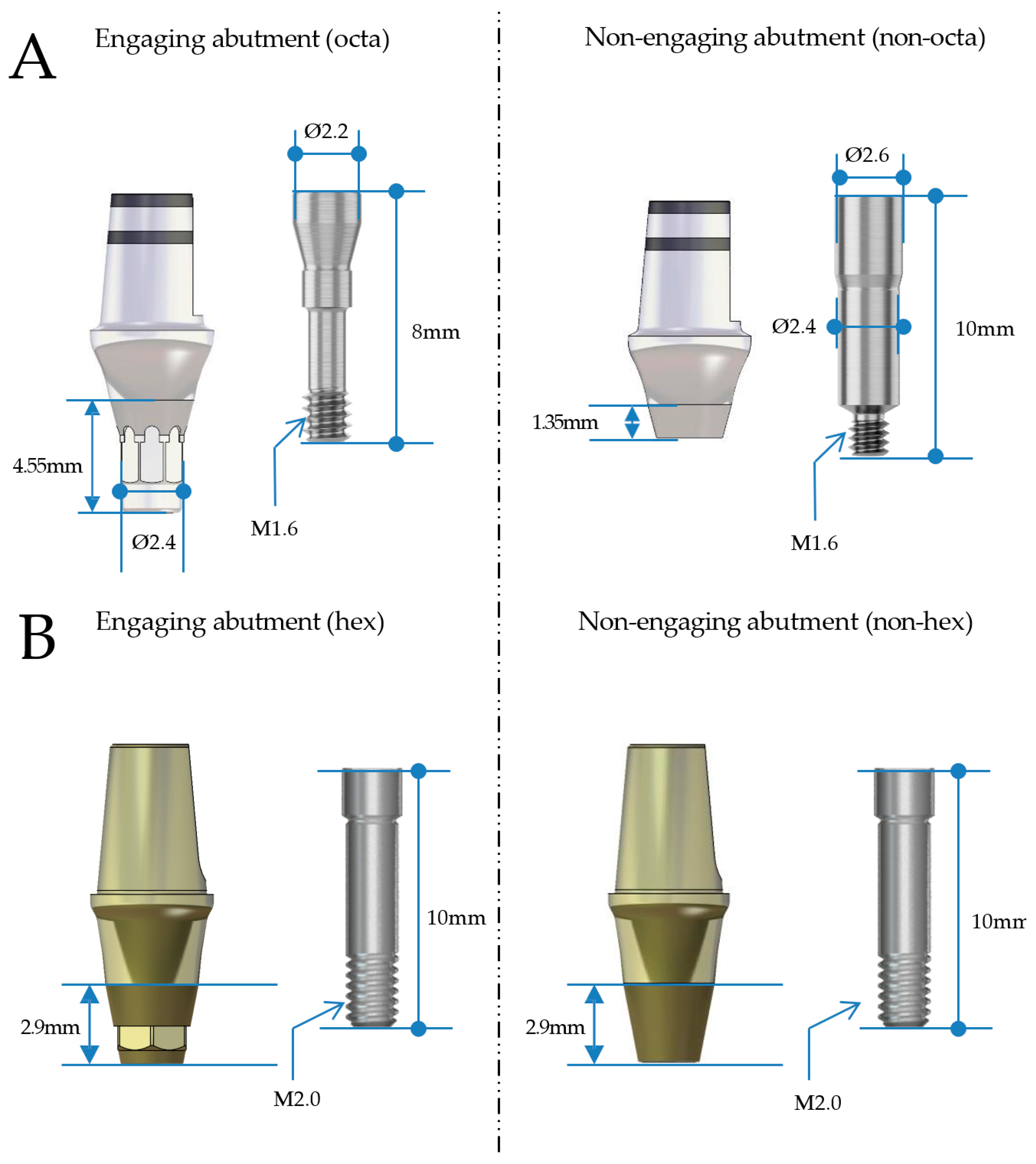

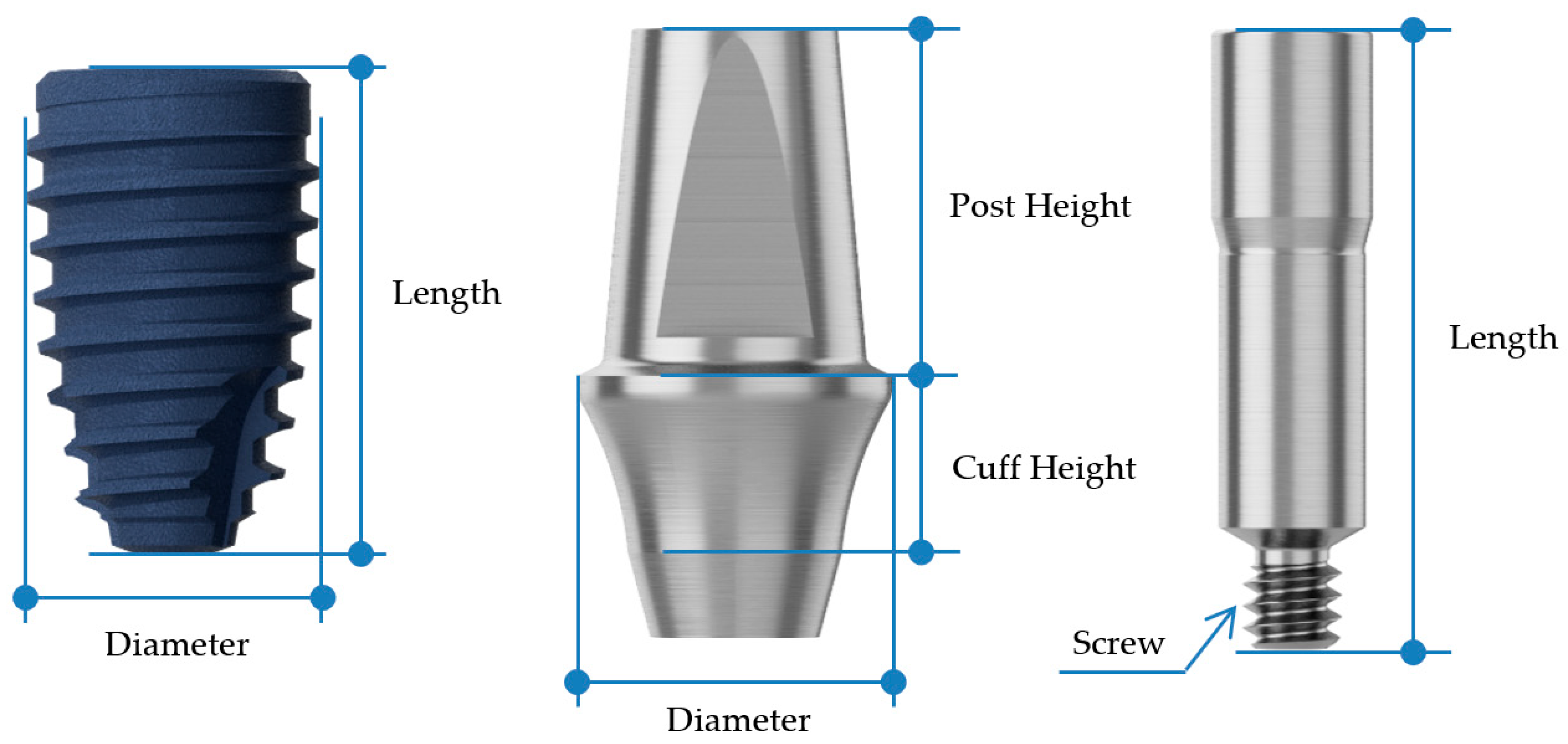
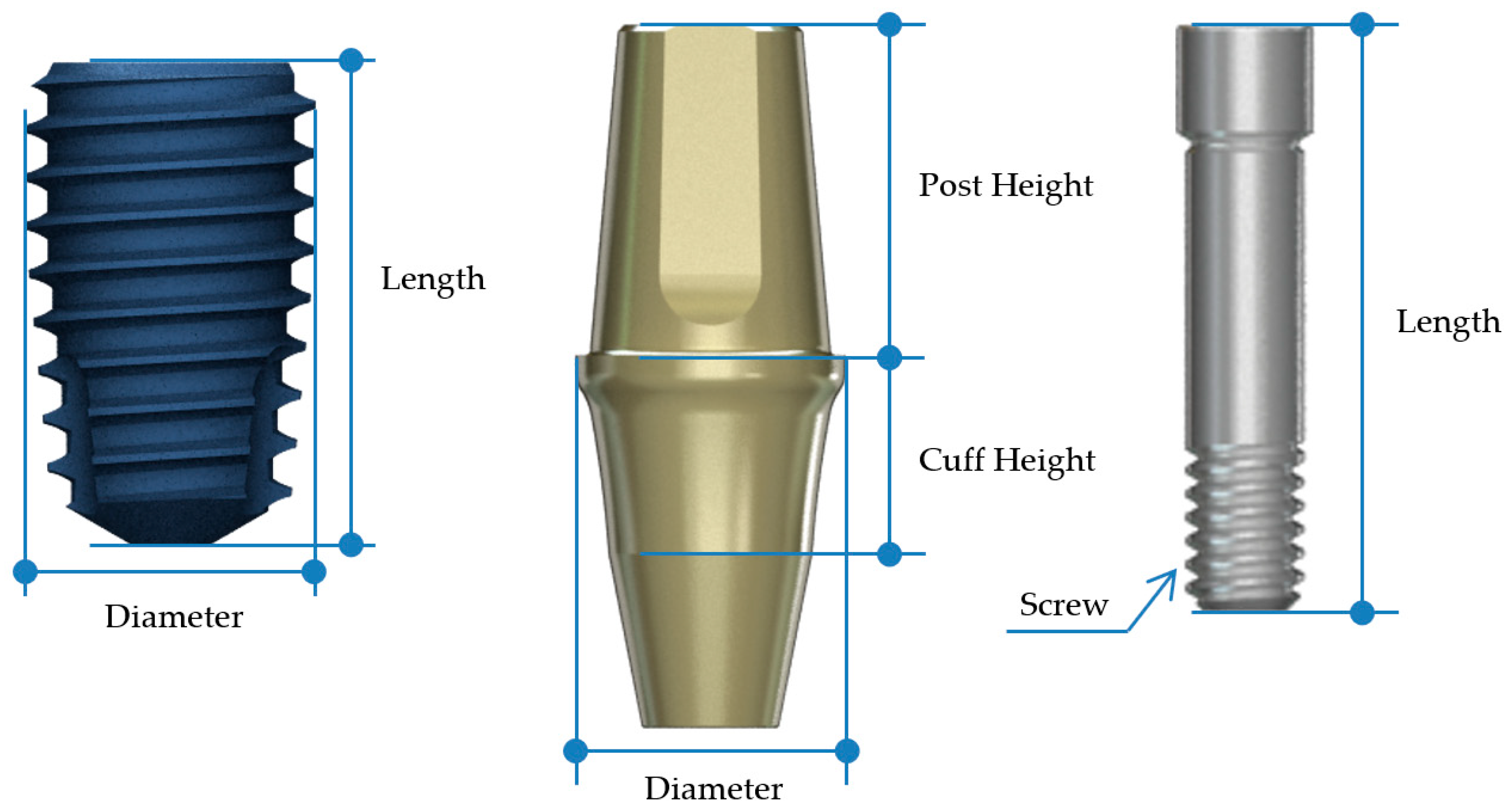




| Fixture | Abutment | Abutment Screw | ||||||
|---|---|---|---|---|---|---|---|---|
| Diameter | Length (mm) | Diameter | Cuff Height (mm) | Post Height (mm) | Screw | Length (mm) | ||
| BD | 2nd premolar | Ø4.4 | 8 | Ø5.0 | 3 | 5.5 | M1.6 | 10 |
| 1st molar | Ø4.8 | 8 | Ø7.0 | 3 | 5.5 | |||
| AO | 2nd premolar | Ø4.5 | 8 | Ø4.5 | 3 | 5.5 | M2.0 | 10 |
| 1st molar | Ø5.0 | 8 | Ø6.5 | 3 | 5.5 | |||
| BD (kgf) | AO (kgf) | |
|---|---|---|
| 1 | 309.98 | 218.41 |
| 2 | 351.75 | 238.66 |
| 3 | 346.87 | 225.15 |
| 4 | 310.25 | 227.96 |
| 5 | 312.75 | 246.92 |
| mean ± std | 326.32 ± 21.09 | 231.82 ± 11.33 |
| t-value | 8.88 | |
| p-value | <0.001 | |
| BD | ||||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | |
| Load (N) | 2534.59 | 2027.67 | 1622.1 | 1297.72 | 1038.21 | 1141.94 |
| Specimen 1 (cycles) | 50 | 997 | 11,580 | 19,849 | 5.0 × 106 | 5.0 × 106 |
| Specimen 2 (cycles) | 100 | 1028 | 10,367 | 21,733 | 5.0 × 106 | 5.0 × 106 |
| Specimen 3 (cycles) | - | - | - | - | 5.0 × 106 | 5.0 × 106 |
| AO | ||||||
| 1st | 2nd | 3rd | 4th | 5th | 6th | |
| Load (N) | 1807.83 | 1446.26 | 1156.99 | 925.61 | 1018.12 | - |
| Specimen 1 (cycles) | 30 | 4304 | 31,081 | 5.0 × 106 | 590,184 | - |
| Specimen 2 (cycles) | 18 | 4268 | 31,570 | 5.0 × 106 | 583,092 | - |
| Specimen 3 (cycles) | - | - | - | 5.0 × 106 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.-M.; Byun, S.-H.; Ahn, S.-Y.; Han, H.-S.; On, S.-W.; Park, S.-Y.; Yi, S.-M.; Park, I.-Y.; Yang, B.-E.; Kim, L.-K. Biomechanical Evaluation of a Novel Non-Engaging Abutment and Screw in Internal Implant Systems: Comparative Fatigue and Load Testing. J. Funct. Biomater. 2025, 16, 107. https://doi.org/10.3390/jfb16030107
Cho S-M, Byun S-H, Ahn S-Y, Han H-S, On S-W, Park S-Y, Yi S-M, Park I-Y, Yang B-E, Kim L-K. Biomechanical Evaluation of a Novel Non-Engaging Abutment and Screw in Internal Implant Systems: Comparative Fatigue and Load Testing. Journal of Functional Biomaterials. 2025; 16(3):107. https://doi.org/10.3390/jfb16030107
Chicago/Turabian StyleCho, Su-Min, Soo-Hwan Byun, So-Yee Ahn, Hyun-Sook Han, Sung-Woon On, Sang-Yoon Park, Sang-Min Yi, In-Young Park, Byoung-Eun Yang, and Lee-Kyoung Kim. 2025. "Biomechanical Evaluation of a Novel Non-Engaging Abutment and Screw in Internal Implant Systems: Comparative Fatigue and Load Testing" Journal of Functional Biomaterials 16, no. 3: 107. https://doi.org/10.3390/jfb16030107
APA StyleCho, S.-M., Byun, S.-H., Ahn, S.-Y., Han, H.-S., On, S.-W., Park, S.-Y., Yi, S.-M., Park, I.-Y., Yang, B.-E., & Kim, L.-K. (2025). Biomechanical Evaluation of a Novel Non-Engaging Abutment and Screw in Internal Implant Systems: Comparative Fatigue and Load Testing. Journal of Functional Biomaterials, 16(3), 107. https://doi.org/10.3390/jfb16030107








