Evaluating the Impact of Pontic Geometry on Load to Failure and Displacement in Implant-Supported Monolithic Zirconia Prostheses: An In Vitro Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Experimental Procedure
2.3. Thermal and Mechanical Cycling Fatigue
2.4. Fracture Load Test
2.5. Statistical Tests
3. Results
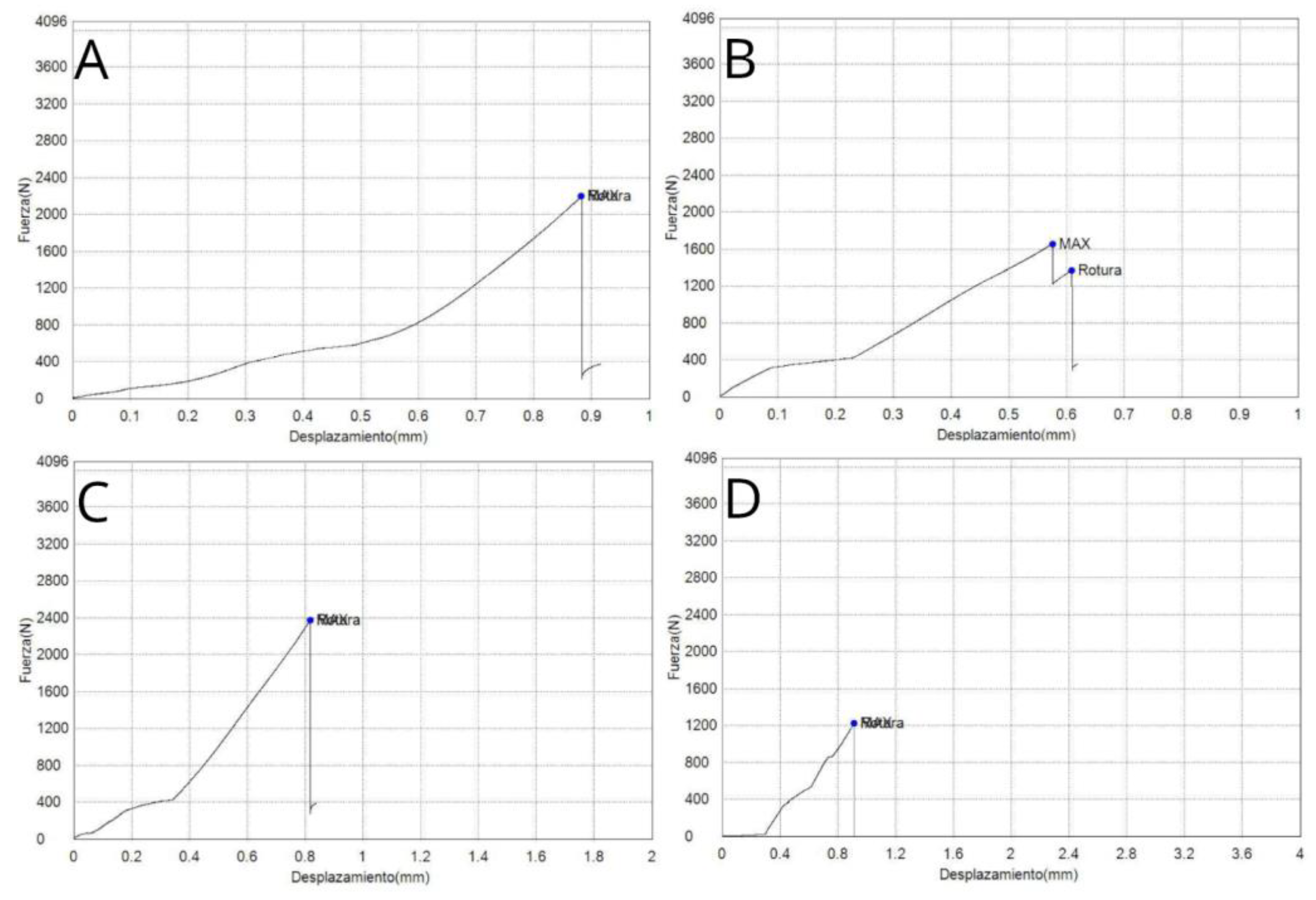
3.1. Load to Failure
3.2. Displacement Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, R.E.; Zembic, A.; Pjetursson, B.E.; Zwahlen, M.; Thoma, D.S. Systematic review of the survival rate and the incidence of biological, technical, and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin. Oral. Implants Res. 2012, 23 (Suppl. 6), 2–21. [Google Scholar] [CrossRef] [PubMed]
- Kokich, V.G.; Nappen, D.L.; Shapiro, P.A. Gingival contour and clinical crown lengththeir effect on the esthetic appearance of maxillary anterior teeth. Am. J. Orthod. 1984, 86, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Thanissorn, C.; Guo, J.; Jing Ying Chan, D.; Koyi, B.; Kujan, O.; Khzam, N.; Miranda, L.A. Success Rates and Complications Associated with Single Immediate Implants: A Systematic Review. Dent. J. 2022, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.R.; Scaini, R.; Chackartchi, T.; Solderer, A.; Schmidlin, P.R.; Testori, T. Soft tissue-related complications around anterior implants: Commentary and clinical checklist. Quintessence Int. 2023, 54, 302–318. [Google Scholar] [CrossRef]
- Nakamura, K.; Kanno, T.; Milleding, P.; Ortengren, U. Zirconia as a dental implant abutment material: A systematic review. Int. J. Prosthodont. 2010, 23, 299–309. [Google Scholar] [PubMed]
- Sailer, I.; Philipp, A.; Zembic, A.; Pjetursson, B.E.; Hämmerle, C.H.; Zwahlen, M. A systematic review of the performance of ceramic and metal implant abutments supporting fixed implant reconstructions. Clin. Oral. Implants Res. 2009, 20 (Suppl. 4), 4–31. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Tomasi, C.; Stellini, E.; Sivolella, S.; Favero, G.; Berglundh, T. Implant- supported mandibular overdentures: A cross-sectional study. Clin. Oral. Implants Res. 2012, 23, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.; Jung, R.E.; Yaman, D.; Lang, N.P. Ridge augmentation by applying bioresorbable membranes and deproteinized bovine bone mineral: A report of twelve consecutive cases. Clin. Oral. Implants Res. 2008, 19, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Prestipino, V.; Ingber, A. Esthetic high-strength implant abutments. Part I. J. Esthet. Dent. 1993, 5, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kongkiatkamon, S.; Rokaya, D.; Kengtanyakich, S.; Peampring, C. Current classification of zirconia in dentistry: An updated review. PeerJ 2023, 11, e15669. [Google Scholar] [CrossRef]
- Bergler, M.; Holst, S.; Blatz, M.B.; Eitner, S.; Wichmann, M. CAD/CAM and telescopic technology: Design options for implant-supported overdentures. Eur. J. Esthet. Dent. 2008, 3, 66–88. [Google Scholar] [PubMed]
- De Angelis, P.; Gasparini, G.; Rella, E.; De Angelis, S.; Grippaudo, C.; D’Addona, A.; Manicone, P.F. Patient Satisfaction with Implant-Supported Monolithic and Partially Veneered Zirconia Restorations. Biomed. Res. Int. 2021, 2021, 6692939. [Google Scholar] [CrossRef] [PubMed]
- Kongkiatkamon, S.; Booranasophone, K.; Tongtaksin, A.; Kiatthanakorn, V.; Rokaya, D. Comparison of fracture load of the four translucent zirconia crowns. Molecules 2021, 26, 5308. [Google Scholar] [CrossRef] [PubMed]
- Koutayasa, S.O.; Vagkopouloub, T.; Pelekanosc, S.; Koidisd, P.; Strube, J.R. Zirconia in dentistry: Evidence-based clinical breakthrough. Eur. J. Esthet. Dent. 2009, 4, 348–380. [Google Scholar]
- Zhang, Y.; Lawn, B.R. Evaluating dental zirconia. Dent. Mater. 2019, 35, 15–23. [Google Scholar] [CrossRef] [PubMed Central]
- Kelly, N.; Lamont, T. Are zirconia crowns the superior choice when restoring primary posterior molars? Evid. Based Dent. 2022, 23, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Ban, S. Classification and Properties of Dental Zirconia as Implant Fixtures and Superstructures. Materials 2021, 14, 4879. [Google Scholar] [CrossRef]
- Araújo, A.M.M.; Januário, A.B.D.N.; Moura, D.M.D.; Tribst, J.P.M.; Özcan, M.; Souza, R.O.A. Can the Application of Multi-Mode Adhesive be a Substitute to Silicatized/Silanized Y-TZP Ceramics? Braz. Dent. J. 2018, 29, 275–281. [Google Scholar] [CrossRef]
- Heboyan, A.; Vardanyan, A.; Karobari, M.I.; Marya, A.; Avagyan, T.; Tebyaniyan, H.; Mustafa, M.; Rokaya, D.; Avetisyan, A. Dental Luting Cements: An Updated Comprehensive Review. Molecules 2023, 28, 1619. [Google Scholar] [CrossRef]
- Kontonasaki, E.; Rigos, A.E.; Ilia, C.; Istantsos, T. Monolithic Zirconia: An Update to Current Knowledge. Optical Properties, Wear, and Clinical Performance. Dent. J. 2019, 7, 90. [Google Scholar] [CrossRef]
- Belli, R.; Hurle, K.; Schürrlen, J.; Petschelt, A.; Werbach, K.; Peterlik, H.; Rabe, T.; Mieller, B.; Lohbauer, U. A revised relationship between fracture toughness and Y2O3 content in modern dental zirconias. J. Eur. Ceram. Soc. 2021, 41, 7771–7782. [Google Scholar] [CrossRef]
- Liao, Y.; Gruber, M.; Lukic, H.; McLees, J.; Chen, S.; Boghosian, A.; Megremis, S. Survey of the mechanical and physical behaviors of yttria-stabilized zirconia from multiple dental laboratories. JADA Found. Sci. 2023, 2, 100018. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, X.; Wang, H.; Liu, B. Clinical evaluation of monolithic zirconia crowns for posterior teeth restorations. Medicine 2019, 98, e17385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Batson, E.R.; Cooper, L.F.; Duqum, I.; Mendonça, G. Clinical outcomes of three different crown systems with CAD/CAM technology. J. Prosthet. Dent. 2014, 112, 770–777. [Google Scholar] [CrossRef]
- Lawson, N.C.; Jurado, C.A.; Huang, C.T.; Morris, G.P.; Burgess, J.O.; Liu, P.R.; Kinderknecht, K.E.; Lin, C.P.; Givan, D.A. Effect of Surface Treatment and Cement on Fracture Load of Traditional Zirconia (3Y), Translucent Zirconia (5Y), and Lithium DisilicateCrowns. J. Prosthodont. 2019, 28, 659–665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patnana, A.K.; Chugh, V.K.; Chugh, A.; Vanga, N.R.V.; Kumar, P. Effectiveness of zirconia crowns compared with stainless steel crowns in primary posterior teeth rehabilitation: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2022, 153, 158–166.e5. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Brägger, U.; Lang, N.P.; Zwahlen, M. Comparison of survival and complication rates of tooth-supported fixed dental prostheses (FDPs) and implant-supported FDPs and single crowns (SCs). Clin. Oral. Implants Res. 2007, 18 (Suppl. 3), 97–113. [Google Scholar] [CrossRef]
- Chander, N.G.; Reddy, D.R. Comparing the influence of cuspal angulation, occlusal loading, and connector widths between tooth- and implant-supported zirconia fixed dental prosthesis. Med. J. Armed Forces India. 2024, 80, 442–448. [Google Scholar] [CrossRef]
- Luft, R.L.; da Rosa, L.S.; Machado, P.S.; Valandro, L.F.; Sarkis-Onofre, R.; Pereira, G.K.R.; Bacchi, A. Influence of connector cross-sectional geometry on the load-bearing capacity under fatigue of implant-supported zirconia fixed partial prosthesis. J. Prosthet. Dent. 2022, 128, 1335.e1–1335.e8. [Google Scholar] [CrossRef]
- Selva-Otaolaurruchi, E.J.; Fernández-Estevan, L.; Solá-Ruiz, M.F.; García-Sala-Bonmati, F.; Selva-Ribera, I.; Agustín-Panadero, R. Graphene-Doped Polymethyl Methacrylate (PMMA) as a New Restorative Material in Implant-Prosthetics: In Vitro Analysis of Resistance to Mechanical Fatigue. J. Clin. Med. 2023, 12, 1269. [Google Scholar] [CrossRef]
- Rosentritt, M.; Behr, M.; van der Zel, J.M.; Feilzer, A.J. Approach for valuating the influence of laboratory simulation. Dent. Mater. 2009, 25, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Spitznagel, F.A.; Hoppe, J.S.; Bonfante, E.A.; Campos, T.M.B.; Langner, R.; Gierthmuehlen, P.C. Failure Load and Fatigue Behavior of Monolithic and Bi-Layer Zirconia Fixed Dental Prostheses Bonded to One-Piece Zirconia Implants. Materials 2022, 15, 8465. [Google Scholar] [CrossRef] [PubMed]
- García-Sala Bonmatí, F.; Bustamante-Hernández, N.; Alonso Pérez-Barquero, J.; Maneiro-Lojo, J.; Fons-Badal, C.; Labaig-Caturla, C.; Fernández-Estevan, L.; Agustín-Panadero, R. Implant-Supported Fixed Partial Dentures with Posterior Cantilevers: In Vitro Study of Mechanical Behavior. Materials 2023, 16, 6805. [Google Scholar] [CrossRef]
- Marchetti, E.; Ratta, S.; Mummolo, S.; Tecco, S.; Pecci, R.; Bedini, R.; Marzo, G. Evaluation of an endosseous oral implant system according to UNI EN ISO 14801 fatigue test protocol. Implant Dent. 2014, 23, 665–671. [Google Scholar] [CrossRef]
- Muhsin, S.A.; Mohammed, E.K.; Bander, K. Finite Element Analysis: Connector Designs and Pontic Stress Distribution of Fixed Partial Denture Implant-Supported Metal Framework. J. Long. Term. Eff. Med. Implants 2024, 34, 33–47. [Google Scholar] [CrossRef]
- Jung, Y.G.; Peterson, I.M.; Kim, D.K.; Lawn, B.R. Lifetime-limiting strength degradation from contact fatigue in dental ceramics. J. Dent. Res. 2000, 79, 722–731. [Google Scholar] [CrossRef]
- Strub, J.R.; Gerds, T. Fracture strength and failure mode of five different single-tooth implant-abutment combinations. Int. J. Prosthodont. 2003, 16, 167–171. [Google Scholar]
- Attia, A.; Kern, M. Influence of cyclic loading and luting agents on the fracture load of two all-ceramic crown systems. J. Prosthet. Dent. 2004, 92, 551–556. [Google Scholar] [CrossRef]
- Sundh, A.; Molin, M.; Sjögren, G. Fracture resistance of yttrium oxide partially-stabilized zirconia all-ceramic bridges after veneering and mechanical fatigue testing. Dent. Mater. 2005, 21, 476–482. [Google Scholar] [CrossRef]
- Zhang, Y.; Lawn, B.R.; Malament, K.A.; Van Thompson, P.; Rekow, E.D. Damage accumulation and fatigue life of particle-abraded ceramics. Int. J. Prosthodont. 2006, 19, 442–448. [Google Scholar]
- Studart, A.R.; Filser, F.; Kocher, P.; Gauckler, L.J. Fatigue of zirconia under cyclic loading in water and its implications for the design of dental bridges. Dent. Mater. 2007, 23, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kohorst, P.; Dittmer, M.P.; Borchers, L.; Stiesch-Scholz, M. Influence of cyclic fatigue in water on the load-bearing capacity of dental bridges made of zirconia. Acta Biomater. 2008, 4, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Nothdurft, F.P.; Doppler, K.E.; Erdelt, K.J.; Knauber, A.W.; Pospiech, P.R. Influence of artificial aging on the load-bearing capability of straight or angulated zirconia abutments in implant/tooth-supported fixed partial dentures. Int. J. Oral. Maxillofac. Implants. 2010, 25, 991–998. [Google Scholar] [PubMed]
- Nothdurft, F.P.; Doppler, K.E.; Erdelt, K.J.; Knauber, A.W.; Pospiech, P.R. Fracture behavior of straight or angulated zirconia implant abutments supporting anterior single crowns. Clin. Oral. Investig. 2011, 15, 157–163. [Google Scholar] [CrossRef]
- Kohorst, P.; Borchers, L.; Strempel, J.; Stiesch, M.; Hassel, T.; Bach, F.W.; Hübsch, C. Low-temperature degradation of different zirconia ceramics for dental applications. Acta Biomater. 2012, 8, 1213–1220. [Google Scholar] [CrossRef]
- Iijima, T.; Homma, S.; Sekine, H.; Sasaki, H.; Yajima, Y.; Yoshinari, M. Influence of surface treatment of yttria-stabilized tetragonal zirconia polycrystal with hot isostatic pressing on cyclic fatigue strength. Dent. Mater. J. 2013, 32, 274–280. [Google Scholar] [CrossRef]
- Spies, B.C.; Sauter, C.; Wolkewitz, M.; Kohal, R.J. Alumina reinforced zirconia implants: Effects of cyclic loading and abutment modification on fracture resistance. Dent. Mater. 2015, 31, 262–272. [Google Scholar] [CrossRef]
- Gehrke, P.; Johannson, D.; Fischer, C.; Stawarczyk, B.; Beuer, F. In vitro fatigue and fracture resistance of one- and two-piece CAD/CAM zirconia implant abutments. Int. J. Oral. Maxillofac. Implants. 2015, 30, 546–554. [Google Scholar] [CrossRef]
- Rosentritt, M.; Siavikis, G.; Behr, M.; Kolbeck, C.; Handel, G. Approach for valuating the significance of laboratory simulation. J. Dent. 2008, 36, 1048–1053. [Google Scholar] [CrossRef]
- Mühlemann, S.; Truninger, T.C.; Stawarczyk, B.; Hämmerle, C.H.; Sailer, I. Bending moments of zirconia and titanium implant abutments supporting all-ceramic crowns after aging. Clin. Oral. Implants Res. 2014, 25, 74–81. [Google Scholar] [CrossRef]
- Butz, F.; Heydecke, G.; Okutan, M.; Strub, J.R. Survival rate, fracture strength and failure mode of ceramic implant abutments after chewing simulation. J. Oral. Rehabil. 2005, 32, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Homaei, E.; Farhangdoost, K.; Tsoi, J.K.H.; Matinlinna, J.P.; Pow, E.H.N. Static and fatigue mechanical behavior of three dental CAD/CAM ceramics. J. Mech. Behav. Biomed. Mater. 2016, 59, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, D.J.; Linderoth, E.H.; Vult Von Steyern, P. The influence of support properties and complexity on fracture strength and fracture mode of all-ceramic fixed dental prostheses. Acta Odontol. Scand. 2011, 69, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, S.S.; de Rijk, W.G. The fracture resistance of all-ceramic crowns on supporting structures with different elastic moduli. Int. J. Prosthodont. 1993, 6, 462–467. [Google Scholar] [PubMed]
- Hadzhigaev, V.; Vlahova, A.; Mitov, G.; Zlatev, S. Fracture resistance of 3-unitmonolithic ZrO2 ceramics FPDs with different preparation designs ofthe distal abutment—An in-vitro study. Folia Med. 2023, 65, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.H.; Huh, Y.H.; Park, C.J.; Cho, L.R. Effect of materials on axial displacement and internal discrepancy of cement-retained implant-supported prostheses. J. Prosthet. Dent. 2022, 127, 462–469. [Google Scholar] [CrossRef]
- Gonzaga, C.C.; Garcia, P.P.; Wambier, L.M.; Prochnow, F.H.O.; Madeira, L.; Cesar, P.F. Dotooth-supported zirconia restorations present more technical failures related to fracture or loss of retention? Systematic review and meta-analysis. Clin. Oral. Investig. 2022, 26, 5129–5142. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Bonachela, W.C.; Lopes Moreno, J.M.; Orlato Rossetti, P.H.; Cortellari, G.C.; Dedavid, B.A.; Calvo-Guirado, J.L. Zirconium Oxide Three-Unit Fixed Partial Denture Frameworks Supported by Dental Implants in Acceptable and Reduced Interocclusal Space Possibilities: Pilot In Vitro Fracture Strength and Fractographic Analyses. Int. J. Oral. Maxillofac. Implants. 2019, 34, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Kihara, H.; Sugawara, S.; Yokota, J.; Takafuji, K.; Fukazawa, S.; Tamada, A.; Hatakeyama, W.; Kondo, H. Applications of three-dimensional printers in prosthetic dentistry. J. Oral. Sci. 2021, 63, 212–216. [Google Scholar] [CrossRef]
- Leitão, C.I.M.B.; Fernandes, G.V.O.; Azevedo, L.P.P.; Araújo, F.M.; Donato, H.; Correia, A.R.M. Clinical performance of monolithic CAD/CAM tooth-supported zirconia restorations: Systematic review and meta-analysis. J. Prosthodont. Res. 2022, 66, 374–384. [Google Scholar] [CrossRef]
- Honda, J.; Komine, F.; Kusaba, K.; Kitani, J.; Matsushima, K.; Matsumura, H. Fracture loads of screw-retained implant-supported zirconia prostheses after thermal and mechanical stress. J. Prosthodont. Res. 2020, 64, 313–318. [Google Scholar] [CrossRef]
- Matta, R.E.; Eitner, S.; Stelzer, S.P.; Reich, S.; Wichmann, M.; Berger, L. Ten-year clinical performance of zirconia posterior fixed partial dentures. J. Oral. Rehabil. 2022, 49, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Refaie, A.; Bourauel, C.; Fouda, A.M.; Keilig, L.; Singer, L. The effect of cyclic loading on the fracture resistance of 3D-printed and CAD/CAM milled zirconia crowns-an in vitro study. Clin. Oral. Investig. 2023, 27, 6125–6133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, W.; Li, X.C.; Bidra, A.S. Clinical outcomes of implant-supported monolithiczirconia crowns and fixed partial dentures: A systematic review. J. Prosthodont. 2023, 32, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, T.A.; Abdulmajeed, A.A.; Delgado, A.; Donovan, T.E. Fracture rate of 188695lithium disilicate and zirconia ceramic restorations after up to 7.5 years ofclinical service: A dental laboratory survey. J. Prosthet Dent. 2020, 123, 807–810. [Google Scholar] [CrossRef] [PubMed]





| Tests for Normality | ||||
|---|---|---|---|---|
| Test | Statistic | p-Value | ||
| Shapiro–Wilk | W | 0.98 | p | 0.719 |
| Study Group | n | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| Concave + Wide | 10 | 2217.09 | 129.40 | 2003.53 | 2417.63 |
| Flat + Narrow | 10 | 1458.33 | 798.13 | 521.28 | 2376.41 |
| Flat + Wide | 10 | 2259.75 | 519.70 | 1539.07 | 3281.16 |
| Concave + Narrow | 10 | 1486.56 | 397.26 | 960.86 | 2015.05 |
| Tests for Normality | ||||
|---|---|---|---|---|
| Test | Statistic | p-Value | ||
| Shapiro–Wilk | W | 0.96 | p | 0.242 |
| Study Group | n | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| Concave + Wide | 10 | 0.80 | 0.09 | 0.69 | 0.97 |
| Flat + Narrow | 10 | 0.55 | 0.25 | 0.26 | 0.94 |
| Flat + Wide | 10 | 0.83 | 0.09 | 0.68 | 0.95 |
| Concave + Narrow | 10 | 0.79 | 0.10 | 0.69 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Jiménez, S.d.l.; Martínez-Alcaraz, P.; Flores-Fraile, J.; Agustín-Panadero, R.; Lobo-Galindo, A.B.; Carbonell-López, C.; Zubizarreta-Macho, Á. Evaluating the Impact of Pontic Geometry on Load to Failure and Displacement in Implant-Supported Monolithic Zirconia Prostheses: An In Vitro Analysis. J. Funct. Biomater. 2025, 16, 76. https://doi.org/10.3390/jfb16030076
Cruz-Jiménez Sdl, Martínez-Alcaraz P, Flores-Fraile J, Agustín-Panadero R, Lobo-Galindo AB, Carbonell-López C, Zubizarreta-Macho Á. Evaluating the Impact of Pontic Geometry on Load to Failure and Displacement in Implant-Supported Monolithic Zirconia Prostheses: An In Vitro Analysis. Journal of Functional Biomaterials. 2025; 16(3):76. https://doi.org/10.3390/jfb16030076
Chicago/Turabian StyleCruz-Jiménez, Silvia de la, Paloma Martínez-Alcaraz, Javier Flores-Fraile, Rubén Agustín-Panadero, Ana Belén Lobo-Galindo, Concepción Carbonell-López, and Álvaro Zubizarreta-Macho. 2025. "Evaluating the Impact of Pontic Geometry on Load to Failure and Displacement in Implant-Supported Monolithic Zirconia Prostheses: An In Vitro Analysis" Journal of Functional Biomaterials 16, no. 3: 76. https://doi.org/10.3390/jfb16030076
APA StyleCruz-Jiménez, S. d. l., Martínez-Alcaraz, P., Flores-Fraile, J., Agustín-Panadero, R., Lobo-Galindo, A. B., Carbonell-López, C., & Zubizarreta-Macho, Á. (2025). Evaluating the Impact of Pontic Geometry on Load to Failure and Displacement in Implant-Supported Monolithic Zirconia Prostheses: An In Vitro Analysis. Journal of Functional Biomaterials, 16(3), 76. https://doi.org/10.3390/jfb16030076










