Antibiotic Action, Drug Delivery, Biodegradability, and Wound Regeneration Characteristics of Surgical Sutures and Cutting-Edge Surgical Suture Manufacturing Technologies
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Cutting-Edge Characteristics of Surgical Sutures
3.1.1. Antibacterial Characteristics of Surgical Sutures
3.1.2. Drug Delivery Characteristics of Surgical Sutures
3.1.3. Biodegradability Characteristics of Surgical Sutures
3.1.4. Wound Regeneration Characteristics of Surgical Sutures
3.2. Latest Manufacturing Technologies for Surgical Sutures
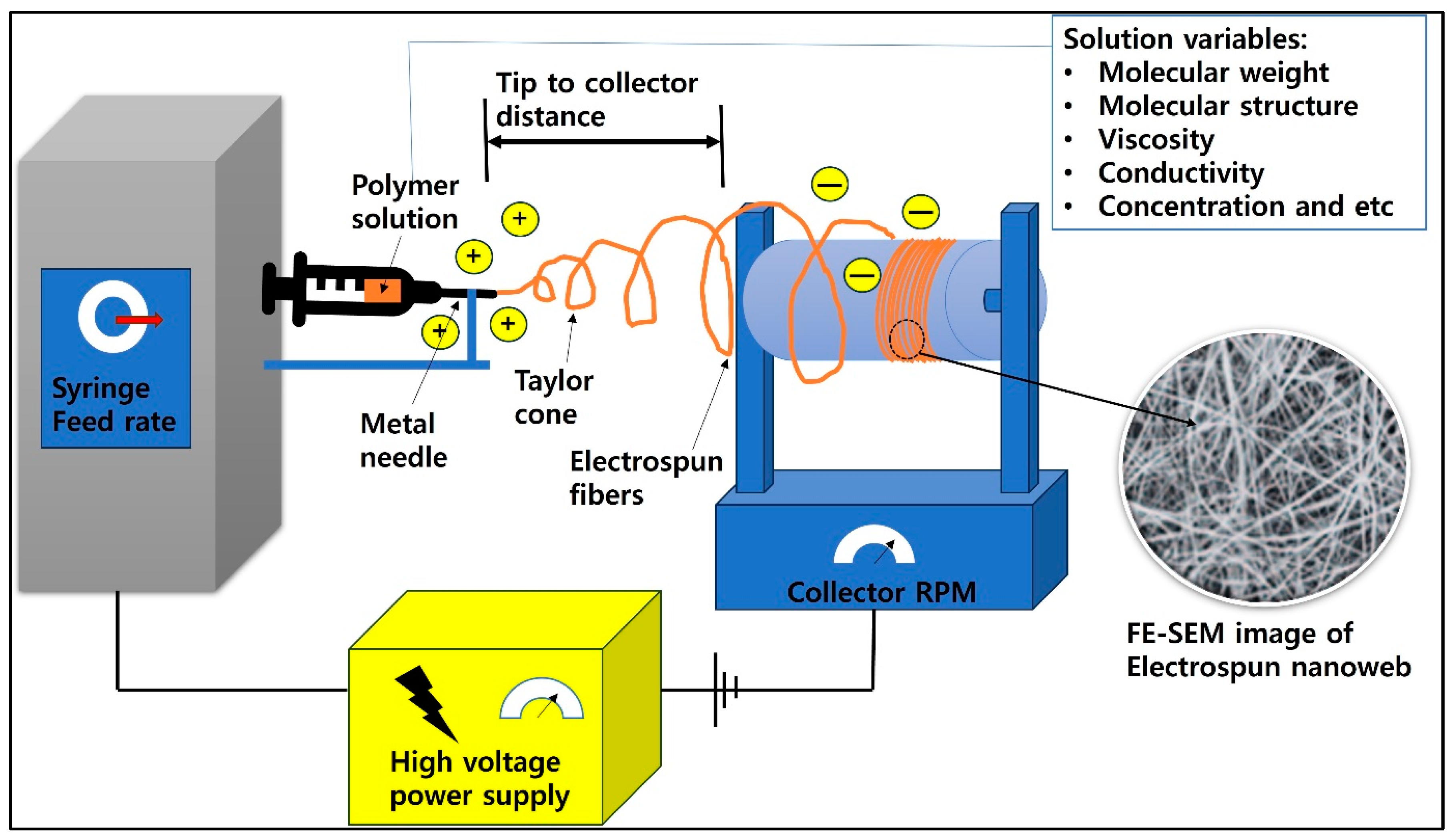
3.2.1. Electrospinning
3.2.2. Three-Dimensional Printing
4. Conclusions
- Current status
- The main obstacles to be addressed
- Future perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Pajnik, J.; Milovanovic, S.; Stojanovic, D.; Dimitrijevic-Brankovic, S.; Jankovic-Častvan, I.; Uskokovic, P. Utilization of supercritical carbon dioxide for development of antibacterial surgical sutures. J. Supercrit. Fluids 2022, 181, 105490. [Google Scholar] [CrossRef]
- James, B.; Ramakrishnan, R.; Aprem, A.S. Development of environmentally safe biodegradable, antibacterial surgical sutures using nanosilver particles. J. Polym. Environ. 2021, 29, 2282–2288. [Google Scholar] [CrossRef]
- Ornaghi, H.L.; Monticeli, F.M.; Agnol, L.D. A Review on Polymers for Biomedical Applications on Hard and Soft Tissues and Prosthetic Limbs. Polymers 2023, 15, 4034. [Google Scholar] [CrossRef]
- Li, Y.; Kumar, K.N.; Dabkowski, J.M.; Corrigan, M.; Scott, R.W.; Nüsslein, K.; Tew, G.N. New Bactericidal Surgical Suture Coating. Langmuir 2012, 28, 12134–12139. [Google Scholar] [CrossRef]
- Faris, A.; Khalid, L.; Hashim, M.; Yaghi, S.; Magde, T.; Bouresly, W.; Hamdoon, Z.; Uthman, A.T.; Marei, H.; Al-Rawi, N. Characteristics of suture materials used in oral surgery: Systematic review. Int. Dent. J. 2022, 72, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Z.; Qiu, H.; Wang, C.; Dong, X.; Du, J.; Li, X.; Yang, X.; Fang, H.; Ding, Y. A robust bio-based polyurethane employed as surgical suture with help to promote skin wound healing. Biomater. Adv. 2025, 166, 214048. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.; Bakmiwewa, S.; Suthananthan, A.; Idrees, M. Suture material in pancreticojejunal anastomosis: A systematic review. ANZ J. Surg. 2025, 95, 321–327. [Google Scholar] [PubMed]
- Paladini, F.; Panico, A.; Masi, A.; Russo, F.; Sannino, A.; Pollini, M. Silver-Treated Sutures for the Prevention of Biofilm-Associated Surgical Site Infections. Antibiotics 2025, 14, 49. [Google Scholar] [CrossRef]
- Rineksa, G.; Whulanza, Y.; Gozan, M. Mechanical characteristics of thermoplastic sago starch-based biopolymer composite reinforced with microcrystalline cellulose (MCC) as a potential surgical suture material. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2025. [Google Scholar]
- Das, S.; Ghosh, D.; Khatib, M.N.; Balaraman, A.K.; Roopashree, R.; Kaur, M.; Srivastava, M.; Barwal, A.; Prasad, G.S.; Rajput, P. Bioelectric surgical sutures: Advancing wound healing through mechano-electrical stimulation. Int. J. Surg. Open 2025, 63, 28–34. [Google Scholar]
- Makrygiannis, I.H.; Nikolaidis, A.K.; Tilaveridis, I.; Kouvelas, A.D.; Lykakis, I.Ν.; Venetis, G. Coated sutures for use in oral surgery: A comprehensive review. Clin. Oral Investig. 2025, 29, 109. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Yang, M.; Song, G.; Wang, Y.; Sun, H.; Yuan, T.; Rao, J.; Lü, B.; Yao, C. Bio-inspired helical-hollow bacterial cellulose fiber for suture materials. Chem. Eng. J. 2025, 505, 159670. [Google Scholar] [CrossRef]
- Abdulagatov, I.M.; Khanaliev, V.Y.; Ragimov, R.M.; Maksumova, A.M.; Khamidov, M.A.; Abdullaeva, N.M.; Mollaeva, N.R. Atomic-layer-deposition application for antibacterial coating of biomedical materials: Surgical sutures. Biomed. Mater. 2025, 20, 025012. [Google Scholar] [CrossRef] [PubMed]
- Bibire, T.; Yilmaz, O.; Ghiciuc, C.M.; Bibire, N.; Dănilă, R. Biopolymers for surgical applications. Coatings 2022, 12, 211. [Google Scholar] [CrossRef]
- Zhang, Q.; Qiao, Y.; Zhu, J.; Li, Y.; Li, C.; Lin, J.; Li, X.; Han, H.; Mao, J.; Wang, F. Electroactive and antibacterial surgical sutures based on chitosan-gelatin/tannic acid/polypyrrole composite coating. Compos. Part B Eng. 2021, 223, 109140. [Google Scholar] [CrossRef]
- Lou, C.-W.; Hung, C.-Y.; Wei, M.; Li, T.; Shiu, B.-C.; Lin, J.-H. Antibacterial Surgical Sutures Developed Using Electrostatic Yarn Wrapping Technology. J. Funct. Biomater. 2023, 14, 248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zou, Y.; Tang, L.; Liu, X.; Hu, M.; Han, H.; Li, Y.; Wang, F.; Wang, L.; Mao, J. Stage-controlled antibacterial surgical sutures based on curcumin@ ZIF-8 functional coating for improved wound healing. Prog. Org. Coat. 2023, 184, 107829. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Yang, X.; Zhang, F.; Pan, Z. Sustainable Antibacterial Surgical Suture Based on Recycled Silk Resource by an Internal Combination of Inorganic Nanomaterials. ACS Appl. Mater. Interfaces 2023, 15, 29971–29981. [Google Scholar] [CrossRef]
- Wang, X.; Liu, P.; Wu, Q.; Zheng, Z.; Xie, M.; Chen, G.; Yu, J.; Wang, X.; Li, G.; Kaplan, D. Sustainable antibacterial and anti-inflammatory silk suture with surface modification of combined-therapy drugs for surgical site infection. ACS Appl. Mater. Interfaces 2022, 14, 11177–11191. [Google Scholar] [CrossRef]
- Altun, E.; Bayram, C.; Gultekinoglu, M.; Matharu, R.; Delbusso, A.; Homer-Vanniasinkam, S.; Edirisinghe, M. Pressure-Spun Fibrous Surgical Sutures for Localized Antibacterial Delivery: Development, Characterization, and In Vitro Evaluation. ACS Appl. Mater. Interfaces 2023, 15, 45561–45573. [Google Scholar] [CrossRef] [PubMed]
- Syukri, D.M.; Nwabor, O.F.; Singh, S.; Voravuthikunchai, S.P. Antibacterial functionalization of nylon monofilament surgical sutures through in situ deposition of biogenic silver nanoparticles. Surf. Coat. Technol. 2021, 413, 127090. [Google Scholar] [CrossRef]
- Rakhmatullayeva, D.; Ospanova, A.; Bekissanova, Z.; Jumagaziyeva, A.; Savdenbekova, B.; Seidulayeva, A.; Sailau, A. Development and characterization of antibacterial coatings on surgical sutures based on sodium carboxymethyl cellulose/chitosan/chlorhexidine. Int. J. Biol. Macromol. 2023, 236, 124024. [Google Scholar]
- La Rosa, G.R.M.; Scapellato, S.; Cicciù, M.; Pedullà, E. Antimicrobial Activity of Antibacterial Sutures in Oral Surgery: A Scoping Review. Int. Dent. J. 2024, 74, 688–695. [Google Scholar] [PubMed]
- Wu, Q.; He, C.; Wang, X.; Zhang, S.; Zhang, L.; Xie, R.; Li, Y.; Wang, X.; Han, Z.; Zheng, Z. Sustainable antibacterial surgical suture using a facile scalable silk-fibroin-based berberine loading system. ACS Biomater. Sci. Eng. 2021, 7, 2845–2857. [Google Scholar]
- Li, H.; Wang, Z.; Robledo-Lara, J.A.; He, J.; Huang, Y.; Cheng, F. Antimicrobial surgical sutures: Fabrication and application of infection prevention and wound healing. Fibers Polym. 2021, 22, 2355–2367. [Google Scholar] [CrossRef]
- Al-Sawarees, D.K.; Darwish, R.M.; Abu-Zurayk, R.; Masri, M.A. Assessing silver nanoparticle and antimicrobial combinations for antibacterial activity and biofilm prevention on surgical sutures. J. Appl. Microbiol. 2024, 135, lxae063. [Google Scholar] [CrossRef]
- Jamshaid, H.; Mishra, R.; Hussain, U.; Rajput, A.W.; Tichy, M.; Muller, M. Natural Fiber Based Antibacterial, Wound Healing Surgical Sutures by the Application of Herbal Antimicrobial Compounds. J. Nat. Fibers 2022, 19, 9531–9546. [Google Scholar]
- Chen, Y.-G.; Li, C.-X.; Zhang, Y.; Qi, Y.-D.; Liu, X.-H.; Feng, J.; Zhang, X.-Z. Hybrid suture coating for dual-staged control over antibacterial actions to match well wound healing progression. Mater. Horiz. 2022, 9, 2824–2834. [Google Scholar] [CrossRef]
- Chua, R.; Lim, S.K.; Chee, C.F.; Chin, S.P.; Kiew, L.V.; Sim, K.S.; Tay, S.T. Surgical site infection and development of antimicrobial sutures: A review. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 828–845. [Google Scholar] [PubMed]
- Matz, D.; Engelhardt, S.; Wiencierz, A.; Soysal, S.D.; Misteli, H.; Kirchhoff, P.; Heizmann, O. Do Antibacterial Skin Sutures Reduce Surgical Site Infections After Elective Open Abdominal Surgery?—A Prospective, Randomized Controlled Single-Center Trial. J. Clin. Med. 2024, 13, 6803. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Cheng, M.; Wan, K.; Yan, S.; Peng, W.; Duan, G.; Wu, Y.; Wen, L. MoO3-X nanodots coated suture for combating surgical site infection via antibacterial and anti-inflammatory properties. Nanomed. Nanotechnol. Biol. Med. 2024, 60, 102757. [Google Scholar]
- Liu, M.; Zhang, Y.; Liu, K.; Zhang, G.; Mao, Y.; Chen, L.; Peng, Y.; Tao, T.H. Biomimicking antibacterial opto-electro sensing sutures made of regenerated silk proteins. Adv. Mater. 2021, 33, 2004733. [Google Scholar]
- Baygar, T.; Ugur, A.; Karaca, I.R.; Kilinc, Y.; Gultekin, S.E.; Sarac, N. Fabrication of a Biocompatible Nanoantimicrobial Suture for Rapid Wound Healing after Surgery. ACS Omega 2024, 9, 22573–22580. [Google Scholar] [PubMed]
- Zlobina, O.; Bugaeva, I.; Glukhova, I.; Glukhova, A. Pichkhidze SYa. Experimental modification and investigation of antibacterial surgical suture material. Главный редактoр 2023, 1, 51–56. [Google Scholar]
- Bhouri, N.; Debbabi, F.; Lassoued, M.A.; Abderrahmen, M.; Abdessalem, S.B. Wound infections preventing using antibacterial chitosan/Laurus nobilis essential oil emulsion on PET braided surgical sutures. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128678. [Google Scholar] [CrossRef]
- Schmitz, N.-D.; Ovington, L.; Berlin, J.; Zhang, S.; Collier, J. Optimal usage of antibacterial sutures for wound closure in clinical trials addressing SSI. Lancet 2023, 401, 1497–1498. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Kumar, K.V.; Prabhu, A.; Shastry, R.P.; Rajesh, K. Braided silk sutures coated with photoreduced silver nanoparticles for eradicating Staphylococcus aureus and Streptococcus mutans infections. J. Microbiol. Methods 2024, 220, 106923. [Google Scholar] [CrossRef]
- Basov, A.; Dzhimak, S.; Sokolov, M.; Malyshko, V.; Moiseev, A.; Butina, E.; Elkina, A.; Baryshev, M. Changes in number and antibacterial activity of silver nanoparticles on the surface of suture materials during cyclic freezing. Nanomaterials 2022, 12, 1164. [Google Scholar] [CrossRef]
- Schäfer, S.; Aavani, F.; Köpf, M.; Drinic, A.; Stürmer, E.K.; Fuest, S.; Grust, A.L.C.; Gosau, M.; Smeets, R. Silk proteins in reconstructive surgery: Do they possess an inherent antibacterial activity? A systematic review. Wound Repair Regen. 2023, 31, 99–110. [Google Scholar] [CrossRef]
- Edis, Z.; Bloukh, S.H. Thymol, a Monoterpenoid within Polymeric Iodophor Formulations and Their Antimicrobial Activities. Int. J. Mol. Sci. 2024, 25, 4949. [Google Scholar] [CrossRef]
- Chen, Y.-G.; Li, C.-X.; Zhang, Y.; Qi, Y.-D.; Feng, J.; Zhang, X.-Z. Antibacterial sutures coated with smooth chitosan layer by gradient deposition. Chin. J. Polym. Sci. 2022, 40, 1050–1061. [Google Scholar] [CrossRef]
- Kandathil, A.M.; Aslam, S.A.; Abidha, R.; Cherian, M.P.; Sudarsanan, M. Evaluation of Microbial Adherence on Antibacterial Suture Materials during Intraoral Wound Healing: A Prospective Comparative Study. J. Contemp. Dent. Pract. 2023, 24, 515–520. [Google Scholar] [PubMed]
- Ranjbar-Mohammadi, M.; Sa’di, V.; Moezzi, M.; Saghafi, R. Fabrication and characterization of antibacterial suture yarns containing PLA/tetracycline hydrochloride-PVA/chitosan nanofibers. Fibers Polym. 2022, 23, 1538–1547. [Google Scholar] [CrossRef]
- Deng, X.; Qasim, M.; Ali, A. Engineering and polymeric composition of drug-eluting suture: A review. J. Biomed. Mater. Res. Part A 2021, 109, 2065–2081. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, H.; Kim, Y.; Noh, S.; Chun, B.; Kim, J.; Park, C.; Choi, M.; Park, K.; Lee, J. A multifunctional electronic suture for continuous strain monitoring and on-demand drug release. Nanoscale 2021, 13, 18112–18124. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Gould, M.; Ali, M.A. Fabrication and characterisation of melt-extruded chitosan/keratin/PCL/PEG drug-eluting sutures designed for wound healing. Mater. Sci. Eng. C 2021, 120, 111696. [Google Scholar] [CrossRef]
- Mendez, K.; Whyte, W.; Freedman, B.R.; Fan, Y.; Varela, C.E.; Singh, M.; Cintron-Cruz, J.C.; Rothenbücher, S.E.; Li, J.; Mooney, D.J. Mechanoresponsive Drug Release from a Flexible, Tissue-Adherent, Hybrid Hydrogel Actuator. Adv. Mater. 2024, 36, 2303301. [Google Scholar] [CrossRef]
- Parikh, K.S.; Omiadze, R.; Josyula, A.; Shi, R.; Anders, N.M.; He, P.; Yazdi, Y.; McDonnell, P.J.; Ensign, L.M.; Hanes, J. Ultra-thin, high strength, antibiotic-eluting sutures for prevention of ophthalmic infection. Bioeng. Transl. Med. 2021, 6, e10204. [Google Scholar] [CrossRef]
- Manoukian, O.S.; Rudraiah, S.; Arul, M.R.; Bartley, J.M.; Baker, J.T.; Yu, X.; Kumbar, S.G. Biopolymer-nanotube nerve guidance conduit drug delivery for peripheral nerve regeneration: In vivo structural and functional assessment. Bioact. Mater. 2021, 6, 2881–2893. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Liang, X.; Shen, C.; Pei, Y.; Wu, B.; He, Z. Carbohydrates used in polymeric systems for drug delivery: From structures to applications. Pharmaceutics 2022, 14, 739. [Google Scholar] [CrossRef] [PubMed]
- Anup, N.; Chavan, T.; Chavan, S.; Polaka, S.; Kalyane, D.; Abed, S.N.; Venugopala, K.N.; Kalia, K.; Tekade, R.K. Reinforced electrospun nanofiber composites for drug delivery applications. J. Biomed. Mater. Res. Part A 2021, 109, 2036–2064. [Google Scholar] [CrossRef]
- Cazorla-Luna, R.; Martín-Illana, A.; Notario-Pérez, F.; Ruiz-Caro, R.; Veiga, M.-D. Naturally occurring polyelectrolytes and their use for the development of complex-based mucoadhesive drug delivery systems: An overview. Polymers 2021, 13, 2241. [Google Scholar] [CrossRef]
- Mu, J.; Luo, D.; Li, W.; Ding, Y. Multiscale polymeric fibers for drug delivery and tissue engineering. Biomed. Technol. 2024, 5, 60–72. [Google Scholar] [CrossRef]
- Arun, Y.; Ghosh, R.; Domb, A.J. Biodegradable hydrophobic injectable polymers for drug delivery and regenerative medicine. Adv. Funct. Mater. 2021, 31, 2010284. [Google Scholar]
- Osi, B.; Khoder, M.; Al-Kinani, A.A.; Alany, R.G. Pharmaceutical, biomedical and ophthalmic applications of biodegradable polymers (BDPs): Literature and patent review. Pharm. Dev. Technol. 2022, 27, 341–356. [Google Scholar]
- Lee, J.; Jang, E.H.; Kim, J.H.; Park, S.; Kang, Y.; Park, S.; Lee, K.; Kim, J.-H.; Youn, Y.-N.; Ryu, W. Highly flexible and porous silk fibroin microneedle wraps for perivascular drug delivery. J. Control. Release 2021, 340, 125–135. [Google Scholar] [PubMed]
- Pires, P.C.; Mascarenhas-Melo, F.; Pedrosa, K.; Lopes, D.; Lopes, J.; Macário-Soares, A.; Peixoto, D.; Giram, P.S.; Veiga, F.; Paiva-Santos, A.C. Polymer-based biomaterials for pharmaceutical and biomedical applications: A focus on topical drug administration. Eur. Polym. J. 2023, 187, 111868. [Google Scholar]
- Rostamitabar, M.; Abdelgawad, A.M.; Jockenhoevel, S.; Ghazanfari, S. Drug-eluting medical textiles: From fiber production and textile fabrication to drug loading and delivery. Macromol. Biosci. 2021, 21, 2100021. [Google Scholar] [CrossRef]
- Wu, S.; Dong, T.; Li, Y.; Sun, M.; Qi, Y.; Liu, J.; Kuss, M.A.; Chen, S.; Duan, B. State-of-the-art review of advanced electrospun nanofiber yarn-based textiles for biomedical applications. Appl. Mater. Today 2022, 27, 101473. [Google Scholar] [PubMed]
- Naser, M.A.; Sayed, A.M.; Abdelmoez, W.; El-Wakad, M.T.; Abdo, M.S. Biodegradable suture development-based albumin composites for tissue engineering applications. Sci. Rep. 2024, 14, 7912. [Google Scholar]
- Antoniac, I.; Antoniac, A.; Gheorghita, D.; Gradinaru, S. In vitro study on biodegradation of absorbable suture Materials used for surgical applications. Mater. Plast. 2021, 58, 130–139. [Google Scholar] [CrossRef]
- Nouri, A.; Shirvan, A.R.; Li, Y.; Wen, C. Biodegradable metallic suture anchors: A review. Smart Mater. Manuf. 2023, 1, 100005. [Google Scholar]
- Szabelski, J.; Karpiński, R. Short-Term Hydrolytic Degradation of Mechanical Properties of Absorbable Surgical Sutures: A Comparative Study. J. Funct. Biomater. 2024, 15, 273. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Guo, T.; Zhou, F.; Bu, J.; Yang, S.; Dai, Z.; Teng, C.; Ouyang, H.; Wei, W. Surface coating prolongs the degradation and maintains the mechanical strength of surgical suture in vivo. Colloids Surf. B Biointerfaces 2022, 209, 112214. [Google Scholar]
- Li, Y.; Meng, Q.; Chen, S.; Ling, P.; Kuss, M.A.; Duan, B.; Wu, S. Advances, challenges, and prospects for surgical suture materials. Acta Biomater. 2023, 168, 78–112. [Google Scholar]
- Alhulaybi, Z.A. Fabrication and Characterization of Poly (lactic acid)-Based Biopolymer for Surgical Sutures. ChemEngineering 2023, 7, 98. [Google Scholar] [CrossRef]
- Mukherjee, C.; Varghese, D.; Krishna, J.; Boominathan, T.; Rakeshkumar, R.; Dineshkumar, S.; Rao, C.B.; Sivaramakrishna, A. Recent advances in biodegradable polymers–properties, applications and future prospects. Eur. Polym. J. 2023, 192, 112068. [Google Scholar]
- Al-Shalawi, F.D.; Hanim, M.A.; Ariffin, M.; Kim, C.L.S.; Brabazon, D.; Calin, R.; Al-Osaimi, M.O. Biodegradable synthetic polymer in orthopaedic application: A review. Mater. Today Proc. 2023, 74, 540–546. [Google Scholar] [CrossRef]
- Sun, S.; Chen, C.; Zhang, J.; Hu, J. Biodegradable smart materials with self-healing and shape memory function for wound healing. RSC Adv. 2023, 13, 3155–3163. [Google Scholar] [CrossRef] [PubMed]
- Zarinkolah, Z.; Hosseinkhani, S.; Nikkhah, M. Investigation on the mechanical, thermal, bio-degradation, and bio-compatibility properties of poly (lactic acid)/poly (ethylene glycol) blend. IIUM Eng. J. 2021, 22, 223–233. [Google Scholar]
- Liu, W.-C.; Chang, C.-H.; Chen, C.-H.; Lu, C.-K.; Ma, C.-H.; Huang, S.-I.; Fan, W.-L.; Shen, H.-H.; Tsai, P.-I.; Yang, K.-Y. 3D-Printed double-helical biodegradable iron suture anchor: A rabbit rotator cuff tear model. Materials 2022, 15, 2801. [Google Scholar]
- Chen, Y.; Sun, Y.; Wu, X.; Lou, J.; Zhang, X.; Peng, Z. Rotator cuff repair with biodegradable high-purity magnesium suture anchor in sheep model. J. Orthop. Transl. 2022, 35, 62–71. [Google Scholar]
- Yang, N.; Venezuela, J.; Zhang, J.; Wang, A.; Almathami, S.; Dargusch, M. Evolution of degradation mechanism and fixation strength of biodegradable Zn–Cu wire as sternum closure suture: An in vitro study. J. Mech. Behav. Biomed. Mater. 2023, 138, 105658. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Zhou, W.; Yu, D. Electrospun Medical Sutures for Wound Healing: A Review. Polymers 2022, 14, 1637. [Google Scholar] [CrossRef] [PubMed]
- Akombaetwa, N.; Bwanga, A.; Makoni, P.A.; Witika, B.A. Applications of electrospun drug-eluting nanofibers in wound healing: Current and future perspectives. Polymers 2022, 14, 2931. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Tang, L.; Li, Y.; Li, Y.; Bi, M.; Wang, J.; Wang, F.; Wang, L.; Mao, J. A multifunctional surgical suture with electroactivity assisted by oligochitosan/gelatin-tannic acid for promoting skin wound healing and controlling scar proliferation. Carbohydr. Polym. 2023, 320, 121236. [Google Scholar] [CrossRef]
- Zhu, J.; Jin, Q.; Zhao, H.; Zhu, W.; Liu, Z.; Chen, Q. Reactive oxygen species scavenging sutures for enhanced wound sealing and repair. Small Struct. 2021, 2, 2100002. [Google Scholar] [CrossRef]
- Öksüz, K.E.; Kurt, B.m.; Şahin İnan, Z.D.; Hepokur, C. Novel bioactive glass/graphene oxide-coated surgical sutures for soft tissue regeneration. ACS Omega 2023, 8, 21628–21641. [Google Scholar] [CrossRef]
- Kim, K.; Kim, H.; Do, S.; Kim, H. Potential of Aligned Electrospun PLGA/SIS Blended Nanofibrous Membrane for Tendon Tissue Engineering. Polymers 2023, 15, 2313. [Google Scholar] [CrossRef] [PubMed]
- Teno, J.; Pardo-Figuerez, M.; Evtoski, Z.; Prieto, C.; Cabedo, L.; Lagaron, J.M. Development of Ciprofloxacin-Loaded Electrospun Yarns of Application Interest as Antimicrobial Surgical Suture Materials. Pharmaceutics 2024, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Madheswaran, D.; Sivan, M.; Valtera, J.; Kostakova, E.K.; Egghe, T.; Asadian, M.; Novotny, V.; Nguyen, N.H.; Sevcu, A.; Morent, R. Composite yarns with antibacterial nanofibrous sheaths produced by collectorless alternating-current electrospinning for suture applications. J. Appl. Polym. Sci. 2022, 139, 51851. [Google Scholar] [CrossRef]
- Rivera, P.; Villegas, C.; Cabezas, R.; Pérez, B.; Torres, A.; de Dicastillo, C.L.; Garrido, L.; Galvez, P.; Araya, C.; Romero, J. Development of PLA suture materials by extrusion, electrospinning and supercritical CO2 impregnation of ibuprofen and naproxen. J. Supercrit. Fluids 2023, 194, 105854. [Google Scholar] [CrossRef]
- Mohamadinooripoor, R.; Kashanian, S.; Omidfar, K. Poly-ε-Caprolactone/Propolis Electrospun Yarns as Suture. Fibers Polym. 2023, 24, 2641–2651. [Google Scholar] [CrossRef]
- Li, Y.; Luo, H.; Li, Y.; Huang, P.; Xu, J.; Zhang, J.; Cai, P.; He, H.; Wu, J.; Li, X. Surface biofunctional bFGF-loaded electrospun suture accelerates incisional wound healing. Mater. Des. 2023, 225, 111451. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, S.; Li, J.; Wu, G.; Zhang, M.; Li, F.; Jia, L.; Zhang, Y.; Li, H.; Liu, X. Study on Preparation of Core-Spun Yarn Surgical Sutures by Compositing Drug-Loaded Nanofiber Membrane with PLA and Its Controllable Drug Release Performance. Fibers Polym. 2023, 24, 4181–4193. [Google Scholar] [CrossRef]
- Madheswaran, D.; Sivan, M.; Hauzerova, S.; Kostakova, E.K.; Jencova, V.; Valtera, J.; Behalek, L.; Mullerova, J.; Nguyen, N.H.; Capek, L. Continuous fabrication of braided composite nanofibrous surgical yarns using advanced AC electrospinning and braiding technology. Compos. Commun. 2024, 48, 101932. [Google Scholar] [CrossRef]
- Abhari, R.E.; Snelling, S.J.; Augustynak, E.; Davis, S.; Fischer, R.; Carr, A.J.; Mouthuy, P.-A. A hybrid electrospun-extruded polydioxanone suture for tendon tissue regeneration. Tissue Eng. Part A 2024, 30, 214–224. [Google Scholar] [CrossRef]
- Jin, D.; Yang, S.; Wu, S.; Yin, M.; Kuang, H. A functional PVA aerogel-based membrane obtaining sutureability through modified electrospinning technology and achieving promising anti-adhesion effect after cardiac surgery. Bioact. Mater. 2022, 10, 355–366. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, P.; Arya, D.K.; Anjum, M.M.; Poonguzhali, S.; Kumar, A.; Gupta, R.; Rajamanickam, V.M.; Singh, S.; Chaurasia, S. Biomimicking dual drug eluting twisted electrospun nanofiber yarns for post-operative wound healing. Biomed. Mater. 2023, 18, 035006. [Google Scholar] [CrossRef]
- Dang, J.; Huang, S.; Li, S.; Liu, J.; Chen, Z.; Wang, L.; Wang, J.; Chen, H.; Xu, S. Effects of the Biomimetic Microstructure in Electrospun Fiber Sutures and Mechanical Tension on Tissue Repair. ACS Appl. Mater. Interfaces 2024, 16, 29087–29097. [Google Scholar] [PubMed]
- Pisani, S.; Croce, S.; Mauramati, S.; Marmonti, M.; Cobianchi, L.; Herman, I.; Dorati, R.; Avanzini, M.A.; Genta, I.; Benazzo, M.; et al. Engineered Full Thickness Electrospun Scaffold for Esophageal Tissue Regeneration: From In Vitro to In Vivo Approach. Pharmaceutics 2022, 14, 252. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Dhingra, S.; Banerjee, A.; Saha, S.; Bhattacharyya, J.; Satapathy, B.K. Designing suture-proof cell-attachable copolymer-mediated and curcumin-β-cyclodextrin inclusion complex loaded aliphatic polyester-based electrospun antibacterial constructs. Int. J. Biol. Macromol. 2022, 216, 397–413. [Google Scholar]
- Ye, Y.; Zhou, Y.; Jing, Z.; Xu, Y.; Yin, D. Electrospun heparin-loaded nano-fiber sutures for the amelioration of achilles tendon rupture regeneration: In vivo evaluation. J. Mater. Chem. B 2021, 9, 4154–4168. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Satapathy, B.K. Understanding release kinetics and collapse proof suture retention response of curcumin loaded electrospun mats based on aliphatic polyesters and their blends. J. Mech. Behav. Biomed. Mater. 2021, 120, 104556. [Google Scholar]
- Nezhentsev, A.; Abhari, R.E.; Baldwin, M.J.; Mimpen, J.Y.; Augustyniak, E.; Isaacs, M.; Mouthuy, P.A.; Carr, A.J.; Snelling, S.J. In vitro evaluation of the response of human tendon-derived stromal cells to a novel electrospun suture for tendon repair. Transl. Sports Med. 2021, 4, 409–418. [Google Scholar] [PubMed]
- Li, Y.; Wang, J.; Wang, Y.; Cui, W. Advanced electrospun hydrogel fibers for wound healing. Compos. Part B Eng. 2021, 223, 109101. [Google Scholar] [CrossRef]
- Reddy, B.; In, K.H.; Panigrahi, B.B.; Paturi, U.M.R.; Cho, K.; Reddy, N. Modeling tensile strength and suture retention of polycaprolactone electrospun nanofibrous scaffolds by artificial neural networks. Mater. Today Commun. 2021, 26, 102115. [Google Scholar]
- Li, A.; Wang, L.; Qin, X. Manufacturing and Application of Electrospinning Nanofiber Yarn. Electrospinning Fundam. Methods Appl. 2024, 45–69. [Google Scholar] [CrossRef]
- Zhang, H.; Lan, D.; Wu, B.; Chen, X.; Li, X.; Li, Z.; Dai, F. Electrospun piezoelectric scaffold with external mechanical stimulation for promoting regeneration of peripheral nerve injury. Biomacromolecules 2023, 24, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Barcena, A.J.R.; Ravi, P.; Kundu, S.; Tappa, K. Emerging Biomedical and Clinical Applications of 3D-Printed Poly(Lactic Acid)-Based Devices and Delivery Systems. Bioengineering 2024, 11, 705. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Peng, C.; Ladani, R.; Tran, P. Analysing fracture properties of bio-inspired 3D printed suture structures. Thin-Walled Struct. 2022, 176, 109317. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Do, T.; Tran, P. Flexural behavior of 3D printed bio-inspired interlocking suture structures. Mater. Sci. Addit. Manuf. 2022, 1, 9. [Google Scholar] [CrossRef]
- Nash, R.J.; Li, Y. Experimental and numerical analysis of 3D printed suture joints under shearing load. Eng. Fract. Mech. 2021, 253, 107912. [Google Scholar] [CrossRef]
- Choi, D.J.; Choi, K.; Park, S.J.; Kim, Y.-J.; Chung, S.; Kim, C.-H. Suture fiber reinforcement of a 3D printed gelatin scaffold for its potential application in soft tissue engineering. Int. J. Mol. Sci. 2021, 22, 11600. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Y.; Tu, Q.; Wang, J. A 3D printing mold method for rapid fabrication of artificial blood vessels. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 130952. [Google Scholar] [CrossRef]
- Xu, X.; Yu, S.; Ma, L.; Mao, J.; Chen, H.; Zhu, Z.; Wang, L.; Lin, H.; Zhang, J.; Wang, Z. Multifunctional high-simulation 3D-printed hydrogel model manufacturing engineering for surgical training. Int. J. Bioprinting 2023, 9, 355–368. [Google Scholar] [CrossRef]
- Altuntas, U.; Coker, D.; Yavas, D. Creating tougher interfaces via suture morphology in 3D-printed multi-material polymer composites by fused filament fabrication. Addit. Manuf. 2023, 61, 103359. [Google Scholar] [CrossRef]
- Ye, T.; Chai, M.; Wang, Z.; Shao, T.; Liu, J.; Shi, X. 3D-Printed hydrogels with engineered nanocrystalline domains as functional vascular constructs. ACS Nano 2024, 18, 25765–25777. [Google Scholar] [CrossRef]
- Ghazi, A. A call for change. Can 3D printing replace cadavers for surgical training? Urol. Clin. 2022, 49, 39–56. [Google Scholar] [CrossRef]
- RG, A.P.; Bajaj, G.; John, A.E.; Chandran, S.; Kumar, V.V.; Ramakrishna, S. A review on the recent applications of synthetic biopolymers in 3D printing for biomedical applications. J. Mater. Sci. Mater. Med. 2023, 34, 62. [Google Scholar]
- Marconi, S.; Mauri, V.; Negrello, E.; Pugliese, L.; Pietrabissa, A.; Auricchio, F. Quantitative Assessment of 3D printed blood vessels produced with J750™ digital anatomy™ for suture simulation. bioRxiv 2022. [Google Scholar] [CrossRef]
- Wu, W.; Dong, Y.; Liu, H.; Jiang, X.; Yang, L.; Luo, J.; Hu, Y.; Gou, M. 3D printed elastic hydrogel conduits with 7, 8-dihydroxyflavone release for peripheral nerve repair. Mater. Today Bio 2023, 20, 100652. [Google Scholar] [CrossRef] [PubMed]
- Laliève, L.; Adam, J.; Nataf, P.; Khonsari, R.H. 3D-printed suture guide for thoracic and cardiovascular surgery produced during the COVID19 pandemic. Ann. 3d Print. Med. 2021, 1, 100005. [Google Scholar] [CrossRef] [PubMed]



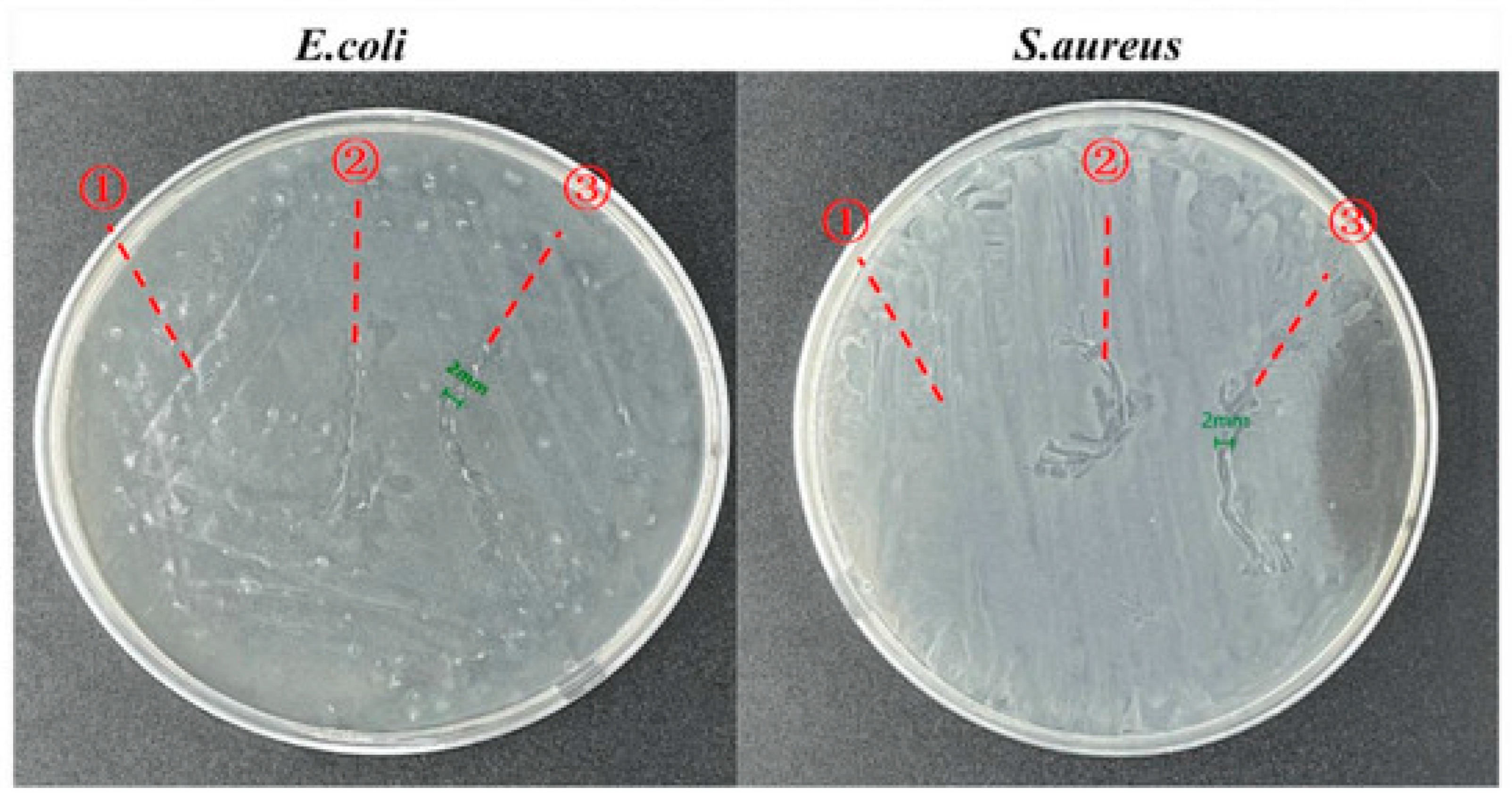

| Authors | Title | Main Contents of Antibacterial Characteristics | Effect of the Research | Bibliographic Source |
|---|---|---|---|---|
| James, B et al. | Development of environmentally safe biodegradable, antibacterial surgical sutures using nanosilver particles. | Antibacterial surgical sutures, coating a biopolymer, nano silver particles, environmentally safe, biodegradable | Antimicrobial properties were obtained by coating a biopolymer polycaprolactone material with nanosilver on the suture surface. Biocompatible polyethylene glycol was selected as the nanosilver particle dispersion solvent to improve the mechanical properties of the suture. | Journal of Polymers and the Environment, 2021. 29(7): p. 2282–2288 [2]. |
| Zhang, Q et al. | Stage-controlled antibacterial surgical sutures based on curcumin@ ZIF-8 functional coating for improved wound healing. | Antibacterial surgical sutures, E. coli and S. aureus, curcumin@ ZIF-8, SZC, wound healing, organic coatings | SZC exhibited excellent antimicrobial properties against E. coli and S. aureus. SZC has excellent mechanical properties and handling performance. | Progress in Organic Coatings, 2023. 184: p. 107829 [17]. |
| Zhang, X et al. | Sustainable Antibacterial Surgical Suture Based on Recycled Silk Resource by an Internal Combination of Inorganic Nanomaterials. | Antibacterial surgical sutures, recycled silk resources, inorganic nanomaterials, wound healing. | The surgical suture with 1.25 wt% TiO2 had a 2.40 N knot strength (diameter 143 μm) and achieved a sustainable antibacterial effect of 93.58%. Surprisingly, this suture significantly reduced the inflammatory response and promoted wound healing. | ACS applied materials & interfaces, 2023. 15(25): p. 29971–29981 [18]. |
| Wang, X et al. | Sustainable antibacterial and anti-inflammatory silk suture with surface modification of combined-therapy drugs for surgical site infection. | Antibacterial, anti-inflammatory, Sprague Dawley (SD) mice, silk suture, surgical site infection. | In vivo evaluation using Sprague Dawley (SD) mice showed that the coating reduced the expression of the inflammatory cytokines interleukin-10 (IL-10) and tumor necrosis factor-α (TNF-α), thus shortening the duration of inflammation and promoting angiogenesis. The results showed that these novel sutures exhibited stable structure, good biocompatibility, sustainable antimicrobial, and anti-inflammatory functions, and had surgical applicability. | ACS Applied Materials & Interfaces, 2022. 14(9): p. 11177–11191 [19]. |
| Pajnik, J et al. | Utilization of supercritical carbon dioxide for development of antibacterial surgical sutures. | Antibacterial, E. coli, Staphylococcus aureus, supercritical carbon dioxide, antibacterial surgical sutures. | The SSI process allowed the manufacture of surgical sutures effective against E. coli and Staphylococcus aureus. Mild process conditions (35 °C, 10 MPa, 1–6 h) allowed up to 5.9% of thymol loading. | The Journal of Supercritical Fluids, 2022. 181: p. 105490 [1]. |
| Altun, E et al. | Pressure-Spun Fibrous Surgical Sutures for Localized Antibacterial Delivery: Development, Characterization, and In Vitro Evaluation. | Pressure-spun fibrous surgical sutures, antibacterial delivery, vitro evaluation. | After 24 h of in vitro antimicrobial testing, sutures with 285 ± 12 μg/mg Tri loading exhibited antimicrobial activity against all tested bacterial strains. | ACS applied materials & interfaces, 2023. 15(39): p. 45561–45573 [20]. |
| Zhang, Q et al. | Electroactive and antibacterial surgical sutures based on chitosan-gelatin/tannic acid/polypyrrole composite coating. | Antibacterial surgical sutures, E. coli and Staphylococcus aureus, CS-GE/TA/PPy, electroactive, chitosan–gelatin/tannic acid/polypyrrole | Functional sutures showed excellent antibacterial properties against E. coli and Staphylococcus aureus. Functional sutures are conductive even when knotted, ensuring stable electrical signal transmission. | Composites Part B: Engineering, 2021. 223: p. 109140 [15]. |
| Authors | Title | Main Contents of Drug Delivery Characteristics | Effect of the Research | Bibliographic Source |
|---|---|---|---|---|
| Deng, X et al. | Engineering and polymeric composition of drug-eluting suture: a review. | Drug-eluting, polymeric composition, wound healing | Natural polymers such as lagen, silk, nylon, and cotton, and synthetic polymers such as polycaprolactone, poly(lactic-co-glycolic acid), and poly(p-deoxanone) provide a solid foundation for drug elution suture engineering. Manufacturing processes and polymer materials can effectively address wound healing requirements by controlling drug elution profiles. | Journal of Biomedical Materials Research Part A, 2021. 109(10): p. 2065–2081 [44]. |
| Lee, Y et al. | A multifunctional electronic suture for continuous strain monitoring and on-demand drug release. | Drug release, multifunctional electronic suture, continuous strain monitoring | A thermoreactive shell layer consisting of flexible poly(vinyl alcohol) (PVA) grafted onto poly(N-isopropylacrylamide) (PNIPAm) facilitates on-demand drug release through Joule heating. In vitro scratch analysis demonstrated the efficacy of DRESS in stimulation response treatment by reducing the wound area by 66% upon heat activation after 48 h, facilitating cell migration. | Nanoscale, 2021. 13(43): p. 18112–18124 [45]. |
| Deng, X et al. | Fabrication and characterisation of melt-extruded chitosan/keratin/PCL/PEG drug-eluting sutures designed for wound healing. | Drug-eluting, melt-extruded, chitosan/keratin/PCL/PEG, wound healing | A literature study comparing drug release in pure PCL and PCL/PEG blends found that the pure PCL matrix released only 4% of the drug over a 7-day period. In contrast, in the case of a polymer blend with a hydrophilic component of 30%, more than 80% of the drug was released within 72 h. In the current study, up to 20% of PEG was used, and the duration of rapid release of the drug was related to the time point at which the PEG began to decompose. | Materials Science and Engineering: C, 2021. 120: p. 111696 [46]. |
| Mendez, K et al. | Mechanoresponsive Drug Release from a Flexible, Tissue-Adherent, Hybrid Hydrogel Actuator. | Drug release, hybrid hydrogel actuator, flexible, tissue-adherent | In this study, a new type of hybrid hydrogel actuator (HHA) was introduced to facilitate enhanced drug delivery. Multi-material soft actuators can induce controllable mechanoreactive release of charged drugs in the alginate/acrylamide hydrogel layer through temporal control. | Advanced Materials, 2024. 36(43): p. 2303301 [47]. |
| Parikh, K.S et al. | Ultra-thin, high strength, antibiotic-eluting sutures for prevention of ophthalmic infection. | Antibiotic-eluting sutures, prevention of ophthalmic infection, ultra-thin, high strength. | Nanofiber-based sutures maintained strength even when loaded with a wide range of drugs, administered antibiotics for 30 days in the eyes of mice, and prevented eye infections in a bacterial keratitis mouse model. The nanofiber-based polycaprolactone sutures did not decrease in strength when loaded with 8% levofloxacin, whereas the monofilament sutures decreased in strength by more than 50%. | Bioengineering & Translational Medicine, 2021. 6(2): p. e10204 [48]. |
| Manoukian, O.S et al. | Biopolymer-nanotube nerve guidance conduit drug delivery for peripheral nerve regeneration: In vivo structural and functional assessment. | Drug delivery, biopolymer-nanotube, in vivo structural peripheral nerve regeneration. | Continuous 4-aminopyridine release amplifies neurotrophic factor release by Schwann cells, promoting axon regeneration. The TGA derivative curve shows a drug loading of 7.69% by weight within the range of 5–10% established by successful drug loading of HNT, which has not been modified in the literature. NGC was able to provide a continuous release of 4-AP for 8 weeks. | Bioactive Materials, 2021. 6(9): p. 2881–2893 [49]. |
| Bibire, T et al. | Biopolymers for surgical applications. | Drug delivery, biopolymer, excellent wet adhesion performance, antimicrobial activity, cell viability | Various combinations of PEG/PCL/chitosan/keratin mixtures achieved rapid and sustained drug release rates. PCL/PEG/chitosan–keratin complex with 30% of diclophenac potassium by weight showed high cell viability and wound healing rates during in vitro cytotoxicity testing. | Coatings, 2022. 12(2): p. 211 [14]. |
| Authors | Title | Main Contents of Biodegradability Characteristics | Effect of the Research | Bibliographic Source |
|---|---|---|---|---|
| Antoniac, I et al. | In vitro study on biodegradation of absorbable suture Materials used for surgical applications. | Biodegradation, in vitro study, absorbable suture materials, surgical applications | Depending on the pH value, it is observed that at pH = 7.4, the sample decomposes faster than under acidic conditions. In addition, important aspects were highlighted for samples P2 (lactic acid-co-glycolic acid) and P5 (poly(glycolide-co-ε-caprolactone). | Mater. Plast, 2021. 58: p. 130–139 [61]. |
| Nouri, A et al. | Biodegradable metallic suture anchors: A review. | Biodegradable, metallic suture anchors, MG, implant | Since AM technology was only recently introduced in orthopedics, the issues related to its effectiveness in bone anchor manufacturing are still unresolved. Only a small number of natural polymers, including collagen, keratin, and chitosan, have been found as suitable biodegradable implant materials. | Smart Materials in Manufacturing, 2023. 1: p. 100005 [62]. |
| Naser, M.A et al. | Biodegradable suture development-based albumin composites for tissue engineering applications. | Biodegradable suture, tissue engineering, albumin composites | Preliminary tensile tests described the mechanical profile of filament sutures (FSs), with tensile strengths ranging from 1.3 to 9.616 MPa and fracture elongation rates ranging from 11.5 to 146.64%. These results reveal the mechanical diversity of sutures, suggesting their applicability to a wide range of medical interventions. | Scientific Reports, 2024. 14(1): p. 7912 [60]. |
| Szabelski, J et al. | Short-Term Hydrolytic Degradation of Mechanical Properties of Absorbable Surgical Sutures: A Comparative Study. | Hydrolytic degradation, absorbable surgical sutures, protein composites | SafilQuick+ and MonosynQuick sutures lost their strength within 9 to 12 days, as evidenced by statistically significant changes in tensile strength. Surgical sutures play an important role in wound sutures and facilitate tissue healing processes in various medical fields. | Journal of Functional Biomaterials, 2024. 15(9): p. 273 [63]. |
| Wu, H et al. | Surface coating prolongs the degradation and maintains the mechanical strength of surgical suture in vivo. | Degradation, surgical sutures in vivo, surface coating, mechanical strength | Absorbable sutures undergo decomposition and absorption during tissue repair, whereas nonabsorbable sutures maintain tensile strength and resist absorption in combination with healing. In anterior cruciate ligament repair, which requires a long time to heal, nonabsorbable sutures outperformed absorbent sutures after 15 weeks of observation. | Colloids and Surfaces B: Biointerfaces, 2022. 209: p. 112214 [64]. |
| Alhulaybi, Z.A et al. | Fabrication and Characterization of Poly(lactic acid)-Based Biopolymer for Surgical Sutures. | Biodegradable, PLA, surgical Sutures, biopolymer | According to decomposition studies, the sutures manufactured in this study were demonstrated to degrade in physiological saline. After 15 days, the sutures lost 50% of their weight, and their pH decreased from 6.49 to 4.42. | ChemEngineering, 2023. 7(5): p. 98 [66]. |
| Authors | Title | Main Contents of Wound Regeneration Characteristics | Effect of the Research | Bibliographic Source |
|---|---|---|---|---|
| Öksüz, K.E., et al. | Novel Bioactive Glass/Graphene Oxide-Coated Surgical Sutures for Soft Tissue Regeneration | Soft tissue regeneration, bioactive glass/graphene oxide, surgical sutures | The highest cell activity was observed in the S + BGNs (95.5 ± 1.23%) and S + BGNs/GO (86.73 ± 2.83%) groups. However, there was a noticeable difference in cell activity in the S group (80.87 ± 1.93%), and consequently, it proved beneficial for cell growth because of the presence of a mixed polycrystalline hydroxyl–carbonate–apatite (HCA) layer on the suture surface. | ACS omega, 2023. 8(24): p. 21628–21641 [78]. |
| Zhu, J et al. | Reactive Oxygen Species Scavenging Sutures for Enhanced Wound Sealing and Repair | Reactive oxygen species, scavenging sutures, wound sealing and repair | The obtained sutures coated with GANP can effectively promote wound closure by maintaining tension around the wound and reducing the ROS level. Specifically, the GANP coated on the sutures can effectively eliminate ROS, upregulate anti-inflammatory molecules, and polarize macrophages around the wound site by M2 phenotype due to the efficient antioxidant activity of GA, a type of low molecular weight difference polyphenol. | Small Structures, 2021. 2(7): p. 2100002 [77]. |
| Chen, Y.-G et al. | Hybrid suture coating for dual-staged control over antibacterial actions to match well wound healing progression | Dual-staged control, wound healing progression, hybrid suture coating, antibacterial | Acidic conditions induce the explosive release of antimicrobial TA, mostly from adsorbed TA monomers. In the later stages, TA release is fully tuned to pH conditions, which depend on the degree of healing of the wound, enabling continuous antimicrobial prevention in a biologically controllable manner. | Materials Horizons, 2022. 9(11): p. 2824–2834 [28]. |
| Han, H et al. | A multifunctional surgical suture with electroactivity assisted by oligochitosan/gelatin-tannic acid for promoting skin wound healing and controlling scar proliferation | Electroactivity assisted, oligochitosan/gelatin–tannic acid, skin wound healing, controlling scar proliferation | Through in vivo experiments, S@LC@CGTP was able to alleviate inflammation and promote scar-free wound healing following the suppression of invasive inflammatory cells, downregulation of TGF-β1 and type I collagen expression, and improved collagen arrangement. Cumulatively, we have shown that S@LC@CGTP suture materials have great potential in promoting optimal, almost scar-free healing of surgical incisions. | Carbohydrate Polymers, 2023. 320: p. 121236 [76]. |
| Akombaetwa, N et al. | Applications of Electrospun Drug-Eluting Nanofibers in Wound Healing: Current and Future Perspectives | Wound healing, drug-eluting, electrospun nanofibers | Running sutures were inserted into the non-abdominal muscles of male Wistar mice at a concentration of 0.1 or 1 µg. VEGF/poly(l-lactide)(PLA) blend coatings showed increased biological activity and cell viability. | Polymers, 2022. 14(14): p. 2931 [75]. |
| Authors | Title | Main Content | Bibliographic Source |
|---|---|---|---|
| Xu, L et al. | Electrospun medical sutures for wound healing: A review. | Electrospinning, wound healing, medical sutures | Polymers, 2022. 14(9): p. 1637 [74]. |
| Teno, J et al. | Development of Ciprofloxacin-Loaded Electrospun Yarns of Application Interest as Antimicrobial Surgical Suture Materials. | Electrospun yarns, ciprofloxacin, antimicrobial, surgical suture materials | Pharmaceutics, 2024. 16(2): p. 220 [80]. |
| Madheswaran, D et al. | Composite yarns with antibacterial nanofibrous sheaths produced by collectorless alternating-current electrospinning for suture applications. | Electrospinning, composite yarns, antibacterial nanofibrous sheaths, suture applications. | Journal of Applied Polymer Science, 2022. 139(13): p. 51851 [81]. |
| Rivera, P et al. | Development of PLA suture materials by extrusion, electrospinning and supercritical CO2 impregnation of ibuprofen and naproxen. | Electrospinning, PLA suture materials, supercritical CO2 impregnation, ibuprofen, naproxen | The Journal of Supercritical Fluids, 2023. 194: p. 105854 [82]. |
| Wu, S et al. | State-of-the-art review of advanced electrospun nanofiber yarn-based textiles for biomedical applications. | Electrospun nanofiber yarn, biomedical applications, double-layered coverspun NYs, multilayered coverspun | Applied Materials Today, 2022. 27: p. 101473 [59]. |
| Mohamadinooripoor, R et al. | Poly-ε-Caprolactone/Propolis Electrospun Yarns as Suture. | Electrospun yarns, Poly-ε-Caprolactone, propolis, sutures | Fibers and Polymers, 2023. 24(8): p. 2641–2651 [83]. |
| Li, Y et al. | Surface biofunctional bFGF-loaded electrospun suture accelerates incisional wound healing. | Electrospun suture, incisional wound healing, surface biofunctional bFGF | Materials & Design, 2023. 225: p. 111451 [84]. |
| Authors | Title | Main Content | Bibliographic Source |
|---|---|---|---|
| Wickramasinghe, S et al. | Analysing fracture properties of bio-inspired 3D printed suture structures. | 3D printing, bio-inspired structure fracture properties | Thin-Walled Structures, 2022. 176: p. 109317 [101]. |
| Wickramasinghe, S., T et al. | Flexural behavior of 3D printed bio-inspired interlocking suture structures. | 3D printing, bio-inspired, interlocking suture structures, flexural behavior | Mater. Sci. Addit. Manuf, 2022. 1(9) [102]. |
| Nash, R.J et al. | Experimental and numerical analysis of 3D printed suture joints under shearing load. | 3D printing, suture joints, shearing load | Engineering Fracture Mechanics, 2021. 253: p. 107912 [103]. |
| Choi, D.J et al. | Suture fiber reinforcement of a 3D printed gelatin scaffold for its potential application in soft tissue engineering. | 3D printing, soft tissue engineering, suture fiber, gelatin scaffold | International journal of molecular sciences, 2021. 22(21): p. 11600 [104]. |
| Zhou, L et al. | A 3D printing mold method for rapid fabrication of artificial blood vessels. | 3D printing, artificial blood vessels, polyacrylamide/N,N′-methylenebisacrylamide/sodium alginate (PMSA) hydrogel tube | Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2023. 662: p. 130952 [105]. |
| Xu, X., et al. et al. | Multifunctional high-simulation 3D-printed hydrogel model manufacturing engineering for surgical training. | 3D printing, hydrogel model, manufacturing engineering for surgical training | International Journal of Bioprinting, 2023. 9(5) [106]. |
| Altuntas, U., D. Coker et al. | Creating tougher interfaces via suture morphology in 3D-printed multi-material polymer composites by fused filament fabrication. | 3D printing, multi-material polymer composites, suture morphology, fused filament fabrication. | Additive Manufacturing, 2023. 61: p. 103359 [107]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.-R. Antibiotic Action, Drug Delivery, Biodegradability, and Wound Regeneration Characteristics of Surgical Sutures and Cutting-Edge Surgical Suture Manufacturing Technologies. J. Funct. Biomater. 2025, 16, 135. https://doi.org/10.3390/jfb16040135
Han H-R. Antibiotic Action, Drug Delivery, Biodegradability, and Wound Regeneration Characteristics of Surgical Sutures and Cutting-Edge Surgical Suture Manufacturing Technologies. Journal of Functional Biomaterials. 2025; 16(4):135. https://doi.org/10.3390/jfb16040135
Chicago/Turabian StyleHan, Hye-Ree. 2025. "Antibiotic Action, Drug Delivery, Biodegradability, and Wound Regeneration Characteristics of Surgical Sutures and Cutting-Edge Surgical Suture Manufacturing Technologies" Journal of Functional Biomaterials 16, no. 4: 135. https://doi.org/10.3390/jfb16040135
APA StyleHan, H.-R. (2025). Antibiotic Action, Drug Delivery, Biodegradability, and Wound Regeneration Characteristics of Surgical Sutures and Cutting-Edge Surgical Suture Manufacturing Technologies. Journal of Functional Biomaterials, 16(4), 135. https://doi.org/10.3390/jfb16040135






