Abstract
Peripheral nerve injuries (PNIs) are a significant clinical challenge, often resulting in persistent sensory and motor deficits despite surgical repair. Autologous nerve grafts remain the gold standard for repair; however, outcomes are frequently suboptimal due to donor site morbidity and inconsistent functional recovery. A major obstacle in nerve regeneration is the formation of postoperative adhesions and fibrosis, which impede healing and necessitate revision surgeries. Nerve protectors from biological, synthetic, and hybrid materials offer a promising tissue engineering strategy to enhance nerve regeneration. These protectors are applied as a protective barrier when a nerve is severed without the gap, allowing for direct repair. They provide mechanical support and reduce scarring. Biocompatible biological wraps, including vascularized fat flaps, vein wraps, collagen-based materials, human amniotic membrane (hAM), porcine small intestinal submucosa (PSIS), and chitosan, modulate immune responses and promote vascularization. Synthetic alternatives, like polycaprolactone (PCL), provide mechanical stability with controlled degradation. Hybrid wraps, such as PCL-amnion, combine the benefits of both. Despite optimistic results, the heterogeneity of study methodologies hinders direct comparisons and standardization. This review highlights the latest developments in nerve wraps, their clinical applications, limitations, and future potential, guiding clinicians in selecting the most appropriate materials for peripheral nerve repair.
1. Introduction
Peripheral nerve injuries (PNIs) represent a significant clinical challenge affecting millions worldwide, often resulting in long-term disability, including chronic neuropathic pain, sensory loss, and motor deficits [1]. Effective management of PNI is essential for the restoration of function and improved quality of life of the affected patients [2]. However, despite advancements in surgical techniques and the introduction of biomaterials for support of nerve regeneration, managing PNI still remains complex, with patient recovery outcomes varying significantly [3].
Historically, the understanding of PNI was limited; by the late 18th century, it was widely believed that regeneration of damaged peripheral nerves was impossible [4]. Modern microsurgical techniques improve nerve regeneration after trauma; however, still, only half of the patients report satisfactory recovery, whereas one-third of patients may see little or no improvement [5]. Various nerve repair techniques offer distinct advantages and potential complications. This highlights the ongoing need for innovation and optimization in therapeutic strategies for nerve injuries [6].
Scar tissue formation is a common consequence of PNI and surgical interventions, significantly impeding nerve regeneration and predisposing patients to complications such as traction neuropathy. Understanding the mechanisms behind scar formation is crucial for improving treatment outcomes and advancing regenerative strategies. Effective prevention of scar tissue formation remains a key focus in the development of biomaterials and surgical techniques [2].
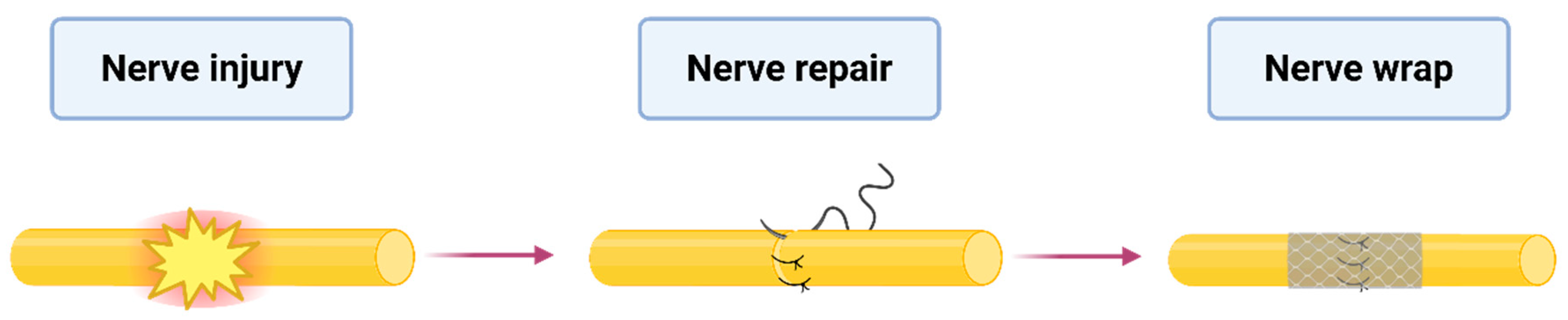
One of the most promising innovations in this field is the use of a nerve wrap—a bioengineered barrier that surrounds the nerve, providing a protective interface [7]. Nerve wraps are used when a nerve is transected without a resulting gap, where direct repair is feasible, and grafting is not required (Figure 1). An ideal nerve protector should be biocompatible, minimize the risk of fibrosis and scarring, and ensure sufficient nerve mobility [8,9]. The concept of nerve wrapping dates back to 1989, when Masear first proposed using an autologous vein as a protective sheath [10]. Since then, a diverse array of biological, synthetic, and hybrid nerve protectors has been introduced, each designed to support the natural regeneration process [9].
Figure 1.
Application of the nerve protector. The figure illustrates the application of nerve-protecting wrap after nerve injury and repair.
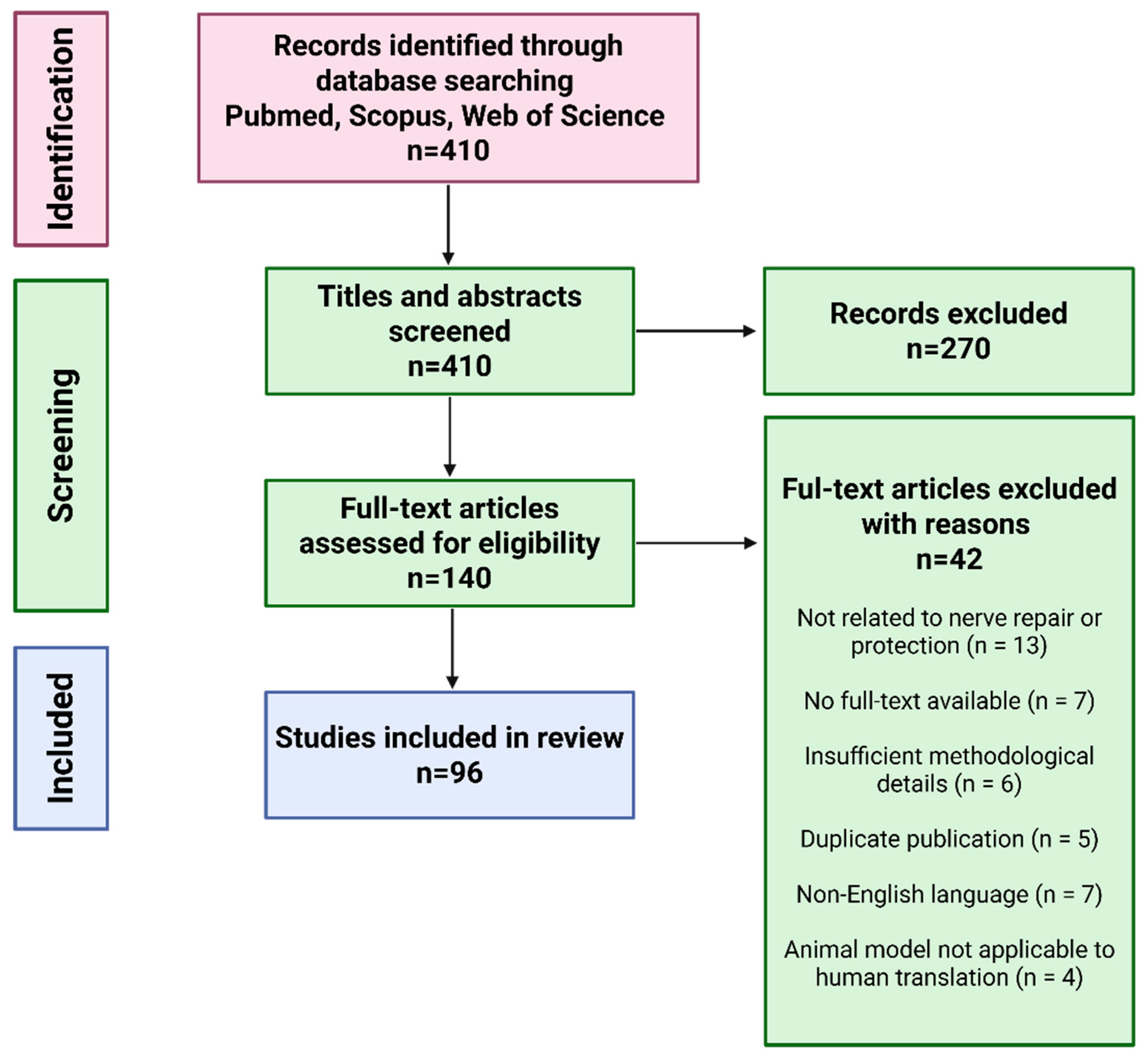
This article presents a narrative review of current strategies and materials used to protect peripheral nerves from scar tissue formation, fibrosis, and inflammation following nerve injuries or surgical procedures. Relevant literature was identified through structured searches of PubMed, Scopus, and Web of Science using keyword combinations such as “peripheral nerve protection,” “nerve protector,” “nerve wrap,” “nerve injury,” “nerve regeneration,” and “regenerative medicine” (Figure 2). Original experimental and clinical studies published in English were included, with priority given to articles from 2020 to 2025 to ensure coverage of the most recent advancements in the field. Additional studies were identified by screening the reference lists of the selected articles. The review is strictly focused on peripheral nerve protection; studies addressing the central nervous system or biomaterials developed exclusively for other tissue types were excluded.
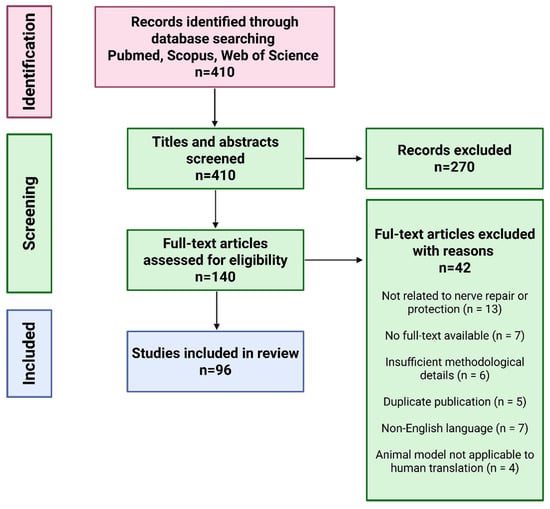
Figure 2.
Flow chart of article preselection, selection, and exclusion for nerve wrapping materials review.
This review provides a comprehensive analysis of the current progress in PNI management, emphasizing cutting-edge surgical techniques and biomaterials. It evaluates their efficacy, limitations, and clinical potential while highlighting their impact on patient outcomes. Additionally, it identifies remaining challenges and suggests future research directions for peripheral nerve repair.
2. Nerve Protection Strategies: Biological vs. Synthetic Nerve Wraps/Protectors
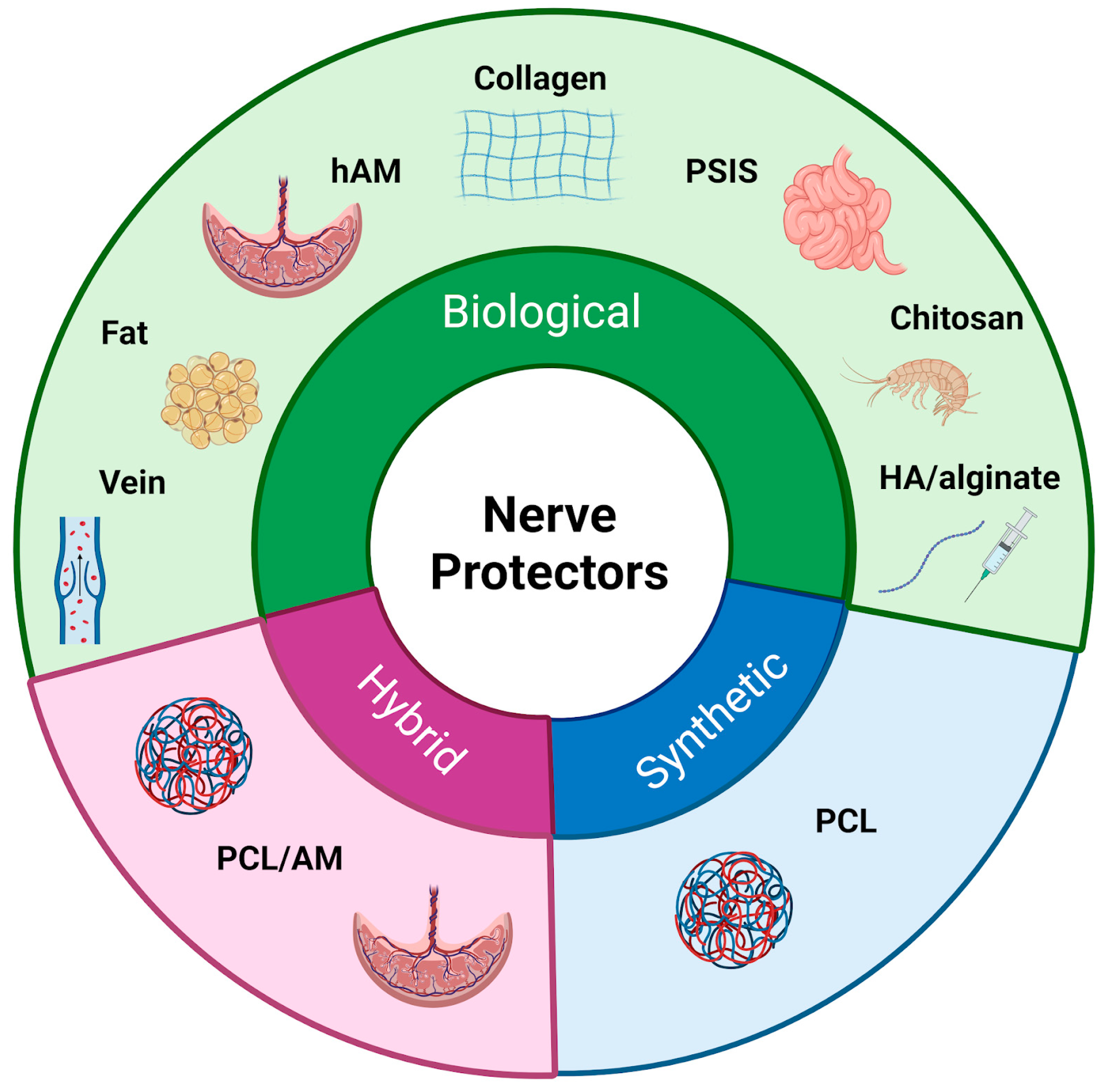
Following the initial exploration of autologous tissues for nerve protection, research has expanded to include other biological materials with regenerative potential [11] (Figure 3) (Table 1). Recognizing that natural tissues might provide an optimal environment for nerve regeneration, researchers have developed biological nerve wraps. By integrating with the surrounding tissue and utilizing intrinsic repair mechanisms, these wraps promote axonal regrowth and regeneration. As the complexity of nerve injuries became more evident, the focus shifted from tissue derivatives to engineered scaffolds, resulting in the development of biological and synthetic nerve wraps [5,12,13]. Despite differences in composition and degradation profiles, both types of materials function as physical barriers, shielding regenerating nerves from external compression and scar tissue formation [11]. The choice between biological versus synthetic wraps depends on various factors, including clinical indications, extent of injury, anatomical location, patient-specific considerations, and surgeon preference [14].
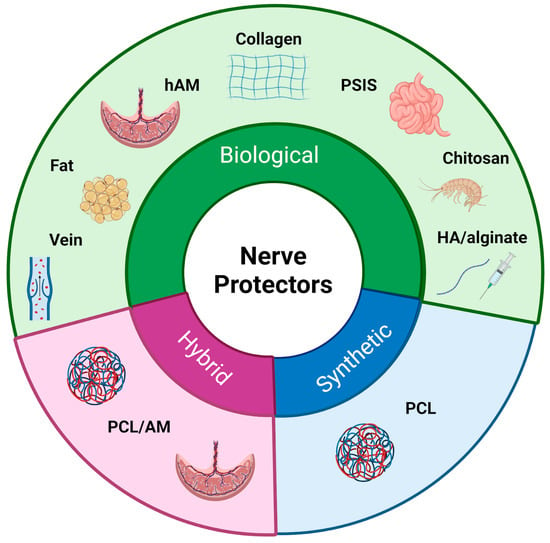
Figure 3.
Classification of nerve protectors. The figure presents a classification of nerve protectors into three main types: biological, synthetic, and hybrid materials.

Table 1.
Comparison of nerve wrapping materials based on key characteristics.
3. Biological Nerve Protectors
Pursuing effective nerve repair strategies has long driven advancements in regenerative medicine, with biological nerve protectors emerging as a cornerstone in this field. Derived from human or animal tissue, they share several key characteristics that contribute to their effectiveness. Their inherent biocompatibility significantly reduces the risk of immune rejection and enhances the likelihood of successful integration into the body [15]. Additionally, their resorbable nature ensures they naturally degrade over time, eliminating the need for surgical removal [16]. Many biological protectors, such as autologous fat flaps and vein wraps, facilitate nerve gliding and reduce adhesions. Others, like collagen-based wraps and human amniotic membrane (hAM), contribute to vascularization and immune modulation, further enhancing nerve recovery. Their anti-inflammatory and antifibrotic properties mitigate perineural scarring. However, they come with certain disadvantages, such as rapid degradation, which may lead to premature loss of structural support before the nerve has fully regenerated [17]. Seven nerve-protecting wraps made of porcine submucosa, collagen, and calcium alginate have received FDA approval for clinical use in the United States [18,19] (Table 2).

Table 2.
FDA-approved nerve protectors.
3.1. Vein Wraps for Enhancement of Nerve Regeneration
Since Masear first introduced vein wrapping (VW) for nerve protection, autologous veins have been utilized to support nerve repair techniques [10] (Table 3). This method employs freshly harvested vein tissue as a protective barrier around nerves, using the vein’s smooth intimal layer to create a gliding surface [20]. VW is accessible and cost effective, making it suitable for addressing both traumatic nerve injuries and chronic compressive neuropathies [21]. Studies suggest that VW may contribute to neuroprotection by promoting the release of basic fibroblast growth factor (bFGF), which triggers the induction of heme oxygenase-1 (HO-1), an enzyme with immunomodulatory and antinociceptive effects. In rat sciatic nerve injury models, this mechanism has shown promise in managing pain [22,23]. Furthermore, vein-derived cytokines such as interleukin-4 (IL-4) and interleukin-10 (IL-10) enhance the expression of M2 macrophage markers like CD206 and Arginase-1 (ARG1), contributing to an anti-inflammatory environment and facilitating tissue regeneration while reducing neuropathic pain [24]. Clinically, VW has been shown to be an effective technique for preventing complications like perineural scarring and neuromas. Application of VW following nerve repair demonstrated improved motor and sensory recovery compared to standard neurorrhaphy [20]. However, the clinical application of VW can present challenges such as donor site morbidity, longer surgery time, and technical difficulties, which must be carefully weighed when considering the application of VW for enhancement of peripheral nerve regeneration [8].

Table 3.
First reports on the use of various materials as nerve protecting wraps.
3.2. Vascularized Fat Flaps for Nerve Protection
Different materials for nerve protection have emerged over the last few decades. Both vascularized and free-fat flaps have been used for peripheral nerve protection. However, the vascularized pedicle flaps have become more popular in recent years. These flaps offer several advantages, such as revascularizing nerve tissue, thereby reducing fibrosis, preventing nerve adherence to surrounding structures, and allowing nerve gliding within its natural surroundings [32]. They have gained attention due to their easy availability and abundance. Adipose tissue is rich in adipose-derived stem cells (ADSCs), which can differentiate into various cell types, particularly Schwann-like cells, and guide regenerating axons [33,34,35]. The rich capillary network within adipose tissue helps sustain Schwann cells and other supporting cellular components, ensuring a steady influx of growth factors. The procedure is minimally invasive, safe, and effective in ameliorating persistent neuropathic pain and promoting nerve recovery, particularly in cases of chronic nerve injury or recalcitrant carpal tunnel syndrome [36]. As a result, there is a reduced need for antineuropathic medications, thus enhancing patients’ quality of life while minimizing drug side effects [37]. Clinical studies have shown that the application of a vascularized fat flap during the anterior subcutaneous transposition of the ulnar nerve reduces perineural scarring and supports nerve regeneration by mimicking the natural fatty surroundings of peripheral nerves [38]. However, despite many advantages, there are still challenges, such as donor site morbidity and extended operative time due to the fat flap harvest, which must be considered when choosing fat flaps as the protective barrier against scar and adhesion formation [39].
3.3. Human Amniotic Membrane as the Protective Barrier
The human amniotic membrane (hAM), well recognized for its wound-healing properties, has emerged as an effective biomaterial for the enhancement of peripheral nerve regeneration. Derived from the placenta, this non-immunogenic tissue is rich in collagen, growth factors, and glycoproteins [40]. Its extracellular matrix is designed to promote wound healing and modulate cellular processes. hAM’s regenerative potential is further enhanced by its anti-inflammatory cytokines, along with its antifibrotic and antibacterial properties that facilitate nerve healing and reduce the incidence of postoperative adhesions [41,42]. One of the key advantages of hAM is its lack of human leukocyte antigen (HLA) expression, significantly reducing the risk of immune rejection and enabling broad clinical applicability without complications typically associated with foreign tissue materials [43]. In a rat model of complete common peroneal nerve transection, the effect of amniotic membrane (AM) wrapping was evaluated by comparing two groups: the suture group (nerve transection with end-to-end repair) and the AM wrap group (nerve transection with end-to-end repair followed by two-layer AM wrapping). AM application significantly enhanced functional recovery, as evidenced by improved nerve conduction velocity and axonal transport. In contrast, the suture group without wrapping showed limited muscle regeneration and persistent perineural fibrosis [44]. Similar benefits were observed in clinical settings, where patients undergoing cubital tunnel decompression supported by wrapping the ulnar nerve with hAM experienced a reduced recurrence of paresthesias compared to those without hAM application [45]. The widespread availability, cost-effectiveness, and potent biological activity of hAM make it a highly advantageous tool in neuroprotection strategies. As research continues to support its clinical applications, hAM is positioned as a leading choice for enhancing nerve recovery and reducing post-surgical complications following PNI [46].
3.4. Bioresorbable Collagen for Nerve Regeneration
Once regarded as a simple structural protein, collagen has now become one of the key materials used to support peripheral nerve repair due to its biocompatibility and low immunogenicity [47]. As a natural component of the extracellular matrix (ECM), collagen provides a conducive scaffold for cellular attachment, Schwann cell adhesion, proliferation, and migration, supporting the alignment of regenerating axons [48]. Unlike synthetic materials, which may provoke chronic foreign body reactions, purified collagen exhibits minimal antigenicity, particularly when enzymatically treated to remove immunogenic telopeptides or chemically crosslinked to modulate degradation kinetics [49]. The bioresorbable nature of collagen ensures gradual degradation, reducing the risks associated with foreign body materials and allowing for controlled healing without the need for surgical removal [50,51]. Preclinical studies using rat sciatic nerve models confirmed that the selective permeability of the collagen Type 1 wrap allowed the diffusion of essential micronutrients and growth factors while preventing infiltration by fibroblasts [52]. Clinically, collagen nerve wraps are effective in preserving nerve tissue homeostasis in conditions such as carpal and cubital tunnel syndrome [53,54]. Commercially distributed, FDA-approved products, such as NeuraWrap™ (Integra LifeSciences Corporation, Plainsboro, NJ, USA) and NeuroMend™ Wrap (Stryker Corp., Kalamazoo, MI, USA), are designed to unroll and naturally conform to the shape of the injured nerve, ensuring an optimal fit without compression [55,56]. NeuraWrap™ and NeuroMend™ mainly differ in their resorption rates, with NeuraWrap™ breaking down over 36 to 48 months, while NeuroMend™ degrades within 4 to 8 months [57]. Despite their advantages, the differences in resorption rates, influenced by crosslinking and host enzymatic activity factors, may lead to nerve compression [58]. Furthermore, while inflammatory responses are generally mild, occasional foreign body reactions have been reported, necessitating careful material selection and post-implantation monitoring [59].
3.5. Porcine-Derived Biomaterials for Nerve Regeneration
In the complex landscape of nerve repair, where meticulous surgical technique and biocompatibility of the applied materials dictate the success of regeneration, porcine small intestine submucosa (PSIS) has emerged as a compelling biomaterial. With its structural resemblance to the native human extracellular matrix (ECM), PSIS provides a supportive microenvironment for nerve healing. In addition, it fully degrades within 3 months following application [60,61]. Advances in decellularization techniques have optimized the safety profile of these matrices by reducing immunogenicity while preserving their bioactive components, such as collagen (Types 1 and 3), laminin, and fibronectin, key factors in axonal growth, Schwann cell migration, and myelination [62]. Pre-clinical studies have demonstrated encouraging results, including revascularization and remodeling into connective tissue similar to the nerve epineurium after implantation [28]. Clinically, PSIS wraps have gained attention, particularly in recurrent and persistent carpal tunnel syndrome and cubital tunnel syndrome, where chronic nerve compression is a major concern [63,64]. Their application in upper extremity surgeries has been associated with reduced pain and improved functional outcomes [65]. Investigations into PSIS use in ulnar nerve revisions and other peripheral nerve conditions highlight its ability to reduce inflammatory reactions [66]. This anti-inflammatory effect has been attributed to the material’s ability to modulate the local immune response, including the downregulation of pro-inflammatory cytokines, such as TNF-α and IL-1β, and the promotion of a macrophage phenotypic shift from the pro-inflammatory M1 to the pro-regenerative M2 subtype, thereby creating a microenvironment favorable for nerve healing and axonal regeneration [67]. In the commercial space, three variants of PSIS nerve repair solutions are available, including Axoguard® Nerve Protector (Axogen, Inc. Alachua, FL, USA), the more advanced Axoguard® HA+ Nerve Protector (Axogen, Inc. Alachua, FL, USA), which incorporates a resorbable hyaluronate–alginate gel layer, and Nerve Tape® (BioCircuit Technologies, Inc, Atlanta, GA, USA), a suture-less coaptation device [68]. Nerve Tape utilizes microhooks embedded in a flexible, biocompatible material to enable quick and precise alignment of nerve ends without sutures, providing an effective alternative to traditional microsuture neurorrhaphy [69,70]. The presented innovations reflect a paradigm shift in peripheral nerve repair techniques, where the application of biomaterials bridges the gap between surgical intervention and natural regeneration. While PSIS-based products are generally well tolerated, rare immune responses have been reported and should be taken into consideration when making choices between different biomaterials used for the enhancement of nerve regeneration [71].
3.6. Chitosan-Based Nerve Protectors
As the quest for innovative biomaterials in nerve repair continues, chitosan nerve sheets emerge as an alternative in regenerative medicine. Derived from chitin, a natural polysaccharide from the exoskeletons of crustaceans, chitosan is a biodegradable and biocompatible material [72]. It is rich in deacetylated chitin and has shown potential in inhibiting extraneural scarring and promoting neural regeneration, as demonstrated in experimental studies on rat tibial nerves [73]. Research suggests it effectively supports nerve regeneration in sheets and as a drug carrier, increasing its clinical potential. In particular, its ability to reduce scarring and support Schwann cell migration indicates its possible use as an alternative to currently used approaches [74]. Chitosan’s inherent antimicrobial properties further reduce the risk of infection [75,76]. However, the degradation time of chitosan-based wraps can vary depending on factors such as the degree of acetylation and the specific formulation used. Some studies have reported that chitosan nerve wraps did not show significant degradation after extended periods, suggesting that the material’s persistence may offer prolonged support during nerve regeneration [77]. Currently, NeuroShield® (Checkpoint Surgical, Inc. Cleveland, OH, USA) is the only commercially available chitosan-based nerve wrap, and there is a limited number of preclinical and clinical studies evaluating its efficacy [78].
3.7. Human Epineural Patch, a Novel Strategy for Nerve Protection
One of the novel approaches explored in our laboratory is the human Epineural Patch (hEP), a nerve protector derived from the human epineurium—the outermost layer of peripheral nerves [79]. In this study, we investigated the efficacy of the hEP in a complex rat sciatic nerve injury model that included crush injury, transection, and end-to-end repair, conditions designed to replicate clinically relevant nerve trauma. Unlike other biological wraps, which often degrade prematurely or lack sufficient structural integrity, our findings demonstrate that the hEP provides a durable protective effect supporting nerve healing without inducing an immune response [79]. Following sciatic nerve repair, the application of the hEP to the injury site enhanced nerve regeneration and vascularization, as evidenced by increased expression of vasculogenic (VEGF and vFV) and neurogenic (laminin B, NGF, and S 100) markers. These results confirm the hEP’s potential to promote nerve regeneration. Furthermore, compared to the human amnion membrane (hAM), hEP application significantly improved long-term functional outcomes, as validated by standard functional tests. Thus, the hEP presents a novel and valuable protective option for peripheral nerve reconstruction, offering an alternative tool in regenerative medicine [79].
In our previous research, we have explored the various applications of the human epineurium, including epineural jackets, sheaths, and conduits. These studies have confirmed the neuroregenerative, anti-inflammatory, and immunomodulatory potential of epineurium-based products [80,81,82,83,84,85]. Given its unique neuroregenerative properties, the hEP represents a valuable addition to the repertoire of biological nerve wraps, making it a strong candidate for clinical translation. These findings are promising; however, the clinical potential of the hEP should be further validated through clinical studies.
3.8. A New Perspective on Biological Nerve Protection
Nerve protectors are not limited to traditional wrap formats. New products, such as the VersaWrap (Alafair Biosciences, Inc, Austin, TX, USA), initially designed for tendon, ligament, and skeletal muscle protection, represent a bioresorbable plant-based hydrogel composed of hyaluronic acid (HA) and alginate [86]. To expand these applications, recent studies have explored its potential in enhancing nerve regeneration following PNI. VersaWrap can be implanted as either a sheet or gel via a syringe, offering an innovative approach to nerve protection [29,87]. It features a nano-porous structure that allows the passage of small molecules, including growth factors while acting as a barrier to larger proteins and fibroblasts. The hydrogel gradually resorbs within 3 to 6 months following implantation, though residual biopolymer traces may persist for up to two years, ensuring extended protection during the critical phases of nerve regeneration and healing [29]. Preliminary clinical data support its safety and efficacy in patients undergoing revision surgery for upper-extremity compressive neuropathies. Despite the study’s limitations—such as a small sample size and retrospective design—all patients experienced symptomatic improvement, expressed postoperative satisfaction, and did not require further surgical intervention within the observed postoperative period [87].
4. Synthetic Nerve Protectors
While biological wraps leverage nature’s regenerative mechanisms, the development of synthetic nerve protectors arose from the need to overcome the limitations of biological wraps, marking the beginning of research on synthetic products to enhance nerve regeneration [88]. In recent years, biodegradable polymers have been increasingly utilized in the fabrication of scaffolds for neural tissue engineering, with polycaprolactone (PCL) being the most extensively studied for nerve wrap applications [89]. In contrast, polymers, such as polyglycolic acid (PGA), polylactic acid (PLA), and their copolymer poly(lactic-co-glycolic) acid (PLGA), degrade into acidic byproducts, which can induce localized inflammatory responses and create a suboptimal microenvironment, ultimately impairing peripheral nerve regeneration. PCL-based wraps offer controlled degradation rates, allowing them to gradually degrade over time as the nerve undergoes recovery [90]. Furthermore, their mechanical properties can be tailored to match the physiological needs of the regenerating nerve, providing a scaffold that guides axonal outgrowth while minimizing perineural fibrosis.
Polycaprolactone (PCL) Wraps for Nerve Protection
Initially developed for controlled drug delivery and tissue engineering, poly-ε-caprolactone (PCL) has found a vital role in nerve repair [30]. Its biodegradable nature, along with its ability to mimic the extracellular matrix and integrate with various coatings and polymers, improves biocompatibility and mechanical strength [91]. Its widespread use is further driven by its affordability, natural resorption over several months, and nonimmunogenic properties, making it a practical choice for biomedical applications. However, despite advantages it should be noted that synthetic polymers such as PCL are inherently hydrophobic, which can impair cellular adhesion and potentially trigger inflammatory or immune responses. Recent advances in electrospinning techniques have enabled the creation of PCL scaffolds with interconnected macropores, forming nerve wraps that reduce scarring and promote healing with minimal inflammation [92]. In a study by Sarhane et al., an innovative nanofiber PCL wrap was evaluated in a rat model involving primary nerve transection followed by direct repair. At five weeks post-surgery, the PCL wrap demonstrated efficacy in enhancing neuroregeneration. The macroporous structure of the wrap facilitated controlled tissue ingrowth and preserved nerve continuity. Histological assessments revealed minimal scarring and smooth integration with surrounding tissues, with no signs of nerve damage or adverse inflammatory responses. In contrast, the control group exhibited significant fibrosis, compromising neural integrity [30]. The effectiveness of PCL can be further improved when it is coated with different materials. A study by Harley-Troxe et al. showed that PCL fibers coated with graphene oxide promote axonal growth in a rat model of sciatic nerve injury, emphasizing the promise of these composite materials in nerve regeneration applications [89]. However, clinical evidence remains limited, and further research is necessary to validate their efficacy in human applications.
5. Hybrid Synthetic-Biological Wraps as the Protective Barrier
Hybrid wraps represent a bridge between biological and synthetic nerve protection options, addressing unfavorable outcomes of peripheral nerve injury, such as axonal escape and excessive scarring, which often compromise functional recovery. Currently, the only reported hybrid constructs are nanofibrous membranes composed of polycaprolactone (PCL) and amniotic membrane (AM), which have shown significant potential in addressing the neurobiology of an injury by modulating macrophage polarization and regulating the inflammatory microenvironment [93]. PCL/AM hybrid wraps are known to reduce fibrosis and preserve the regenerative pathway. PCL provides mechanical stability, while AM fosters biological integration, together creating an optimal framework for axonal regrowth and Schwann cell migration. Evidence from preclinical studies using rat sciatic nerve injury models further supports the therapeutic potential of these materials. PCL-amnion nanofibrous membranes decrease the expression of pro-inflammatory cytokines such as IL-6 and TNF-α while increasing levels of anti-inflammatory cytokines like IL-10 and IL-13, thereby promoting an M2 macrophage-dominant healing response. This immunomodulatory effect contributes to a reduction in fibrotic tissue formation by suppressing the excessive extracellular matrix deposition, ultimately preventing scar-related inhibition of axonal regrowth. [31]. At 16 weeks post-implantation, these scaffolds have been shown to enhance functional recovery by facilitating Schwann cell proliferation, accelerating axon regeneration, and reducing muscle denervation. Moreover, they contribute to higher axon numbers, increased myelin sheath thickness, and improved overall nerve maturity [94].
6. Limitations of Commercial Nerve Wraps
Although significant advancements have been made in the development of biological and synthetic wraps and materials for nerve protection and regeneration, no standardized guidelines exist for their optimal application in acute versus chronic nerve injuries. While nerve protectors serve as a barrier shielding injured nerves from surrounding tissues, they also present certain limitations (Table 4). Biocompatibility concerns, particularly with animal-derived materials, may trigger immune reactions or be unsuitable for patients due to ethical or religious considerations. Even biocompatible options can induce low-grade inflammatory responses over time, and their biodegradation may be slower than expected, potentially leading to fibrosis or foreign body reactions [95,96]. Additionally, standardized sizes may not accommodate all nerve repairs, resulting in mismatches that can cause iatrogenic constriction or slippage, thereby reducing effectiveness [11]. Proper placement of nerve wraps and protectors is crucial, as excessive tension can hinder nerve regeneration and increase the risk of scarring, fibrosis, and adhesion formation [2].

Table 4.
Advantages and disadvantages of nerve wrapping materials.
7. Future Perspectives
Looking ahead, the next generation of nerve protectors must move beyond the limitations of current materials. A comprehensive, multilevel intervention can substantially improve functional recovery and, consequently, the long-term quality of life of patients with peripheral nerve injuries. Future strategies may involve the integration of nerve-protecting wraps with advanced therapeutic approaches such as stem cell therapies and controlled delivery of the growth factors. Incorporation of stem cells into nerve wraps may enhance nerve regeneration leading to cell differentiation into neural lineages, including motor neurons and Schwann cells. Moreover, stem cells can promote axonal growth by secreting paracrine factors, modulating the inflammatory milieu, facilitating tissue remodeling, reducing fibrosis, and enhancing repair quality. Furthermore, the release of neurogenic and angiogenic growth factors, such as NGF, BDNF, VEGF, and PDGF, respectively, in a controlled and sustained manner will further promote axonal growth and enhance neuronal survival while mitigating trauma-induced muscle atrophy. To be effective, therapeutic constructs must be both durable and capable of adapting to the dynamic nature of nerve regeneration. Therefore, future innovative approaches should prioritize the development of personalized and/or customizable nerve wraps and protectors that would address these challenges effectively.
8. Conclusion
Nerve protectors, including biological and synthetic wraps, have significantly advanced the management of peripheral nerve injuries (PNIs). They serve as an adjuvant treatment option in severe cases, where creating a barrier between the repaired nerves and surrounding tissues is essential to prevent fibrosis and tissue adhesions. Depending on the type and severity of the PNI, the application of nerve protective materials should be tailored to the individual patient’s needs to achieve optimal outcomes. While nerve protectors enhance nerve regeneration, challenges such as biocompatibility and immune reactions remain. Further research is required to assess long-term neuroregenerative effects and optimize clinical applications of nerve wraps. Continued innovation and rigorous clinical evaluations are essential to ensuring their effectiveness and achieving consistent, reliable outcomes.
Author Contributions
Conceptualization, M.S.; data curation, W.R. and W.N.; writing—original draft preparation, W.R. and W.N.; writing—review and editing, M.S.; visualization, M.S.; supervision, M.S.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PNIs | Peripheral nerve injuries |
| hAM | Human amniotic membrane |
| PSIS | Porcine small intestinal submucosa |
| PCL | Poly-ε-caprolactone |
| VW | Vein wrapping |
| bFGF | Basic fibroblast growth factor |
| HO-1 | Heme oxygenase-1 |
| IL-4 | Interleukin-4 |
| IL-10 | Interleukin-10 |
| ARG1 | Arginase-1 |
| ADSCs | Adipose-derived stem cells |
| HLA | Human leukocyte antigen |
| ECM | Extracellular matrix |
| VEGF | Vascular endothelial growth factor |
| NGF | Nerve growth factor |
| FDA | Food and Drug Administration |
| PGA | Polyglycolic acid |
| PLA | Polylactic acid |
| PLGA | Poly(lactic-co-glycolic) acid |
| TNF-α | Tumor necrosis factor-alpha |
| hEP | Human Epineural Patch |
| HA | Hyaluronic acid |
| NGF | Nerve growth factor |
| BDNF | Brain-derived neurotrophic factor |
| VEGF | Vascular endothelial growth factor |
| PDGF | Platelet-derived growth factor |
References
- Aman, M.; Zimmermann, K.S.; Thielen, M.; Thomas, B.; Daeschler, S.; Boecker, A.H.; Stolle, A.; Bigdeli, A.K.; Kneser, U.; Harhaus, L. An Epidemiological and Etiological Analysis of 5026 Peripheral Nerve Lesions from a European Level I Trauma Center. J. Pers. Med. 2022, 12, 1673. [Google Scholar] [CrossRef]
- Siemionow, M.; Brzezicki, G. Current techniques and concepts in peripheral nerve repair. Int. Rev. Neurobiol. 2009, 87, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Bateman, E.A.; Pripotnev, S.; Larocerie-Salgado, J.; Ross, D.C.; Miller, T.A. Assessment, management, and rehabilitation of traumatic peripheral nerve injuries for non-surgeons. Muscle Nerve 2024, 71, 696–714. [Google Scholar] [CrossRef]
- Dellon, E.S.; Dellon, A.L. The first nerve graft, Vulpian, and the nineteenth-century neural regeneration controversy. J. Hand Surg. Am. 1993, 18, 369–372. [Google Scholar] [CrossRef]
- Thomson, S.E.; Ng, N.Y.; Riehle, M.O.; Kingham, P.J.; Dahlin, L.B.; Wiberg, M.; Hart, A.M. Bioengineered nerve conduits and wraps for peripheral nerve repair of the upper limb. Cochrane Database Syst. Rev. 2022, 12, CD012574. [Google Scholar] [CrossRef]
- Rath, S.; Green, C.J. Selectivity of distal reinnervation of regenerating mixed motor and sensory nerve fibers across muscle grafts in rats. Br. J. Plast. Surg. 1991, 44, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Supra, R.; Agrawal, D.K. Peripheral nerve regeneration: Opportunities and challenges. J. Spine Res. Surg. 2023, 5, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Dy, C.J.; Aunins, B.; Brogan, D.M. Barriers to epineural scarring: Role in treatment of traumatic nerve injury and chronic compressive neuropathy. J. Hand Surg. Am. 2018, 43, 360–367. [Google Scholar] [CrossRef]
- Thakker, A.; Sharma, S.C.; Hussain, N.M.; Devani, P.; Lahiri, A. Nerve wrapping for recurrent compression neuropathy: A systematic review. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 549–559. [Google Scholar] [CrossRef]
- Masear, V. Venous wrapping of nerves to prevent scarring. In Proceedings of the 44th Annual Meeting of the American Society for Surgery of the Hand, Seattle, WA, USA, 9 September 1989. [Google Scholar]
- Mayrhofer-Schmid, M.; Klemm, T.T.; Aman, M.; Kneser, U.; Eberlin, K.R.; Harhaus, L.; Boecker, A.H. Shielding the Nerve: A Systematic Review of Nerve Wrapping to Prevent Adhesions in the Rat Sciatic Nerve Model. J. Pers. Med. 2023, 13, 1431. [Google Scholar] [CrossRef]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA-approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Fakhr, M.J.; Kavakebian, F.; Ababzadeh, S.; Rezapour, A. Challenges and Advances in Peripheral Nerve Tissue Engineering Critical Factors Affecting Nerve Regeneration. J. Tissue Eng. Regen. Med. 2024, 2024, 8868411. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.S.; Haycock, J.W. Biomaterials and scaffolds for repair of the peripheral nervous system. In Peripheral Nerve Tissue Engineering and Regeneration; Phillips, J.B., Hercher, D., Hausner, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Fornasari, B.E.; Carta, G.; Gambarotta, G.; Raimondo, S. Natural-based biomaterials for peripheral nerve injury repair. Front. Bioeng. Biotechnol. 2020, 8, 554257. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Duan, X. Mechanisms and treatments of peripheral nerve injury. Ann. Plast. Surg. 2023, 91, 313–318. [Google Scholar] [CrossRef]
- Crabtree, J.R.; Mulenga, C.M.; Tran, K.; Feinberg, K.; Santerre, J.P.; Borschel, G.H. Biohacking Nerve Repair: Novel Biomaterials, Local Drug Delivery, Electrical Stimulation, and Allografts to Aid Surgical Repair. Bioengineering 2024, 11, 776. [Google Scholar] [CrossRef]
- Lam, T.C.; Leung, Y.Y. Innovations in peripheral nerve regeneration. Bioengineering 2024, 11, 444. [Google Scholar] [CrossRef]
- Arkansas Blue Cross Blue Shield. Peripheral Nerve Repair and Reconstruction. Available online: https://journals.lww.com/jbjsjournal/abstract/2013/12040/peripheral_nerve_repair_and_reconstruction.9.aspx (accessed on 19 March 2025).
- Mukai, M.; Uchida, K.; Hirosawa, N.; Murakami, K.; Inoue, G.; Miyagi, M.; Shiga, Y.; Sekiguchi, H.; Inage, K.; Orita, S.; et al. Frozen vein wrapping for chronic nerve constriction injury reduces sciatic nerve allodynia in a rat model. BMC Neurosci. 2022, 23, 1–6. [Google Scholar] [CrossRef]
- Papatheodorou, L.K.; Sotereanos, D.G. Vein wrapping of peripheral nerves: Surgical technique. In Compressive Neuropathies of the Upper Extremity; Sotereanos, D.G., Papatheodorou, L.K., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 247–252. [Google Scholar] [CrossRef]
- Hirosawa, N.; Uchida, K.; Kuniyoshi, K.; Murakami, K.; Inoue, G.; Miyagi, M.; Matsuura, Y.; Orita, S.; Inage, K.; Suzuki, T.; et al. Vein wrapping facilitates basic fibroblast growth factor-induced heme oxygenase-1 expression following chronic nerve constriction injury. J. Orthop. Res. 2017, 36, 898–905. [Google Scholar] [CrossRef]
- Mukai, M.; Uchida, K.; Hirosawa, N.; Murakami, K.; Kuniyoshi, K.; Inoue, G.; Miyagi, M.; Sekiguchi, H.; Shiga, Y.; Inage, K.; et al. Wrapping With Basic Fibroblast Growth Factor-Impregnated Collagen Sheet Reduces Rat Sciatic Nerve Allodynia. J. Orthop. Res. 2019, 37, 2258–2263. [Google Scholar] [CrossRef]
- Hirosawa, N.; Uchida, K.; Kuniyoshi, K.; Murakami, K.; Inoue, G.; Miyagi, M.; Matsuura, Y.; Orita, S.; Inage, K.; Suzuki, T.; et al. Vein wrapping promotes M2 macrophage polarization in a rat chronic constriction injury model. J. Orthop. Res. 2018, 36, 2210–2217. [Google Scholar] [CrossRef]
- Strickland, J.W.; Idler, R.S.; Lourie, G.M.; Plancher, K.D. The hypothenar fat pad flap for management of recalcitrant carpal tunnel syndrome. J. Hand Surg. 1996, 21, 840–848. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.C.; Randolph, M.A.; Bujold, K.E.; Kochevar, I.E.; Redmond, R.W.; Winograd, J.M. Photochemical Sealing Improves Outcome Following Peripheral Neurorrhaphy. J. Surg. Res. 2009, 151, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.D.; Hayes, A.; Amin, F.; Akelina, Y.; Hays, A.P.; Rosenwasser, M.P. Collagen nerve protector in rat sciatic nerve repair: A morphometric and histological analysis. Microsurgery 2010, 30, 392–396. [Google Scholar] [CrossRef]
- Kokkalis, Z.T.; Pu, C.; Small, G.; Weiser, R.W.; Venouziou, A.; Sotereanos, D.G. Assessment of Processed Porcine Extracellular Matrix as a Protective Barrier in a Rabbit Nerve Wrap Model. J. Reconstr. Microsurg. 2010, 27, 019–028. [Google Scholar] [CrossRef]
- Hones, K.M.; Nichols, D.S.; Barker, H.; Cox, E.; Hones, J.A.; Chim, H. Outcomes following use of VersaWrap nerve protector in treatment of patients with recurrent compressive neuropathies. Front. Surg. 2023, 10, 1123375. [Google Scholar] [CrossRef]
- Sarhane, K.A.; Ibrahim, Z.; Martin, R.; Krick, K.; Cashman, C.R.; Tuffaha, S.H.; Broyles, J.M.; Prasad, N.; Yao, Z.-C.; Cooney, D.S.; et al. Macroporous nanofiber wraps promote axonal regeneration and functional recovery in nerve repair by limiting fibrosis. Acta Biomater. 2019, 88, 332–345. [Google Scholar] [CrossRef]
- Liu, C.; Liu, D.; Zhang, X.; Hui, L.; Zhao, L. Nanofibrous polycaprolactone/amniotic membrane facilitates peripheral nerve regeneration by promoting macrophage polarization and regulating inflammatory microenvironment. Int. Immunopharmacol. 2023, 121, 110507. [Google Scholar] [CrossRef] [PubMed]
- Langdell, H.C.; Zeng, S.L.; Pidgeon, T.S.; Mithani, S.K. Recalcitrant Neuropathies in the Upper Extremity. J. Hand Surg. Glob. Online 2023, 5, 503–509. [Google Scholar] [CrossRef]
- Oh, S.H.; Chung, J.I. Oblique axis hypothenar free flaps: Tips for harvesting larger flaps with minimal donor site morbidity. Arch. Plast. Surg. 2023, 50, 279–287. [Google Scholar] [CrossRef]
- Shin, A.; Saffari, T.; Saffari, S.; Vyas, K.; Mardini, S. Role of adipose tissue grafting and adipose-derived stem cells in peripheral nerve surgery. Neural Regen. Res. 2022, 17, 2179–2184. [Google Scholar] [CrossRef]
- Danoff, J.R.; Lombardi, J.M.; Rosenwasser, M.P. Use of a pedicled adipose flap as a sling for anterior subcutaneous transposition of the ulnar nerve. J. Hand Surg. Am. 2014, 39, 552–555. [Google Scholar] [CrossRef]
- Dibbs, R.P.; Ali, K.; Sarrami, S.M.; Koshy, J.C. Revision peripheral nerve surgery of the upper extremity. Semin. Plast. Surg. 2021, 35, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Ching, R.C.; Wiberg, M.; Kingham, P.J. Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cell Res. Ther. 2018, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Riccio, M.; Gravina, P.; Pangrazi, P.P.; Cecconato, V.; Gigante, A.; De Francesco, F. Ulnar nerve anteposition with adipofascial flap, an alternative treatment for severe cubital syndrome. BMC Surg. 2023, 23, 268. [Google Scholar] [CrossRef]
- Mamede, A.M.A.; Botelho, A.C. Amniotic Membrane: Origin, Characterization, and Medical Applications; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Wolfe, E.M.; Mathis, S.A.; Muñoz, N.d.l.O.; Ovadia, S.A.; Panthaki, Z.J. Comparison of human amniotic membrane and collagen nerve wraps around sciatic nerve reverse autografts in a rat model. Biomater. Biosyst. 2022, 6, 100048. [Google Scholar] [CrossRef]
- Leal-Marin, S.; Kern, T.; Hofmann, N.; Pogozhykh, O.; Framme, C.; Börgel, M.; Figueiredo, C.; Glasmacher, B.; Gryshkov, O. Human Amniotic Membrane: A review on tissue engineering, application, and storage. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 109, 1198–1215. [Google Scholar] [CrossRef]
- McClendon, D.C.; Su, J.; Smith, D.W. Human amniotic allograft in hand surgery. J. Hand Surg. Am. 2023, 48, 388–395. [Google Scholar] [CrossRef]
- Yu, L.-M.; Yu, C.-Y.; Zhang, Z.-Y.; Yang, J.; Fan, Z.-H.; Wang, D.-L.; Wang, Y.-Y.; Zhang, T. Fresh human amniotic membrane effectively promotes the repair of injured common peroneal nerve. Neural Regen. Res. 2019, 14, 2199–2208. [Google Scholar] [CrossRef]
- Mirzayan, R.; Russo, F.; Yang, S.-J.T.; Lowe, N.; Shean, C.J.; Harness, N.G. Human Amniotic Membrane Wrapping of the Ulnar Nerve During Cubital Tunnel Surgery Reduces Recurrence of Symptoms. Arch. Bone Jt. Surg. 2022, 10, 969–975. [Google Scholar] [CrossRef]
- Fénelon, M.; Catros, S.; Meyer, C.; Fricain, J.-C.; Obert, L.; Auber, F.; Louvrier, A.; Gindraux, F. Applications of Human Amniotic Membrane for Tissue Engineering. Membranes 2021, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Hao, M.; Wang, D.; Jiang, Z.; Sun, L.; Gao, Y.; Jin, Y.; Lei, P.; Zhuo, Y. The application of collagen in the repair of peripheral nerve defect. Front. Bioeng. Biotechnol. 2022, 10, 973301. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, M.; Liu, N. Interactions between Schwann cell and extracellular matrix in peripheral nerve regeneration. Front. Neurol. 2024, 15, 1372168. [Google Scholar] [CrossRef] [PubMed]
- Hardin-Young, J.; Carr, R.M.; Downing, G.J.; Condon, K.D.; Termin, P.L. Modification of native collagen reduces antigenicity but preserves cell compatibility. Biotechnol. Bioeng. 2000, 49, 675–682. [Google Scholar] [CrossRef]
- Eleftheriadou, D.; Phillips, J.B. Collagen biomaterials for nerve tissue engineering. In Peripheral Nerve Tissue Engineering and Regeneration; Phillips, J.B., Hercher, D., Hausner, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Abedi, M.; Shafiee, M.; Afshari, F.; Mohammadi, H.; Ghasemi, Y. Collagen-Based Medical Devices for Regenerative Medicine and Tissue Engineering. Appl. Biochem. Biotechnol. 2023, 196, 5563–5603. [Google Scholar] [CrossRef]
- Chen, S.; Gao, Y.-B.; Liu, Z.-G.; Lin, G.-D.; Guo, Y.; Chen, L.; Huang, B.-T.; Yin, Y.-B.; Yang, C.; Sun, L.-Y.; et al. Safety and efficacy of a nerve matrix membrane as a collagen nerve wrapping: A randomized, single-blind, multicenter clinical trial. Neural Regen. Res. 2021, 16, 1652–1659. [Google Scholar] [CrossRef]
- Wong, G.C.; Chung, K.C. Bioengineered nerve conduits and wraps. Hand Clin. 2024, 40, 379–387. [Google Scholar] [CrossRef]
- Spielman, A.F.; Sankaranarayanan, S.; Skowronski, P.; Lessard, A.-S.; Panthaki, Z. Recurrent and persistent carpal tunnel syndrome: “Triple-therapy approach”. J. Orthop. 2020, 22, 431–435. [Google Scholar] [CrossRef]
- Integra LifeSciences Corporation. NeuraWrap™ Nerve Protector. Available online: https://www.integranerve.com/nerve-protector (accessed on 19 March 2025).
- Stryker Corporation. NeuroMend™ Wrap. Available online: https://www.stryker.com/us/en/trauma-and-extremities/products/neuromend.html (accessed on 19 March 2025).
- Downey, M.S. A guide to nerve wrapping for tarsal tunnel surgery. Podiatry Today 2015, 28, 32. [Google Scholar]
- Rault, I.; Frei, V.; Herbage, D.; Abdul-Malak, N.; Huc, A. Evaluation of different chemical methods for cros-linking collagen gel, films and sponges. J. Mater. Sci. Mater. Med. 1996, 7, 215–221. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef]
- Hanwright, P.J.; Rath, J.B.; von Guionneau, N.; Slavin, B.; Pinni, S.; Zlotolow, D.; Shores, J.; Dellon, A.L.; Tuffaha, S.H. The Effects of a Porcine Extracellular Matrix Nerve Wrap as an Adjunct to Primary Epineurial Repair. J. Hand Surg. 2021, 46, 813.e1–813.e8. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.-S.; Lee, H.-J.; Lee, H.-J.; Lee, I.-W.; Yang, J.-H. Rat Peripheral Nerve Regeneration Using Nerve Guidance Channel by Porcine Small Intestinal Submucosa. J. Korean Neurosurg. Soc. 2013, 53, 65–71. [Google Scholar] [CrossRef]
- Li, T.; Javed, R.; Ao, Q. Xenogeneic decellularized extracellular matrix-based biomaterials for peripheral nerve repair and regeneration. Curr. Neuropharmacol. 2021, 19, 2152–2163. [Google Scholar] [CrossRef]
- Grandizio, L.C.; Maschke, S.; Evans, P.J. The management of persistent and recurrent cubital tunnel syndrome. J. Hand Surg. Am. 2018, 43, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Imran, R.; George, S.; Jose, R.; Shirley, C.; Power, D.M. Clinical outcomes following neurolysis and porcine collagen extracellular matrix wrapping of scarred nerves in revision carpal tunnel decompression. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 2802–2808. [Google Scholar] [CrossRef] [PubMed]
- Fones, L.; DePascal, M.; Ilyas, A.M. Use of nerve wraps in the upper extremity. SurgiColl 2024, 2. [Google Scholar] [CrossRef]
- Burahee, A.S.; Duraku, L.S.; Bosman, R.; Shirley, C.; van der Oest, M.J.; Zuidam, M.J.; Power, D.M. Porcine submucosal extracellular matrix wrapping of the ulnar nerve in revision cubital tunnel surgery. J. Plast. Reconstr. Aesthetic Surg. 2024, 98, 176–183. [Google Scholar] [CrossRef]
- Fujii, M.; Tanaka, R. Porcine Small Intestinal Submucosa Alters the Biochemical Properties of Wound Healing: A Narrative Review. Biomedicines 2022, 10, 2213. [Google Scholar] [CrossRef]
- Jordaan, P.; Uhiara, O.; Power, D. Management of the scarred nerve using porcine submucosa extracellular matrix nerve wraps. J. Musculoskelet. Surg. Res. 2019, 3, 128. [Google Scholar] [CrossRef]
- Eberlin, K.R.; Safa, B.; Buntic, R.; Rekant, M.S.; Richard, M.J.; Styron, J.F.; Bendale, G.; Isaacs, J. Usability of Nerve Tape: A Novel Sutureless Nerve Coaptation Device. J. Hand Surg. 2024, 49, 346–353. [Google Scholar] [CrossRef]
- Bendale, G.S.; Sonntag, M.; Clements, I.P.; Isaacs, J.E. Biomechanical Testing of a Novel Device for Sutureless Nerve Repair. Tissue Eng. Part C Methods 2022, 28, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Osterman, M.; Stern, P.J. Inflammatory reactions to xenogenic nerve wraps: A report of three cases. JBJS Case Connect. 2019, 9, e0302. [Google Scholar] [CrossRef]
- Zhang, M.; An, H.; Zhang, F.; Jiang, H.; Wan, T.; Wen, Y.; Han, N.; Zhang, P. Prospects of Using Chitosan-Based Biopolymers in the Treatment of Peripheral Nerve Injuries. Int. J. Mol. Sci. 2023, 24, 12956. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Li, P.; Jiang, J.J.; Li, H.Y. Anastomotic stoma coated with chitosan film as a betamethasone dipropionate carrier for peripheral nerve regeneration. Neural Regen. Res. 2018, 13, 309–316. [Google Scholar] [CrossRef]
- Bąk, M.; Gutlowska, O.N.; Wagner, E.; Gosk, J. The role of chitin and chitosan in peripheral nerve reconstruction. Polym. Med. 2017, 47, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, M.; Jankowska, K.; Klak, M.; Wszoła, M. Chitosan as an Underrated Polymer in Modern Tissue Engineering. Nanomaterials 2021, 11, 3019. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Jain, N.; Murchinson, M.; Rounds, A.; Bourland, B. Hemoclip migration after revision carpal tunnel release: A case report. J. Surg. Case Rep. 2023, 2023, rjad548. [Google Scholar] [CrossRef]
- Siemionow, M.; Radecka, W.; Kozlowska, K.; Chambily, L.; Brodowska, S.; Kuc, D.; Filipek, G.; Budzynska, K. Protective Effect of the Human Epineural Patch Application after Sciatic Nerve Crush Injury Followed by Nerve Transection and End-to-End Repair. Arch. Immunol. Ther. Exp. 2025, 73. [Google Scholar] [CrossRef] [PubMed]
- Siemionow, M.; Uygur, S.; Madajka, M. Application of epineural sheath as a novel approach for fat volume maintenance. Ann Plast Surg. 2017, 79, 606–612. [Google Scholar] [CrossRef]
- Siemionow, M.; Cwykiel, J.; Uygur, S.; Kwiecien, G.; Oztürk, C.; Szopinski, J.; Madajka, M. Application of epineural sheath conduit for restoration of 6-cm long nerve defects in a sheep median nerve model. Microsurgery 2018, 39, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Siemionow, M.; Demir, Y.; Mukherjee, A.L. Repair of peripheral nerve defects with epineural sheath grafts. Ann. Plast. Surg. 2010, 65, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Siemionow, M.; Duggan, W.; Brzezicki, G.; Klimczak, A.; Grykien, C.; Gatherwright, J.; Nair, D. Peripheral Nerve Defect Repair With Epineural Tubes Supported With Bone Marrow Stromal Cells: A preliminary report. Ann. Plast. Surg. 2011, 67, 73–84. [Google Scholar] [CrossRef]
- Siemionow, M.; Strojny, M.M.; Kozlowska, K.; Brodowska, S.; Grau-Kazmierczak, W.; Cwykiel, J. Application of Human Epineural Conduit Supported with Human Mesenchymal Stem Cells as a Novel Therapy for Enhancement of Nerve Gap Regeneration. Stem Cell Rev. Rep. 2021, 18, 642–659. [Google Scholar] [CrossRef]
- Siemionow, M.; Tetik, C.; Ozer, K.; Ayhan, S.; Siemionow, K.; Browne, E. Epineural sleeve neurorrhaphy: Surgical technique and functional results—A preliminary report. Ann. Plast. Surg. 2002, 48, 281–285. [Google Scholar] [CrossRef]
- Wan, R.; Zhao, G.; Adam, E.A.; Selim, O.A.; Sarcon, A.K.; Reisdorf, R.L.; Meves, A.; Zhao, C.; Moran, S.L. Evaluating the Effectiveness of Commercially Available Antiadhesion Tendon Protector Sheets in Tendon Repair Surgery Versus Tendon Repair Surgery Alone: A Preclinical Model Study. J. Hand Surg. 2024. [Google Scholar] [CrossRef]
- Adu, Y.; Harder, J.; Cox, C.; Baum, G.; Hernandez, E.J.; MacKay, B.J. Evaluating the effect of VersaWrap tendon protector on functional outcomes in operative tendon repairs. Front. Surg. 2024, 11, 1447515. [Google Scholar] [CrossRef]
- Zhang, M.; Li, C.; Zhou, L.P.; Pi, W.; Zhang, P.X. Polymer scaffolds for biomedical applications in peripheral nerve reconstruction. Molecules 2021, 26, 2712. [Google Scholar] [CrossRef]
- Harley-Troxell, M.E.; Steiner, R.; Newby, S.D.; Bow, A.J.; Masi, T.J.; Millis, N.; Matavosian, A.A.; Crouch, D.; Stephenson, S.; Anderson, D.E.; et al. Electrospun PCL Nerve Wrap Coated with Graphene Oxide Supports Axonal Growth in a Rat Sciatic Nerve Injury Model. Pharmaceutics 2024, 16, 1254. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tang, X.; Li, T.; Ling, J.; Yang, Y. The success of biomaterial-based tissue engineering strategies for peripheral nerve regeneration. Front. Bioeng. Biotechnol. 2022, 10, 1039777. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bártolo, P.J.; Granja, P.L. Electrospun Polycaprolactone (PCL) Degradation: An In Vitro and In Vivo Study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef]
- Lopez, J.; Xin, K.; Quan, A.; Xiang, S.; Barone, A.A.L.; Budihardjo, J.; Musavi, L.; Mulla, S.; Redett, R.; Martin, R.; et al. Poly(ε-Caprolactone) Nanofiber Wrap Improves Nerve Regeneration and Functional Outcomes after Delayed Nerve Repair. Plast. Reconstr. Surg. 2019, 144, 48e–57e. [Google Scholar] [CrossRef]
- Bai, J.; Liu, C.; Kong, L.; Tian, S.; Yu, K.; Tian, D. Electrospun Polycaprolactone (PCL)-Amnion Nanofibrous Membrane Promotes Nerve Regeneration and Prevents Fibrosis in a Rat Sciatic Nerve Transection Model. Front. Surg. 2022, 9, 842540. [Google Scholar] [CrossRef]
- Dong, R.; Tian, S.; Bai, J.; Yu, K.; Liu, C.; Liu, L.; Tian, D. Electrospun Polycaprolactone (PCL)-Amnion Nanofibrous Membrane Promotes Nerve Repair after Neurolysis. J. Biomater. Appl. 2022, 36, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, C.F.; Corvi, J.J.; Zheng, Y.; Park, K.H.; Akelina, Y.; Engemann, A.; Strauch, R.J. Resorbable Nerve Wraps: Can They Be Overtightened? J. Reconstr. Microsurg. 2022, 38, 694–702. [Google Scholar] [CrossRef]
- Koenig, Z.A.; Burns, J.C.; Hayes, J.D. Necrotic granulomatous inflammation after use of small intestine submucosa matrix for recurrent compression neuropathy. Plast. Reconstr. Surg. Glob. Open. 2022, 10, e4378. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).