Injectable Stem Cell-Based Therapies for Myocardial Regeneration: A Review of the Literature
Abstract
1. Introduction
2. Methods of Stem Cell Delivery
2.1. Intramyocardial Injection
2.2. Intravenous Infusion
2.3. Intracoronary Administration
2.4. Timing of Administration
3. Sources of Stem Cells for Differentiation into Cardiomyocytes
3.1. Cardiac Stem Cells and Cardiac Progenitor Cells
3.2. Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs)
3.3. Adipose-Derived Stem Cells (ADSCs)
3.4. Umbilical Cord-Derived Mesenchymal Stem Cells (UC-MSCs)
3.5. Amniotic Stem Cells
3.6. Embryonic Stem Cells (ESCs)
3.7. Induced Pluripotent Stem Cells (iPSCs)
4. Tissue Engineering Strategies to Enhance Cell Engraftment and Survival
4.1. Injectable Biomaterials
4.2. Cell Combinations and Aggregates
5. Tissue Engineering Strategies to Enhance Treatment Efficacy
5.1. Stem Cell Preconditioning
5.2. Genetic Modification of Stem Cells
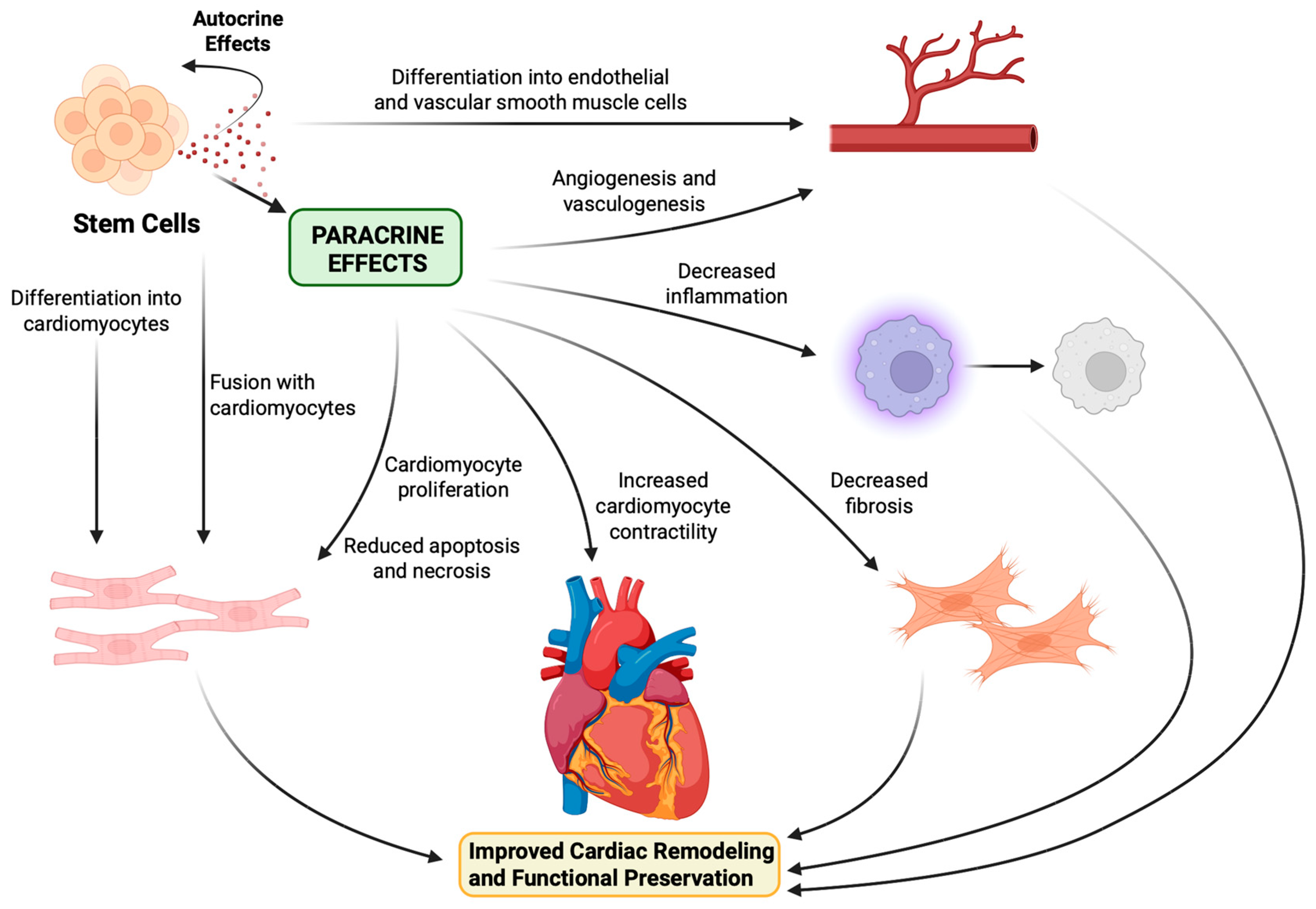
6. Stem Cell Mechanism of Action
7. Alternative Cell-Free Therapies
7.1. Exosome Therapy
7.2. Direct In Vivo Reprogramming
8. Comment
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADSC | Adipose-derived stem cell |
| AEC | Amniotic epithelial cell |
| AFSC | Amniotic fluid-derived stem cell |
| bFGF | Basic fibroblast growth factor |
| BM-MSC | Bone marrow-derived mesenchymal stem cell |
| CABG | Coronary artery bypass graft surgery |
| CCS | Canadian Cardiovascular Society |
| CDC | Cardiosphere-derived cell |
| CM | Cardiomyocyte |
| CSF2Rβ | Colony-stimulating factor 2 receptor β |
| CPC | Cardiac progenitor cell |
| CSC | Cardiac stem cell |
| DNA | Deoxyribonucleic acid |
| EC | Endothelial cell |
| ESC | Embryonic stem cell |
| FGF-2 | Fibroblast growth factor 2 |
| hESC | Human embryonic stem cell |
| hESC-CM | Human embryonic stem cell-derived cardiomyocyte |
| HGF | Hepatocyte growth factor |
| hiPSC | Human induced pluripotent stem cell |
| hiPSC-CM | Human induced pluripotent stem cell-derived cardiomyocyte |
| HO-1 | Heme oxygenase 1 |
| IGF-1 | Insulin-like growth factor 1 |
| IL-6 | Interleukin 6 |
| IL-1⍺ | Interleukin 1⍺ |
| IL-1β | Interleukin 1β |
| iPSC | Induced pluripotent stem cell |
| iPSC-CM | Induced pluripotent stem cell-derived cardiomyocyte |
| Isl-1 Insulin | gene enhancer protein 1 |
| LAD | Left anterior descending artery |
| LV | Left ventricle |
| LVEF | Left ventricular ejection fraction |
| LVESV | Left ventricular end-systolic volume |
| MI | Myocardial Infarction |
| miRNA | Micro-ribonucleic acid |
| MLHFQ | Minnesota Living with Heart Failure Questionnaire |
| MN-BMC | Mononuclear bone marrow cell |
| MSC | Mesenchymal stem cell |
| MVO2 | Myocardial volume oxygen |
| NYHA | New York Heart Association |
| PCI | Percutaneous coronary intervention |
| PDGF | Platelet-derived growth factor |
| RCT | Randomized controlled trial |
| SDF-1 | Stromal cell-derived factor 1 |
| SMC | Smooth muscle cell |
| SSEA-1 | Stage-specific embryonic antigen 1 |
| Tβ4 | Thymosin β4 |
| TGF-⍺ | Transforming growth factor ⍺ |
| TGF-β | Transforming growth factor β |
| TIMP-1 | Tissue inhibitor of metalloproteinase 1 |
| TNF-⍺ | Tumor necrosis factor ⍺ |
| UC-MSC | Umbilical cord-derived mesenchymal stem cell |
| VEGF | Vascular endothelial growth factor |
| VEGF-A | Vascular endothelial growth factor A |
References
- Pastena, P.; Frye, J.T.; Ho, C.; Goldschmidt, M.E.; Kalogeropoulos, A.P. Ischemic cardiomyopathy: Epidemiology, pathophysiology, outcomes, and therapeutic options. Heart Fail. Rev. 2024, 29, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Fumarulo, I.; Stefanini, A.; Masarone, D.; Burzotta, F.; Cameli, M.; Aspromonte, N. Cardiac replacement therapy: Critical issues and future perspectives of heart transplantation and artificial heart. Curr. Probl. Cardiol. 2025, 50, 102971. [Google Scholar] [CrossRef] [PubMed]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K.; et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003, 114, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Hatayama, N.; Guo, M.; Yuhara, S.; Shinoka, T. Bridging the Gap: Advances and Challenges in Heart Regeneration from In Vitro to In Vivo Applications. Bioengineering 2024, 11, 954. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Youssef, E.A.-S.; Brinton, T.J.; Zhang, P.; Rogers, P.; Price, E.T.; Yeung, A.C.; Johnstone, B.H.; Yock, P.G.; March, K.L. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: Implications for current clinical trials. Circulation 2005, 112 (Suppl. S9), I150–I156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Sun, Y.; Xia, L.; Chen, A.; Wang, Z. Randomized study of mononuclear bone marrow cell transplantation in patients with coronary surgery. Ann. Thorac. Surg. 2008, 86, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Karantalis, V.; DiFede, D.L.; Gerstenblith, G.; Pham, S.; Symes, J.; Zambrano, J.P.; Fishman, J.; Pattany, P.; McNiece, I.; Conte, J.; et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ. Res. 2014, 114, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Pätilä, T.; Lehtinen, M.; Vento, A.; Schildt, J.; Sinisalo, J.; Laine, M.; Hämmäinen, P.; Nihtinen, A.; Alitalo, R.; Nikkinen, P.; et al. Autologous bone marrow mononuclear cell transplantation in ischemic heart failure: A prospective, controlled, randomized, double-blind study of cell transplantation combined with coronary bypass. J. Heart Lung Transplant. 2014, 33, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, G.; Steinhoff, G.; Liebold, A.; Pesce, M.; Alamanni, F.; Capogrossi, M.C.; Biglioli, P. Direct minimally invasive intramyocardial injection of bone marrow-derived AC133+ stem cells in patients with refractory ischemia: Preliminary results. Thorac. Cardiovasc. Surg. 2008, 56, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.M.; Fishman, J.E.; Gerstenblith, G.; Velazquez, D.L.D.; Zambrano, J.P.; Suncion, V.Y.; Tracy, M.; Ghersin, E.; Johnston, P.V.; Brinker, J.A.; et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA 2012, 308, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; Willerson, J.T.; Pepine, C.J.; Henry, T.D.; Ellis, S.G.; Zhao, D.X.M.; Silva, G.V.; Lai, D.; Thomas, J.D.; Kronenberg, M.W.; et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: The FOCUS-CCTRN trial. JAMA 2012, 307, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Bartunek, J.; Behfar, A.; Dolatabadi, D.; Vanderheyden, M.; Ostojic, M.; Dens, J.; El Nakadi, B.; Banovic, M.; Beleslin, B.; Vrolix, M.; et al. Cardiopoietic stem cell therapy in heart failure: The C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J. Am. Coll. Cardiol. 2013, 61, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Heldman, A.W.; DiFede, D.L.; Fishman, J.E.; Zambrano, J.P.; Trachtenberg, B.H.; Karantalis, V.; Mushtaq, M.; Williams, A.R.; Suncion, V.Y.; McNiece, I.K.; et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The TAC-HFT randomized trial. JAMA 2014, 311, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Santoso, T.; Siu, C.-W.; Irawan, C.; Chan, W.-S.; Alwi, I.; Yiu, K.-H.; Aziz, A.; Kwong, Y.-L.; Tse, H.-F. Endomyocardial implantation of autologous bone marrow mononuclear cells in advanced ischemic heart failure: A randomized placebo-controlled trial (END-HF). J. Cardiovasc. Transl. Res. 2014, 7, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Mathiasen, A.B.; Qayyum, A.A.; Jørgensen, E.; Helqvist, S.; Kofoed, K.F.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, J. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: Final 4-year follow-up of the MSC-HF trial. Eur. J. Heart Fail. 2020, 22, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.A.; Nasseri, B.A.; Makower, J.; Pomeraniseva, I.; Lamson, T.; Chang, J.Y.; McGarry, M.; Fishman, M.C.; Vacanti, J.P.; Oesterie, S.N. Percutaneous transvenous cellular cardiomyoplasty. A novel nonsurgical approach for myocardial cell transplantation. J. Am. Coll. Cardiol. 2003, 41, 1964–1971. [Google Scholar] [CrossRef] [PubMed]
- George, J.C.; Goldberg, J.; Joseph, M.; Abdulhameed, N.; Crist, J.; DAS, H.; Pompili, V.J. Transvenous intramyocardial cellular delivery increases retention in comparison to intracoronary delivery in a porcine model of acute myocardial infarction. J. Interv. Cardiol. 2008, 21, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Halkos, M.E.; Zhao, Z.-Q.; Kerendi, F.; Wang, N.-P.; Jiang, R.; Schmarkey, L.S.; Martin, B.J.; Quyyumi, A.A.; Few, W.L.; Kin, H.; et al. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic. Res. Cardiol. 2008, 103, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Rogers, P.I.; Kihlken, J.; Warfel, J.; Bull, C.; Deuter-Reinhard, M.; Feng, D.; Xie, J.; Kyle, A.; Merfeld-Clauss, S.; et al. Intravenous xenogeneic transplantation of human adipose-derived stem cells improves left ventricular function and microvascular integrity in swine myocardial infarction model. Catheter. Cardiovasc. Interv. 2015, 86, E38–E48. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Wang, W.; Liang, L.; Han, Z.-B.; Li, Z.; Geng, J.; Zhao, M.; Jia, H.; Feng, J.; Wei, Z.; et al. Intravenous injection of allogeneic umbilical cord-derived multipotent mesenchymal stromal cells reduces the infarct area and ameliorates cardiac function in a porcine model of acute myocardial infarction. Stem Cell Res. Ther. 2018, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.M.; Traverse, J.H.; Henry, T.D.; Dib, N.; Strumpf, R.K.; Schulman, S.P.; Gerstenblith, G.; DeMaria, A.N.; Denktas, A.E.; Gammon, R.S.; et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009, 54, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Wollert, K.C.; Meyer, G.P.; Menke, A.; Arseniev, L.; Hertenstein, B.; Ganser, A.; Knapp, W.H.; Drexler, H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation 2005, 111, 2198–2202. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.J.; Kang, H.-J.; Kim, H.-S.; Chung, J.-K.; Lee, M.C.; Lee, D.S. Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J. Nucl. Med. 2006, 47, 1295–1301. [Google Scholar]
- Tao, Z.; Tan, S.; Chen, W.; Chen, X. Stem Cell Homing: A Potential Therapeutic Strategy Unproven for Treatment of Myocardial Injury. J. Cardiovasc. Transl. Res. 2018, 11, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Xu, X.; Guo, Y.; Xia, Y.; Peng, L.; Li, C.; Ding, F.; Gao, C.; Fan, M.; Yu, M.; et al. CSF2RB overexpression promotes the protective effects of mesenchymal stromal cells against ischemic heart injury. Theranostics 2023, 13, 1759–1773. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Nakamura, S.; Li, Q.; Wysoczynski, M.; Gumpert, A.M.; Wu, W.; Hunt, G.; Stowers, H.; Ou, Q.; Bolli, R. Repeated Administrations of Cardiac Progenitor Cells Are Superior to a Single Administration of an Equivalent Cumulative Dose. J. Am. Heart Assoc. 2018, 7, e007400. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.-Y.; Li, Q.; Hu, M.-J.; Huang, P.-S.; Xu, J.-Y.; Tian, X.-Q.; Jin, C.; Liu, J.-D.; Qian, L.; Yang, Y.-J. Optimization of Timing and Times for Administration of Atorvastatin-Pretreated Mesenchymal Stem Cells in a Preclinical Model of Acute Myocardial Infarction. Stem Cells Transl. Med. 2019, 8, 1068–1083. [Google Scholar] [CrossRef] [PubMed]
- Strauer, B.E.; Brehm, M.; Zeus, T.; Köstering, M.; Hernandez, A.; Sorg, R.V.; Kögler, G.; Wernet, P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002, 106, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Lunde, K.; Solheim, S.; Aakhus, S.; Arnesen, H.; Abdelnoor, M.; Egeland, T.; Endresen, K.; Ilebekk, A.; Mangschau, A.; Fjeld, J.G.; et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N. Engl. J. Med. 2006, 355, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Schächinger, V.; Assmus, B.; Erbs, S.; Elsässer, A.; Haberbosch, W.; Hambrecht, R.; Yu, J.; Corti, R.; Mathey, D.G.; Hamm, C.W.; et al. Intracoronary infusion of bone marrow-derived mononuclear cells abrogates adverse left ventricular remodelling post-acute myocardial infarction: Insights from the reinfusion of enriched progenitor cells and infarct remodelling in acute myocardial infarction (REPAIR-AMI) trial. Eur. J. Heart Fail. 2009, 11, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Assmus, B.; Rolf, A.; Erbs, S.; Elsässer, A.; Haberbosch, W.; Hambrecht, R.; Tillmanns, H.; Yu, J.; Corti, R.; Mathey, D.G.; et al. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ. Heart Fail. 2010, 3, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.; Nijveldt, R.; van der Vleuten, P.A.; Tijssen, J.G.; van der Giessen, W.J.; Tio, R.A.; Waltenberger, J.; Berg, J.M.T.; Doevendans, P.A.; Aengevaeren, W.R.; et al. Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: Results of the randomized controlled HEBE trial. Eur. Heart J. 2011, 32, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Sürder, D.; Manka, R.; Cicero, V.L.; Moccetti, T.; Rufibach, K.; Soncin, S.; Turchetto, L.; Radrizzani, M.; Astori, G.; Schwitter, J.; et al. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: Effects on global left ventricular function. Circulation 2013, 127, 1968–1979. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Meyer, G.P.; Müller-Ehmsen, J.; Tschöpe, C.; Bonarjee, V.; Larsen, A.I.; May, A.E.; Empen, K.; Chorianopoulos, E.; Tebbe, U.; et al. Intracoronary autologous bone marrow cell transfer after myocardial infarction: The BOOST-2 randomised placebo-controlled clinical trial. Eur. Heart J. 2017, 38, 2936–2943. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.C.; Zhou, L.; Hao, J. Current stem cell delivery methods for myocardial repair. Biomed. Res. Int. 2013, 2013, 547902. [Google Scholar] [CrossRef] [PubMed]
- Traverse, J.H.; Henry, T.D.; Pepine, C.J.; Willerson, J.T.; Chugh, A.; Yang, P.C.; Zhao, D.X.M.; Ellis, S.G.; Forder, J.R.; Perin, E.C.; et al. TIME Trial: Effect of Timing of Stem Cell Delivery Following ST-Elevation Myocardial Infarction on the Recovery of Global and Regional Left Ventricular Function: Final 2-Year Analysis. Circ. Res. 2018, 122, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Tohyama, S.; Kanazawa, H.; Ichimura, H.; Chino, S.; Tanaka, Y.; Suzuki, Y.; Zhao, J.; Shiba, N.; Kadota, S.; et al. Intracoronary transplantation of pluripotent stem cell-derived cardiomyocytes: Inefficient procedure for cardiac regeneration. J. Mol. Cell Cardiol. 2023, 174, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Vicario, J.; Campo, C.; Piva, J.; Faccio, F.; Gerardo, L.; Becker, C.; Ortega, H.; Pierini, A.; Lofeudo, C.; Novero, R.; et al. One-year follow-up of transcoronary sinus administration of autologous bone marrow in patients with chronic refractory angina. Cardiovasc. Revasc Med. 2005, 6, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ge, J.; Zhang, S.; Sun, A.; Shen, J.; Chen, L.; Wang, K.; Zou, Y. Time course of myocardial stromal cell-derived factor 1 expression and beneficial effects of intravenously administered bone marrow stem cells in rats with experimental myocardial infarction. Basic. Res. Cardiol. 2005, 100, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, J.; Chen, J.; Luo, R.; He, A.; Xie, X.; Li, J. Optimal temporal delivery of bone marrow mesenchymal stem cells in rats with myocardial infarction. Eur. J. Cardiothorac. Surg. 2007, 31, 438–443. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Johnsen, H.E.; Mortensen, S.; Bindslev, L.; Ripa, R.S.; Haack-Sørensen, M.; Jørgensen, E.; Fang, W.; Kastrup, J. Changes in circulating mesenchymal stem cells, stem cell homing factor, and vascular growth factors in patients with acute ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart 2006, 92, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, A.; Xu, D.; Yao, K.; Huang, Z.; Jin, H.; Wang, K.; Zou, Y.; Ge, J. Impact of timing on efficacy and safetyof intracoronary autologous bone marrow stem cells transplantation in acute myocardial infarction: A pooled subgroup analysis of randomized controlled trials. Clin. Cardiol. 2009, 32, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Li, N.; Duan, C.-E.; Yang, Y.-J. Cardiac progenitor/stem cells on myocardial infarction or ischemic heart disease: What we have known from current research. Heart Fail. Rev. 2014, 19, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Zwetsloot, P.P.; Végh, A.M.D.; Jansen of Lorkeers, S.J.; van Hout, G.P.; Currie, G.L.; Sena, E.S.; Gremmels, H.; Buikema, J.W.; Goumans, M.-J.; Macleod, M.R. Cardiac Stem Cell Treatment in Myocardial Infarction: A Systematic Review and Meta-Analysis of Preclinical Studies. Circ. Res. 2016, 118, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Chugh, A.R.; Beache, G.M.; Loughran, J.H.; Mewton, N.; Elmore, J.B.; Kajstura, J.; Pappas, P.; Tatooles, A.; Stoddard, M.F.; Lima, J.A.C.; et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: The SCIPIO trial: Surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 2012, 126 (Suppl. S1), S54–S64. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Editors. Retraction-Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet 2019, 393, 1084. [Google Scholar] [CrossRef] [PubMed]
- Malliaras, K.; Makkar, R.R.; Smith, R.R.; Cheng, K.; Wu, E.; Bonow, R.O.; Marbán, L.; Mendizabal, A.; Cingolani, E.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells after myocardial infarction: Evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J. Am. Coll. Cardiol. 2014, 63, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Ostovaneh, M.R.; Makkar, R.R.; Ambale-Venkatesh, B.; Ascheim, D.; Chakravarty, T.; Henry, T.D.; Kowalchuk, G.; Aguirre, F.V.; Kereiakes, D.J.; Povsic, T.J.; et al. Effect of cardiosphere-derived cells on segmental myocardial function after myocardial infarction: ALLSTAR randomised clinical trial. Open Heart 2021, 8, e001614. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, S.; Ohtsuki, S.; Eitoku, T.; Ousaka, D.; Kondo, M.; Kurita, Y.; Hirai, K.; Fukushima, Y.; Baba, K.; Goto, T.; et al. Intracoronary Cardiac Progenitor Cells in Single Ventricle Physiology: The PERSEUS (Cardiac Progenitor Cell Infusion to Treat Univentricular Heart Disease) Randomized Phase 2 Trial. Circ. Res. 2017, 120, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Orlic, D.; Kajstura, J.; Chimenti, S.; Bodine, D.M.; Leri, A.; Anversa, P. Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann. N. Y. Acad. Sci. 2001, 938, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Shake, J.G.; Gruber, P.J.; Baumgartner, W.A.; Senechal, G.; Meyers, J.; Redmond, J.; Pittenger, M.F.; Martin, B.J. Mesenchymal stem cell implantation in a swine myocardial infarct model: Engraftment and functional effects. Ann. Thorac. Surg. 2002, 73, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, B.A.; Ebell, W.; Dandel, M.; Kukucka, M.; Gebker, R.; Doltra, A.; Knosalla, C.; Choi, Y.-H.; Hetzer, R.; Stamm, C. Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: The Cardio133 trial. Eur. Heart J. 2014, 35, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Strem, B.M.; Zhu, M.; Alfonso, Z.; Daniels, E.; Schreiber, R.; Begyui, R.; MacLellan, W.; Hedrick, M.; Fraser, J. Expression of cardiomyocytic markers on adipose tissue-derived cells in a murine model of acute myocardial injury. Cytotherapy 2005, 7, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Wang, X.-D.; Yokoyama, S.-I.; Fukuda, N.; Takakura, N. Cardiac progenitor cells in brown adipose tissue repaired damaged myocardium. Biochem. Biophys. Res. Commun. 2006, 342, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Oñate, B.; Vilahur, G. Adipose-derived Mesenchymal Stem Cells and Their Reparative Potential in Ischemic Heart Disease. Rev. Esp. Cardiol. 2015, 68, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-Z.; Gai, L.-Y.; Liu, H.-W.; Jin, Q.-H.; Huang, J.-H.; Zhu, X.-Y. Transplantation of autologous adipose-derived stem cells ameliorates cardiac function in rabbits with myocardial infarction. Chin. Med. J. 2007, 120, 300–307. [Google Scholar] [CrossRef]
- Hwangbo, S.; Kim, J.; Her, S.; Cho, H.; Lee, J. Therapeutic potential of human adipose stem cells in a rat myocardial infarction model. Yonsei Med. J. 2010, 51, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Soler-Botija, C.; Farré, J.; Sepúlveda, P.; Raya, A.; Roura, S.; Prat-Vidal, C.; Gálvez-Montón, C.; Montero, J.A.; Büscher, D.; et al. Human progenitor cells derived from cardiac adipose tissue ameliorate myocardial infarction in rodents. J. Mol. Cell Cardiol. 2010, 49, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Bagno, L.L.S.; Werneck-De-Castro, J.P.S.; Oliveira, P.F.; Cunha-Abreu, M.S.; Rocha, N.N.; Kasai-Brunswick, T.H.; Lago, V.M.; Goldenberg, R.C.S.; Campos-De-Carvalho, A.C. Adipose-derived stromal cell therapy improves cardiac function after coronary occlusion in rats. Cell Transplant. 2012, 21, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Wang, F.; Xiang, B.; Deng, J.; Liu, S.; Lin, H.-Y.; Natarajan, K.; Li, G.; Wang, L.; Wang, J.; et al. Adipose-derived stem cells from both visceral and subcutaneous fat deposits significantly improve contractile function of infarcted rat hearts. Cell Transplant. 2015, 24, 2337–2351. [Google Scholar] [CrossRef] [PubMed]
- Karpov, A.A.; Uspenskaya, Y.K.; Minasian, S.M.; Puzanov, M.V.; Dmitrieva, R.I.; Bilibina, A.A.; Anisimov, S.V.; Galagudza, M.M. The effect of bone marrow- and adipose tissue-derived mesenchymal stem cell transplantation on myocardial remodelling in the rat model of ischaemic heart failure. Int. J. Exp. Pathol. 2013, 94, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Mazo, M.; Hernández, S.; Gavira, J.J.; Abizanda, G.; Araña, M.; López-Martínez, T.; Moreno, C.; Merino, J.; Martino-Rodríguez, A.; Uixeira, A.; et al. Treatment of reperfused ischemia with adipose-derived stem cells in a preclinical Swine model of myocardial infarction. Cell Transplant. 2012, 21, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Schenke-Layland, K.; Strem, B.M.; Jordan, M.C.; DeEmedio, M.T.; Hedrick, M.H.; Roos, K.P.; Fraser, J.K.; MacLellan, W.R. Adipose tissue-derived cells improve cardiac function following myocardial infarction. J. Surg. Res. 2009, 153, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Johnstone, B.H.; Cook, T.G.; Tan, J.; Fishbein, M.C.; Chen, P.-S.; March, K.L. IFATS collection: Human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells 2009, 27, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yan, Y.; Coleman, M.; Wu, G.; Rabinovich, B.; Seidensticker, M.; Alt, E. Tracking long-term survival of intramyocardially delivered human adipose tissue-derived stem cells using bioluminescence imaging. Mol. Imaging Biol. 2011, 13, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; Sanz-Ruiz, R.; Sánchez, P.L.; Lasso, J.; Pérez-Cano, R.; Alonso-Farto, J.C.; Pérez-David, E.; Fernández-Santos, M.E.; Serruys, P.W.; Duckers, H.J.; et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am. Heart J. 2014, 168, 88–95.e2. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-C.; Chang, Y.-H.; Shyu, W.-C.; Lin, S.-Z. Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transplant. 2015, 24, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, D.S.; Mosqueira, D.; Sousa, L.M.; Teixeira, M.; Filipe, M.; Resende, T.P.; Araújo, A.F.; Valente, M.; Almeida, J.; Martins, J.P.; et al. Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling after myocardial infarction by proangiogenic, antiapoptotic, and endogenous cell-activation mechanisms. Stem Cell Res. Ther. 2014, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Yannarelli, G.; Dayan, V.; Pacienza, N.; Lee, C.-J.; Medin, J.; Keating, A. Human umbilical cord perivascular cells exhibit enhanced cardiomyocyte reprogramming and cardiac function after experimental acute myocardial infarction. Cell Transplant. 2013, 22, 1651–1666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, X.-C.; Yang, L.; Zhu, D.-L.; Zhang, Y.-D.; Chen, Y.; Zhang, H.-Y. Wharton’s jelly-derived mesenchymal stem cells promote myocardial regeneration and cardiac repair after miniswine acute myocardial infarction. Coron. Artery Dis. 2013, 24, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, J.G.; Verdugo, F.J.; González, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.G.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure. Circ. Res. 2017, 121, 1192–1204. [Google Scholar] [CrossRef]
- Weber, B.; Emmert, M.Y.; Behr, L.; Schoenauer, R.; Brokopp, C.; Drögemüller, C.; Modregger, P.; Stampanoni, M.; Vats, D.; Rudin, M.; et al. Prenatally engineered autologous amniotic fluid stem cell-based heart valves in the fetal circulation. Biomaterials 2012, 33, 4031–4043. [Google Scholar] [CrossRef] [PubMed]
- Chiavegato, A.; Bollini, S.; Pozzobon, M.; Callegari, A.; Gasparotto, L.; Taiani, J.; Piccoli, M.; Lenzini, E.; Gerosa, G.; Vendramin, I.; et al. Human amniotic fluid-derived stem cells are rejected after transplantation in the myocardium of normal, ischemic, immuno-suppressed or immuno-deficient rat. J. Mol. Cell Cardiol. 2007, 42, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Ise, H.; Hongo, M.; Ota, M.; Konishi, I.; Nikaido, T. Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation 2005, 79, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-C.; Wei, H.-J.; Lee, W.-Y.; Yu, C.-L.; Chang, Y.; Hsu, L.-W.; Chung, M.-F.; Tsai, M.-S.; Hwang, S.-M.; Sung, H.-W. Cellular cardiomyoplasty with human amniotic fluid stem cells: In vitro and in vivo studies. Tissue Eng. Part. A 2010, 16, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-H.; Jin, J.; Joe, J.-H.; Song, Y.-S.; So, B.-I.; Lim, S.M.; Cheon, G.J.; Woo, S.-K.; Ra, J.-C.; Lee, Y.-Y.; et al. In vivo differentiation of human amniotic epithelial cells into cardiomyocyte-like cells and cell transplantation effect on myocardial infarction in rats: Comparison with cord blood and adipose tissue-derived mesenchymal stem cells. Cell Transplant. 2012, 21, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Takov, K.; He, Z.; Johnston, H.E.; Timms, J.F.; Guillot, P.V.; Yellon, D.M.; Davidson, S.M. Small extracellular vesicles secreted from human amniotic fluid mesenchymal stromal cells possess cardioprotective and promigratory potential. Basic. Res. Cardiol. 2020, 115, 26. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, X.; Li, P.; Lu, X.; Yan, J.; Tan, H.; Zhang, C. Exosomes derived from human amniotic fluid mesenchymal stem cells alleviate cardiac fibrosis via enhancing angiogenesis in vivo and in vitro. Cardiovasc. Diagn. Ther. 2021, 11, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Blin, G.; Nury, D.; Stefanovic, S.; Neri, T.; Guillevic, O.; Brinon, B.; Bellamy, V.; Rücker-Martin, C.; Barbry, P.; Bel, A.; et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J. Clin. Investig. 2010, 120, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Ménard, C.; Hagège, A.A.; Agbulut, O.; Barro, M.; Morichetti, M.C.; Brasselet, C.; Bel, A.; Messas, E.; Bissery, A.; Bruneval, P.; et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: A preclinical study. Lancet 2005, 366, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Caspi, O.; Huber, I.; Kehat, I.; Habib, M.; Arbel, G.; Gepstein, A.; Yankelson, L.; Aronson, D.; Beyar, R.; Gepstein, L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J. Am. Coll. Cardiol. 2007, 50, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Kehat, I.; Khimovich, L.; Caspi, O.; Gepstein, A.; Shofti, R.; Arbel, G.; Huber, I.; Satin, J.; Itskovitz-Eldor, J.; Gepstein, L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat. Biotechnol. 2004, 22, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Cho, H.C.; Akar, F.G.; Tsang, S.-Y.; Jones, S.P.; Marbán, E.; Tomaselli, G.F.; Li, R.A. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: Insights into the development of cell-based pacemakers. Circulation 2005, 111, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, M.A.; Chen, K.Y.; Naumova, A.V.; Muskheli, V.; Fugate, J.A.; Dupras, S.K.; Reinecke, H.; Xu, C.; Hassanipour, M.; Police, S.; et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007, 25, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.J.H.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018, 36, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wu, Q.; Ni, C.; Zhang, P.; Zhong, Z.; Wu, Y.; Wang, Y.; Xu, Y.; Kong, M.; Cheng, H.; et al. Lack of Remuscularization Following Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitor Cells in Infarcted Nonhuman Primates. Circ. Res. 2018, 122, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, S.; Miki, K.; Takaki, T.; Okubo, C.; Hatani, T.; Chonabayashi, K.; Nishikawa, M.; Takei, I.; Oishi, A.; Narita, M.; et al. Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep. 2016, 6, 19111. [Google Scholar] [CrossRef] [PubMed]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K.; et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Marchiano, S.; Nakamura, K.; Reinecke, H.; Neidig, L.; Lai, M.; Kadota, S.; Perbellini, F.; Yang, X.; Klaiman, J.M.; Blakely, L.P.; et al. Gene editing to prevent ventricular arrhythmias associated with cardiomyocyte cell therapy. Cell Stem Cell. 2023, 30, 741. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-C.; Hsieh, M.L.; Lin, C.-J.; Chang, C.M.; Huang, C.-Y.; Puntney, R.; Moy, A.W.; Ting, C.-Y.; Chan, D.Z.H.; Nicholson, M.W.; et al. Combined Treatment of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes and Endothelial Cells Regenerate the Infarcted Heart in Mice and Non-Human Primates. Circulation 2023, 148, 1395–1409. [Google Scholar] [CrossRef] [PubMed]
- Deuse, T.; Hu, X.; Gravina, A.; Wang, D.; Tediashvili, G.; De, C.; Thayer, W.O.; Wahl, A.; Garcia, J.V.; Reichenspurner, H.; et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 2019, 37, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, X.; Wang, W.E.; Zeng, C. How to Improve the Survival of Transplanted Mesenchymal Stem Cell in Ischemic Heart? Stem Cells Int. 2016, 2016, 9682757. [Google Scholar] [CrossRef] [PubMed]
- Collantes, M.; Pelacho, B.; García-Velloso, M.J.; Gavira, J.J.; Abizanda, G.; Palacios, I.; Rodriguez-Borlado, L.; Álvarez, V.; Prieto, E.; Ecay, M.; et al. Non-invasive in vivo imaging of cardiac stem/progenitor cell biodistribution and retention after intracoronary and intramyocardial delivery in a swine model of chronic ischemia reperfusion injury. J. Transl. Med. 2017, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.F.; Yu, J.; Sievers, R.; Li, S.; Lee, R.J. Injectable biopolymers enhance angiogenesis after myocardial infarction. Tissue Eng. 2005, 11, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Danoviz, M.E.; Nakamuta, J.S.; Marques, F.L.N.; dos Santos, L.; Alvarenga, E.C.; dos Santos, A.A.; Antonio, E.L.; Schettert, I.T.; Tucci, P.J.; Krieger, J.E. Rat adipose tissue-derived stem cells transplantation attenuates cardiac dysfunction post infarction and biopolymers enhance cell retention. PLoS ONE 2010, 5, e12077. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, C.; Li, C.; Chen, J.; Li, Y.; Xie, H.; Lin, C.; Fan, M.; Guo, Y.; Gao, E.; et al. Tailorable Hydrogel Improves Retention and Cardioprotection of Intramyocardial Transplanted Mesenchymal Stem Cells for the Treatment of Acute Myocardial Infarction in Mice. J. Am. Heart Assoc. 2020, 9, e013784. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, J.; Wang, Y.; Yin, Y.; Wang, L.; Liu, J.; Liu, Z.; Duan, C.; Zhu, P.; Wang, C. Promotion of cardiac differentiation of brown adipose derived stem cells by chitosan hydrogel for repair after myocardial infarction. Biomaterials 2014, 35, 3986–3998. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, H.; Wang, Y.; Lin, Q.; Yao, A.; Cao, F.; Li, D.; Zhou, J.; Duan, C.; Du, Z.; et al. The influence of chitosan hydrogel on stem cell engraftment, survival and homing in the ischemic myocardial microenvironment. Biomaterials 2012, 33, 3093–3106. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Tian, L.; Li, Y.; Zhang, J.; Wei, Y.; Jin, Z.; Liu, Z.; Liu, H. Combining ECM Hydrogels of Cardiac Bioactivity with Stem Cells of High Cardiomyogenic Potential for Myocardial Repair. Stem Cells Int. 2019, 2019, 6708435. [Google Scholar] [CrossRef] [PubMed]
- Takehara, N.; Tsutsumi, Y.; Tateishi, K.; Ogata, T.; Tanaka, H.; Ueyama, T.; Takahashi, T.; Takamatsu, T.; Fukushima, M.; Komeda, M.; et al. Controlled delivery of basic fibroblast growth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. J. Am. Coll. Cardiol. 2008, 52, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Bargehr, J.; Ong, L.; Colzani, M.; Davaapil, H.; Hofsteen, P.; Bhandari, S.; Gambardella, L.; Le Novère, N.; Iyer, D.; Sampaziotis, F.; et al. Epicardial cells derived from human embryonic stem cells augment cardiomyocyte-driven heart regeneration. Nat. Biotechnol. 2019, 37, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chang, Y.-H.; Xiong, Q.; Zhang, P.; Zhang, L.; Somasundaram, P.; Lepley, M.; Swingen, C.; Su, L.; Wendel, J.S.; et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 2014, 15, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-H.; Kang, S.-W.; Park, S.-J.; Bae, D.; Kim, S.J.; Lee, H.-A.; Kim, K.S.; Hong, K.-S.; Kim, J.S.; Do, J.T.; et al. The use of aggregates of purified cardiomyocytes derived from human ESCs for functional engraftment after myocardial infarction. Biomaterials 2013, 34, 4013–4026. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Soma, Y.; Nakajima, K.; Kanazawa, H.; Tohyama, S.; Tabei, R.; Hirano, A.; Handa, N.; Yamada, Y.; Okuda, S.; et al. Intramyocardial Transplantation of Human iPS Cell-Derived Cardiac Spheroids Improves Cardiac Function in Heart Failure Animals. JACC Basic. Transl. Sci. 2021, 6, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Tohyama, S.; Ichimura, H.; Ohashi, N.; Chino, S.; Soma, Y.; Tani, H.; Tanaka, Y.; Yang, X.; Shiba, N.; et al. Regeneration of Nonhuman Primate Hearts With Human Induced Pluripotent Stem Cell-Derived Cardiac Spheroids. Circulation 2024, 150, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.C.; Vu, D.-T.; Wang, J.; Lilyanna, S.; Ling, L.H.; Gan, S.U.; Tan, A.L.; Phan, T.T.; Lee, C.N.; Kofidis, T. Grafts enriched with subamnion-cord-lining mesenchymal stem cell angiogenic spheroids induce post-ischemic myocardial revascularization and preserve cardiac function in failing rat hearts. Stem Cells Dev. 2013, 22, 3087–3099. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Coyle, R.C.; Barrs, R.W.; Silver, S.E.; Li, M.; Richards, D.J.; Lin, Y.; Jiang, Y.; Wang, H.; Menick, D.R.; et al. Nanowired human cardiac organoid transplantation enables highly efficient and effective recovery of infarcted hearts. Sci Adv. 2023, 9, eadf2898. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yu, S.P.; Fraser, J.L.; Lu, Z.; Ogle, M.E.; Wang, J.-A.; Wei, L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J. Thorac. Cardiovasc. Surg. 2008, 135, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Jaussaud, J.; Biais, M.; Calderon, J.; Chevaleyre, J.; Duchez, P.; Ivanovic, Z.; Couffinhal, T.; Barandon, L. Hypoxia-preconditioned mesenchymal stromal cells improve cardiac function in a swine model of chronic myocardial ischaemia. Eur. J. Cardiothorac. Surg. 2013, 43, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.-Q.; Yang, Y.-J.; Li, Q.; Xu, J.; Huang, P.-S.; Xiong, Y.-Y.; Li, X.-D.; Jin, C.; Qi, K.; Jiang, L.-P.; et al. Combined therapy with atorvastatin and atorvastatin-pretreated mesenchymal stem cells enhances cardiac performance after acute myocardial infarction by activating SDF-1/CXCR4 axis. Am. J. Transl. Res. 2019, 11, 4214–4231. [Google Scholar]
- Pasha, Z.; Wang, Y.; Sheikh, R.; Zhang, D.; Zhao, T.; Ashraf, M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc. Res. 2008, 77, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.L.; Wang, Y.; Abarbanell, A.M.; Weil, B.R.; Tan, J.; Meldrum, D.R. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock 2010, 33, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Guo, Y.; Teng, L.; Nong, Y.; Tan, M.; Book, M.J.; Zhu, X.; Wang, X.-L.; Du, J.; Wu, W.-J.; et al. Preconditioning Human Cardiac Stem Cells with an HO-1 Inducer Exerts Beneficial Effects After Cell Transplantation in the Infarcted Murine Heart. Stem Cells 2015, 33, 3596–3607. [Google Scholar] [CrossRef] [PubMed]
- Mangi, A.A.; Noiseux, N.; Kong, D.; He, H.; Rezvani, M.; Ingwall, J.S.; Dzau, V.J. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003, 9, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Kim, Y.S.; Ahn, Y.; Jeong, M.H.; Hong, M.H.; Joo, S.Y.; Nam, K.I.; Cho, J.G.; Kang, P.M.; Park, J.C. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc. Res. 2006, 70, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Karpov, A.A.; Udalova, D.V.; Pliss, M.G.; Galagudza, M.M. Can the outcomes of mesenchymal stem cell-based therapy for myocardial infarction be improved? Providing weapons and armour to cells. Cell Prolif. 2017, 50, e12316. [Google Scholar] [CrossRef] [PubMed]
- Lemcke, H.; Voronina, N.; Steinhoff, G.; David, R. Recent Progress in Stem Cell Modification for Cardiac Regeneration. Stem Cells Int. 2018, 2018, 1909346. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guan, Q.; Dai, S.; Wei, W.; Zhang, Y. Integrin β1 Increases Stem Cell Survival and Cardiac Function after Myocardial Infarction. Front. Pharmacol. 2017, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Nollet, E.; Hoymans, V.Y.; Van Craenenbroeck, A.H.; Vrints, C.J.; Van Craenenbroeck, E.M. Improving stem cell therapy in cardiovascular diseases: The potential role of microRNA. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H207–H218. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Luo, R.; Yu, X.; Dong, W.; Hao, G.; Hu, D.; Yu, Z.; Liu, M.; Lu, T.; Wang, X.; et al. Enhanced human adipose-derived stem cells with VEGFA and bFGF mRNA promote stable vascular regeneration and improve cardiac function following myocardial infarction. Clin. Transl. Med. 2025, 15, e70250. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.U.; Guo, Y.; Li, Q.-H.; Cao, P.; Al-Maqtari, T.; Vajravelu, B.N.; Du, J.; Book, M.J.; Zhu, X.; Nong, Y.; et al. c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS ONE 2014, 9, e96725. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-L.; Li, Q.; Rokosh, G.; Sanganalmath, S.K.; Chen, N.; Ou, Q.; Stowers, H.; Hunt, G.; Bolli, R. Long-Term Outcome of Administration of c-kit(POS) Cardiac Progenitor Cells After Acute Myocardial Infarction: Transplanted Cells Do not Become Cardiomyocytes, but Structural and Functional Improvement and Proliferation of Endogenous Cells Persist for at Least One Year. Circ. Res. 2016, 118, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006, 20, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yu, Y.; Hu, S.; Chen, Y.; Shen, Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Mirotsou, M.; Jayawardena, T.M.; Schmeckpeper, J.; Gnecchi, M.; Dzau, V.J. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J. Mol. Cell Cardiol. 2011, 50, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Uemura, R.; Xu, M.; Ahmad, N.; Ashraf, M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ. Res. 2006, 98, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Kamihata, H.; Matsubara, H.; Nishiue, T.; Fujiyama, S.; Tsutsumi, Y.; Ozono, R.; Masaki, H.; Mori, Y.; Iba, O.; Tateishi, E.; et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 2001, 104, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, Z.; Xu, Y.; Cui, G. Effects of mesenchymal stem cell transplantation on extracellular matrix after myocardial infarction in rats. Coron. Artery Dis. 2005, 16, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, N.; Kangawa, K.; Itoh, T.; Iwase, T.; Murakami, S.; Miyahara, Y.; Fujii, T.; Uematsu, M.; Ohgushi, H.; Yamagishi, M.; et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation 2005, 112, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Lin, G.-S.; Bao, C.-Y.; Hu, Z.-M.; Hu, M.-Y. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation 2007, 30, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, A.; Santoso, M.R.; Mahmoudi, M.; Shukla, P.; Wang, L.; Bennett, M.; Goldstone, A.B.; Wang, M.; Fukushi, M.; Ebert, A.D.; et al. Paracrine Effects of the Pluripotent Stem Cell-Derived Cardiac Myocytes Salvage the Injured Myocardium. Circ. Res. 2017, 121, e22–e36. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, Y.; Yin, L.; Lin, X.; Luo, Z.; Wang, S.; Yuan, L.; Liang, P.; Jiang, B. Mesenchymal stem cell-derived exosomes in myocardial infarction: Therapeutic potential and application. J. Gene Med. 2024, 26, e3596. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Cheng, W.; Wang, L.; Chen, S.; Teng, Y.; Lu, Z.; Li, Y.; Zhao, M. Mesenchymal Stem Cell Exosomes in the Treatment of Myocardial Infarction: A Systematic Review of Preclinical In Vivo Studies. J. Cardiovasc. Transl. Res. 2022, 15, 317–339. [Google Scholar] [CrossRef] [PubMed]
- Spannbauer, A.; Mester-Tonczar, J.; Traxler, D.; Kastner, N.; Zlabinger, K.; Hašimbegović, E.; Riesenhuber, M.; Pavo, N.; Goliasch, G.; Gyöngyösi, M. Large Animal Models of Cell-Free Cardiac Regeneration. Biomolecules 2020, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Shao, A.; Potel, K.N.; So, S.W.; Swingen, C.M.; Wright, C.A.; Stone, L.L.H.; McFalls, E.O.; Butterick, T.A.; Kelly, R.F. Stem cell-derived exosome patch with coronary artery bypass graft restores cardiac function in chronically ischemic porcine myocardium. J. Thorac. Cardiovasc. Surg. 2023, 166, e512–e530. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Yan, J.; Yao, Z.; Zhang, C.; Li, X.; Mao, H.-Q. Effects of Mesenchymal Stem Cell-Derived Paracrine Signals and Their Delivery Strategies. Adv. Healthc. Mater. 2021, 10, e2001689. [Google Scholar] [CrossRef] [PubMed]
- Femminò, S.; Bonelli, F.; Brizzi, M.F. Extracellular vesicles in cardiac repair and regeneration: Beyond stem-cell-based approaches. Front. Cell Dev. Biol. 2022, 10, 996887. [Google Scholar] [CrossRef] [PubMed]
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014, 103, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, F.; Chai, R.; Zhou, W.; Hu, M.; Liu, B.; Chen, X.; Liu, M.; Xu, Q.; Liu, N.; et al. Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. J. Cell Mol. Med. 2019, 23, 7617–7631. [Google Scholar] [CrossRef] [PubMed]
- Lima Correa, B.; El Harane, N.; Gomez, I.; Rachid Hocine, H.; Vilar, J.; Desgres, M.; Bellamy, V.; Keirththana, K.; Guillas, C.; Perotto, M.; et al. Extracellular vesicles from human cardiovascular progenitors trigger a reparative immune response in infarcted hearts. Cardiovasc. Res. 2021, 117, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; Vanni, R.; Iacono, M.L.; Rastaldo, R.; Giachino, C. Direct Reprogramming of Resident Non-Myocyte Cells and Its Potential for In Vivo Cardiac Regeneration. Cells 2023, 12, 1166. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, X.; Chung, M.; Cai, S.; Pan, Y. Direct fibroblast reprogramming: An emerging strategy for treating organic fibrosis. J. Transl. Med. 2025, 23, 240. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiang, S.; Lin, Z.; Li, E.N.; Yagi, H.; Cao, G.; Yocum, L.; Li, L.; Hao, T.; Bruce, K.K. Direct in vivo reprogramming with non-viral sequential targeting nanoparticles promotes cardiac regeneration. Biomaterials 2021, 276, 121028. [Google Scholar] [CrossRef] [PubMed]

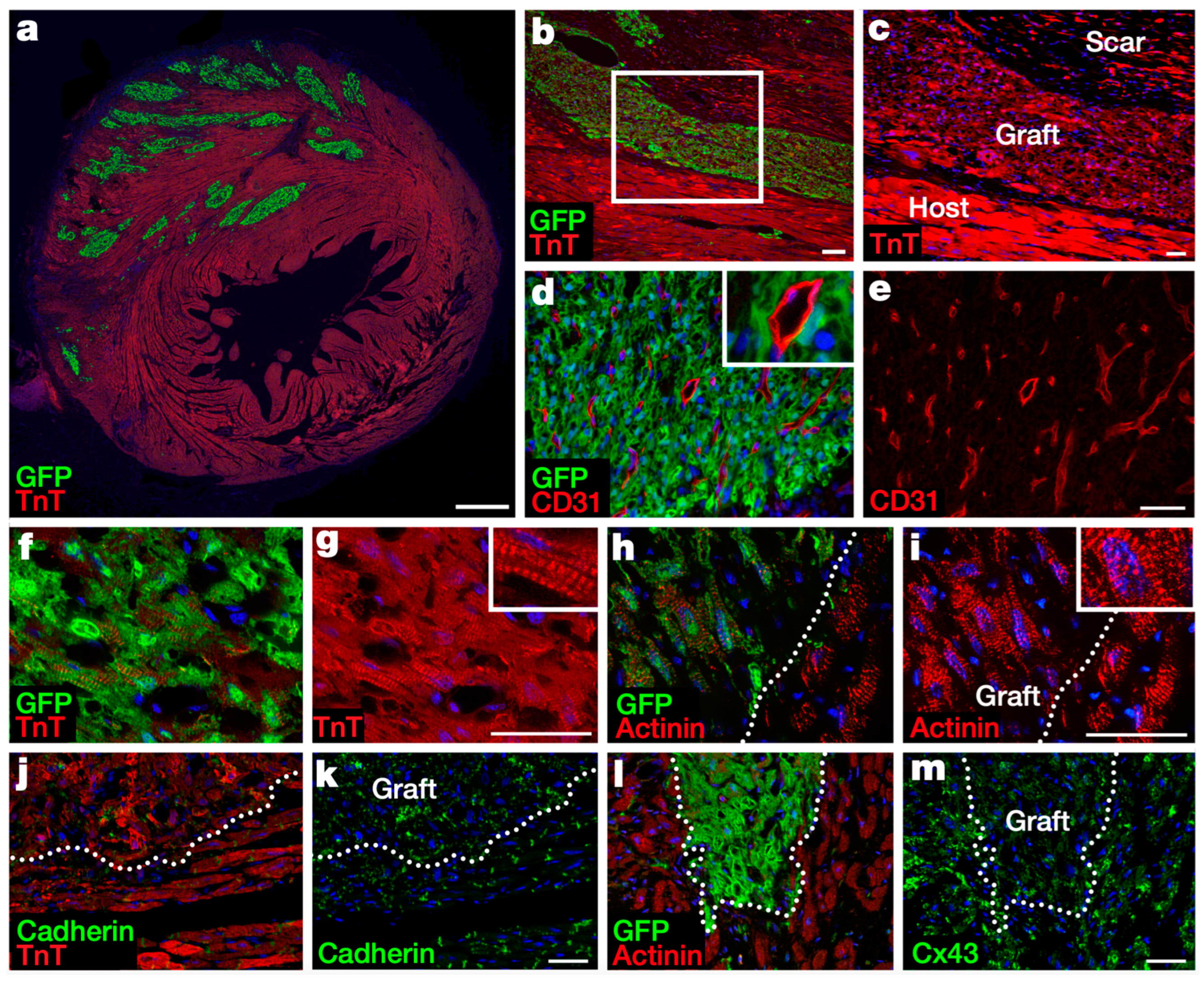
| Author | Year of Publication | Trial Name | Country | Study Design | Experimental Group Sample Size | Cell Origin | Route of Administration | Follow-Up Duration | |
|---|---|---|---|---|---|---|---|---|---|
| Zhao et al. [8] | 2008 | -- | China | Phase I RCT | 18 | Autologous MN-BMCs | Intracoronary infusion during CABG | 6 months | |
| Chugh et al. [47] * | 2012 | SCIPIO | United States | Phase I RCT | 20 | Autologous CSCs from the right atrial appendage | Intracoronary infusion during CABG | 4 and 12 months | |
| Nasseri et al. [55] | 2014 | Cardio133 | Germany | Phase II RCT | 30 | Autologous BM-MSCs | Intracoronary infusion during CABG | 6 months | |
| Karantalis et al. [9] | 2014 | PROMETHEUS | United States | Phase I RCT | 6 | Autologous BM-MSCs | Intramyocardial injection during CABG | 18 months | |
| Pätilä et al. [10] | 2014 | -- | Finland | Phase II RCT | 20 | Autologous MN-BMCs | Intramyocardial injection during CABG | 12 months | |
| Hare et al. [12] | 2012 | POSEIDON | United States | Phase I/II RCT | 27 | Autologous and allogeneic BM-MSCs | Transendocardial injection | 13 months | |
| Perin et al. [13] | 2012 | FOCUS-CCTRN | United States | Phase II RCT | 61 | Autologous MN-BMCs | Transendocardial injection | 6 months | |
| Bartunek et al. [14] | 2013 | C-CURE | Belgium | Phase I RCT | 32 | Autologous BM-MSCs | Transendocardial injection | 6 months | |
| Santoso et al. [16] | 2014 | END-HF | Indonesia/Hong Kong | Phase I RCT | 19 | Autologous MN-BMCs | Transendocardial injection | 6 months | |
| Perin et al. [69] | 2014 | PRECISE | Spain | Phase I RCT | 21 | Autologous ADSCs | Transendocardial injection | 6 and 18 months | |
| Heldman et al. [15] | 2014 | TAC-HFT | United States | Phase I/II RCT | 38 | Autologous BM-MSCs and MN-BMCs | Transendocardial injection | 12 months | |
| Mathiasen et al. [17] | 2020 | MSC-HF | Denmark | Phase I RCT | 40 | Autologous BM-MSCs | Transendocardial injection | 12 months | |
| Hare et al. [23] | 2009 | -- | United States | Phase I RCT | 39 | Allogeneic BM-MSCs | Intravenous infusion | 6 months | |
| Bartolucci et al. [74] | 2017 | RIMECARD | United States | Phase I RCT | 15 | Allogeneic UC-MSCs | Intravenous infusion | 12 months | |
| Strauer et al. [30] | 2002 | -- | Germany | Non-randomized phase I controlled trial | 10 | Autologous MN-BMCs | Intracoronary infusion via PCI | 3 months | |
| Ge et al. [148] | 2006 | TCT-STAMI | China | Phase I RCT | 10 | Autologous MN-BMCs | Intracoronary infusion via PCI | 6 months | |
| Janssens et al. [147] | 2006 | -- | Belgium | Phase I/II RCT | 33 | Autologous BM-MSCs | Intracoronary infusion via PCI | 4 months | |
| Lunde et al. [31] | 2006 | ASTAMI | Norway | Phase I/II RCT | 47 | Autologous MN-BMCs | Intracoronary infusion via PCI | 6 months | |
| Assmus et al. [33] | 2010 | REPAIR-AMI | Germany | Phase II RCT | 101 | Autologous MN-BMCs | Intracoronary infusion via PCI | 24 months | |
| Hirsch et al. [34] | 2011 | HEBE | Netherlands | Phase II RCT | 69 | Autologous MN-BMCs | Intracoronary infusion via PCI | 4 months | |
| Sürder et al. [35] | 2013 | SWISS-AMI | Switzerland | Phase II RCT | 128 | Autologous MN-BMCs | Intracoronary infusion via PCI | 4 months | |
| Malliaras et al. [49] | 2014 | CADUCEUS | United States | Phase I RCT | 17 | Autologous CDCs obtained from endomyocardial biopsy | Intracoronary infusion via PCI | 13.4 months | |
| Wollert et al. [36] | 2017 | BOOST-2 | Germany | Phase II RCT | 127 | Autologous MN-BMCs | Intracoronary infusion via PCI | 6 months | |
| Traverse et al. [38] | 2018 | TIME | United States | Phase I/II RCT | 58 | Autologous MN-BMCs | Intracoronary infusion via PCI | 24 months | |
| Ostovaneh et al. [50] | 2021 | ALLSTAR | United States | Phase II RCT | 124 | Allogeneic CDCs | Intracoronary infusion via PCI | 6 months | |
| Outcome | |||||||||
| LVEF | LVESV | Segmental Myocardial Circumferential Strain | Infarct Size | LV Viable Mass | NYHA Class | MLHFQ | 6-Minute Walk Test | MVO2 | |
| Zhao et al. [8] | ↑ compared to control | ↓ compared to control | -- | -- | ↑ compared to control | Improved compared to control | -- | -- | -- |
| Chugh et al. [47] * | ↑ compared to baseline | -- | -- | ↓ compared to baseline | ↑ compared to baseline | Improved compared to baseline | Improved compared to baseline | -- | -- |
| Nasseri et al. [55] | No difference compared to control | No difference compared to control | Improved compared to control (inferior and posterior segments only) | No difference compared to control | -- | Worse compared to control | No difference compared to control | No difference compared to control | No difference compared to control |
| Karantalis et al. [9] | ↑ compared to baseline | ↓ compared to baseline | No significant change | ↓ compared to baseline | ↑ compared to baseline | -- | -- | -- | -- |
| Pätilä et al. [10] | No difference compared to control | No difference compared to control | -- | ↓ compared to baseline | No difference compared to control | -- | -- | -- | -- |
| Hare et al. [12] | No difference compared to baseline | No difference compared to baseline | -- | ↓ compared to baseline | ↑ compared to baseline | No difference from baseline | Improved compared to baseline | Improved compared to baseline | No difference compared to baseline |
| Perin et al. [13] | ↑ compared to baseline | No difference compared to control | -- | No difference compared to control | -- | Improved compared to baseline | -- | -- | No difference compared to control |
| Bartunek et al. [14] | ↑ compared to control | ↓ compared to control | -- | -- | -- | No difference compared to control | No difference compared to control | Improved compared to control | No difference compared to control |
| Santoso et al. [16] | No difference compared to control | No difference compared to control | -- | No difference compared to control | -- | No difference compared to control | -- | No difference compared to control | -- |
| Perin et al. [69] | -- | -- | -- | No difference compared to control | ↑ compared to baseline | No difference compared to control | -- | -- | Improved compared to control |
| Heldman et al. [15] | No difference compared to control | No difference compared to control | Improved compared to control (MSCs only) | ↓ compared to control (MSCs only) | ↑ compared to control (MSCs only) | No difference compared to control | Improved compared to control (MSCs only) | Improved compared to control (MSCs only) | No difference compared to control |
| Mathiasen et al. [17] | ↑ compared to control | ↓ compared to control | -- | No difference compared to control | ↑ compared to control | No difference compared to control | -- | No difference compared to control | -- |
| Hare et al. [23] | ↑ compared to baseline, no significant difference compared to control | No difference compared to baseline | -- | -- | -- | -- | -- | Improved compared to control | -- |
| Bartolucci et al. [74] | No difference compared to control | No difference compared to control | -- | -- | -- | No difference compared to control | No difference compared to control | -- | No difference compared to control |
| Strauer et al. [30] | No difference compared to baseline | ↓ compared to baseline | -- | ↓ compared to control | -- | -- | -- | -- | -- |
| Ge et al. [148] | ↑ compared to control | -- | -- | -- | -- | -- | -- | -- | -- |
| Janssens et al. [147] | No difference compared to control | No difference compared to control | -- | No difference compared to control | No difference compared to control | -- | -- | -- | -- |
| Lunde et al. [31] | No difference compared to control | -- | -- | No difference compared to control | -- | -- | -- | -- | -- |
| Assmus et al. [33] | ↑ compared to control | No difference compared to control | -- | -- | -- | -- | -- | -- | -- |
| Hirsch et al. [34] | No difference compared to control | No difference compared to control | -- | No difference compared to control | No difference compared to control | -- | -- | -- | -- |
| Sürder et al. [35] | No difference compared to control | ↓ compared to control (late treatment only) | -- | No difference compared to control | No difference compared to control | No difference compared to control | -- | -- | -- |
| Malliaras et al. [49] | No difference compared to control | No difference compared to control | Improved compared to control | ↓ compared to control | ↑ compared to control | No difference compared to control | No difference compared to control | No difference compared to baseline | No difference compared to baseline |
| Wollert et al. [36] | No difference compared to control | No difference compared to control | -- | No difference compared to control | -- | -- | -- | -- | -- |
| Traverse et al. [38] | No difference compared to control | No difference compared to control | -- | ↑ compared to control | No difference compared to control | -- | -- | -- | -- |
| Ostovaneh et al. [50] | No difference compared to control | ↓ compared to control | Improved compared to control | No difference compared to control | No difference compared to control | -- | -- | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Watanabe, T.; Shinoka, T. Injectable Stem Cell-Based Therapies for Myocardial Regeneration: A Review of the Literature. J. Funct. Biomater. 2025, 16, 152. https://doi.org/10.3390/jfb16050152
Guo M, Watanabe T, Shinoka T. Injectable Stem Cell-Based Therapies for Myocardial Regeneration: A Review of the Literature. Journal of Functional Biomaterials. 2025; 16(5):152. https://doi.org/10.3390/jfb16050152
Chicago/Turabian StyleGuo, Marissa, Tatsuya Watanabe, and Toshiharu Shinoka. 2025. "Injectable Stem Cell-Based Therapies for Myocardial Regeneration: A Review of the Literature" Journal of Functional Biomaterials 16, no. 5: 152. https://doi.org/10.3390/jfb16050152
APA StyleGuo, M., Watanabe, T., & Shinoka, T. (2025). Injectable Stem Cell-Based Therapies for Myocardial Regeneration: A Review of the Literature. Journal of Functional Biomaterials, 16(5), 152. https://doi.org/10.3390/jfb16050152






