Fibroblastic Transformation of Corneal Keratocytes by Rac Inhibition is Modulated by Extracellular Matrix Structure and Stiffness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Substrates
2.2.1. Standard (Uncompressed) Collagen Matrices
2.2.2. Compressed Collagen Matrices
2.3. Culture Conditions
2.4. Imaging
3. Results
3.1. Cells within Uncompressed Collagen Matrices

| Culture media | 1 day of culture | 4 days of culture | ||
|---|---|---|---|---|
| Control | NSC23766 | Control | NSC23766 | |
| Serum-Free | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0 ± 0% |
| PDGF | 0 ± 0% | 0 ± 0% | 0 ± 0% | 3 ± 6% |
| IGF | 0 ± 0% | 5 ± 8% | 0 ± 0% | 5 ± 8% |
| FGF | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0 ± 0% |
| TGFβ1 | 100 ± 0% | 100 ± 0% | 100 ± 0% | 97 ± 6% |
3.2. Cells on Bottom of Uncompressed Collagen Matrices


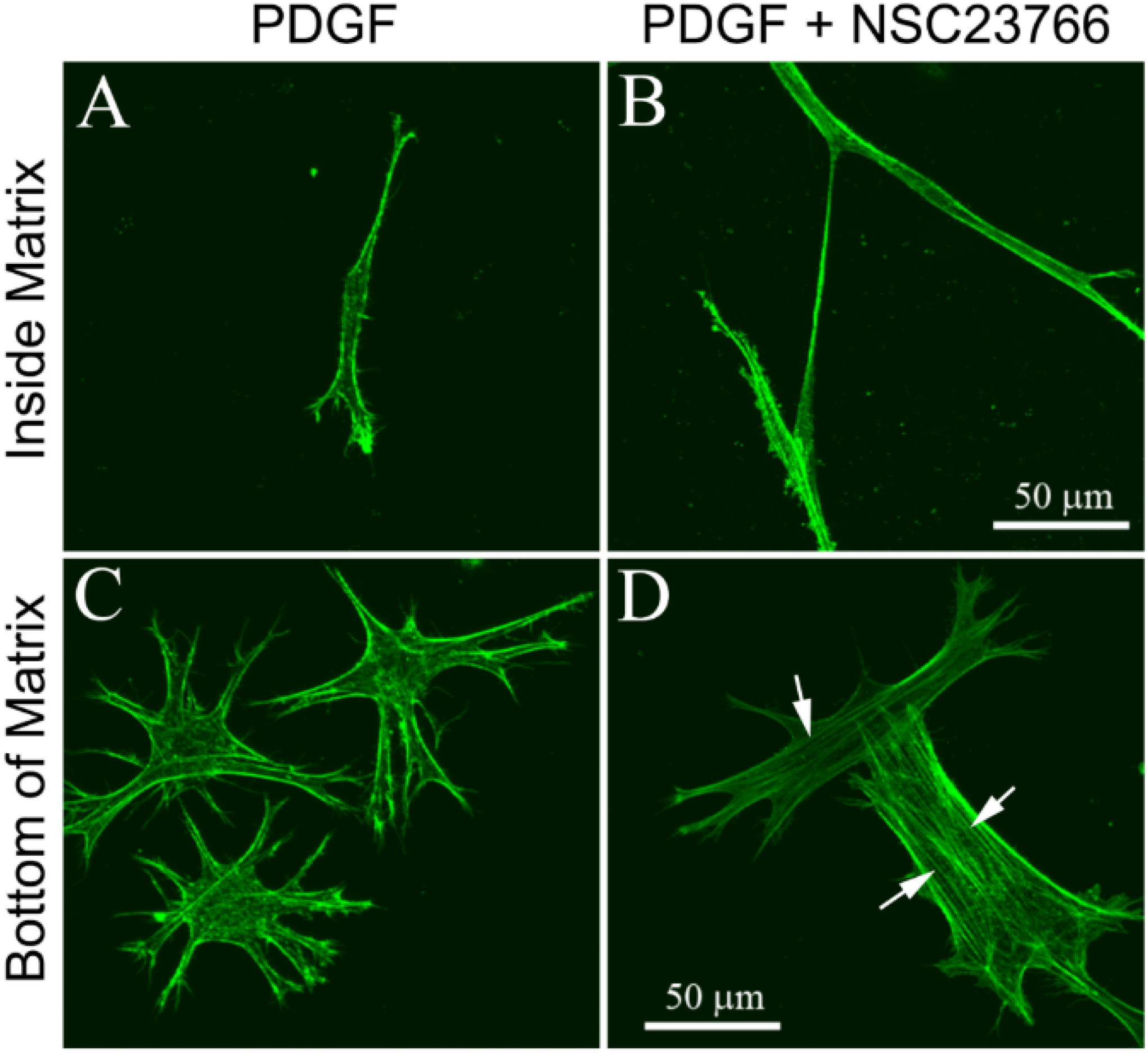
3.3. Cells within Compressed Collagen Matrices

| Culture media | 1 day of culture | 4 days of culture | ||||
|---|---|---|---|---|---|---|
| Control | NSC23766 | NSC + Y-27632 | Control | NSC23766 | NSC + Y-27632 | |
| Serum-Free | 15 ± 13% | 78 ± 25% * | 0 ± 0% ** | 8 ± 10% | 80 ± 19% * | 0 ± 0% ** |
| PDGF | 7 ± 12% | 58 ± 37% * | 0 ± 0% ** | 9 ± 10% | 85 ± 13% * | 0 ± 0% ** |
| IGF | 19 ± 2% | 57 ± 25% * | 0 ± 0% ** | 12 ± 11% | 81 ± 13% * | 0 ± 0% ** |
| FGF | 95 ± 8% | 100 ± 0% | 0 ± 0% ** | 79 ± 26% | 97 ± 6% * | 0 ± 0% ** |
| TGFβ1 | 100 ± 0% | 100 ± 0% | 0 ± 0% ** | 100 ± 0% | 100 ± 0% | 0 ± 0% ** |
4. Discussion
| Culture media | Uncompressed 3-D collagen ECM | Fibrillar collagen on glass (2-D) | Compressed 3-D collagen ECM | |||
|---|---|---|---|---|---|---|
| (↓ Stiffness, ↓ Collagen density) | (↑ Stiffness, ↓ Collagen density) | (↑ Stiffness, ↑ Collagen density) | ||||
| Control | Rac1 inhibitor | Control | Rac1 inhibitor | Control | Rac1 inhibitor | |
| Serum-Free | No | No | No | No | No | Yes |
| PDGF | No | No | No | Yes | No | Yes |
| IGF | No | No | No | No | No | Yes |
| FGF | No | No | Yes | Yes | Yes | Yes |
| TGFβ1 | Yes | Yes | Yes | Yes | Yes | Yes |
Supplementary Files
Supplementary File 1Author Contributions
Conflict of Interests
References
- Hassell, J.R.; Birk, D.E. The molecular basis of corneal transparency. Exp. Eye Res. 2010, 91, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, S.; Petroll, W.M.; Hassell, J.; Jester, J.V.; Lass, J.H.; Paul, J.; Birk, D.E. Corneal opacity in lumican-null mice: Defects in collagen fibril structure and packing in the posterior stroma. Invest. Ophthalmol. Vis. Sci. 2000, 41, 3365–3373. [Google Scholar] [PubMed]
- Funderburgh, J.L.; Mann, M.M.; Funderburgh, M.L. Keratocyte phenotype mediates proteoglycan structure: A role for fibroblasts in corneal fibrosis. J. Biol. Chem. 2003, 278, 45629–45637. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Barry, P.A.; Lind, G.J.; Petroll, W.M.; Garana, R.; Cavanagh, H.D. Corneal keratocytes: In situ and in vitro organization of cytoskeletal contractile proteins. Invest. Ophthalmol. Vis. Sci. 1994, 35, 730–743. [Google Scholar] [PubMed]
- Lakshman, N.; Kim, A.; Petroll, W.M. Characterization of corneal keratocyte morphology and mechanical activity within 3-D collagen matrices. Exp. Eye. Res. 2010, 90, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Netto, M.V.; Mohan, R.R.; Ambrosio, R., Jr.; Hutcheon, A.E.; Zieske, J.D.; Wilson, S.E. Wound healing in the cornea: A review of refractive surgery complications and new prospects for therapy. Cornea 2005, 24, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Petroll, W.M.; Cavanagh, H.D. Corneal stromal wound healing in refractive surgery: The role of the myofibroblast. Prog. Retinal. Eye. Res. 1999, 18, 311–356. [Google Scholar] [CrossRef]
- Stramer, B.M.; Zieske, J.D.; Jung, J.-C.; Austin, J.S.; Fini, M.E. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest. Ophthalmol. Vis. Sci. 2003, 44, 4237–4246. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Huang, J.; Barry-Lane, P.A.; Kao, W.W.; Petroll, W.M.; Cavanagh, H.D. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest. Ophthalmol. Vis. Sci. 1999, 40, 1959–1967. [Google Scholar] [PubMed]
- Blalock, T.D.; Duncan, M.R.; Varela, J.C.; Goldstein, M.H.; Tuli, M.H.; Grotensdorst, G.R.; Schultz, G.S. Connective tissue growth factor expression and action in human corneal fibroblast cultures and rat corneas after photorefractive keratectomy. Invest. Ophthalmol. Vis. Sci. 2003, 44, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Moller-Pedersen, T.; Cavanagh, H.D.; Petroll, W.M.; Jester, J.V. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: A 1-year confocal microscopic study. Ophthalmology 2000, 107, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Dupps, W.J.; Wilson, S.E. Biomechanics and wound healing in the cornea. Exp. Eye Res. 2006, 83, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Hall, A. Rho GTPases and the control of cell behaviour. Biochem. Soc. Trans. 2005, 33, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell. Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Zhong, Y. Rho/Rho-associated kinase pathway in glaucoma (Review). Int. J. Oncol. 2013, 43, 1357–1367. [Google Scholar] [PubMed]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010, 67, 545–554. [Google Scholar] [CrossRef]
- Sander, E.E.; ten Klooster, J.P.; van Delft, S.; van der Kammen, R.A.; Collard, J.G. Rac downregulates Rho activity: Reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell. Biol. 1999, 147, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Parizi, M.; Howard, E.W.; Tomasek, J.J. Regulation of LPA-promoted myofibroblast contraction: Role of Rho, myosin light chain kinase, and myosin light chain phosphatase. Exp. Cell. Res. 2000, 254, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Totsukawa, G.; Yamakita, Y.; Yamashiro, S.; Hartshorne, D.J.; Sasaki, Y. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J. Cell. Biol. 2000, 150, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Rottner, K.; Hall, A.; Small, J.V. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 1999, 9, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; DiCesare, L.; Tan, I.; Leung, T.; SundarRaj, N. Rho-mediated assembly of stress fibers is differentially regulated in corneal fibroblasts and myofibroblasts. Exp. Cell. Res. 2004, 298, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Chang, J.-H. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp. Eye Res. 2003, 77, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Ito, M.; Kimura, K.; Fukata, Y.; Chihara, K.; Nakano, T.; Matsuura, Y.; Kaibuchi, K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 1996, 271, 20246–20249. [Google Scholar] [CrossRef] [PubMed]
- Svitkina, T.M.; Borisy, G.G. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell. Biol. 1999, 145, 1009–1026. [Google Scholar] [CrossRef] [PubMed]
- Demali, K.A.; Burridge, K. Coupling membrane protrusion and cell adhesion. J. Cell. Sci. 2003, 116, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Dreier, B.; Thomasy, S.M.; Mendonsa, R.; Raghunathan, V.K.; Russell, P.; Murphy, C.J. Substratum compliance modulates corneal fibroblast to myofibroblast transformation. Invest. Ophthalmol. Vis. Sci. 2013, 54, 5901–5907. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, N.; Petroll, W.M. Growth factor regulation of corneal keratocyte mechanical phenotypes in 3-D collagen matrices. Invest. Ophthalmol. Vis. Sci. 2012, 53, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Huang, J.; Fisher, S.; Spiekerman, J.; Chang, J.H.; Wright, W.E.; Shay, J.W. Myofibroblast differentiation of normal human keratocytes and hTERT, extended-life, human corneal fibroblasts. Invest. Ophthalmol. Vis. Sci. 2003, 44, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Petroll, W.M.; Ma, L.; Kim, A.; Ly, L.; Vishwanath, M. Dynamic assessment of fibroblast mechanical activity during Rac-induced cell spreading in 3-D culture. J. Cell. Physiol. 2008, 217, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.; Wiseman, M.; Chuo, C.-B.; Cheema, U.; Nazhat, S.N. Ultrarapid engineering of biomimetic materials and tissues: Fabrication of nano- and microstructures by plastic compression. Adv. Funct. Mater. 2005, 15, 1762–1770. [Google Scholar] [CrossRef]
- Neel, E.A.A.; Cheema, U.; Knowles, J.C.; Brown, R.A.; Nazhat, S.N. Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft. Matter. 2006, 2, 986–992. [Google Scholar] [CrossRef]
- Kim, A.; Lakshman, N.; Karamichos, D.; Petroll, W.M. Growth factor regulation of corneal keratocyte differentiation and migration in compressed collagen matrices. Invest. Ophthalmol. Vis. Sci. 2010, 51, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Dickerson, J.B.; Guo, F.; Zheng, J.; Zheng, Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. USA 2004, 101, 7618–7623. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Lakshman, N.; Petroll, W.M. Quantitative assessment of local collagen matrix remodeling in 3-D culture: The role of Rho kinase. Exp. Cell. Res. 2006, 312, 3683–3692. [Google Scholar] [CrossRef] [PubMed]
- Tovell, V.E.; Chau, C.Y.; Khaw, P.T.; Bailly, M. Rac1 inhibition prevents tissue contraction and MMP mediated matrix remodeling in the conjunctiva. Invest. Ophthalmol. Vis. Sci. 2012, 53, 4682–4691. [Google Scholar] [CrossRef] [PubMed]
- San Miguel, S.M.; Opperman, L.A.; Allen, E.P.; Zielinski, J.; Svoboda, K.K. Antioxidants counteract nicotine and promote migration via RacGTP in oral fibroblast cells. J. Periodontol. 2010, 81, 1675–1690. [Google Scholar]
- Petroll, W.M.; Ma, L.; Ly, L.; Vishwanath, M. Analysis of the pattern of sub-cellular force generation by corneal fibroblasts following Rho activation. Eye Contact Lens 2008, 34, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Petroll, W.M.; Cavanagh, H.D.; Jester, J.V. Exertion of tractional force requires the coordinated upregulation of cell contractility and adhesion. Cell. Motil. Cytoskel. 1999, 43, 23–34. [Google Scholar] [CrossRef]
- Vishwanath, M.; Ma, L.; Jester, J.V.; Otey, C.A.; Petroll, W.M. Modulation of corneal fibroblast contractility within fibrillar collagen matrices. Invest. Ophthalmol. Vis. Sci. 2003, 44, 4724–4735. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guerriero, E.; Sado, Y.; SundarRaj, N. Rho-mediated regulation of TGF-beta1- and FGF-2-induced activation of corneal stromal keratocytes. Invest. Ophthalmol. Vis. Sci. 2009, 50, 3662–3670. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-J.; Mohan, R.R.; Mohan, R.R.; Wilson, S.E. Effect of PDGF, IL-1α, and BMP2/4 on corneal fibroblast chemotaxis: Expression of the platelet-derived growht factor system in the cornea. Invest. Ophthalmol. Vis. Sci. 1999, 40, 1364–1372. [Google Scholar] [PubMed]
- Musselmann, K.; Kane, B.P.; Alexandrou, B.; Hassell, J.R. IGF-II is present in bovine corneal stroma and activates keratocytes to proliferate in vitro. Exp. Eye Res. 2008, 86, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.R.; Moshayedi, P.; Schoen, T.J.; Jones, B.E.; Chader, G.J.; Waldbillig, R.J. Distribution of IGF-I and -II, IGF binding proteins (IGFBPs) and IGFBP mRNA in ocular fluids and tissues: Potential sites of synthesis of IGFBPs in aqueous and vitreous. Exp. Eye Res. 1993, 56, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, I.S.; Tervo, T.M.; Teppo, A.M.; Valle, T.U.; Gronhagen-Riska, C.; Vesaluoma, M.H. Human tear fluid PDGF-BB, TNF-alpha and TGF-beta1 vs corneal haze and regeneration of corneal epithelium and subbasal nerve plexus after PRK. Exp. Eye Res. 2001, 72, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Etheredge, L.; Kane, B.P.; Hassell, J.R. The effect of growth factor signaling on keratocytes in vitro and its relationship to the phases of stromal wound repair. Invest. Ophthalmol. Vis. Sci. 2009, 50, 3128–3136. [Google Scholar] [CrossRef] [PubMed]
- Shyy, J.Y.-J.; Chien, S. Role of integrins in endothelial mechanosensing of shear stress. Circ. Res. 2002, 91, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Sadoshima, J.; Izumo, S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu. Rev. Physiol. 1997, 59, 551–571. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Tanswell, A.K.; Post, M. Mechanical force-induced signal transduction in lung cells. Am. J. Physiol. 1999, 277, L667–L683. [Google Scholar] [PubMed]
- Brown, T.D. Techniques for mechanical stimulation of cells in vitro: A review. J. Biomech. 2000, 33, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Tummina, S.J.; Mitton, K.P.; Arora, J.; Zelenka, P.; Epstein, D.L.; Russell, P. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 1998, 39, 1361–1371. [Google Scholar] [PubMed]
- Ingber, D.E.; Folkman, J. Mechanochemical switching between growth and differentiation during fibroblast growth factor stimulated angiogenesis in vitro: Role of extracellular matrix. J. Cell. Biol. 1989, 109, 317–330. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Grinnell, F. Stress relaxation of fibroblasts activates a cyclic AMP signaling pathway. J. Cell. Biol 1994, 126, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Van Bockxmeer, F.M.; Martin, C.E.; Constable, I.J. Effect of cyclic AMP on cellular contractility and DNA synthesis in chorioretinal fibroblasts maintained in collagen matrices. Exp. Cell. Res. 1984, 155, 413–421. [Google Scholar]
- Pelham, R.J.; Wang, Y.L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 1997, 94, 13661–13665. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell. Motil. Cytoskel. 2005, 60, 24–34. [Google Scholar] [CrossRef]
- Miron-Mendoza, M.; Seemann, J.; Grinnell, F. The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials 2010, 31, 6425–6435. [Google Scholar] [CrossRef] [PubMed]
- Kolodney, M.S.; Wysolmerski, R.B. Isometric contraction by fibroblasts and endothelial cells in tissue culture. J. Cell. Biol. 1992, 117, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Takakuda, K.; Miyairi, H. Tensile behavior of fibroblasts cultured in collagen gel. Biomaterials 1996, 17, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, T.; Elson, E.L. Reciprocal interactions between cells and extracellular matrix during remodeling of tissue constructs. Biophys. Chem. 2003, 100, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chang, T.R.; Aggarwal, A.; Lee, R.C.; Ehrlich, H.P. Mechanisms and dynamics of mechanical strengthening in ligament-equivalent fibroblast populated collagen matrices. Ann. Biomed. Eng. 1993, 21, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Cheema, U.; Yang, S.-Y.; Mudera, V.; Goldspink, G.G.; Brown, R.A. 3-D in vitro model of early skeletal muscle development. Cell. Motil. Cytoskel. 2003, 54, 226–236. [Google Scholar] [CrossRef]
- Eastwood, M.; McGrouther, D.A.; Brown, R.A. A culture force monitor for measurement of contraction forces generated in human dermal fibroblast cultures: Evidence for cell matrix mechanical signalling. Biochim. Biophys. Acta 1994, 1201, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, M.; Mudera, V.C.; McGrouther, D.A.; Brown, R.A. Effect of prcise mechanical loading on fibroblast populated collagen lattices: Morphological changes. Cell. Motil. Cytoskel. 1998, 40, 13–21. [Google Scholar] [CrossRef]
- Mudera, V.C.; Pleass, R.; Eastwood, M.; Tarnuzzer, R.; Schultz, G.; Khaw, P.; McGrouther, D.A.; Brown, R.A. Molecular responses of human dermal fibroblasts to dual cues: Contact guidance and mechanical load. Cell. Motil. Cytoskel. 2000, 45, 1–9. [Google Scholar] [CrossRef]
- Karamichos, D.; Lakshman, N.; Petroll, W.M. Regulation of corneal fibroblast morphology and collagen reorganization by extracellular matrix mechanical properties. Invest. Ophthalmol. Vis. Sci. 2007, 48, 5030–5037. [Google Scholar] [CrossRef] [PubMed]
- Barocas, V.H.; Moon, A.G.; Tranquillo, R.T. The fibroblast-populated collagen microsphere assay of cell traction force—Part 2: Measurement of the cell traction parameter. J. Biomech. Eng. 1995, 117, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.Y.; Tian, D.; Brangwynne, C.P.; Weitz, D.A.; Tschumperlin, D.J. A new microrheometric approach reveals individual and cooperative roles for TGF-beta1 and IL-1beta in fibroblast-mediated stiffening of collagen gels. FASEB J. 2007, 21, 2064–2073. [Google Scholar] [CrossRef] [PubMed]
- Miron-Mendoza, M.; Lin, X.; Ma, L.; Ririe, P.; Petroll, W.M. Individual versus collective fibroblast spreading and migration: Regulation by matrix composition in 3D culture. Exp. Eye Res. 2012, 99, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayi, E.; Mudera, V.; Brown, R.A. Guding cell migration in 3D: A collagen matrix with graded directional stiffness. Cell. Motil. Cytoskel. 2009, 66, 121–129. [Google Scholar] [CrossRef]
- Grinnell, F. Fibroblast mechanics in three-dimensional collagen matrices. J. Bodyw. Mov. Ther. 2008, 12, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Zhou, C.; Lakshman, N.; Petroll, W.M. Corneal stromal cells use both high- and low-contractility migration mechanisms in 3-D collagen matrices. Exp. Cell. Res. 2012, 318, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Levay, M.; Krobert, K.A.; Wittig, K.; Voigt, N.; Bermudez, M.; Wolber, G.; Dobrev, D.; Levy, F.O.; Wieland, T. NSC23766, a widely used inhibitor of Rac1 activation, additionally acts as a competitive antagonist at muscarinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2013, 347, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Myrna, K.E.; Mendonsa, R.; Russell, P.; Pot, S.A.; Liliensiek, S.J.; Jester, J.V.; Nealey, P.F.; Brown, D.; Murphy, C.J. Substratum topography modulates corneal fibroblast to myofibroblast transformation. Invest. Ophthalmol. Vis. Sci. 2012, 53, 811–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grinnell, F.; Petroll, W.M. Cell motility and mechanics in three-dimensional collagen matrices. Annu. Rev. Cell. Dev. Biol. 2010, 26, 335–361. [Google Scholar] [CrossRef] [PubMed]
- Arthur, W.T.; Burridge, K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell. 2001, 12, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Bustos, R.I.; Forget, M.A.; Settleman, J.E.; Hansen, S.H. Coordination of Rho and Rac GTPase function via p190B RhoGAP. Curr. Biol. 2008, 18, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- Sailem, H.; Bousgouni, V.; Cooper, S.; Bakal, C. Cross-talk between Rho and Rac GTPases drives deterministic exploration of cellular shape space and morphological heterogeneity. Open Biol. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Ruberti, J.W.; Roy, A.S.; Roberts, C.J. Corneal biomechanics and biomaterials. Annu. Rev. Biomed. Eng. 2011, 13, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, T.T.; Simonsen, A.H.; Oxlund, H. Biomechanical properties of keratoconus and normal corneas. Exp. Eye Res. 1980, 31, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Edmund, C. Corneal topography and elasticity in normal and keratoconic eyes. A methodological study concerning the pathogenesis of keratoconus. Acta Ophthalmol. Suppl. 1989, 193, 1–36. [Google Scholar] [PubMed]
- Ali, N.Q.; Patel, D.V.; McGhee, C.N. Biomechanical responses of healthy and keratoconic corneas measured using a noncontact scheimpflug-based tonometer. Invest. Ophthalmol. Vis. Sci. 2014, 55, 3651–3659. [Google Scholar] [CrossRef] [PubMed]
- Morishige, N.; Wahlert, A.J.; Kenney, M.C.; Brown, D.J.; Kawamoto, K.; Chikama, T.; Nishida, T.; Jester, J.V. Second-harmonic imaging microscopy of normal human and keratoconus cornea. Invest. Ophthalmol. Vis. Sci. 2007, 48, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, R.; Toussaint, K.C., Jr.; Wagoner Johnson, A. The effect of keratoconus on the structural, mechanical, and optical properties of the cornea. J. Mech. Behav. Biomed. Mater. 2011, 4, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Beshtawi, I.M.; O’Donnell, C.; Radhakrishnan, H. Biomechanical properties of corneal tissue after ultraviolet-A-riboflavin crosslinking. J. Cataract. Refract. Surg. 2013, 39, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.; Chai, D.; Kriling, S.; Nien, C.J.; Brown, D.J.; Jester, B.; Juhasz, T.; Jester, J.V. Nonlinear optical macroscopic assessment of 3-D corneal collagen organization and axial biomechanics. Invest. Ophthalmol. Vis. Sci. 2011, 52, 8818–8827. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petroll, W.M.; Lakshman, N. Fibroblastic Transformation of Corneal Keratocytes by Rac Inhibition is Modulated by Extracellular Matrix Structure and Stiffness. J. Funct. Biomater. 2015, 6, 222-240. https://doi.org/10.3390/jfb6020222
Petroll WM, Lakshman N. Fibroblastic Transformation of Corneal Keratocytes by Rac Inhibition is Modulated by Extracellular Matrix Structure and Stiffness. Journal of Functional Biomaterials. 2015; 6(2):222-240. https://doi.org/10.3390/jfb6020222
Chicago/Turabian StylePetroll, W. Matthew, and Neema Lakshman. 2015. "Fibroblastic Transformation of Corneal Keratocytes by Rac Inhibition is Modulated by Extracellular Matrix Structure and Stiffness" Journal of Functional Biomaterials 6, no. 2: 222-240. https://doi.org/10.3390/jfb6020222
APA StylePetroll, W. M., & Lakshman, N. (2015). Fibroblastic Transformation of Corneal Keratocytes by Rac Inhibition is Modulated by Extracellular Matrix Structure and Stiffness. Journal of Functional Biomaterials, 6(2), 222-240. https://doi.org/10.3390/jfb6020222






