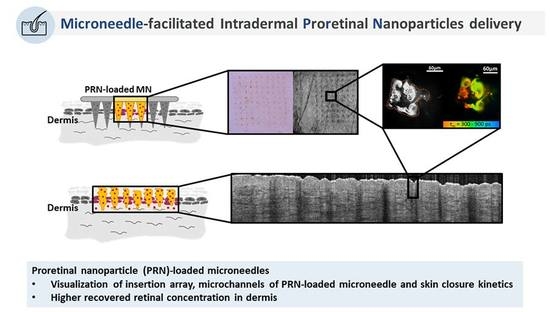
Microneedle-Facilitated Intradermal Proretinal Nanoparticle Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Dissolving PRN-Loaded Microneedles
2.2. Experimental Design of Topical Applications
2.3. Optical Methods to Study Microchannel Formation and Kinetics and PRN Release after the Application of PRN-Loaded MN
2.3.1. Dermoscopy
2.3.2. Optical Coherence Tomography (OCT)
2.3.3. Multiphoton Tomography (MPT) with Fluorescence Lifetime Imaging Microscopy (FLIM)
2.4. Skin Penetration of Retinal from PRN-Loaded MN, PRN and Conv. RAL
2.4.1. Extraction of Epidermis and Dermis
2.4.2. UV-VIS Spectroscopy for Quantification of Retinal in the Skin
2.5. Statistical Analysis
3. Results
3.1. Morphology of PRN-Loaded MN
3.2. Optical Methods to Study Microchannel Formation and Kinetics and PRN Release after the Application of PRN-Loaded MN
3.2.1. Dermoscopy
3.2.2. Optical Coherence Tomography (OCT)
3.2.3. Multiphoton Tomography (MPT) with Fluorescence Lifetime Imaging (FLIM)
3.3. Skin Deposition of Retinal from PRN-Loaded MN, PRN and Conv. RAL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Griffiths, C.E.; Russman, A.N.; Majmudar, G.; Singer, R.S.; Hamilton, T.A.; Voorhees, J.J. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid). N. Engl. J. Med. 1993, 329, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Cui, Y.; Fisher, G.J.; Wang, X.; Chen, Y.; Schneider, L.M.; Majmudar, G. A comparative study of the effects of retinol and retinoic acid on histological, molecular, and clinical properties of human skin. J. Cosmet. Dermatol. 2016, 15, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sass, J.O.; Didierjean, L.; Carraux, P.; Plum, C.; Nau, H.; Saurat, J.H. Metabolism of topical retinaldehyde and retinol by mouse skin in vivo: Predominant formation of retinyl esters and identification of 14-hydroxy-4, 14-retro-retinol. Exp. Dermatol. 1996, 5, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Lee, J.H.; Kim, G.M.; Bae, J.M. Efficacy and safety of retinaldehyde 0.1% and 0.05% creams used to treat photoaged skin: A randomized double-blind controlled trial. J. Cosmet. Dermatol. 2018, 17, 471–476. [Google Scholar] [CrossRef]
- Afra, T.P.; Razmi, T.M.; Narang, T.; Dogra, S.; Kumar, A. Topical tazarotene gel, 0.1%, as a novel treatment approach for atrophic postacne scars: A randomized active-controlled clinical trial. JAMA Facial Plast. Surg. 2019, 21, 125–132. [Google Scholar] [CrossRef]
- Loss, M.J.; Leung, S.; Chien, A.; Kerrouche, N.; Fischer, A.H.; Kang, S. Adapalene 0.3% gel shows efficacy for the treatment of atrophic acne scars. Dermatol. Ther. 2018, 8, 245–257. [Google Scholar] [CrossRef] [Green Version]
- Kligman, L.H.; Duo, C.H.; Kligman, A.M. Topical retinoic acid enhances the repair of ultraviolet damaged dermal connective tissue. Connect. Tissue Res. 1984, 12, 139–150. [Google Scholar] [CrossRef]
- Geria, A.N.; Lawson, C.N.; Halder, R.M. Topical retinoids for pigmented skin. J. Drugs Dermatol. 2011, 10, 483–489. [Google Scholar]
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the treatment of skin aging: An overview of clinical efficacy and safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef]
- Dreno, B.; Katsambas, A.; Pelfini, C.; Plantier, D.; Jancovici, E.; Ribet, V.; Nocera, T.; Morinet, P.; Khammari, A. Combined 0.1% retinaldehyde/6% glycolic acid cream in prophylaxis and treatment of acne scarring. Dermatology 2007, 214, 260–267. [Google Scholar] [CrossRef]
- Schaefer, H.; Zesch, A. Penetration of vitamin A acid into human skin. Acta Derm. Venereol. Suppl. 1975, 74, 50–55. [Google Scholar]
- Sinico, C.; Manconi, M.; Peppi, M.; Lai, F.; Valenti, D.; Fadda, A.M. Liposomes as carriers for dermal delivery of tretinoin: In vitro evaluation of drug permeation and vesicle–skin interaction. J. Control. Release 2005, 103, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Wiechers, J.W. The barrier function of the skin in relation to percutaneous absorption of drugs. Pharm. Weekbl. Sci. 1989, 11, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.; Schleusener, J.; Lademann, J.; Darvin, M.E. Human skin in vivo has a higher skin barrier function than porcine skin ex vivo—Comprehensive raman microscopic study of the stratum corneum. J. Biophotonics 2018, 11, e201700355. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.; Lademann, J.; Darvin, M.E. Analysis of human and porcine skin in vivo/ex vivo for penetration of selected oils by confocal raman microscopy. Ski. Pharm. Physiol. 2015, 28, 318–330. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Raj Singh, T.R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207. [Google Scholar] [CrossRef] [Green Version]
- Hiraishi, Y.; Nakagawa, T.; Quan, Y.-S.; Kamiyama, F.; Hirobe, S.; Okada, N.; Nakagawa, S. Performance and characteristics evaluation of a sodium hyaluronate-based microneedle patch for a transcutaneous drug delivery system. Int. J. Pharm. 2013, 441, 570–579. [Google Scholar] [CrossRef]
- Matsuo, K.; Yokota, Y.; Zhai, Y.; Quan, Y.-S.; Kamiyama, F.; Mukai, Y.; Okada, N.; Nakagawa, S. A low-invasive and effective transcutaneous immunization system using a novel dissolving microneedle array for soluble and particulate antigens. J. Control. Release 2012, 161, 10–17. [Google Scholar] [CrossRef]
- Guo, L.; Chen, J.; Qiu, Y.; Zhang, S.; Xu, B.; Gao, Y. Enhanced transcutaneous immunization via dissolving microneedle array loaded with liposome encapsulated antigen and adjuvant. Int. J. Pharm. 2013, 447, 22–30. [Google Scholar] [CrossRef]
- Zhu, Y.; Choe, C.-S.; Ahlberg, S.; Meinke, M.C.; Ulrike, A.; Lademann, J.M.; Darvin, M.E. Penetration of silver nanoparticles into porcine skin ex vivo using fluorescence lifetime imaging microscopy, raman microscopy, and surface-enhanced raman scattering microscopy. J. Biomed. Opt. 2014, 20, 051006. [Google Scholar] [CrossRef]
- Chu, L.Y.; Choi, S.-O.; Prausnitz, M.R. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: Bubble and pedestal microneedle designs. J. Pharm. Sci. 2010, 99, 4228–4238. [Google Scholar] [CrossRef] [PubMed]
- Pisetpackdeekul, P.; Supmuang, P.; Pan-In, P.; Banlunara, W.; Limcharoen, B.; Kokpol, C.; Wanichwecharungruang, S. Proretinal nanoparticles: Stability, release, efficacy, and irritation. Int. J. Nanomed. 2016, 11, 3277–3286. [Google Scholar]
- Limcharoen, B.; Toprangkobsin, P.; Banlunara, W.; Wanichwecharungruang, S.; Richter, H.; Lademann, J.; Patzelt, A. Increasing the percutaneous absorption and follicular penetration of retinal by topical application of proretinal nanoparticles. Eur. J. Pharm. Biopharm. 2019, 139, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine ear skin: An in vitro model for human skin. Ski. Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef]
- Wanichwecharungruang, S.; Rujwaree, T. A Dissolvable Microneedle. Pct International Application no.Pct/th2019/000031, 18 September 2018. [Google Scholar]
- Becker, W.; Bergmann, A.; Biscotti, G.; Koenig, K.; Riemann, I.; Kelbauskas, L.; Biskup, C. High-Speed Flim Data Acquisition by Time-Correlated Single-Photon Counting; SPIE: Washington, DA, USA, 2004; Volume 5323. [Google Scholar]
- Shirshin, E.A.; Gurfinkel, Y.I.; Priezzhev, A.V.; Fadeev, V.V.; Lademann, J.; Darvin, M.E. Two-photon autofluorescence lifetime imaging of human skin papillary dermis in vivo: Assessment of blood capillaries and structural proteins localization. Sci. Rep. 2017, 7, 1171. [Google Scholar] [CrossRef] [Green Version]
- Lau, W.M.; Ng, K.W.; Sakenyte, K.; Heard, C.M. Distribution of esterase activity in porcine ear skin, and the effects of freezing and heat separation. Int. J. Pharm. 2012, 433, 10–15. [Google Scholar] [CrossRef]
- Gerstel, M.S.; Place, V.A. Drug Delivery Device. U.S. Patent 3,964,482, 22 June 1976. [Google Scholar]
- Moothanchery, M.; Seeni, R.Z.; Xu, C.; Pramanik, M. In vivo studies of transdermal nanoparticle delivery with microneedles using photoacoustic microscopy. Biomed. Opt. Express. 2017, 8, 5483–5492. [Google Scholar] [CrossRef] [Green Version]
- Niu, L.; Chu, L.Y.; Burton, S.A.; Hansen, K.J.; Panyam, J. Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. J. Control. Release 2019, 294, 268–278. [Google Scholar] [CrossRef]
- Lan, X.; She, J.; Lin, D.A.; Xu, Y.; Li, X.; Yang, W.F.; Lui, V.W.Y.; Jin, L.; Xie, X.; Su, Y.X. Microneedle-mediated delivery of lipid-coated cisplatin nanoparticles for efficient and safe cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 33060–33069. [Google Scholar] [CrossRef]
- Hiraishi, Y.; Hirobe, S.; Iioka, H.; Quan, Y.-S.; Kamiyama, F.; Asada, H.; Okada, N.; Nakagawa, S. Development of a novel therapeutic approach using a retinoic acid-loaded microneedle patch for seborrheic keratosis treatment and safety study in humans. J. Control. Release 2013, 171, 93–103. [Google Scholar] [CrossRef]
- Hirobe, S.; Otsuka, R.; Iioka, H.; Quan, Y.-S.; Kamiyama, F.; Asada, H.; Okada, N.; Nakagawa, S. Clinical study of a retinoic acid-loaded microneedle patch for seborrheic keratosis or senile lentigo. Life Sci. 2017, 168, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Park, J.-H.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, G.; Warner, K.S.; Zhang, J.; Sharma, S.; Gale, B.K. Evaluation needle length and density of microneedle arrays in the pretreatment of skin for transdermal drug delivery. Int. J. Pharm. 2010, 391, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Rattanapak, T.; Birchall, J.; Young, K.; Ishii, M.; Meglinski, I.; Rades, T.; Hook, S. Transcutaneous immunization using microneedles and cubosomes: Mechanistic investigations using optical coherence tomography and two-photon microscopy. J. Control. Release 2013, 172, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Gill, H.S.; Andrews, S.N.; Prausnitz, M.R. Kinetics of skin resealing after insertion of microneedles in human subjects. J. Control. Release 2011, 154, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samant, P.P.; Prausnitz, M.R. Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proc. Natl. Acad. Sci. USA 2018, 115, 4583. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Allen, M.G.; Prausnitz, M.R. Polymer microneedles for controlled-release drug delivery. Pharm. Res. 2006, 23, 1008–1019. [Google Scholar] [CrossRef]
- Arayachukeat, S.; Wanichwecharungruang, S.P.; Tree-Udom, T. Retinyl acetate-loaded nanoparticles: Dermal penetration and release of the retinyl acetate. Int. J. Pharm. 2011, 404, 281–288. [Google Scholar] [CrossRef]
- Jenning, V.; Schäfer-Korting, M.; Gohla, S. Vitamin a-loaded solid lipid nanoparticles for topical use: Drug release properties. J. Control. Release 2000, 66, 115–126. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Kang, M.-K.; Sun, H.-S.; Kang, S.-S.; Kim, H.-W.; Moon, K.-S.; Lee, K.-J.; Kim, S.-H.; Jung, S. All-trans-retinoic acid release from core-shell type nanoparticles of poly(ε-caprolactone)/poly(ethylene glycol) diblock copolymer. Int. J. Pharm. 2004, 273, 95–107. [Google Scholar] [CrossRef]
- Liu, J.; Hu, W.; Chen, H.; Ni, Q.; Xu, H.; Yang, X. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int. J. Pharm. 2007, 328, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Morrow, D.I.J.; Fay, F.; Scott, C.J.; Abdelghany, S.; Singh, R.R.T.; Garland, M.J.; David Woolfson, A. Microneedle-mediated intradermal nanoparticle delivery: Potential for enhanced local administration of hydrophobic pre-formed photosensitisers. Photodiagn. Photodyn. 2010, 7, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; König, K.; Kellner-Hoefer, M.; Breunig, H.G.; Werncke, W.; Meinke, M.C.; Patzelt, A.; Sterry, W.; Lademann, J. Safety assessment by multiphoton fluorescence/second harmonic generation/hyper-rayleigh scattering tomography of zno nanoparticles used in cosmetic products. Ski. Pharm. Physiol. 2012, 25, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Richter, H.; Teichmann, A.; Otberg, N.; Blume-Peytavi, U.; Luengo, J.; Weiß, B.; Schaefer, U.F.; Lehr, C.-M.; Wepf, R.; et al. Nanoparticles—An efficient carrier for drug delivery into the hair follicles. Eur. J. Pharm. Biopharm. 2007, 66, 159–164. [Google Scholar] [CrossRef]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Skin penetration and distribution of polymeric nanoparticles. J. Control. Release 2004, 99, 53–62. [Google Scholar] [CrossRef]
- Patzelt, A.; Richter, H.; Dähne, L.; Walden, P.; Wiesmüller, K.-H.; Wank, U.; Sterry, W.; Lademann, J. Influence of the vehicle on the penetration of particles into hair follicles. Pharmaceutics 2011, 3, 307–314. [Google Scholar] [CrossRef]
- Patzelt, A.; Richter, H.; Knorr, F.; Schafer, U.; Lehr, C.M.; Dahne, L.; Sterry, W.; Lademann, J. Selective follicular targeting by modification of the particle sizes. J. Control. Release 2011, 150, 45–48. [Google Scholar] [CrossRef]
- Kimura, E.; Kawano, Y.; Todo, H.; Ikarashi, Y.; Sugibayashi, K. Measurement of skin permeation/penetration of nanoparticles for their safety evaluation. Biol. Pharm. Bull. 2012, 35, 1476–1486. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limcharoen, B.; Toprangkobsin, P.; Kröger, M.; Darvin, M.E.; Sansureerungsikul, T.; Rutwaree, T.; Wanichwecharungruang, S.; Banlunara, W.; Lademann, J.; Patzelt, A. Microneedle-Facilitated Intradermal Proretinal Nanoparticle Delivery. Nanomaterials 2020, 10, 368. https://doi.org/10.3390/nano10020368
Limcharoen B, Toprangkobsin P, Kröger M, Darvin ME, Sansureerungsikul T, Rutwaree T, Wanichwecharungruang S, Banlunara W, Lademann J, Patzelt A. Microneedle-Facilitated Intradermal Proretinal Nanoparticle Delivery. Nanomaterials. 2020; 10(2):368. https://doi.org/10.3390/nano10020368
Chicago/Turabian StyleLimcharoen, Benchaphorn, Pattrawadee Toprangkobsin, Marius Kröger, Maxim E. Darvin, Titiporn Sansureerungsikul, Teeranut Rutwaree, Supason Wanichwecharungruang, Wijit Banlunara, Jürgen Lademann, and Alexa Patzelt. 2020. "Microneedle-Facilitated Intradermal Proretinal Nanoparticle Delivery" Nanomaterials 10, no. 2: 368. https://doi.org/10.3390/nano10020368