Removal of Emerging Pollutants from Water Using Environmentally Friendly Processes: Photocatalysts Preparation, Characterization, Intermediates Identification and Toxicity Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of TiO2, ZnO and MgO Nanopowders
2.2. Materials Characterization
2.3. Measurements of Photocatalytic Activity
2.4. Germination and Toxicity Measurements
3. Results and Discussion
3.1. Structural Characteristics
3.2. Removal Efficiency
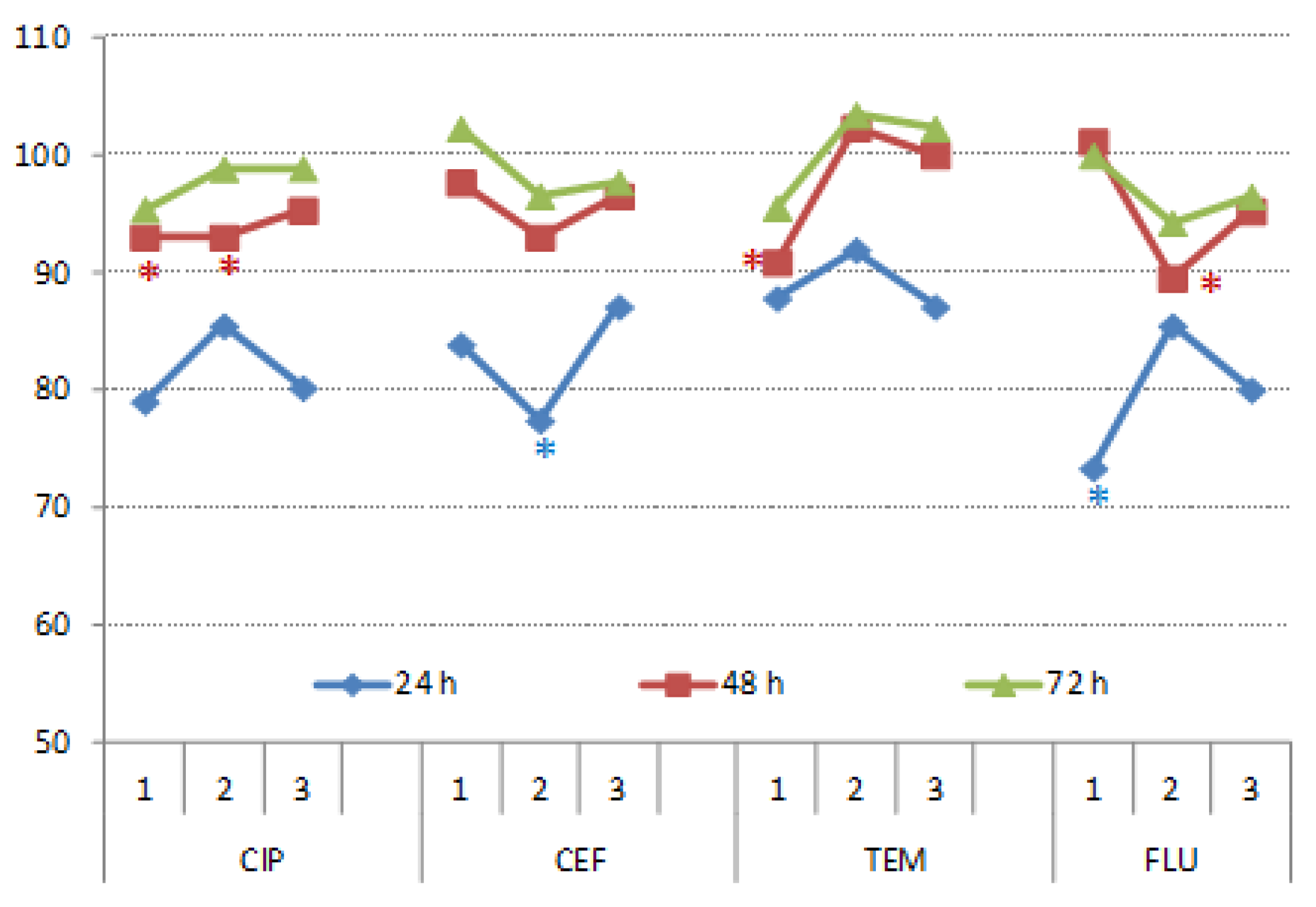
3.3. Impact on Wheat Germination and Growth
3.3.1. Germination
3.3.2. Plant Growth
3.3.3. Photosynthetic Pigments
3.3.4. Concentration of Malondyaldehide in Roots and Shoots of Wheat
3.4. LC/MS Identification of Pollutant Degradation Intermediates
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [Green Version]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef]
- Guo, X.; Kang, C.; Huang, H.; Chang, Y.; Zhong, C. Exploration of functional MOFs for efficient removal of fluoroquinolone antibiotics from water. Microporous Mesoporous Mater. 2019, 286, 84–91. [Google Scholar] [CrossRef]
- Zhang, H.; Kumar Khanal, S.; Ji, Y.; Song, S.; Lu, H. Fundamental insights into ciprofloxacin adsorption by sulfate-reducing bacteria sludge: Mechanisms and thermodynamics. Chem. Eng. J. 2019, 378, 122103. [Google Scholar] [CrossRef]
- Fei, Y.; Yong, L.; Sheng, H.; Jie, M. Adsorptive removal of ciprofloxacin by sodium alginate/graphene oxide composite beads from aqueous solution. J. Colloid Interface Sci. 2016, 484, 196–204. [Google Scholar] [CrossRef]
- Shokri, M.; Isapour, G.; Shamsvand, S.; Kavousi, B. Photocatalytic degradation of ceftriaxone in aqueous solutions by immobilized TiO2 and ZnO nanoparticles: Investigating operational parameters. J. Mater. Environ. Sci. 2016, 7, 2843–2851. [Google Scholar]
- De Diego Glaria, M.; Moscciati, G.G.; Ramos, R.G.; Riquelme, M.M. Stability of ceftriaxone in water and cerebrospinal fluid determined by high-performance liquid chromatography. J. Sep. Sci. 2003, 26, 939–942. [Google Scholar] [CrossRef]
- De Diego, M.; Godoy, G.; Mennickent, S. Chemical stability of ceftriaxone by a validated stability-indicating liquid chromatographic method. J. Chil. Chem. Soc. 2010, 55, 335–337. [Google Scholar] [CrossRef] [Green Version]
- Abramović, B.F.; Uzelac, M.M.; Finčur, N.L. Photocatalytic degradation of thiotriazinone, stable hydrolysis product of antibiotic ceftriaxone. Acta Period. Technol. 2019, 50, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Joshua, D.I.; Praveenkumarreddy, Y.; Prabhasankar, V.P.; D’Souza, A.P.; Yamashita, N.; Balakrishna, K. First report of pharmaceuticals and personal care products in two tropical rivers of southwestern India. Environ. Monit. Assess. 2020, 192, 529. [Google Scholar] [CrossRef] [PubMed]
- Diwan, V.; Tamhankar, A.J.; Aggarwal, M.; Sen, S.; Khandal, R.K.; Lundborg, C.S. Detection of antibiotics in hospital effluents in India. Curr. Sci. India 2009, 97, 1752–1755. [Google Scholar]
- Opris, O.; Soran, M.-L.; Coman, V.; Copaciu, F.; Ristoiu, D. Determination of some frequently used antibiotics in waste waters using solid phase extraction followed by high performance liquid chromatography with diode array and mass spectrometry detection. Cent. Eur. J. Chem. 2013, 11, 1343–1351. [Google Scholar] [CrossRef]
- Yang, B.; Zuo, J.; Li, P.; Wang, K.; Yu, X.; Zhang, M. Effective ultrasound electrochemical degradation of biological toxicity and refractory cephalosporin pharmaceutical wastewater. Chem. Eng. J. 2016, 287, 30–37. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of parameters on the heterogeneous photocatalytic degradation of herbicides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumas, E.; Giraudo, M.; Goujon, E.; Halma, M.; Knhili, E.; Stauffert, M.; Batisson, I.; Besse-Hoggan, P.; Bohatier, J.; Bouchard, P.; et al. Fate and ecotoxicological impact of new generation herbicides from the triketone family: An overview to assess the environmental risks. J. Hazard. Mater. 2017, 325, 136–156. [Google Scholar] [CrossRef]
- US-EPA-United States Environmental Protection Agency. EFED Risk Assessment for the Registration of the New Chemical Tembotrione; US-EPA: Washington, DC, USA, 2007; p. 26.
- Tawk, A.; Deborde, M.; Labanowski, J.; Gallard, H. Chlorination of the β-triketone herbicides tembotrione and sulcotrione: Kinetic and mechanistic study, transformation products identification and toxicity. Water Res. 2015, 76, 132–142. [Google Scholar] [CrossRef]
- Scheurer, M.; Nodler, K.; Freeling, F.; Janda, J.; Happel, O.; Riegel, M.; Müller, U.; Storck, F.R.; Fleig, M.; Lange, F.T.; et al. Small, mobile, persistent: Trifluoroacetate in the water cycle–Overlooked sources, pathways, and consequences for drinking water supply. Water Res. 2017, 126, 460–471. [Google Scholar] [CrossRef]
- Chorbadjian, R.; Kogan, M. Interaction between glyphosate and fluroxypyr improve mallow control. Crop Protect. 2002, 21, 689–692. [Google Scholar] [CrossRef]
- Hu, J.; Wang, T.; Long, J.; Chen, Y. Hydrolysis, aqueous photolysis and soil degradation of fluroxypyr. Int. J. Environ. Anal. Chem. 2014, 94, 211–222. [Google Scholar] [CrossRef]
- EPA Fact Sheet for Fluroxypyr. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-128959_30-Sep-98.pdf (accessed on 12 November 2020).
- Wu, G.L.; Cui, J.; Tao, L.; Yang, H. Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicology 2010, 19, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Sirés, I.; Brillas, E. Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review. Environ. Int. 2012, 40, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic degradation for environmental applications–a review. J. Chem. Technol. Biotechnol. 2001, 77, 102–116. [Google Scholar] [CrossRef]
- Yun, E.-T.; Yoo, H.-Y.; Bae, H.; Kim, H.-I.; Lee, J. Exploring the role of persulfate in the activation process: Radical precursor versus electron acceptor. Environ. Sci. Technol. 2017, 51, 10090–10099. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ghosh, S.; Misra, A.J.; Tamhankar, A.J.; Jyoti Mishra, A.; Stålsby Lundborg, C.; Tripathy, S.K. Sunlight assisted photocatalytic degradation of ciprofloxacin in water using Fe doped ZnO nanoparticles for potential public health applications. Int. J. Environ. Res. Public Health 2018, 15, 2440. [Google Scholar] [CrossRef] [Green Version]
- Budavari, S. The Merck Index, 13th ed.; Merck & Co. Inc.: Rahway, NJ, USA, 2001; p. 335. [Google Scholar]
- Pastrana-Martínez, L.M.; López-Ramón, M.V.; Moreno-Castilla, C. Adsorption and thermal desorption of the herbicide fluroxypyr on activated carbon fibers and cloth at different pH values. J. Colloid Interface Sci. 2009, 331, 2–7. [Google Scholar] [CrossRef]
- Abramović, B.F.; Despotović, V.N.; Šojić, D.V.; Orčić, D.Z.; Csanádi, J.J.; Četojević-Simin, D.D. Photocatalytic degradation of the herbicide clomazone in natural water using TiO2: Kinetics, mechanism, and toxicity of degradation products. Chemosphere 2013, 93, 166–171. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Bull. 1950, 347, 1–32. [Google Scholar]
- Holm, G. Chlorophyll mutations in barley. Acta Agric. Scand. 1954, 4, 457–471. [Google Scholar] [CrossRef]
- Von Wettstein, D. Cloropill-letale und submikroskoppische formwechsel der plastiden. Exp. Cell Res. 1957, 12, 427–433. [Google Scholar] [CrossRef]
- Devasagayam, T.P.A.; Boloor, K.K.; Ramasarma, T. Methods for estimating lipid peroxidation: An analysis of merits and demerits. Indian J. Biochem. Biophys. 2003, 40, 300–308. [Google Scholar] [PubMed]
- Rezaei, M.; Khajenoori, M.; Nematollahi, B. Synthesis of high surface area nanocrystalline MgO by pluronic P123 triblock copolymer surfactant. Powder Technol. 2011, 205, 112–116. [Google Scholar] [CrossRef]
- Szanyi, J.; Kwak, J.H. Dissecting the steps of CO2 reduction: 1. The interaction of CO and CO2 with γ-Al2O3: An in situ FTIR study. Phys. Chem. Chem. Phys. 2014, 16, 15117–15125. [Google Scholar] [CrossRef]
- Jayarambabu, N.; Siva Kumari, B.; Venkateswara Rao, K.; Prabhu, Y.T. Enhancement of growth in maize by biogenic-synthesized MgO nanoparticles. Int. J. Pure Appl. Zool. 2016, 4, 258–270. [Google Scholar]
- Morterra, C.; Magnacca, G. A case study: Surface chemistry and surface structure of catalytic aluminas, as studied by vibrational spectroscopy of adsorbed species. Catal. Today 1996, 27, 497–532. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: New York, NY, USA, 2004. [Google Scholar]
- Thommes, M.; Cychosz, K.A. Physical adsorption characterization of nanoporous materials: Progress and challenges. Adsorption 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Kamagate, M.; Assadi, A.A.; Kone, T.; Coulibaly, L.; Hanna, K. Activation of persulfate by irradiated laterite for removal of fluoroquinolones in multi-component systems. J. Hazard. Mater. 2018, 346, 159–166. [Google Scholar] [CrossRef]
- Despotović, V.N.; Abramović, B.F.; Šojić, D.V.; Kler, S.J.; Dalmacija, M.B.; Bjelica, L.J.; Orčić, D.Z. Photocatalytic degradation of herbicide quinmerac in various types of natural water. Water Air Soil Pollut. 2012, 223, 3009–3020. [Google Scholar] [CrossRef]
- An, J.; Zhou, Q.; Sun, Y.; Xu, Z. Ecotoxicological effects of typical personal care products on seed germination and seedling development of wheat (Triticum aestivum L.). Chemosphere 2009, 76, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Richardi, V.S.; Bicalho, E.M.; da Rocha, D.C.; Navarro-Silva, M.A.; Soffiatti, P.; Garcia, Q.S.; Sant’Anna-Santos, B.F. Effects of Ciprofloxacin and Roundup on seed germination and root development of maize. Sci. Total Environ. 2019, 651, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Minden, V.; Deloy, A.; Volkert, A.M.; Leonhardt, S.D.; Pufal, G. Antibiotics impact plant traits, even at small concentrations. AoB Plants 2017, 9, plx010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Xu, X.; Shi, C.; Yan, W.; Zhang, L.; Wang, G. Ecotoxicological effects and accumulation of ciprofloxacin in Eichhornia crassipes under hydroponic conditions. Environ. Sci. Pollut. Res. Int. 2019, 26, 30348–30355. [Google Scholar] [CrossRef]
- Pesticides Properties DataBase, Agriculture & Environment Research Unit (AERU), University of Hertfordshire. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/atoz_herb.htm (accessed on 28 November 2020).
- Vaidyanathan, H.; Sivakumar, P.; Chakrabarty, R.; Thomas, G. Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativa L.): Differential response in salt-tolerant and sensitive varieties. Plant Sci. 2003, 165, 1411–1418. [Google Scholar] [CrossRef]
- Lazić, D.; Putnik-Delić, M.; Daničić, M.; Župunski, M.; Arsenov, D.; Vuković, S.; Maksimović, I. Efficiency of Si in alleviating NaCl-induced stress in oilseed rape. Pak. J. Agric. Sci. 2020, 57, 901–907. [Google Scholar]
- Yu, X.; Zhang, J.; Zhang, J.; Niu, J.; Zhao, J.; Wei, Y.; Yao, B. Photocatalytic degradation of ciprofloxacin using Zn-doped Cu2O particles: Analysis of degradation pathways and intermediates. Chem. Eng. J. 2019, 374, 316–327. [Google Scholar] [CrossRef]
- Tawk, A.; Deborde, M.; Labanowski, J.; Thibaudeau, S.; Gallard, H. Transformation of the Β-triketone pesticides tembotrione and sulcotrione by reactions with ozone: Kinetic study, transformation products, toxicity and biodegradability. Ozone Sci. Eng. 2017, 39, 3–13. [Google Scholar] [CrossRef]
- Aramendía, M.A.; Marinas, A.; Marinas, J.M.; Moreno, J.M.; Urbano, F.J. Photocatalytic degradation of herbicide fluroxypyr in aqueous suspension of TiO2. Catal. Today 2005, 101, 187–193. [Google Scholar] [CrossRef]

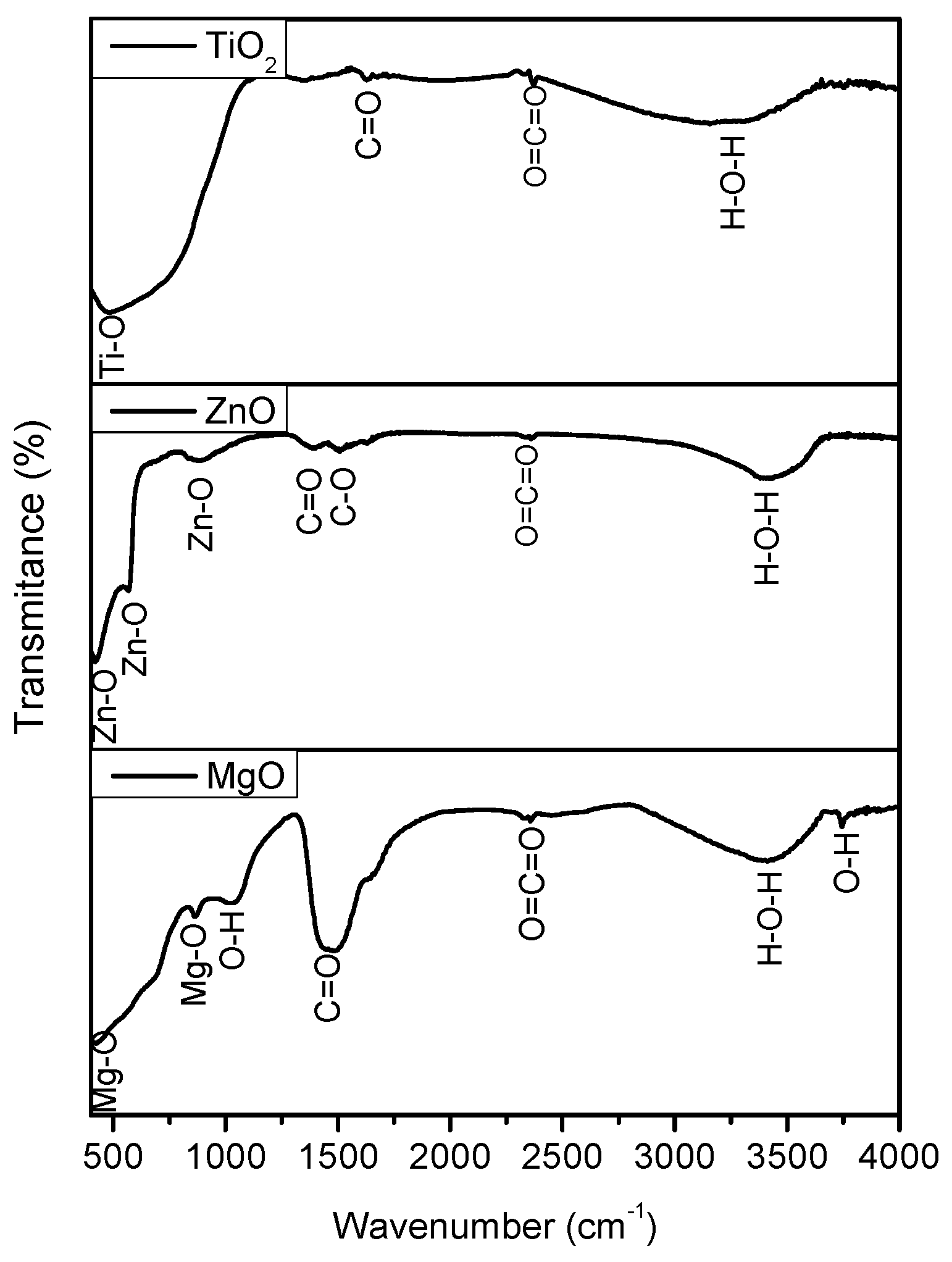






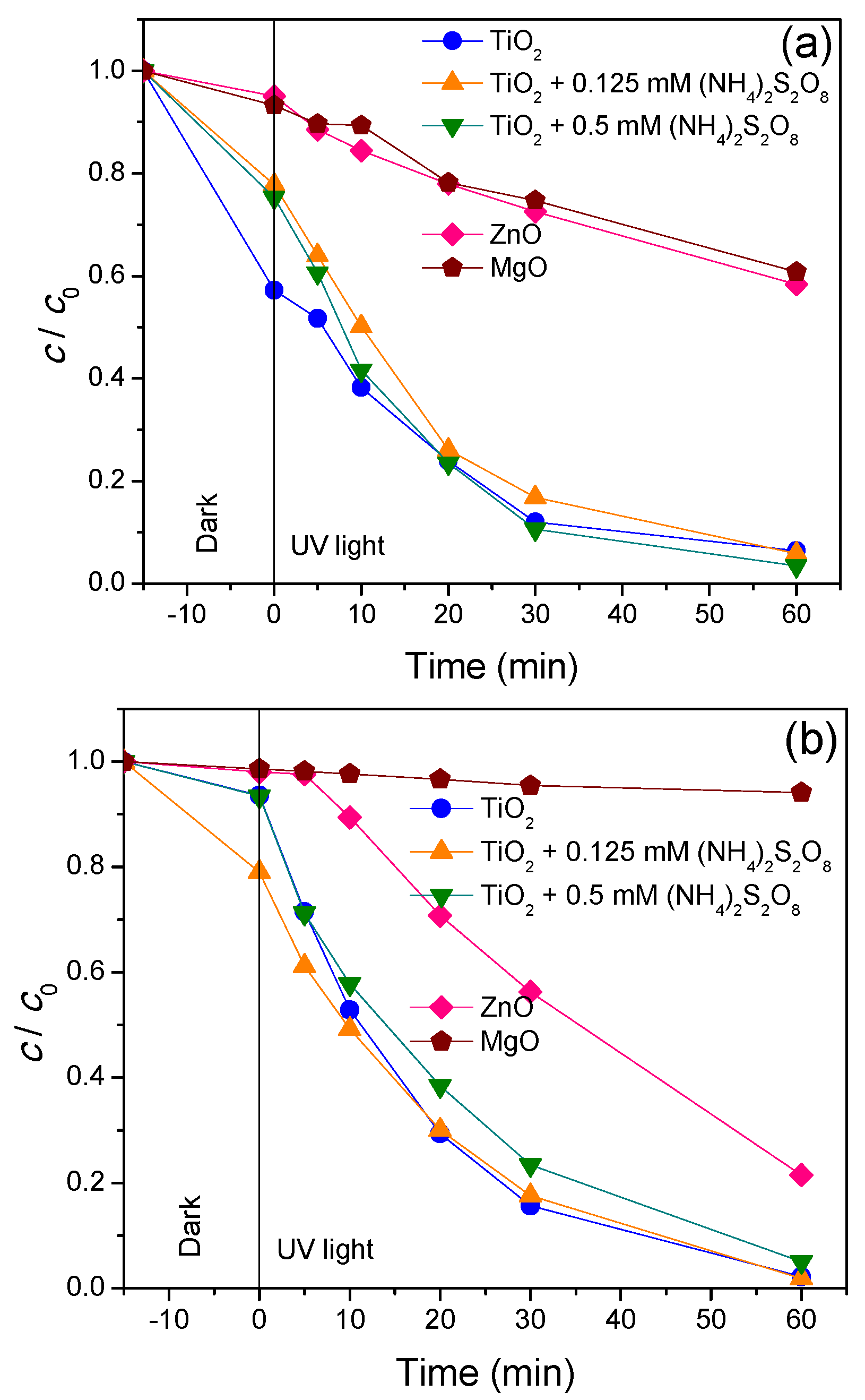








| Pollutant | Property | |||
|---|---|---|---|---|
| Group | Molecular Formula | Molecular Weight (g/mol) | pKa | |
| CIP | Fluoroquinolone antibiotic | C17H18FN3O3 | 331.34 | pKa1 = 6.09 a pKa2 = 8.74 a |
| CEF | Cephalosporin antibiotic | C18H16N8Na2O7S3·3.5H2O | 661.60 | pKa1 ~ 3.0 b pKa2 = 3.2 b pKa3 = 4.1 b |
| TEM | Triketone herbicide | C17H16ClF3O6S | 440.82 | 3.18 (20 °C) c |
| FLU | Synthetic auxins | C7H5Cl2FN2O3 | 255.03 | pKa1 = 3.5 d pKa2 = 10.9 d |
| Sample | Surface Area (m2/g) | Pore Size Distribution BJH Ads (nm) | Pore Size Distribution BJH Des (nm) | Total Pore Volume (cm3/g) |
|---|---|---|---|---|
| TiO2 | 112 | 4.293 | 3.748 | 0.11980 |
| ZnO | 18 | 3.078 | 3.711 | 0.04179 |
| MgO | 75 | 16.826 | 9.720 | 0.33360 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finčur, N.; Sfîrloagă, P.; Putnik, P.; Despotović, V.; Lazarević, M.; Uzelac, M.; Abramović, B.; Vlazan, P.; Ianăși, C.; Alapi, T.; et al. Removal of Emerging Pollutants from Water Using Environmentally Friendly Processes: Photocatalysts Preparation, Characterization, Intermediates Identification and Toxicity Assessment. Nanomaterials 2021, 11, 215. https://doi.org/10.3390/nano11010215
Finčur N, Sfîrloagă P, Putnik P, Despotović V, Lazarević M, Uzelac M, Abramović B, Vlazan P, Ianăși C, Alapi T, et al. Removal of Emerging Pollutants from Water Using Environmentally Friendly Processes: Photocatalysts Preparation, Characterization, Intermediates Identification and Toxicity Assessment. Nanomaterials. 2021; 11(1):215. https://doi.org/10.3390/nano11010215
Chicago/Turabian StyleFinčur, Nina, Paula Sfîrloagă, Predrag Putnik, Vesna Despotović, Marina Lazarević, Maria Uzelac, Biljana Abramović, Paulina Vlazan, Cătălin Ianăși, Tünde Alapi, and et al. 2021. "Removal of Emerging Pollutants from Water Using Environmentally Friendly Processes: Photocatalysts Preparation, Characterization, Intermediates Identification and Toxicity Assessment" Nanomaterials 11, no. 1: 215. https://doi.org/10.3390/nano11010215
APA StyleFinčur, N., Sfîrloagă, P., Putnik, P., Despotović, V., Lazarević, M., Uzelac, M., Abramović, B., Vlazan, P., Ianăși, C., Alapi, T., Náfrádi, M., Maksimović, I., Putnik-Delić, M., & Šojić Merkulov, D. (2021). Removal of Emerging Pollutants from Water Using Environmentally Friendly Processes: Photocatalysts Preparation, Characterization, Intermediates Identification and Toxicity Assessment. Nanomaterials, 11(1), 215. https://doi.org/10.3390/nano11010215












