Evolution of Surface Catalytic Sites on Bimetal Silica-Based Fenton-Like Catalysts for Degradation of Dyes with Different Molecular Charges
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Preparation of the Catalysts
2.3. Materials Characterization
2.4. Catalytic Tests
3. Results and Discussion
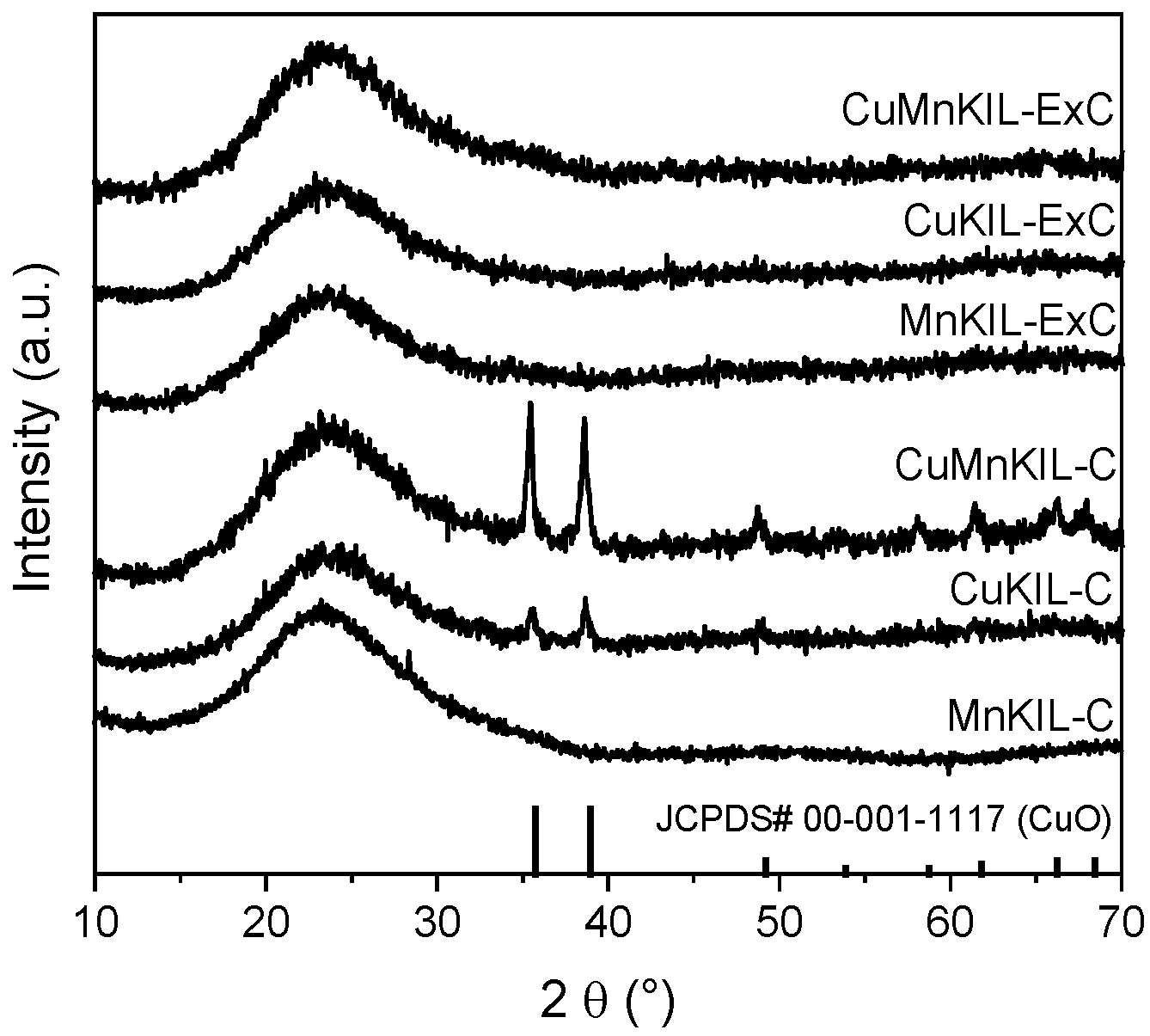
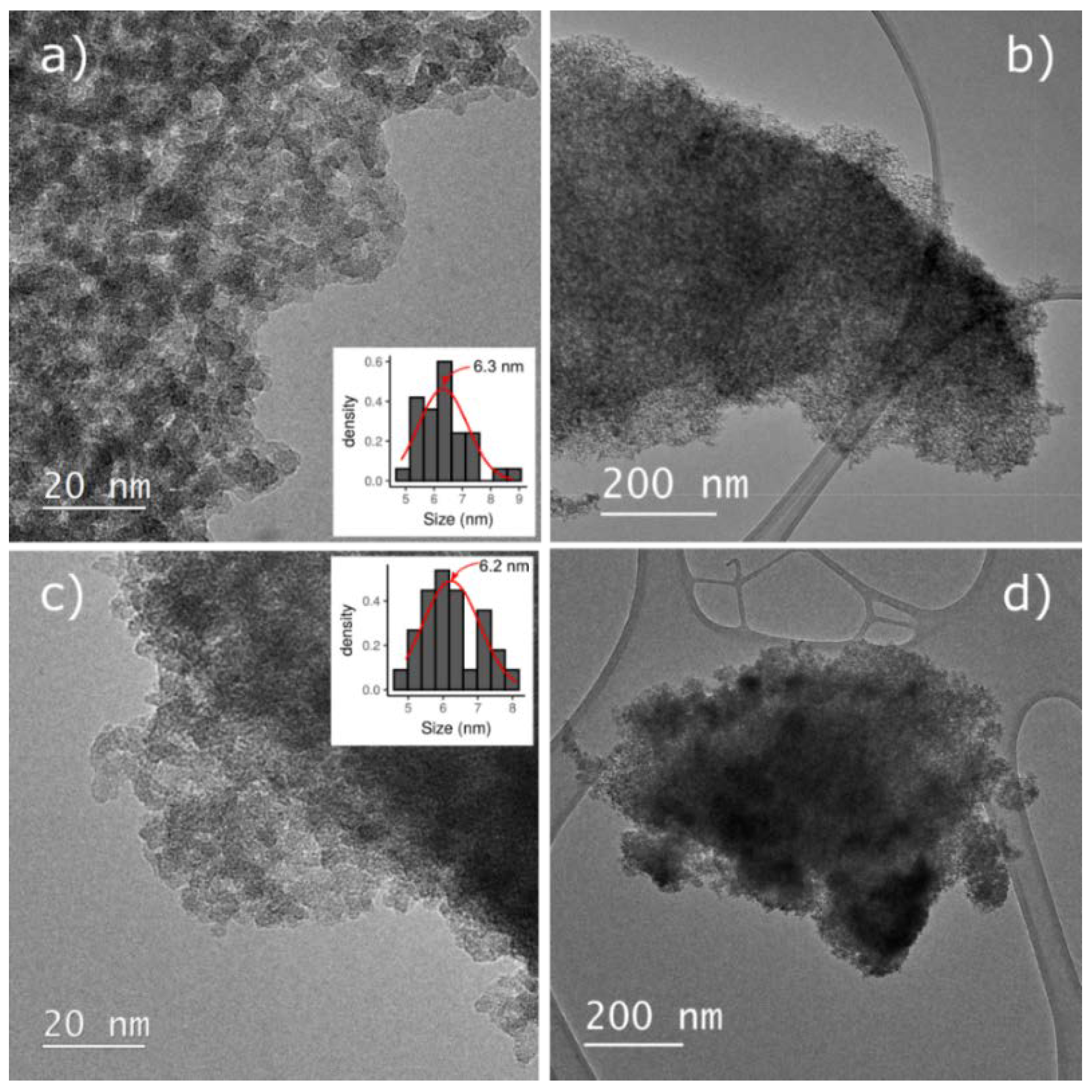
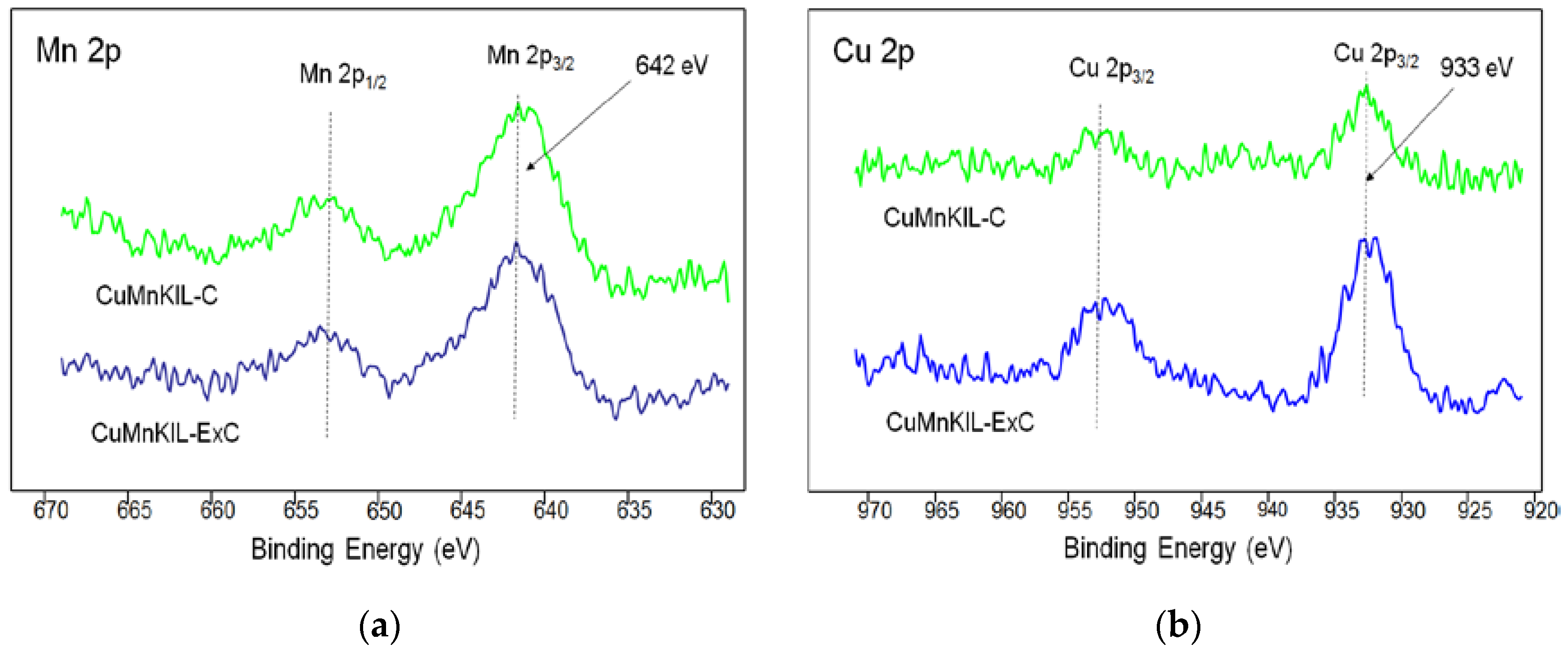
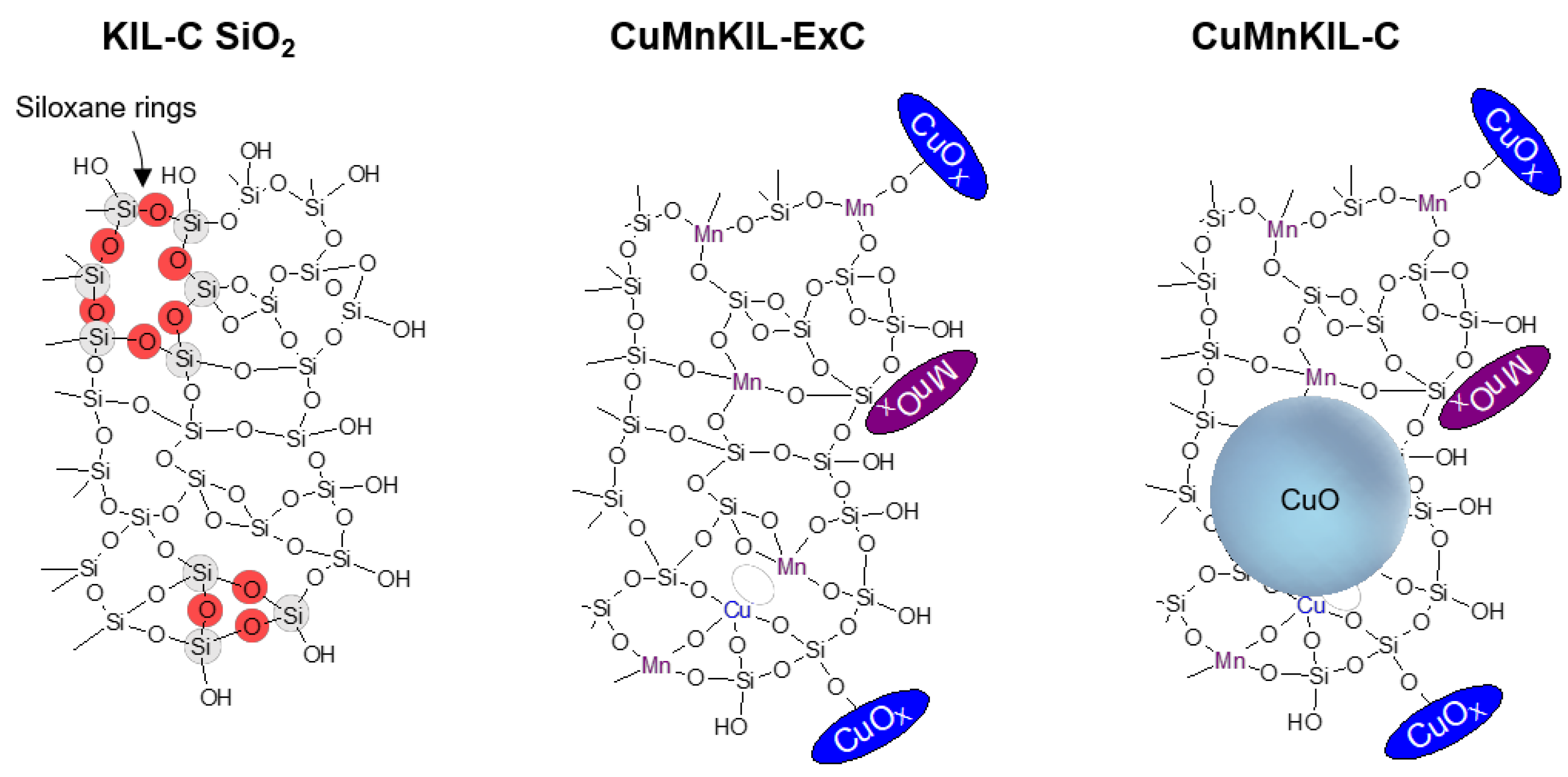
3.1. Materials Characterization
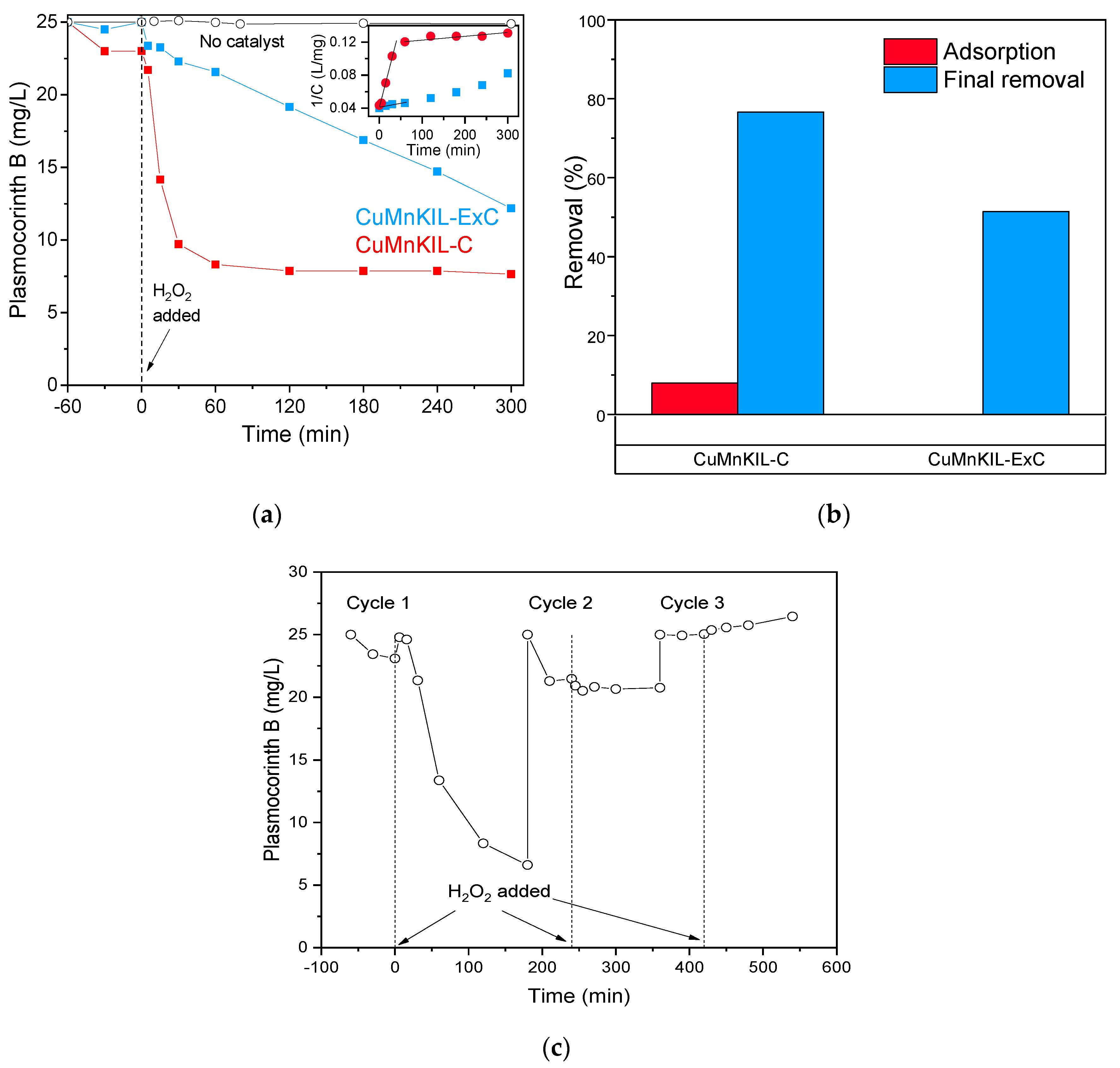
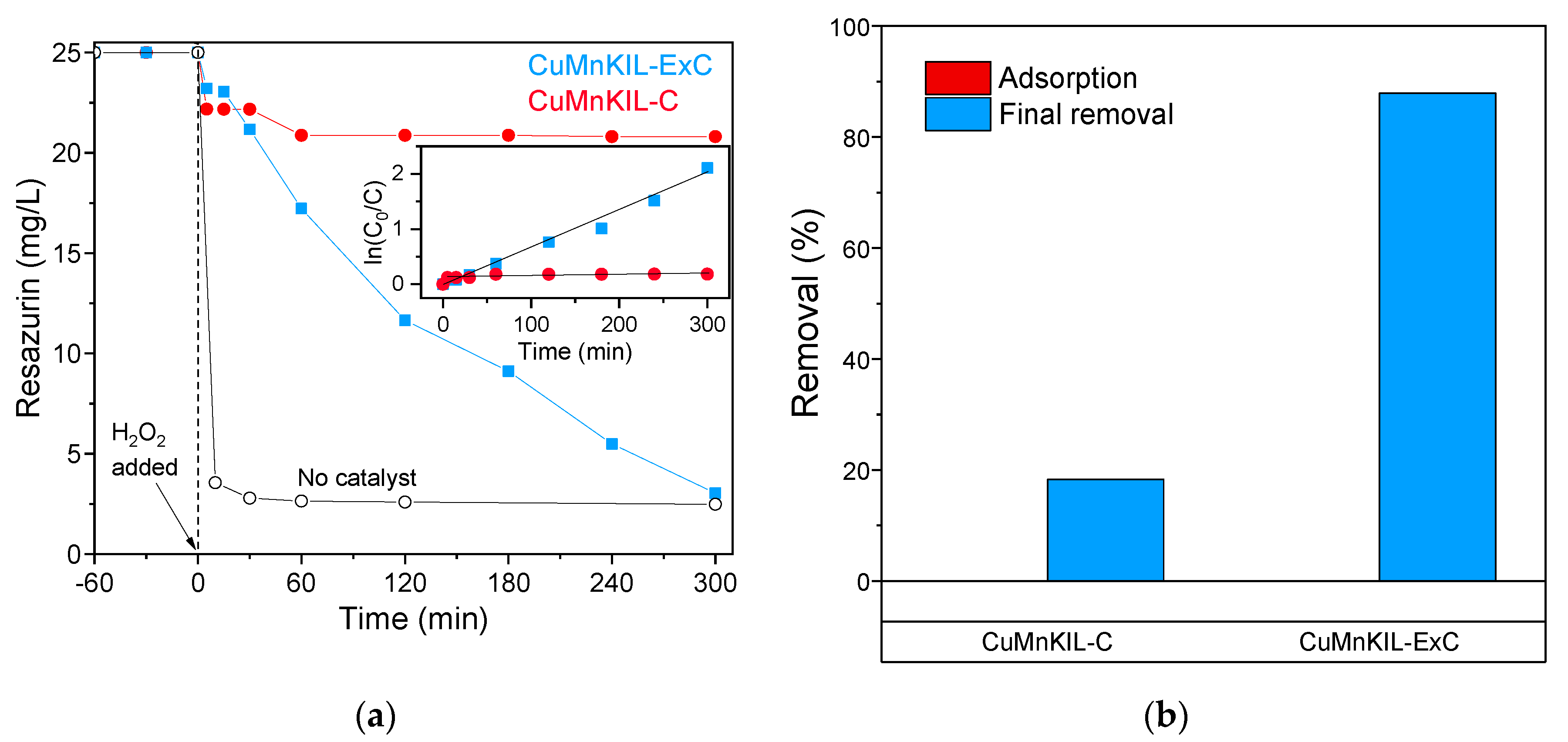
3.2. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, F.; Astruc, D. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord. Chem. Rev. 2020, 408, 213180. [Google Scholar] [CrossRef]
- He, K.; Chen, G.; Zeng, G.; Chen, A.; Huang, Z.; Shi, J.; Huang, T.; Peng, M.; Hu, L. Three-dimensional graphene supported catalysts for organic dyes degradation. Appl. Catal. B Environ. 2018, 228, 19–28. [Google Scholar] [CrossRef]
- Gu, J.; Hu, C.; Zhang, W.; Dichiara, A.B. Reagentless preparation of shape memory cellulose nanofibril aerogels decorated with Pd nanoparticles and their application in dye discoloration. Appl. Catal. B Environ. 2018, 237, 482–490. [Google Scholar] [CrossRef]
- Nidheesh, P.V. Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv. 2015, 5, 40552–40577. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Zang, C.; Hu, S.; Jin, S.; Chen, F. Catalytic Process Optimization of Birnessite-based Fenton-like Reaction with Surface Cu2+ Modification. ChemCatChem 2018, 10, 3576–3582. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, S.; Pachamuthu, M.P.; Isaacs, M.A.; Kumar, S.; Lee, A.F.; Sekaran, G. Cu and Fe oxides dispersed on SBA-15: A Fenton type bimetallic catalyst for N,N-diethyl-p-phenyl diamine degradation. Appl. Catal. B Environ. 2016, 199, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Tušar, N.N.; Maučec, D.; Rangus, M.; Arčon, I.; Mazaj, M.; Cotman, M.; Pintar, A.; Kaučič, V. Manganese functionalized silicate nanoparticles as a Fenton-type catalyst for water purification by Advanced Oxidation Processes (AOP). Adv. Funct. Mater. 2012, 22, 820–826. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Guo, Y. Copper-based non-precious metal heterogeneous catalysts for environmental remediation. Chinese J. Catal. 2018, 39, 566–582. [Google Scholar] [CrossRef]
- Šuligoj, A.; Ristić, A.; Dražić, G.; Pintar, A.; Logar, N.Z.; Tušar, N.N. Bimetal Cu-Mn porous silica-supported catalyst for Fenton-like degradation of organic dyes in wastewater at neutral pH. Catal. Today 2020, 358, 270277. [Google Scholar] [CrossRef]
- Zhao, N.; Hua, W.; Wang, Y.; Yang, C.; Ouyang, X.; Yuan, B.; Bian, F. In-situ GISAXS investigation of the structure evolution mechanism of template removal of ordered mesoporous films prepared via a soft-templating method. Appl. Surf. Sci. 2019, 479, 776–785. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Tang, Y. Rapid detemplation of nanozeolite β: Microwave-assisted Fenton-like oxidation. RSC Adv. 2012, 2, 6036–6041. [Google Scholar] [CrossRef]
- Zhang, Z.; Mayoral, A.; Melián-Cabrera, I. Protocol optimization for the mild detemplation of mesoporous silica nanoparticles resulting in enhanced texture and colloidal stability. Microporous Mesoporous Mater. 2016, 220, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Pérez, L.L.; Ortiz-Iniesta, M.J.; Zhang, Z.; Agirrezabal-Telleria, I.; Santes, M.; Heeres, H.J.; Melián-Cabrera, I. Detemplation of soft mesoporous silica nanoparticles with structural preservation. J. Mater. Chem. A 2013, 1, 4747–4753. [Google Scholar] [CrossRef]
- González-Rivera, J.; Tovar-Rodríguez, J.; Bramanti, E.; Duce, C.; Longo, I.; Fratini, E.; Galindo-Esquivel, I.R.; Ferrari, C. Surfactant recovery from mesoporous metal-modified materials (Sn-, Y-, Ce-, Si-MCM-41), by ultrasound assisted ion-exchange extraction and its re-use for a microwave in situ cheap and eco-friendly MCM-41 synthesis. J. Mater. Chem. A 2014, 2, 7020–7033. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Barczak, M. Template removal from mesoporous silicas using different methods as a tool for adjusting their properties. New J. Chem. 2018, 42, 4182–4191. [Google Scholar] [CrossRef]
- Vázquez, G.; Alvarez, E.; Rendo, R.; Romero, E.; Navaza, J.M. Surface Tension of Aqueous Solutions of Diethanolamine and Triethanolamine from 25 °C to 50 °C. J. Chem. Eng. Data 1996, 41, 806–808. [Google Scholar] [CrossRef]
- Vazquez, G.; Alvarez, E.; Navaza, J.M. Surface Tension of Alcohol Water + Water from 20 to 50. degree.C. J. Chem. Eng. Data 1995, 40, 611–614. [Google Scholar] [CrossRef]
- Kesmez, Ö.; Erdem Çamurlu, H.; Burunkaya, E.; Arpaç, E. Sol-gel preparation and characterization of anti-reflective and self-cleaning SiO2-TiO2 double-layer nanometric films. Sol. Energy Mater. Sol. Cells 2009, 93, 1833–1839. [Google Scholar] [CrossRef]
- Nauert, S.L.; Rosen, A.S.; Kim, H.; Snurr, R.Q.; Stair, P.C.; Notestein, J.M. Evidence for Copper Dimers in Low-Loaded CuOX/SiO2 Catalysts for Cyclohexane Oxidative Dehydrogenation. ACS Catal. 2018, 8, 9775–9789. [Google Scholar] [CrossRef]
- Borodko, Y.; Ager, J.W.; Marti, G.E.; Song, H.; Niesz, K.; Somorjai, G.A. Structure Sensitivity of Vibrational Spectra of Mesoporous Silica SBA-15 and Pt/SBA-15. J. Phys. Chem. B 2005, 109, 17386–17390. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, N.M.; Hu, B.; Das, U.; Kim, H.; Greeley, J.; Curtiss, L.A.; Stair, P.C.; Miller, J.T.; Hock, A.S. Propylene Hydrogenation and Propane Dehydrogenation by a Single-Site Zn 2+ on Silica Catalyst. ACS Catal. 2014, 4, 1091–1098. [Google Scholar] [CrossRef]
- Deng, Y.; Handoko, A.D.; Du, Y.; Xi, S.; Yeo, B.S. In Situ Raman Spectroscopy of Copper and Copper Oxide Surfaces during Electrochemical Oxygen Evolution Reaction: Identification of Cu III Oxides as Catalytically Active Species. ACS Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- Julien, C.; Massot, M.; Poinsignon, C. Lattice vibrations of manganese oxides. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 60, 689–700. [Google Scholar] [CrossRef]
- Ristić, A.; Mazaj, M.; Arčon, I.; Daneu, N.; Zabukovec Logar, N.; Gläser, R.; Tušar, N.N. New Insights into Manganese Local Environment in MnS-1 Nanocrystals. Cryst. Growth Des. 2019, 19, 3130–3138. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.X.; Zhu, Y.J.; Tong, H.; Wang, W.W.; Cheng, G.F. Low temperature synthesis of Mn3O4 polyhedral nanocrystals and magnetic study. J. Solid State Chem. 2006, 179, 1225–1229. [Google Scholar] [CrossRef]
- Tian, Z.Y.; Mountapmbeme Kouotou, P.; Bahlawane, N.; Tchoua Ngamou, P.H. Synthesis of the catalytically active Mn3O4 spinel and its thermal properties. J. Phys. Chem. C 2013, 117, 6218–6224. [Google Scholar] [CrossRef]
- Tang, Q.; Hu, S.; Chen, Y.; Guo, Z.; Hu, Y.; Chen, Y.; Yang, Y. Highly dispersed manganese oxide catalysts grafted on SBA-15: Synthesis, characterization and catalytic application in trans-stilbene epoxidation. Microporous Mesoporous Mater. 2010, 132, 501–509. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Q.; Fei, X.; He, Y.; Zhang, P.; Zhang, G.; Peng, L.; Xie, W. Synthesis of CuO nano- and micro-structures and their Raman spectroscopic studies. CrystEngComm 2010, 12, 2232. [Google Scholar] [CrossRef]
- Peng, G.; Wu, S.; Ellis, J.E.; Xu, X.; Xu, G.; Yu, C.; Star, A. Single-walled carbon nanotubes templated CuO networks for gas sensing. J. Mater. Chem. C 2016, 4, 6575–6580. [Google Scholar] [CrossRef]
- Li, Y.; Qu, J.; Gao, F.; Lv, S.; Shi, L.; He, C.; Sun, J. In situ fabrication of Mn3O4 decorated graphene oxide as a synergistic catalyst for degradation of methylene blue. Appl. Catal. B Environ. 2015, 162, 268–274. [Google Scholar] [CrossRef]
- Czaplinska, J.; Sobczak, I.; Ziolek, M. Bimetallic AgCu/SBA-15 System: The Effect of Metal Loading and Treatment of Catalyst on Surface Properties. J. Phys. Chem. C 2014, 118, 12796–12810. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, S.; Ning, P. Degradation Mechanism of Methylene Blue in a Heterogeneous Fenton-like Reaction Catalyzed by Ferrocene. Ind. Eng. Chem. Res. 2014, 53, 643–649. [Google Scholar] [CrossRef]
- Rhadfi, T.; Piquemal, J.-Y.; Sicard, L.; Herbst, F.; Briot, E.; Benedetti, M.; Atlamsani, A. Polyol-made Mn3O4 nanocrystals as efficient Fenton-like catalysts. Appl. Catal. A Gen. 2010, 386, 132–139. [Google Scholar] [CrossRef]
- Do, S.-H.; Batchelor, B.; Lee, H.-K.; Kong, S.-H. Hydrogen peroxide decomposition on manganese oxide (pyrolusite): Kinetics, intermediates, and mechanism. Chemosphere 2009, 75, 8–12. [Google Scholar] [CrossRef]
- Szpyrkowicz, L.; Juzzolino, C.; Kaul, S.N. A Comparative study on oxidation of disperse dyes by electrochemical process, ozone, hypochlorite and fenton reagent. Water Res. 2001, 35, 2129–2136. [Google Scholar] [CrossRef]
- Guedes, A.M.F.M.; Madeira, L.M.P.; Boaventura, R.A.R.; Costa, C.A.V. Fenton oxidation of cork cooking wastewater—overall kinetic analysis. Water Res. 2003, 37, 3061–3069. [Google Scholar] [CrossRef]
- Sidney Santana, C.; Nicodemos Ramos, M.D.; Vieira Velloso, C.C.; Aguiar, A. Kinetic Evaluation of Dye Decolorization by Fenton Processes in the Presence of 3-Hydroxyanthranilic Acid. Int. J. Environ. Res. Public Health 2019, 16, 1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.Y.; Wang, Y.; Shi, T.N.; Zhao, H.; Tan, Q.; Zhao, B.C.; He, X.L.; Qi, S.Y. Heterogeneous Fenton-like discoloration of methyl orange using Fe3O4/MWCNTs as catalyst: Kinetics and Fenton-like mechanism. Front. Mater. Sci. 2018, 12, 34–44. [Google Scholar] [CrossRef]
- Biswas, S.; Pal, A. Visible light assisted Fenton type degradation of methylene blue by admicelle anchored alumina supported rod shaped manganese oxide. J. Water Process Eng. 2020, 36, 101272. [Google Scholar] [CrossRef]
- Wang, T.; Shi, M.; Zhang, S.; Wu, L.; Jiang, B.; Cai, J.; Li, C. Sacrificial template-directed fabrication of mesoporous manganese oxide based catalysts: Relationship between the ordering degree of pore structure and Fenton-like catalytic activity. Appl. Surf. Sci. 2020, 530, 147304. [Google Scholar] [CrossRef]
- Kim, E.J.; Oh, D.; Lee, C.S.; Gong, J.; Kim, J.; Chang, Y.S. Manganese oxide nanorods as a robust Fenton-like catalyst at neutral pH: Crystal phase-dependent behavior. Catal. Today 2017, 282, 71–76. [Google Scholar] [CrossRef]
- Song, H.; You, J.-A.; Chen, C.; Zhang, H.; Ji, X.Z.; Li, C.; Yang, Y.; Xu, N.; Huang, J. Manganese functionalized mesoporous molecular sieves Ti-HMS as a Fenton-like catalyst for dyes wastewater purification by advanced oxidation processes. J. Environ. Chem. Eng. 2016, 4, 4653–4660. [Google Scholar] [CrossRef]
- European Commission. European Water Framework Directive 2000/60/EC. Off. J. Eur. Communities 2000, 43, 1–72. [Google Scholar]
- WHO (Ed.) Guidelines for Drinking-Water Quality, 4th ed.; WHO Press: Geneva, Switzerland, 2011; ISBN 9789241548151. [Google Scholar]
- Li, Y.; Xu, Z.X.; Ma, H.; Hursthouse, S.A. Removal of Manganese(II) from Acid Mine Wastewater: A Review of the Challenges and Opportunities with Special Emphasis on Mn-Oxidizing Bacteria and Microalgae. Water 2019, 11, 2493. [Google Scholar] [CrossRef] [Green Version]







| Dye | Molar Mass (g/mol) | λmax (nm) | Type | Molecular Structure |
|---|---|---|---|---|
| Methylene blue | 319.85 | 664 | cationic | 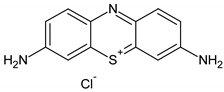 |
| Plasmocorinth B | 518.81 | 550 | anionic |  |
| Resazurin | 229.19 | 600 | zwitterionic | 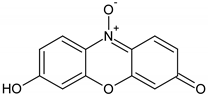 |
| Sample | Mn (at. %) | Cu (at. %) | Mn (wt. %) | Cu (wt. %) | Mn/Si | Cu/Si |
|---|---|---|---|---|---|---|
| CuMnKIL-ExC | 0.8 | 0.9 | 2.2 | 2.9 | 0.03 | 0.03 |
| CuMnKIL-C | 0.9 | 1.4 | 2.4 | 4.4 | 0.03 | 0.05 |
| Sample | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| CuMnKIL-ExC | 967 ± 1 | 1.896 | 9.3 |
| CuMnKIL-C | 734 ± 1 | 1.513 | 11.1 |
| Catalyst | H2O2 (mM) | Pollutant | Pollutant (mg/L) | Efficiency | Reaction Time (min) | Reference |
|---|---|---|---|---|---|---|
| MnOX on Al2O3 | 388 | MB a | 20 | 99% | 400 | [44] |
| Ordered mesoporous MnOX | 19.6 | RhB b | 100 | 90% | 150 | [45] |
| γ-MnO2 | 1450 | MB a | 51 | 99% | 20 | [46] |
| Mn on SiO2 | 97 | MB a | 25 | 97% | 300 | This study |
| Mn on SiO2 | 97 | RZ c | 25 | 90% | 300 | This study |
| Mn on SiO2 | 97 | PC d | 25 | 65% | 300 | This study |
| Mn-Ti-HMS | 10 | MB & RhB | 16 & 24 | 95% | 120 | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trendafilova, I.; Šuligoj, A.; Ristić, A.; Van de Velde, N.; Dražić, G.; Opresnik, M.; Zabukovec Logar, N.; Pintar, A.; Novak Tušar, N. Evolution of Surface Catalytic Sites on Bimetal Silica-Based Fenton-Like Catalysts for Degradation of Dyes with Different Molecular Charges. Nanomaterials 2020, 10, 2419. https://doi.org/10.3390/nano10122419
Trendafilova I, Šuligoj A, Ristić A, Van de Velde N, Dražić G, Opresnik M, Zabukovec Logar N, Pintar A, Novak Tušar N. Evolution of Surface Catalytic Sites on Bimetal Silica-Based Fenton-Like Catalysts for Degradation of Dyes with Different Molecular Charges. Nanomaterials. 2020; 10(12):2419. https://doi.org/10.3390/nano10122419
Chicago/Turabian StyleTrendafilova, Ivalina, Andraž Šuligoj, Alenka Ristić, Nigel Van de Velde, Goran Dražić, Mojca Opresnik, Nataša Zabukovec Logar, Albin Pintar, and Nataša Novak Tušar. 2020. "Evolution of Surface Catalytic Sites on Bimetal Silica-Based Fenton-Like Catalysts for Degradation of Dyes with Different Molecular Charges" Nanomaterials 10, no. 12: 2419. https://doi.org/10.3390/nano10122419











