Inorganic Materials by Atomic Layer Deposition for Perovskite Solar Cells
Abstract
:1. Introduction
2. ALD below the Perovskite Layer
2.1. Charge Transport Layers below the Perovskite Layer
2.1.1. Electron Transport Layers
2.1.2. Hole Transport Layers
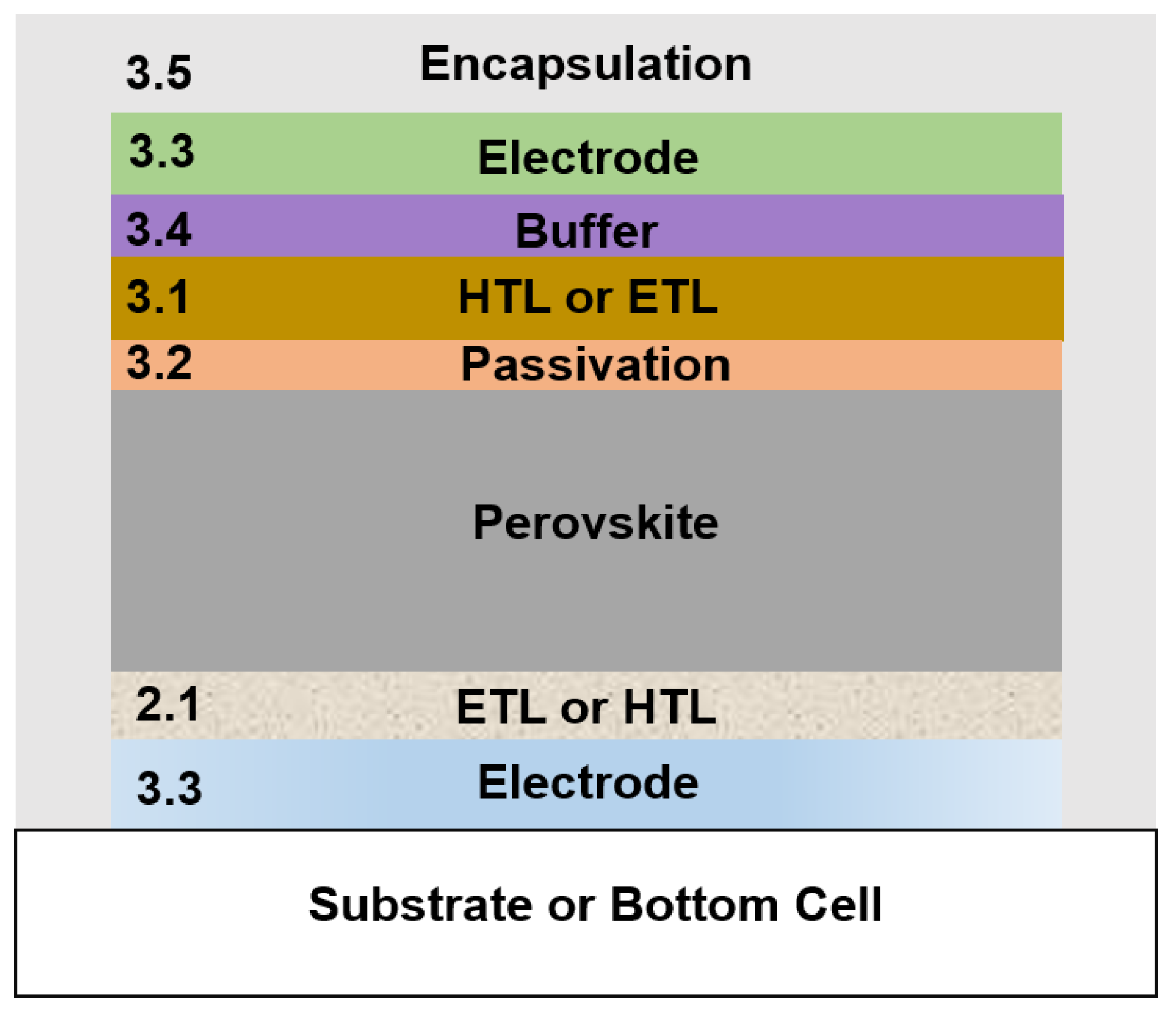
3. ALD above the Perovskite Layer
3.1. Charge Transport Layers above the Perovskite Layer
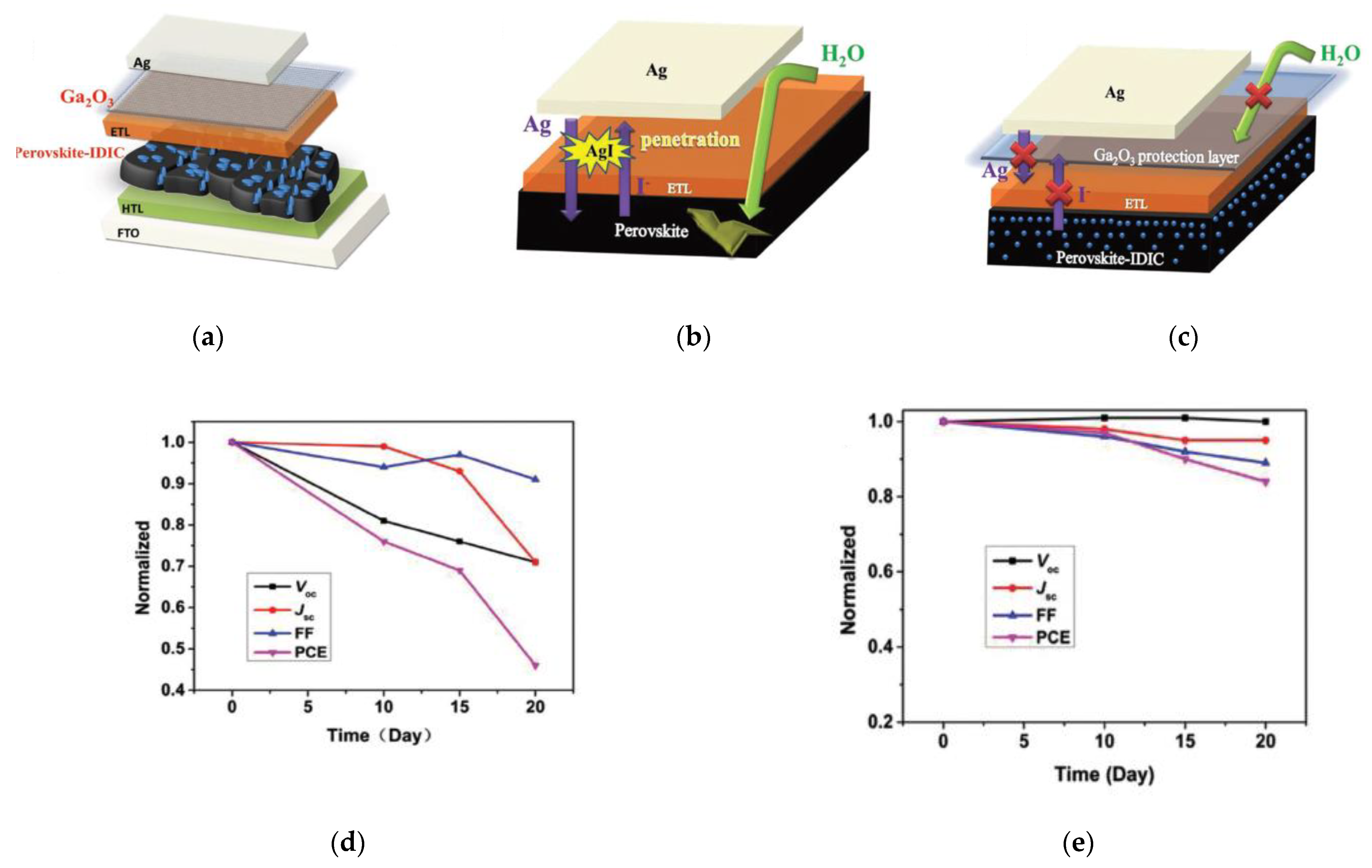
3.2. Passivation or Barrier Layers
3.3. Recombination Layers in Tandem Applications
3.4. Buffer Layers in Semitransparent and Tandem Applications
3.5. Encapsulation
4. ALD in Perovskite Light Emitting Diode Applications
5. Variations of ALD
6. Summary and Future Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- NREL, E.C. Photovoltaic Research . Available online: http://www.nrel.gov/pv/ (accessed on 31 December 2020).
- Lee, B.; Lee, S.; Cho, D.; Kim, J.; Hwang, T.; Kim, K.H.; Hong, S.; Moon, T.; Park, B. Evaluating the Optoelectronic Quality of Hybrid Perovskites by Conductive Atomic Force Microscopy with Noise Spectroscopy. ACS Appl. Mater. Interfaces 2016, 8, 30985–30991. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yun, A.J.; Gil, B.; Lee, Y.; Park, B. Triamine-Based Aromatic Cation as a Novel Stabilizer for Efficient Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1905190. [Google Scholar] [CrossRef] [Green Version]
- Hwang, T.; Lee, B.; Kim, J.; Lee, S.; Gil, B.; Yun, A.J.; Park, B. Organometal Halide Perovskites: From Nanostructural Evolution to Dynamic Interplay of Constituents: Perspectives for Perovskite Solar Cells. Adv. Mater. 2018, 30, 1704208. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.; Cho, D.; Kim, J.; Kim, J.; Lee, S.; Lee, B.; Kim, K.H.; Hong, S.; Kim, C.; Park, B. Park Investigation of chlorine-mediated microstructural evolution of CH3NH3PbI3(Cl) grains for high optoelectronic responses. Nano Energy 2016, 25, 91–99. [Google Scholar] [CrossRef]
- Gunawan, O.; Pae, S.R.; Bishop, D.M.; Lee, Y.S.; Virgus, Y.; Jeon, N.J.; Noh, J.H.; Shao, X.; Todorov, T.; Mitzi, D.B. Carrier-resolved photo hall measurement in world record-quality perovskite and kesterite solar absorbers. Nature 2019, 575, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hwang, T.; Lee, B.; Lee, S.; Park, K.; Park, H.H.; Park, B. An Aromatic Diamine Molecule as the A -Site Solute for Highly Durable and Efficient Perovskite Solar Cells. Small Methods 2019, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Jeon, N.J.; Yang, T.Y.; Park, H.H.; Seo, J.; Nam, D.Y.; Jeong, D.; Hong, S.; Kim, S.H.; Cho, J.M.; Jang, J.J.; et al. Thermally activated, light-induced electronspin-resonance spin density reflected by photocurrents in a perovskite solar cell. Appl. Physics Lett. 2019, 114, 013903. [Google Scholar] [CrossRef]
- Kim, M.; Kang, T.-W.; Kim, S.H.; Jung, E.H.; Park, H.H.; Seo, J.; Lee, S.-J. Antireflective, self-cleaning and protective film by continuous sputtering of a plasma polymer on inorganic multilayer for perovskite solar cells application. Sol. Energy Mater. Sol. Cells 2019, 191, 55–61. [Google Scholar] [CrossRef]
- Kim, G.; Moon, C.S.; Yang, T.-Y.; Kim, Y.Y.; Chung, J.; Jung, E.H.; Shin, T.J.; Jeon, N.J.; Park, H.H.; Seo, J. A Thermally Induced Perovskite Crystal Control Strategy for Efficient and Photostable Wide-Bandgap Perovskite Solar Cells. Sol. RRL 2020, 4. [Google Scholar] [CrossRef]
- Park, H.H.; Larrabee, T.J.; Ruppalt, L.B.; Culbertson, J.C.; Prokes, S.M. Tunable Electrical Properties of Vanadium Oxide by HydrogenPlasma-Treated Atomic Layer Deposition. ACS Omega 2017, 2, 1259–1264. [Google Scholar] [CrossRef]
- Park, H.H.; Jayaraman, A.; Heasley, R.; Yang, C.; Hartle, L.; Mankad, R.; Haight, R.; Mitzi, D.B.; Gunawan, O.; Gordon, R.G. Atomic layer deposition of Al-incorporated Zn(O,S) thin films with tunable electrical properties. Appl. Phys. Lett. 2014, 105, 202101. [Google Scholar] [CrossRef]
- Hejin Park, H.; Heasley, R.; Gordon, R.G. Atomic layer deposition of Zn(O,S) thin films with tunable electrical properties by oxygen annealing. Appl. Phys. Lett. 2013, 102, 132110. [Google Scholar] [CrossRef]
- Raiford, J.A.; Oyakhire, S.T.; Bent, S.F. Applications of atomic layer deposition and chemical vapor deposition for perovskite solar cells. Energy Environ. Sci. 2020, 13, 1997–2023. [Google Scholar] [CrossRef]
- Seo, S.; Jeong, S.; Park, H.; Shin, H.; Park, N.-G. Atomic layer deposition for efficient and stable perovskite solar cells. Chem. Commun. 2019, 55, 2403–2416. [Google Scholar] [CrossRef] [PubMed]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee RA, M.; Jen, T.C. New development of atomic layer deposition: Processes, methods and applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef] [Green Version]
- Hoye, R.; Muñoz-Rojas, D.; Nelson, S.F.; Illiberi, A.; Poodt, P.; Roozeboom, F.F.; MacManus-Driscoll, J.L. Research Update: Atmospheric pressure spatial atomic layer deposition of ZnO thin films: Reactors, doping, and devices. APL Mater. 2015, 3, 040701. [Google Scholar] [CrossRef]
- Steinmann, V.; Jaramillo, R.; Hartman, K.; Chakraborty, R.; Brandt, R.E.; Poindexter, J.R.; Lee, Y.S.; Sun, L.; Polizzotti, A.; Park, H.H.; et al. Buonassisi, 3.88% Effi cient Tin Sulfi de Solar Cells using Congruent Thermal Evaporation. Adv. Mater. 2014, 26, 7488–7492. [Google Scholar] [CrossRef]
- Kim, D.; Jung, H.J.; Park, I.J.; Larson, B.W.; Dunfield, S.P.; Xiao, C.; Kim, J.; Tong, J.; Boonmongkolras, P.; Ji, S.G.; et al. Efficient, stable silicon tandem cells enabled by anion-engineered wide-bandgap perovskites. Science 2020, 368, 155–160. [Google Scholar] [CrossRef]
- Lim, J.; Kim, M.; Park, H.H.; Jung, H.; Lim, S.; Hao, X.; Choi, E.; Park, S.; Lee, M.; Liu, Z.; et al. Kinetics of light-induced degradation in semi-transparent perovskite solar cells. Sol. Energy Mater. Sol. Cells 2021, 219, 110776. [Google Scholar] [CrossRef]
- Lee, B.; Shin, B.; Park, B. Uniform Cs2SnI6 Thin Films for Lead-Free and Stable Perovskite Optoelectronics via Hybrid Deposition Approaches. Electron. Mater. Lett. 2019, 15, 192–200. [Google Scholar] [CrossRef]
- Todorov, T.; Gunawan, O.; Guha, S. A road towards 25% efficiency and beyond: Perovskite tandem solar cells. Mol. Syst. Des. Eng. 2016, 1, 370–376. [Google Scholar] [CrossRef]
- Yun, A.J.; Kim, J.; Hwang, T.; Park, B. Origins of Efficient Perovskite Solar Cells with Low-Temperature Processed SnO2 Electron Transport Layer. ACS Appl. Energy Mater. 2019, 2, 3554–3560. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Tress, W.; Domanski, K.; Anaraki, E.H.; Cruz, S.H.T.; Roose, B.; Boix, P.P.; Grätzel, M.; Saliba, M.; Abate, A.; et al. Identifying and suppressing interfacial recombination to achieve high open-circuit voltage in perovskite solar cells. Energy Environ. Sci. 2017, 10, 1207–1212. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, C.; Yu, Y.; Zhao, D.; Awni, R.A.; Grice, C.R.; Ghimire, K.; Constantinou, I.; Liao, W.; Cimaroli, A.J.; et al. Understanding and Eliminating Hysteresis for Highly Efficient Planar Perovskite Solar Cells. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Guo, W. Optimizing the efficiency of perovskite solar cells by a sub-nanometer compact titanium oxide electron transport layer. Nano Energy 2018, 49, 230–236. [Google Scholar] [CrossRef]
- Lu, H.; Ma, Y.; Gu, B.; Tian, W.; Li, L. Identifying the optimum thickness of electron transport layers for highly efficient perovskite planar solar cells. J. Mater. Chem. A 2015, 3, 16445–16452. [Google Scholar] [CrossRef]
- Sun, H.; Deng, K.; Zhu, Y.; Liao, M.; Xiong, J.; Li, Y.; Li, L. A Novel Conductive Mesoporous Layer with a Dynamic Two-Step Deposition Strategy Boosts Efficiency of Perovskite Solar Cells to 20%. Adv. Mater. 2018, 30, e1801935. [Google Scholar] [CrossRef]
- Chavan, R.D.; Yadav, P.; Tavakoli, M.M.; Prochowicz, D.; Nimbalkar, A.; Bhoite, S.P.; Bhosale, P.N.; Hong, C.K. Double layer mesoscopic electron contact for efficient perovskite solar cells. Sustain. Energy Fuels 2020, 4, 843–851. [Google Scholar] [CrossRef]
- Wei, H.; Wu, J.; Qiu, P.; Liu, S.; He, Y.; Peng, M.; Li, D.; Meng, Q.; Zaera, F.; Zheng, X. Plasma-enhanced atomic-layer-deposited gallium nitride as an electron transport layer for planar perovskite solar cells. J. Mater. Chem. A 2019, 7, 25347–25354. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Steier, L.; Tress, W.; Saliba, M.; Neutzner, S.; Matsui, T.; Giordano, F.; Jacobsson, T.J.; Kandada, A.R.S.; Zakeeruddin, S.M.; et al. Highly efficient planar perovskite solar cells through band alignment engineering. Energy Environ. Sci. 2015, 8, 2928–2934. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Lee, S.; Seo, G.; Paek, S.; Cho, K.T.; Huckaba, A.J.; Calizzi, M.; Choi, D.-W.; Park, J.-S.; Lee, D.; et al. Efficient Planar Perovskite Solar Cells Using Passivated Tin Oxide as an Electron Transport Layer. Adv. Sci. 2018, 5, 1800130. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guan, L.; Zhao, D.; Yu, Y.; Grice, C.R.; Song, Z.; Awni, R.A.; Chen, J.; Wang, J.; Zhao, X.; et al. Water Vapor Treatment of Low-Temperature Deposited SnO2 Electron Selective Layers for Efficient Flexible Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 2118–2124. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, D.; Grice, C.R.; Liao, W.; Yu, Y.; Cimaroli, A.; Shrestha, N.; Roland, P.J.; Chen, J.; Yu, Z.; et al. Low-temperature plasma-enhanced atomic layer deposition of tin oxide electron selective layers for highly efficient planar perovskite solar cells. J. Mater. Chem. A 2016, 4, 12080–12087. [Google Scholar] [CrossRef]
- Li, W.; Dong, H.; Guo, X.; Li, N.; Li, J.; Niu, G.; Wang, L. Graphene oxide as dual functional interface modifier for improving wettability and retarding recombination in hybrid perovskite solar cells. J. Mater. Chem. A 2014, 2, 20105–20111. [Google Scholar] [CrossRef]
- Zhu, W.; Su, A.; Li, X.; Pang, S.; Chang, J.; Xi, H.; Zhang, C.; Zhang, J.; Zhang, C.; Hao, Y. Efficient planar perovskite solar cells with low-temperature atomic layer deposited TiO2 electron transport layer and interfacial modifier. Sol. Energy 2019, 188, 239–246. [Google Scholar] [CrossRef]
- Kim, T.W.; Uchida, S.; Kondo, T.; Segawa, H. Optimization of TiO2 compact layer formed by atomic layer deposition for efficient perovskite solar cells. Appl. Phys. Lett. 2019, 115, 203902. [Google Scholar] [CrossRef]
- Chandiran, A.K.; Yella, A.; Mayer, M.T.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sub-Nanometer Conformal TiO 2 Blocking Layer for High Effi ciency Solid-State Perovskite Absorber Solar Cells. Adv. Mater. 2014, 26, 4309–4312. [Google Scholar] [CrossRef]
- Zardetto, V.; Di Giacomo, F.; Lifka, H.; Verheijen, M.A.; Weijtens, C.H.L.; Black, L.E.; Veenstra, S.; Kessels, W.M.M.; Andriessen, R.; Creatore, M. Surface Fluorination of ALD TiO2 Electron Transport Layer for Efficient Planar Perovskite Solar Cells. Adv. Mater. Interfaces 2018, 5. [Google Scholar] [CrossRef]
- Jin, T.Y.; Li, W.; Li, Y.Q.; Luo, Y.X.; Shen, Y.; Cheng, L.P.; Tang, J.X. High-Performance Flexible Perovskite Solar Cells Enabled by Low-Temperature ALD-Assisted Surface Passivation. Adv. Optical Mater. 2018, 6, 1801153. [Google Scholar] [CrossRef]
- Mali, S.S.; Shim, C.-S.; Park, H.K.; Heo, J.; Patil, P.S.; Hong, C.K. Ultrathin Atomic Layer Deposited TiO2for Surface Passivation of Hydrothermally Grown 1D TiO2Nanorod Arrays for Efficient Solid-State Perovskite Solar Cells. Chem. Mater. 2015, 27, 1541–1551. [Google Scholar] [CrossRef]
- Chavan, R.D.; Tavakoli, M.M.; Prochowicz, D.; Yadav, P.; Lote, S.S.; Bhoite, S.P.; Nimbalkar, A.; Hong, C.K. Atomic Layer Deposition of an Effective Interface Layer of TiN for Efficient and Hysteresis-Free Mesoscopic Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 8098–8106. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Hendricks, O.L.; Meng, A.C.; Braun, M.R.; McGehee, M.D.; Chidsey, C.E.; McIntyre, P.C. Atomic Layer Deposited TiO2–IrOx Alloy as a Hole Transport Material for Perovskite Solar Cells. Adv. Mater. Interfaces 2018, 5, 1800191. [Google Scholar] [CrossRef]
- Cao, B.; Yang, L.; Jiang, S.; Lin, H.; Wang, N.; Li, X. Flexible quintuple cation perovskite solar cells with high efficiency. J. Mater. Chem. A 2019, 7, 4960–4970. [Google Scholar] [CrossRef]
- Zhao, B.; Lee, L.C.; Yang, L.; Pearson, A.J.; Lu, H.; She, X.-J.; Cui, L.; Zhang, K.H.L.; Hoye, R.L.Z.; Karani, A.; et al. In Situ Atmospheric Deposition of Ultrasmooth Nickel Oxide for Efficient Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 41849–41854. [Google Scholar] [CrossRef]
- Koushik, D.; Jošt, M.; Dučinskas, A.; Burgess, C.; Zardetto, V.; Weijtens, C.; Verheijen, M.A.; Kessels, W.M.M.; Albrecht, S.; Creatore, M. Plasma-assisted atomic layer deposition of nickel oxide as hole transport layer for hybrid perovskite solar cells. J. Mater. Chem. C 2019, 7, 12532–12543. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.; Jeong, S.; Bae, C.; Park, N.-G.; Shin, H. Perovskite Solar Cells with Inorganic Electron- and Hole-Transport Layers Exhibiting Long-Term (≈500 h) Stability at 85 °C under Continuous 1 Sun Illumination in Ambient Air. Adv. Mater. 2018, 30, e1801010. [Google Scholar] [CrossRef]
- Chu, S.; Zhao, R.; Liu, R.; Gao, Y.; Wang, X.; Liu, C.; Chen, J.; Zhou, H. Atomic-layer-deposited ultra-thin VOx film as a hole transport layer for perovskite solar cells. Semicond. Sci. Technol. 2018, 33, 115016. [Google Scholar] [CrossRef]
- Si, H.; Liao, Q.; Zhang, Z.; Li, Y.; Yang, X.; Zhang, G.; Kang, Z.; Zhang, Y. An innovative design of perovskite solar cells with Al 2 O 3 inserting at ZnO/perovskite interface for improving the performance and stability. Nano Energy 2016, 22, 223–231. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Yun, A.J.; Gil, B.; Park, B. Interfacial Modification and Defect Passivation by the Cross-Linking Interlayer for Efficient and Stable CuSCN-Based Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 46818–46824. [Google Scholar] [CrossRef]
- Gil, B.; Yun, A.J.; Lee, Y.; Kim, J.; Lee, B.; Park, B. Recent Progress in Inorganic Hole Transport Materials for Efficient and Stable Perovskite Solar Cells. Electron. Mater. Lett. 2019, 15, 505–524. [Google Scholar] [CrossRef]
- Gil, B.; Kim, J.; Yun, A.J.; Park, K.; Cho, J.; Park, M.; Park, B. CuCrO2 Nanoparticles Incorporated into PTAA as a Hole Transport Layer for 85 °C and Light Stabilities in Perovskite Solar Cells. Nanomaterials 2020, 10, 1669. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Martinson, A.B.F. Stabilizing hybrid perovskites against moisture and temperature via non-hydrolytic atomic layer deposited overlayers. J. Mater. Chem. A 2015, 3, 20092–20096. [Google Scholar] [CrossRef]
- Zardetto, V.V.; Ben Williams, B.; Perrotta, A.A.; Di Giacomo, F.; Verheijen, M.M.; Andriessen, R.; Kessels, W.M.M.; Creatore, M. Atomic layer deposition for perovskite solar cells: Research status, opportunities and challenges. Sustain. Energy Fuels 2017, 1, 30–55. [Google Scholar] [CrossRef]
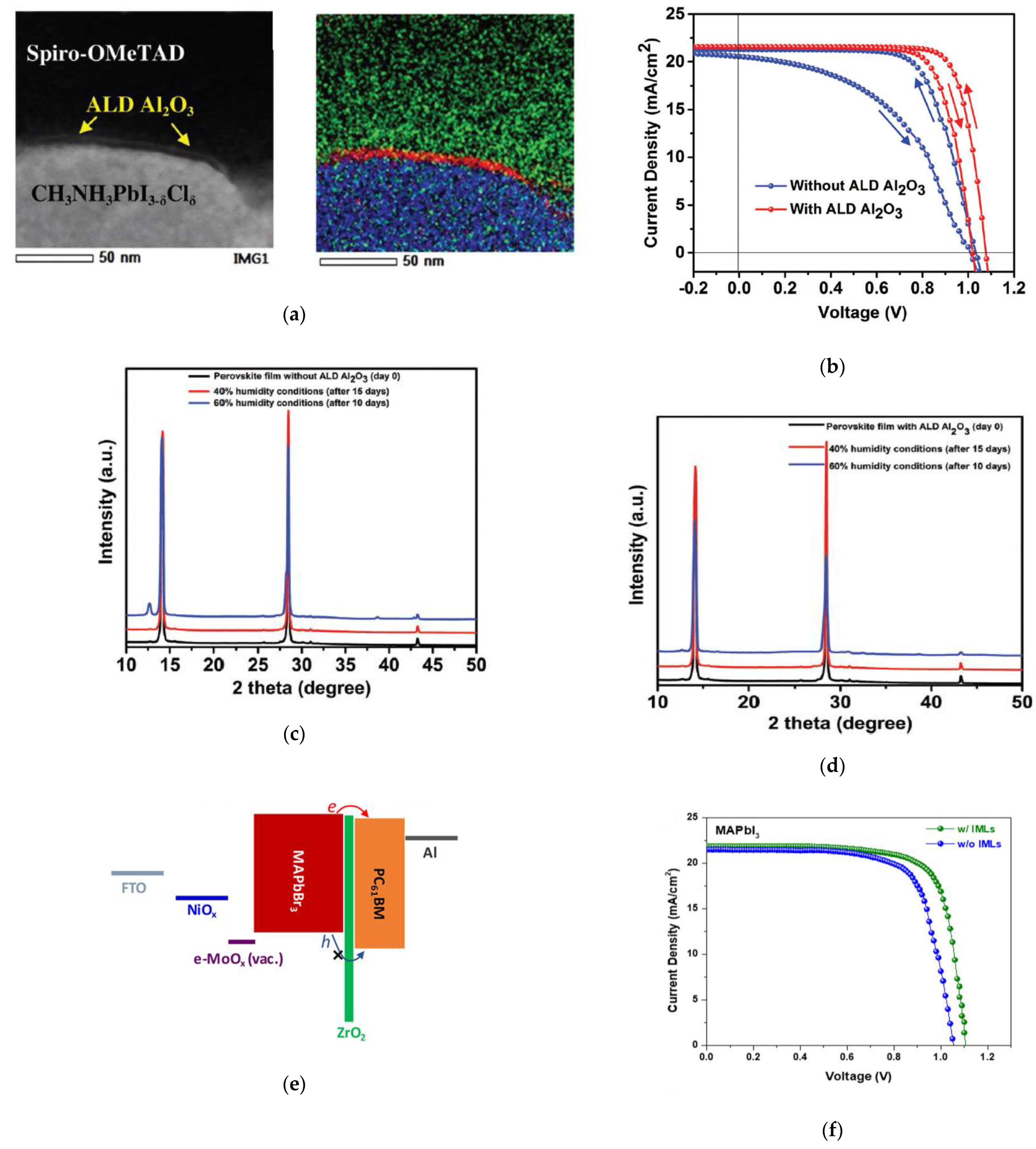
- Koushik, D.; Hazendonk, L.; Zardetto, V.; Vandalon, V.; Verheijen, M.A.; Kessels, W.M.M.; Creatore, M. Chemical Analysis of the Interface between Hybrid Organic–Inorganic Perovskite and Atomic Layer Deposited Al2O3. ACS Appl. Mater. Interfaces 2019, 11, 5526–5535. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Yan, H.; Peng, Q. Reaction Temperature and Partial Pressure Induced Etching of Methylammonium Lead Iodide Perovskite by Trimethylaluminum. Langmuir 2019, 35, 6522–6531. [Google Scholar] [CrossRef]
- Hultqvist, A.; Aitola, K.; Sveinbjörnsson, K.; Saki, Z.; Larsson, F.; Törndahl, T.; Johansson, E.M.J.; Boschloo, G.; Edoff, M. Atomic Layer Deposition of Electron Selective SnOx and ZnO Films on Mixed Halide Perovskite: Compatibility and Performance. ACS Appl. Mater. Interfaces 2017, 9, 29707–29716. [Google Scholar] [CrossRef]
- Palmstrom, A.F.; Raiford, J.A.; Prasanna, R.; Bush, K.A.; Sponseller, M.; Cheacharoen, R.; Minichetti, M.C.; Bergsman, D.S.; Leijtens, T.; Wang, H.-P.; et al. Interfacial Effects of Tin Oxide Atomic Layer Deposition in Metal Halide Perovskite Photovoltaics. Adv. Energy Mater. 2018, 8. [Google Scholar] [CrossRef]
- Tong, J.; Song, Z.; Kim, D.H.; Chen, X.; Chen, C.; Palmstrom, A.F.; Ndione, P.F.; Reese, M.O.; Dunfield, S.P.; Reid, O.G. Carrier lifetimes of >1 ms in Sn-Pb perovskites enable efficient all-perovskite tandem solar cells. Science 2019, 364, 475–479. [Google Scholar] [CrossRef]
- Eom, T.; Kim, S.; Agbenyeke, R.E.; Jung, H.; Shin, S.M.; Lee, Y.K.; Kim, C.G.; Chung, T.; Jeon, N.J.; Park, H.H.; et al. Copper Oxide Buffer Layers by Pulsed-Chemical Vapor Deposition for Semitransparent Perovskite Solar Cells. Adv. Mater. Interfaces 2020, 2020. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, S.; Liu, H.; Feng, F.; Li, W. Improved Efficiency of Perovskite Solar Cells by the Interfacial Modification of the Active Layer. Nanomaterials 2019, 9, 204. [Google Scholar] [CrossRef] [Green Version]
- Koushik, D.; Verhees, W.J.H.; Kuang, Y.; Veenstra, S.; Zhang, D.; Verheijen, M.A.; Creatore, M.; Schropp, R.E.I. High-efficiency humidity-stable planar perovskite solar cells based on atomic layer architecture. Energy Environ. Sci. 2017, 10, 91–100. [Google Scholar] [CrossRef]
- Palmstrom, A.F.; Eperon, G.E.; Leijtens, T.; Prasanna, R.; Habisreutinger, S.N.; Nemeth, W.; Gaulding, E.A.; Dunfield, S.P.; Reese, M.; Nanayakkara, S.; et al. Enabling Flexible All-Perovskite Tandem Solar Cells. Joule 2019, 3, 2193–2204. [Google Scholar] [CrossRef]
- Jagt, R.A.; Huq, T.N.; Hill, S.A.; Thway, M.; Liu, T.; Napari, M.; Roose, B.; Gałkowski, K.; Li, W.; Lin, S.F.; et al. Rapid Vapor-Phase Deposition of High-Mobility p-Type Buffer Layers on Perovskite Photovoltaics for Efficient Semitransparent Devices. ACS Energy Lett. 2020, 5, 2456–2465. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, M.; Chen, C.; Zhu, Z.; Zheng, X.; Chen, Z.; Guo, Y.; Liu, C.; Yan, Y.; Fang, G. Efficient and Stable Nonfullerene-Graded Heterojunction Inverted Perovskite Solar Cells with Inorganic Ga2 O3 Tunneling Protective Nanolayer. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Chen, B.; Yu, Z.; Liu, K.; Zheng, X.; Liu, Y.; Shi, J.; Spronk, D.; Rudd, P.N.; Holman, Z.; Huang, J. Grain Engineering for Perovskite/Silicon Monolithic Tandem Solar Cells with Efficiency of 25.4%. Joule 2019, 3, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Nogay, G.; Sahli, F.; Werner, J.; Monnard, R.; Boccard, M.; Despeisse, M.; Haug, F.-J.; Jeangros, Q.; Ingenito, A.; Ballif, C. 25.1%-Efficient Monolithic Perovskite/Silicon Tandem Solar Cell Based on a p-type Monocrystalline Textured Silicon Wafer and High-Temperature Passivating Contacts. ACS Energy Lett. 2019, 4, 844–845. [Google Scholar] [CrossRef]
- Jošt, M.; Köhnen, E.; Vilches, A.B.M.; Lipovšek, B.; Jäger, K.; Macco, B.; Al-Ashouri, A.; Krč, J.; Korte, L.; Rech, B.; et al. Textured interfaces in monolithic perovskite/silicon tandem solar cells: Advanced light management for improved efficiency and energy yield. Energy Environ. Sci. 2018, 11, 3511–3523. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Muzzillo, C.P.; Tong, J.; Palmstrom, A.F.; Larson, B.W.; Choi, C.; Harvey, S.P.; Glynn, S.; Whitaker, J.B.; Zhang, F.; et al. Bimolecular Additives Improve Wide-BandGap Perovskites for Efficient Tandem Solar Cells with CIGS. Joule 2019, 3, 1734–1745. [Google Scholar] [CrossRef]
- Raiford, J.A.; Boyd, C.C.; Palmstrom, A.F.; Wolf, E.J.; Fearon, B.A.; Berry, J.J.; McGehee, M.D.; Bent, S.F. Enhanced Nucleation of Atomic Layer Deposited Contacts Improves Operational Stability of Perovskite Solar Cells in Air. Adv. Energy Mater. 2019, 9. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, P.; Ren, G.; Chen, F.; Nan, H.; Liu, R.; Wang, D.; Tan, X.; Liu, X.; Zhang, H.; et al. Low-Temperature Atomic Layer Deposition of Metal Oxide Layers for Perovskite Solar Cells with High Efficiency and Stability under Harsh Environmental Conditions. ACS Appl. Mater. Interfaces 2018, 10, 23928–23937. [Google Scholar] [CrossRef]
- Raiford, J.A.; Belisle, R.A.; Bush, K.A.; Prasanna, R.; Palmstrom, A.F.; McGehee, M.D.; Bent, S.F. Atomic layer deposition of vanadium oxide to reduce parasitic absorption and improve stability in n–i–p perovskite solar cells for tandems. Sustain. Energy Fuels 2019, 3, 1517–1525. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Lee, K.-T.; Huang, W.-K.; Siao, H.-Y.; Chang, Y.-C. High-Performance, Air-Stable, Low-Temperature Processed Semitransparent Perovskite Solar Cells Enabled by Atomic Layer Deposition. Chem. Mater. 2015, 27, 5122–5130. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, X.; Xing, X.; Nian, L.; Liu, X.; Huang, R.; Wang, K.; Yip, H.-L.; Zhou, G. Wide-Bandgap Perovskite Solar Cells With Large Open-Circuit Voltage of 1653 mV Through Interfacial Engineering. Sol. RRL 2018, 2. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, W.; Jung, D.R.; Kim, J.; Nam, S.; Kim, H.; Park, B. Optical and electronic properties of post-annealed ZnO:Al thin films. Appl. Phys. Lett. 2010, 96, 171902. [Google Scholar] [CrossRef] [Green Version]
- Park, H.H.; Kim, J.; Kim, G.; Jung, H.; Kim, S.; Moon, C.S.; Lee, S.J.; Shin, S.S.; Hao, X.; Yun, J.S.; et al. Transparent Electrodes Consisting of a Surface-Treated Buffer Layer Based on Tungsten Oxide for Semitransparent Perovskite Solar Cells and Four-Terminal Tandem Applications. Small Methods 2020, 4. [Google Scholar] [CrossRef]
- Hoye, R.L.; Chua, M.R.; Musselman, K.P.; Li, G.; Lai, M.L.; Tan, Z.K.; Greenham, N.C.; MacManus-Driscoll, J.L.; Friend, R.H.; Credgington, D.C. Enhanced Performance in Fluorene-Free Organometal Halide Perovskite Light-Emitting Diodes using Tunable, Low Electron Affi nity Oxide Electron Injectors. Adv. Mater. 2015, 27, 1414–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Xu, Y.-X.; Wang, D.; Chen, F.; Chen, Z.-K. Inorganic perovskite light emitting diodes with ZnO as the electron transport layer by direct atomic layer deposition. Org. Electron. 2018, 57, 60–67. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Chen, K.-T.; Huang, P.-H.; Wu, W.-Y.; Zhang, X.-Y.; Wang, C.; Liang, L.-S.; Gao, P.; Qiu, Y.; Lien, S.-Y.; et al. Effect of Annealing Temperature on Spatial Atomic Layer Deposited Titanium Oxide and Its Application in Perovskite Solar Cells. Nanomaterials 2020, 10, 1322. [Google Scholar] [CrossRef]
- Park, H.H.; Heasley, R.; Sun, L.; Steinmann, V.; Jaramillo, R.; Hartman, K.; Chakraborty, R.; Sinsermsuksakul, P.; Chua, D.; Buonassisi, A.; et al. Co-optimization of SnS absorber and Zn(O,S) buffer materials for improved solar cells. Prog. Photovoltaics Res. Appl. 2015, 23, 901–908. [Google Scholar] [CrossRef] [Green Version]


| Material | Precursors | Temp. (°C) | Application/Structure | Device Stack | JSC (mA/cm2) | VOC (V) | FF (%) | η (%) | Institute, Year [Ref] |
|---|---|---|---|---|---|---|---|---|---|
| SnO2 | TDMASn + O3 | 118 | ETL/ n-i-p | FTO/SnO2 (15 nm)/FA0.85MA0.15Pb(I0.85Br0.15)3/spiro-OMeTAD/Au | 21.3 | 1.14 | 74.0 | 18.4 | EPFL, 2015 [31] |
| SnO2 | TDMASn + O3 | 100–120 | ETL/ n-i-p | FTO/d-TiO2/SnO2/FA0.85MA0.15Pb(I0.85Br0.15)3/PTAA/Au | 22.7 | 1.13 | 78.0 | 20.0 | EPFL, 2018 [32] |
| SnO2 | TDMASn + O3 | 118 | ETL/ n-i-p | FTO/SnO2 (15 nm)/Rb1(FA0.83MA0.17)99Pb(I0.83Br0.17)3/spiro-OMeTAD/Au | 23.0 | 1.17 | 71.0 | 20.0 | EPFL, 2017 [24] |
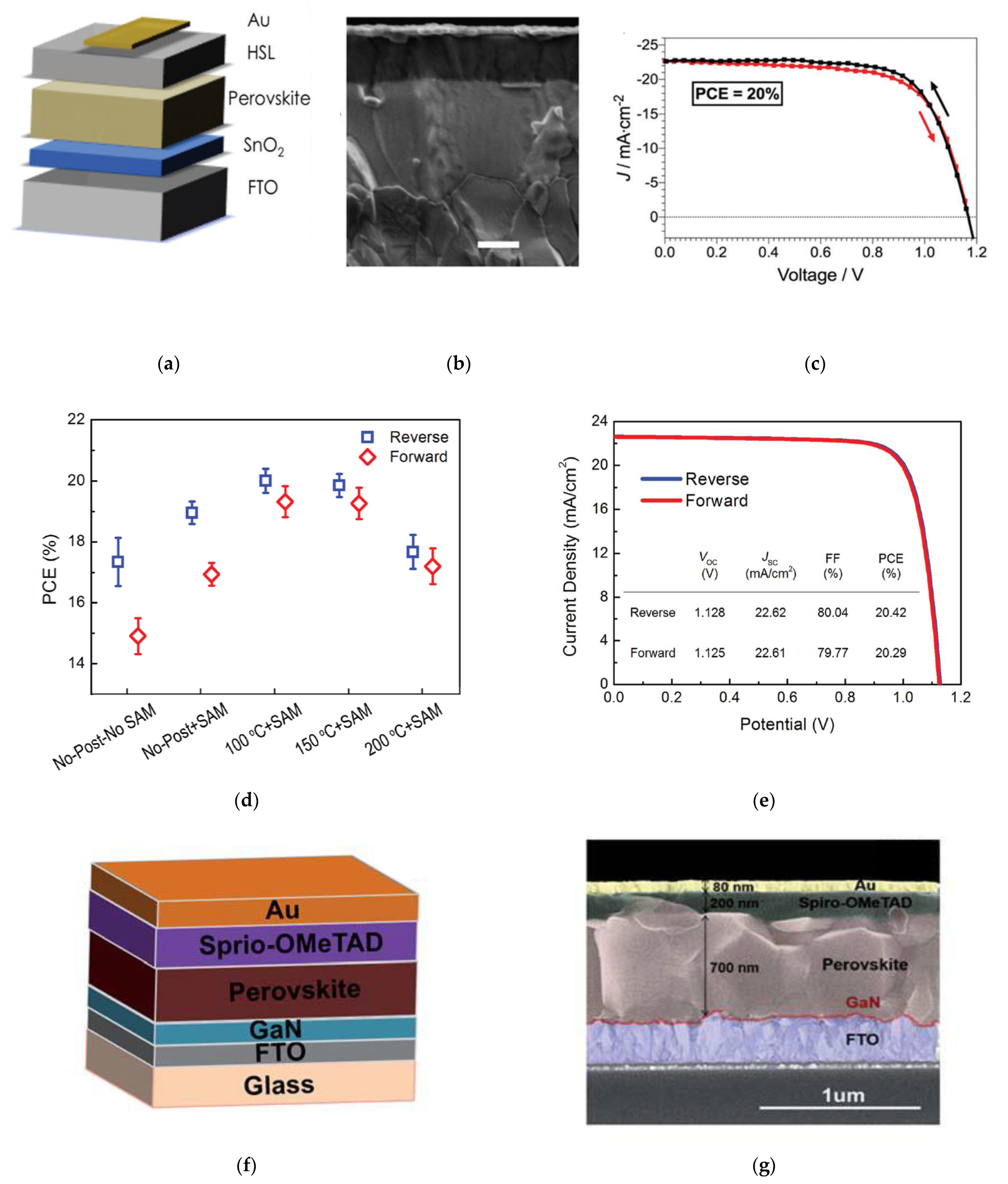
| SnO2 | TDMASn + O2 Plasma | 100 | ETL/ n-i-p | FTO/SnO2/100 °C/C60-SAM/FA0.30MA0.70PbI3/spiro-OMeTAD/Au | 22.6 | 1.13 | 80.0 | 20.4 | Toledo, 2017 [25] |
| SnO2 | TDMASn + O2 Plasma | 100 | ETL/ n-i-p | PET/ITO/SnO2/C60-SAM/FA0.30MA0.70PbI3/spiro-OMeTAD/Au | 22.1 | 1.10 | 75.4 | 18.4 | Toledo, 2017 [33] |
| SnO2 | TDMASn + O2 Plasma | 100 | ETL/ n-i-p | FTO/SnO2/C60-SAM/FA0.30MA0.70PbI3/spiro-OMeTAD/Au | 21.6 | 1.13 | 78.1 | 19.0 | Toledo, 2016 [34] |
| TiO2 | TiCl4 + H2O | 150 | ETL/ n-i-p | FTO/TiO2 (17 nm)/mp-TiO2/MAPbI3/Graphene Oxide/spiro-OMeTAD/Au | 20.2 | 1.04 | 73.0 | 15.1 | Tsinghua, 2014 [35] |
| TiO2 | TDMAT + H2O | 120 | ETL/ n-i-p | FTO/TiO2 (4 nm)/mp-TiO2/MAPbI3/spiro-OMeTAD/Au | 23.1 | 1.08 | 73.4 | 18.3 | Nanjing, 2018 [26] |
| TiO2 | TDMAT + H2O | 150 | ETL/ n-i-p | ITO/TiO2 (10 nm)/np-SnO2/PC61BM/FA0.30MA0.70Pb(I1-xClx)3/spiro-OMeTAD/Au | 23.0 | 1.08 | 78.2 | 19.5 | Xidian, 2019 [36] |
| TiO2 | TDMAT + H2O | 225 | ETL/ n-i-p | FTO/TiO2 (11 nm)/mp-TiO2/MAPbI3 | 22.3 | 1.11 | 74.0 | 18.4 | Tokyo, 2019 [37] |
| TiO2 | TDMAT + H2O | ETL/ n-i-p | FTO/mp-Sb:SnO2/TiO2 (10 nm)/MAPbI3/PTAA/Au | 23.8 | 1.10 | 77.0 | 20.1 | Soochow, 2018 [28] | |
| TiO2 | TDMAT + H2O | 120 | ETL, Passivation/ n-i-p | FTO/np-TiO2/TiO2 (2 nm)/MAPbI3/spiro-OMeTAD/Au | 17.6 | 0.97 | 67.0 | 11.5 | EPFL, 2014 [38] |
| TiO2 | TTIP + O2 Plasma | 130 | ETL/ n-i-p | ITO/CF4 plasma TiO2 (20 nm)/MAPbI3/spiro-OMeTAD/Au | 20.3 | 1.03 | 75.5 | 15.8 | Eindhoven, 2018 [39] |
| TiO2 | Ti(CpMe)(NMe2)3 + H2O | 150 | ETL Passivation/ n-i-p | ITO/ZnO (80 nm)/TiO2 (<3 nm)/Cs0.15FA0.75MA0.10PbI2.9Br0.1/spiro-OMeTAD/MoO3/Au | 22.5 | 1.03 | 74.0 | 17.1 | Soochow, 2018 [40] |
| TiO2 | TTIP + H2O | 250 | Passivation/n-i-p | FTO/c-TiO2/NR-TiO2/TiO2 (4 nm)/MAPbI3/spiro-OMeTAD/Au | 19.8 | 0.95 | 72.0 | 13.5 | CNU, 2015 [41] |
| TiN | TiCl4 + NH3 | 350 | ETL Passivation/ n-i-p | FTO/c-TiO2/mp-TiO2/TiN (<2 nm)/FA0.83MA0.17Pb(I0.83Br0.17)3/PTAA/Au | 22.5 | 1.14 | 75.0 | 19.0 | CNU, 2020 [42] |
| TiO2-IrOx | TDMAT + H2O (EtCp)Ir(CHD) + O3 | 175 | HTL/ p-i-n | ITO/TiO2-IrOx(10 nm)/Cs0.17FA0.83Pb(I0.83Br0.17)3/C60/BCP/Ag | 19.6 | 1.01 | 80.0 | 15.8 | Stanford, 2018 [43] |
| GaN | TEG + Ar/N2/H2 plasma | 280 | ETL/ n-i-p | FTO/GaN (5 nm)/FA0.85MA0.15Pb(I0.85Br0.15)3/spiro-OMeTAD/Au | 22.6 | 0.98 | 68.9 | 15.2 | UST Beijing, 2019 [30] |
| HfO2 | TEMAHf + H2O | 90 | Passivation/ n-i-p | PEN/ITO/HfO2 (<1 nm)/SnO2/Cs0.05(FA0.83MA0.17)0.95Pb(I0.83Br0.17)3 + RbI + KI/spiro-OMeTAD/Au | 21.2 | 1.14 | 79.2 | 19.1 | Xiamen, 2019 [44] |
| Nb2O5 | (tert-butylimido)bis(diethylamino)niobium + O3 | 170 | ETL/ n-i-p | FTO/Nb2O5 (15 nm)/FA0.85MA0.15Pb(I0.85Br0.15)3/spiro-OMeTAD/Au | Very low | EPFL, 2015 [31] | |||
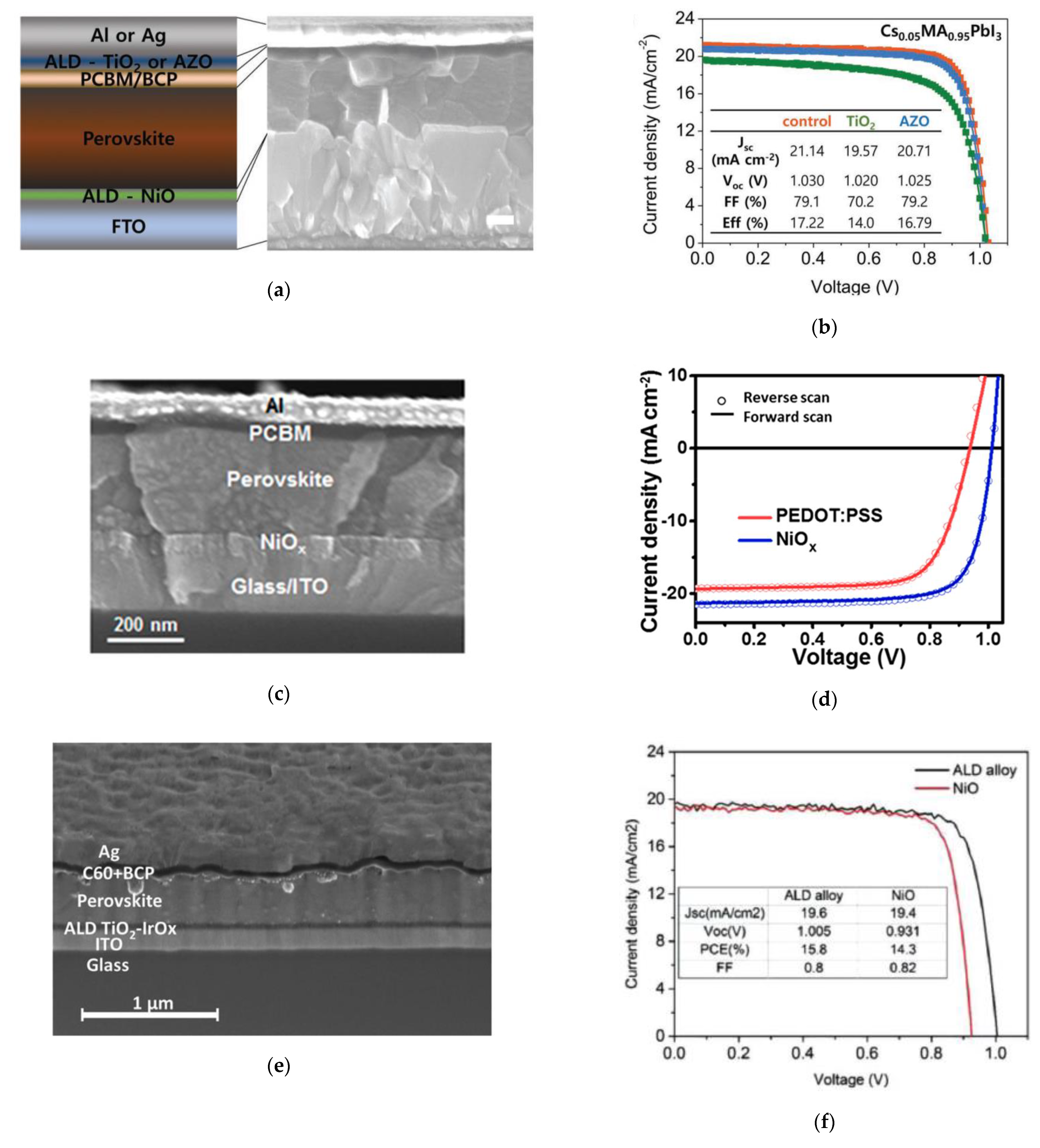
| NiO | Ni(MeCp)2 + O2 | 350 | HTL/ p-i-n | ITO/s-ALD NiOx/FA0.2MA0.8PbI3/PC61BM/Al | 23.0 | 1.08 | 81.0 | 17.1 | Cambridge, 2018 [45] |
| NiO | Ni(MeCp)2 + O2 plasma | 150 | HTL/ p-i-n | ITO/NiO (10 nm)/Cs0.05(FA0.83MA0.17)Pb(I0.83Br0.17)3/C60/BCP/Cu | 21.8 | 1.07 | 73.4 | 17.1 | Eindhoven, 2019 [46] |
| NiO, AZO, Al2O3 | Ni(dmamb)2 + O3, TMA/DEZ + H2O | 200, 100, 100 | ETL/ p-i-n | FTO/NiO (6 nm)/Cs0.05MA0.95PbI3/PCBM/BCP/AZO (40 nm)/Ag/Al2O3 (50 nm) | 22.5 | 1.03 | 80.8 | 18.8 | SKKU, 2018 [47] |
| VOx | V(dma)4 + H2O | 50 | HTL/ p-i-n | ITO/VOx (1 nm)/MAPbI3/PC61BM/BCP/Ag | 17.9 | 0.90 | 71.2 | 11.5 | Peking, 2018 [48] |
| ZnO/Al2O3 | DEZ + H2O | 150 | ETL/ n-i-p | FTO/ZnO (50 nm)/Al2O3 (<1 nm)/mp-TiO2/MAPbI3/spiro-OMeTAD/Au | 18.9 | 1.01 | 62.0 | 15.6 | UST Beijing, 2016 [49] |
| ZnS | DEZ + H2S | 150 | Passivation/ n-i-p | FTO/c-TiO2/mp-TiO2/ZnS (<2 nm)/FA0.85MA0.15Pb(I0.85Br0.15)3/PTAA/Au | 22.5 | 1.13 | 75.0 | 18.8 | CNU, 2020 [29] |
| Material | Precursors | Temp. (°C) | Application/Structure | Device Stack | JSC (mA/cm2) | VOC (V) | FF (%) | η (%) | Institute, Year [Ref] |
|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | TMA + H2O | 100 | Passivation/ n-i-p | ITO/c-TiO2/MAPb(I1-xClx)3/Al2O3 (1 nm)/spiro-OMeTAD/Au | 21.7 | 1.07 | 77.0 | 18.0 | Eindhoven, 2017 [62] |
| NiO, AZO, Al2O3 | Ni(dmamb)2 + O3, TMA/DEZ + H2O | 200, 100, 100 | ETL/ p-i-n | FTO/NiO (6 nm)/Cs0.05MA0.95PbI3/PCBM/BCP/AZO (40 nm)/Ag/Al2O3 (50 nm) | 22.5 | 1.03 | 80.8 | 18.8 | SKKU, 2018 [47] |
| AZO | TMA/DEZ + H2O | 85 | Recombination/ p-i-n | ITO/PolyTPD/PFN/Cs0.30FA0.60MA0.10Pb(I0.80Br0.20)3/LiF/C60/PEIE/AZO (25 nm)/IZO/PEDOT:PSS/Cs0.25FA0.75Sn0.5Pb0.5I3/C60/BCP/Au | 15.6 | 1.82 | 75.0 | 21.3 | NREL, 2019 [63] |
| CuOx | Cu(dmamb)2 + H2O | 100 | Buffer/ n-i-p (ST) | FTO/c-TiO2/mp-TiO2/FA0.95MA0.05Pb(I0.95Br0.05)3/PTAA/pulsed-CVD CuOx (15 nm)/ITO | 21.7 | 1.01 | 71.1 | 15.6 | KRICT, 2020 [60] |
| CuOx | ATHFAACu + H2O | 100 | Buffer/ n-i-p (ST) | FTO/c-TiO2/mp-TiO2/Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3/PTAA/AP-CVD CuOx (3 nm)/ITO/MgF2 | 20.6 | 1.10 | 73.7 | 16.7 | Cambridge, 2020 [64] |
| Ga2O3 | Ga2(NMe2)6 + H2O | 120 | Passivation/ p-i-n | FTO/Li:NiO/MAPbI3/IDIC/PCBM/BCP/Ga2O3 (<2 nm)/Ag | 22.4 | 1.12 | 79.4 | 19.9 | Wuhan, 2018 [65] |
| SnO2 | TDMASn + H2O | 100 | Buffer/ p-i-n (2-T) | Si PV/ITO/PTAA/Cs0.15(FA0.83MA0.17)0.85Pb(I0.7Br0.3)3/ICBA/C60/SnO2/IZO/MgF2 | 17.8 | 1.80 | 79.4 | 25.4 | UNC, 2019 [66] |
| SnO2 | TDMASn + H2O | 100 | Buffer/ p-i-n (2-T) | Si PV/spiro-TTB/CsxFA1-xPb(I1-yBry)3/LiF/C60/SnO2/IZO/MgF2 | 19.5 | 1.74 | 74.7 | 25.4 | EPFL, 2019 [67] |
| SnO2 | TDMASn + H2O | Buffer/ p-i-n (2-T) | Si PV/ITO/PTAA/Cs0.05(FA0.83MA0.17)0.95Pb(I0.83Br0.17)3/C60/SnO2 (20 nm)/IZO/AR foil | 18.5 | 1.76 | 78.5 | 25.5 | HZB, 2018 [68] | |
| SnOx/Zn:SnOx | TDMASn/DEZ + H2O | 85 | Buffer/ p-i-n (ST) | ITO/PTAA/Cs0.05FA0.80MA0.15Pb (I0.85Br0.15)3/C60/BCP/SnOx (6 nm)/Zn:SnOx (2 nm)/IZO | 20.8 | 1.12 | 79.3 | 18.5 | NREL, 2019 [59] |
| SnO2/Zn:SnOx | TDMASn/DEZ + H2O | 85 | Buffer/ p-i-n (ST) | ITO/PTAA/Cs0.15FA0.65MA0.20Pb (I0.80Br0.20)3 + PEAI + Pb(SCN)2/C60/SnOx (6 nm)/Zn:SnOx (2 nm)/IZO | 19.6 | 1.14 | 76.8 | 17.1 | NREL, 2019 [69] |
| SnO2/Zn:SnOx | TDMASn/DEZ + H2O | 90 | ETL/ p-i-n | ITO/Poly-TPD/PFN/Cs0.25FA0.75Pb(I0.80Br0.20)3/LiF/C60/PEIE/SnO2/Zn:SnOx/Au | 19.7 | 1.15 | 81.8 | 18.6 | Stanford, 2019 [70] |
| TiO2 | TDMAT + H2O | 60 | ETL/ p-i-n | ITO/NiO/MAPbI3/PC61BM (40 nm)/TiO2 (2 nm)/Ag | 22.8 | 1.04 | 76.9 | 18.3 | Nanjing, 2018 [71] |
| VOx | VTIP + H2O | 80 | Buffer/ n-i-p (ST) | ITO/np-SnO2/C60/FA0.83MA0.17Pb(I0.83Br0.17)3/spiro-TTB/VOx (9 nm)/ITO | 18.9 | 1.07 | 71.0 | 14.2 | Stanford, 2019 [72] |
| ZnO | DEZ + H2O | 80 | ETL/ p-i-n | ITO/PEDOT:PSS/MAPbI3/ZnO (40 nm)/Ag NWs/ALD Al2O3 (50 nm)-coated PET | 20.7 | 1.02 | 76.4 | 16.2 | Feng Chia, 2015 [73] |
| ZrO2 | TDMAZr + O3 | 80 | Passivation/ p-i-n | FTO/NiOx/e-MoOx (10 nm)/MAPbI3/ZrO2 (<2 nm)/PC61BM/Al | 21.9 | 1.11 | 75.0 | 18.2 | SCN, 2018 [74] |
| Deposition Method | Advantages | Disadvantages |
|---|---|---|
| Conventional ALD |
|
|
| Pulsed-CVD |
|
|
| Spatial ALD |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.H. Inorganic Materials by Atomic Layer Deposition for Perovskite Solar Cells. Nanomaterials 2021, 11, 88. https://doi.org/10.3390/nano11010088
Park HH. Inorganic Materials by Atomic Layer Deposition for Perovskite Solar Cells. Nanomaterials. 2021; 11(1):88. https://doi.org/10.3390/nano11010088
Chicago/Turabian StylePark, Helen Hejin. 2021. "Inorganic Materials by Atomic Layer Deposition for Perovskite Solar Cells" Nanomaterials 11, no. 1: 88. https://doi.org/10.3390/nano11010088




