Carbon Dots: Classification, Properties, Synthesis, Characterization, and Applications in Health Care—An Updated Review (2018–2021)
Abstract
:1. Introduction
Classification of CDs
2. Properties of CDs
2.1. Electrochemical Properties of CDs
- (1)
- (2)
- (3)
- (4)
2.1.1. Electrical Conductivity
2.1.2. Heteroatom Doping- Electronic Structure Arrangement
2.1.3. Stability Enrichment
2.1.4. Defect Sites and Active Center
2.2. Optical Properties of CDs
2.2.1. Absorption Property
2.2.2. Fluorescence Properties
- (a)
- Up-conversion fluorescence: It is the phenomenon, where the excitation wavelength is larger than the emission wavelength. The up-conversion fluorescence property can be observed in the CDs that are synthesized through ultrasonic treatment. Larger excitation wavelength results in the reduction of background autofluorescence, which is significant for the bioimaging application [53,77].
- (b)
- Down-conversion fluorescence: The luminescent mechanism of CDs is yet to be deeply investigated. However, several origins responsible for the fluorescence of CDs usually include multi-emissive centers, free zigzag sites, self-trapped excitons, quantum confinement effects, special edge defects, their conjugated structures, and surface states [55,76,77,78]. Since CDs are 0D quantum confined nanomaterials, their fluorescence can be accredited to the presence of an electron-hole pair in their system [55]. As the size of CDs increases, their energy gap decreases. Therefore, the fluorescence property of CDs can be regulated by altering their quantum confinement effect [55,78,79]. The surface state phenomena due to the existence of surface functional groups and surface oxidation, is one of the other mechanisms for the origin of CDs’ fluorescence [76,80]. The surface oxidation incurred by oxygen-containing groups at the edge of CDs, is responsible for creating the surface defects that results in the fluorescence [76,78].
- (c)
- Emission properties: Different fluorescence emissions of CDs can be obtained by controlling their excitation wavelength, which can be achieved by regulating several physicochemical parameters during CDs’ synthesis. For instance, the fluorescence of CDs is highly influenced by pH, concentration, as well as temperature [77]. The pH-dependent emission is because of the functional group protonation and deprotonation on their surfaces [81]; the concentration-dependent fluorescence is due to the surface state emission; whereas the temperature-dependent emission is the result of non-radiative decay occurring at the surface of CDs [77].
- (d)
- Chemical stability and photobleaching properties: Fluorescence bioimaging or biosensing requires long emission lifetimes and stable fluorescence signal. This can be achieved with the help of CDs, since they have the tendency to produce stable signals when stored in an aqueous environment [82,83]. Furthermore, CDs can emit strong fluorescence for long time (i.e., up to a year). Generally, CDs are resistant to a broad pH range (i.e., from 3 to 12), therefore they demonstrate excellent impedance for photobleaching [81,83].
2.2.3. Phosphorescence
2.2.4. Chemiluminescence
2.2.5. Electrochemiluminescence
3. Strategies for CDs Synthesis
3.1. Top-Down Approach
3.1.1. Arc Discharge Method
3.1.2. Laser Ablation Method
3.1.3. Ultrasonic Method
3.1.4. Electrochemical/Chemical Oxidation Method
3.2. Bottom-Up Approach
3.2.1. Thermal Method
3.2.2. Microwave-Assisted Method
3.2.3. Hydrothermal Method
3.2.4. Template Method
4. Characterization Techniques for CDs
4.1. Characterization of CDs by Microscopy
4.1.1. Atomic Force Microscopy
4.1.2. Transmission Electron Microscopy
4.1.3. Scanning Electron Microscopy
4.2. Characterization of CDs by Mass Spectrometry
4.2.1. Electrospray Ionization Quadrupole Time-Of-Flight Tandem Mass Spectrometry
4.2.2. Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight Mass Spectrometry
4.3. Characterization of CDs by Spectroscopy
4.3.1. Photoluminescence and Ultraviolet-Visible Spectroscopy
4.3.2. Infrared Spectroscopy
4.3.3. Raman Spectroscopy
4.3.4. Energy Dispersive X-ray Spectroscopy
4.3.5. Nuclear Magnetic Resonance Spectroscopy
4.3.6. Dynamic Light Scattering, and Zeta Potential Measurements
4.3.7. X-ray Photoelectron Spectroscopy
4.4. Characterization of CDs by Diffraction Technique
5. Applications of CDs in Electrochemical Sensors
6. Applications of CDs in Optical Sensors
7. Applications of CDs in Bioimaging
8. Applications of CDs in Drug Delivery, and Gene Delivery
8.1. Role of CDs in Drug Delivery
8.2. Role of CDs in Gene Delivery or Gene Therapy
9. Applications of CDs in Photodynamic / Photothermal Therapy
9.1. Role of CDs in Photodynamic Therapy
9.2. Role of CDs in Photothermal Therapy
10. Summary, Main Challenges, and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Molinero-Fernández, Á.; Moreno-Guzmán, M.; López, M.Á.; Escarpa, A. Magnetic bead-based electrochemical immunoassays on-drop and on-chip for procalcitonin determination: Disposable tools for clinical sepsis diagnosis. Biosensors 2020, 10, 66. [Google Scholar] [CrossRef]
- Salvo-Comino, C.; Martin-Pedrosa, F.; Garcia-Cabezon, C.; Rodriguez-Mendez, M.L. Silver nanowires as electron transfer mediators in electrochemical catechol biosensors. Sensors 2021, 21, 899. [Google Scholar] [CrossRef]
- Herrera-Chacón, A.; Cetó, X.; del Valle, M. Molecularly imprinted polymers - towards electrochemical sensors and electronic tongues. Anal. Bioanal. Chem. 2021, 413, 6117–6140. [Google Scholar] [CrossRef]
- John, B. Polymer nanocomposite-based electrochemical sensors and biosensors. In Nanorods and Nanocomposites; Books on Demand: Norderstedt, Germany, 2020. [Google Scholar] [CrossRef] [Green Version]
- Elancheziyan, M.; Senthilkumar, S. Redox-active gold nanoparticle-encapsulated poly(amidoamine) dendrimer for electrochemical sensing of 4-aminophenol. J. Mol. Liq. 2021, 325, 115131. [Google Scholar] [CrossRef]
- Yaqoob, S.B.; Adnan, R.; Rameez Khan, R.M.; Rashid, M. Gold, silver, and palladium nanoparticles: A chemical tool for biomedical applications. Front. Chem. 2020, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Shekhar, S.; Gautam, S.; Sharma, B.; Kumar, A.; Jain, P. Carbon-based Nanomaterials as Novel Nanosensors; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128207024. [Google Scholar]
- Xiao, Y.; Du, J. Superparamagnetic nanoparticles for biomedical applications. J. Mater. Chem. B 2020, 8, 354–367. [Google Scholar] [CrossRef]
- Wu, L.; Ji, Y.; Ouyang, B.; Li, Z.; Yang, Y. Low-temperature induced enhancement of photoelectric performance in semiconducting nanomaterials. Nanomaterials 2021, 11, 1131. [Google Scholar] [CrossRef] [PubMed]
- Kortel, M.; Mansuriya, B.D.; Vargas Santana, N.; Altintas, Z. Graphene quantum dots as flourishing nanomaterials for bio-imaging, therapy development, and micro-supercapacitors. Micromachines 2020, 11, 866. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Park, S.J.; Hedau, B.S.; Ha, T.J. Recent progress in solar cells based on carbon nanomaterials. Sol. Energy 2021, 220, 953–990. [Google Scholar] [CrossRef]
- Otsuka, K.; Fang, N.; Yamashita, D.; Taniguchi, T.; Watanabe, K.; Kato, Y.K. Deterministic transfer of optical-quality carbon nanotubes for atomically defined technology. Nat. Commun. 2021, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, Z.; Ren, Y.; Chen, X.; Zhang, W.; Zhu, X.; Mao, Z.; Shen, J.; Nie, S. Advances in nanomaterials for use in photothermal and photodynamic therapeutics (Review). Mol. Med. Rep. 2019, 20, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.; Mukhopadhyay, S.; Hod, I. Metal–organic framework derived nanomaterials for electrocatalysis: Recent developments for CO2 and N2 reduction. Nano Converg. 2021, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, G.; Luciani, G. Photocatalysis: Activity of nanomaterials. Catalysts 2021, 11, 611. [Google Scholar] [CrossRef]
- Zhao, J.; Lin, M.; Wang, Z.; Cao, X.; Xing, B. Engineered nanomaterials in the environment: Are they safe? Crit. Rev. Environ. Sci. Technol. 2021, 51, 1443–1478. [Google Scholar] [CrossRef]
- Xavier, M.; Parente, I.A.; Rodrigues, P.M.; Cerqueira, M.A.; Pastrana, L.; Gonçalves, C. Safety and fate of nanomaterials in food: The role of in vitro tests. Trends Food Sci. Technol. 2021, 109, 593–607. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Bose, R.J.; Ha, K.; McCarthy, J.R. Bio-inspired nanomaterials as novel options for the treatment of cardiovascular disease. Drug Discov. Today 2021, 26, 1200–1211. [Google Scholar] [CrossRef]
- Abbasi Kajani, A.; Haghjooy Javanmard, S.; Asadnia, M.; Razmjou, A. Recent advances in nanomaterials development for nanomedicine and cancer. ACS Appl. Bio Mater. 2021, 4, 5908–5925. [Google Scholar] [CrossRef]
- Ghaemi, F.; Amiri, A.; Bajuri, M.Y.; Yuhana, N.Y.; Ferrara, M. Role of different types of nanomaterials against diagnosis, prevention and therapy of COVID-19. Sustain. Cities Soc. 2021, 72, 103046. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Hou, Y. Self-assembled magnetic nanomaterials: Versatile theranostics nanoplatforms for cancer. Aggregate 2021, 2, 1–12. [Google Scholar] [CrossRef]
- Cui, M.R.; Gao, F.; Shu, Z.Y.; Ren, S.K.; Zhu, D.; Chao, J. Nucleic acids-based functional nanomaterials for bioimaging. J. Anal. Test. 2021, 5, 142–154. [Google Scholar] [CrossRef]
- Mansuriya, B.D.; Altintas, Z. Applications of graphene quantum dots in biomedical sensors. Sensors 2020, 20, 1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porto, L.S.; Silva, D.N.; de Oliveira, A.E.F.; Pereira, A.C.; Borges, K.B. Carbon nanomaterials: Synthesis and applications to development of electrochemical sensors in determination of drugs and compounds of clinical interest. Rev. Anal. Chem. 2020, 38, 1–16. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, Z. Carbon nanomaterial-based electrochemical biosensors: An overview. Nanoscale 2015, 7, 6420–6431. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, K.N.; Mathan, S.; Yan, W.; Rafieerad, A.; Sekaran, S.; Manego, H.; Dhingra, S. Carbon nanomaterials for cardiovascular theranostics: Promises and challenges. Bioact. Mater. 2021, 6, 2261–2280. [Google Scholar] [CrossRef]
- Hwang, H.S.; Jeong, J.W.; Kim, Y.A.; Chang, M. Carbon nanomaterials as versatile platforms for biosensing applications. Micromachines 2020, 11, 814. [Google Scholar] [CrossRef]
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Review—Recent advances in carbon nanomaterials as electrochemical biosensors. J. Electrochem. Soc. 2020, 167, 037555. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, G.; Zhao, L.; Lai, K.W.C. Carbon nanomaterial-based biosensors: A review of design and applications. IEEE Nanotechnol. Mag. 2019, 13, 4–14. [Google Scholar] [CrossRef]
- Shetti, N.P.; Mishra, A.; Basu, S.; Aminabhavi, T.M. Versatile fullerenes as sensor materials. Mater. Today Chem. 2021, 20, 100454. [Google Scholar] [CrossRef]
- Nurrohman, D.T.; Chiu, N.F. A review of graphene-based surface plasmon resonance and surface-enhanced raman scattering biosensors: Current status and future prospects. Nanomaterials 2021, 11, 216. [Google Scholar] [CrossRef]
- Silva, T.A.; Moraes, F.C.; Janegitz, B.C.; Fatibello-Filho, O.; Ganta, D. Electrochemical biosensors based on nanostructured carbon black: A review. J. Nanomater. 2017, 2017, 4571614. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Tian, J.; Cui, C.; Wang, Y.; Yang, H.; Yuan, M.; Yu, H. A label-free aptasensor for turn-on fluorescent detection of ochratoxin a based on SYBR gold and single walled carbon nanohorns. Food Control 2021, 123, 107741. [Google Scholar] [CrossRef]
- Nie, L.; Zhang, Y.; Li, L.; van Rijn, P.; Schirhagl, R. pH sensitive dextran coated fluorescent nanodiamonds as a biomarker for hela cells endocytic pathway and increased cellular uptake. Nanomaterials 2021, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Temoçin, Z. Designing of a stable and selective glucose biosensor by glucose oxidase immobilization on glassy carbon electrode sensitive to H2O2 via nanofiber interface. J. Appl. Electrochem. 2021, 51, 283–293. [Google Scholar] [CrossRef]
- Molazemhosseini, A.; Viola, F.A.; Berger, F.J.; Zorn, N.F.; Zaumseil, J.; Caironi, M. A rapidly stabilizing water-gated field-effect transistor based on printed single-walled carbon nanotubes for biosensing applications. ACS Appl. Electron. Mater. 2021. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; Dou, Y.; Wang, L.; Song, S. A Carbon-based antifouling nano-biosensing interface for label-free POCT of HbA1c. Biosensors 2021, 11, 118. [Google Scholar] [CrossRef]
- Hu, J.; Sun, Y.; Aryee, A.A.; Qu, L.; Zhang, K.; Li, Z. Mechanisms for carbon dots-based chemosensing, biosensing, and bioimaging: A review. Anal. Chim. Acta 2021, 338885. [Google Scholar] [CrossRef]
- Pirzada, M.; Altintas, Z. Nanomaterials for healthcare biosensing applications. Sensors 2019, 19, 5311. [Google Scholar] [CrossRef] [Green Version]
- Esim, O.; Kurbanoglu, S.; Savaser, A.; Ozkan, S.A.; Ozkan, Y. Nanomaterials for drug delivery systems. New Dev. Nanosensors Pharm. Anal. 2019, 273–301. [Google Scholar] [CrossRef]
- Boakye-Yiadom, K.O.; Kesse, S.; Opoku-Damoah, Y.; Filli, M.S.; Aquib, M.; Joelle, M.M.B.; Farooq, M.A.; Mavlyanova, R.; Raza, F.; Bavi, R.; et al. Carbon dots: Applications in bioimaging and theranostics. Int. J. Pharm. 2019, 564, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; Wu, R.S.; Wei, S.C.; Ross, G.M.; Chang, H.T. The analytical and biomedical applications of carbon dots and their future theranostic potential: A review. J. Food Drug Anal. 2020, 28, 677–695. [Google Scholar] [CrossRef]
- Sagbas, S.; Sahiner, N. Carbon Dots: Preparation, Properties, and Application; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081025093. [Google Scholar]
- Bakirhan, N.K.; Ozkan, S.A. Quantum Dots as a New Generation Nanomaterials and Their Electrochemical Applications in Pharmaceutical Industry; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128133514. [Google Scholar]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and synthesis of carbon dots: From carbon dots to carbonized polymer dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef] [PubMed]
- Mansuriya, B.D.; Altintas, Z. Enzyme-free electrochemical nano-immunosensor based on graphene quantum dots and gold nanoparticles for cardiac biomarker determination. Nanomaterials 2021, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Arora, R.; Dhiman, H.; Pahwa, R. Carbon quantum dots: Synthesis, characterization and biomedical applications. Turkish J. Pharm. Sci. 2018, 15, 219–230. [Google Scholar] [CrossRef]
- Dinç, S.; Günhan, R.S. Carbon dots applications in electrochemical and electrochemiluminescence sensors: Some examples of pathogen sensors. Turkish J. Anal. Chem. 2020, 2, 37–46. [Google Scholar]
- Mansuriya, B.D.; Altintas, Z. Graphene quantum dot-based electrochemical immunosensors for biomedical applications. Materials 2019, 13, 96. [Google Scholar] [CrossRef] [Green Version]
- Sciortino, A.; Cannizzo, A.; Messina, F. Carbon nanodots: A review—From the current understanding of the fundamental photophysics to the full control of the optical response. C 2018, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef] [Green Version]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, properties and biomedical applications of carbon-based quantum dots: An updated review. Biomed. Pharmacother. 2017, 87, 209–222. [Google Scholar] [CrossRef]
- Tuerhong, M.; XU, Y.; YIN, X.B. Review on carbon dots and their applications. Chinese J. Anal. Chem. 2017, 45, 139–150. [Google Scholar] [CrossRef]
- Speranza, G. Carbon nanomaterials: Synthesis, functionalization and sensing applications. Nanomaterials 2021, 11. [Google Scholar] [CrossRef]
- Altintas, Z. Biosensors and Nanotechnology-Applications in Health Care Diagnostics, 1st ed.; Altintas, Z., Ed.; Wiley: New Jersey, NJ, USA, 2018; ISBN 978-1-119-06501-2. [Google Scholar]
- Nocito, G.; Calabrese, G.; Forte, S.; Petralia, S.; Puglisi, C.; Campolo, M.; Esposito, E.; Conoci, S. Carbon dots as promising tools for cancer diagnosis and therapy. Cancers 2021, 13, 1991. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Wang, X.; Bao, Y.; Sun, Z. Recent advance of carbon dots in bio-related applications. JPhys Mater. 2020, 3. [Google Scholar] [CrossRef]
- Koutsogiannis, P.; Thomou, E.; Stamatis, H.; Gournis, D.; Rudolf, P. Advances in fluorescent carbon dots for biomedical applications. Adv. Phys. X 2020, 5. [Google Scholar] [CrossRef]
- Jhonsi, M.A. Carbon quantum dots for bioimaging. In State Art Nano-bioimaging; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yu, S.H. Carbon dots: Large-scale synthesis, sensing and bioimaging. Mater. Today 2016, 19, 382–393. [Google Scholar] [CrossRef]
- Kasouni, A.; Chatzimitakos, T.; Stalikas, C. Bioimaging applications of carbon nanodots: A review. C 2019, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A mini review on carbon quantum dots: Preparation, properties, and electrocatalytic application. Front. Chem. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Hassanvand, Z.; Jalali, F.; Nazari, M.; Parnianchi, F.; Santoro, C. Carbon nanodots in electrochemical sensors and biosensors: A review. ChemElectroChem 2021, 8, 15–35. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Tao, S.; Feng, T.; Zheng, C.; Zhu, S.; Yang, B. Carbonized polymer dots: A brand new perspective to recognize luminescent carbon-based nanomaterials. J. Phys. Chem. Lett. 2019, 10, 5182–5188. [Google Scholar] [CrossRef]
- Sun, J.; Yu, J.; Jiang, Z.; Zhao, Z.; Xia, Y. Fluorescent carbonized polymer dots prepared from sodium alginate based on the CEE effect. ACS Omega 2020, 5, 27514–27521. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Li, Z.; Wang, P.; Zhai, X.; Wang, X.; Li, T. Carbon quantum dots for advanced electrocatalysis. J. Energy Chem. 2021, 55, 279–294. [Google Scholar] [CrossRef]
- Xu, Q.; Kuang, T.; Liu, Y.; Cai, L.; Peng, X.; Sreenivasan Sreeprasad, T.; Zhao, P.; Yu, Z.; Li, N. Heteroatom-doped carbon dots: Synthesis, characterization, properties, photoluminescence mechanism and biological applications. J. Mater. Chem. B 2016, 4, 7204–7219. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Liang, K.; Zhu, J.; Yang, B.; Zhao, D.; Kong, B. Hetero-atom-doped carbon dots: Doping strategies, properties and applications. Nano Today 2020, 33, 100879. [Google Scholar] [CrossRef]
- Jana, J.; Ngo, Y.L.T.; Chung, J.S.; Hur, S.H. Contribution of carbon dot nanoparticles in electrocatalysis: Development in energy conversion process. J. Electrochem. Sci. Technol. 2020, 11, 220–237. [Google Scholar] [CrossRef]
- Bhartiya, P.; Singh, A.; Kumar, H.; Jain, T.; Singh, B.K.; Dutta, P.K. Carbon dots: Chemistry, properties and applications. J. Indian Chem. Soc. 2016, 93, 759–766. [Google Scholar]
- Kou, X.; Jiang, S.; Park, S.J.; Meng, L.Y. A review: Recent advances in preparations and applications of heteroatom-doped carbon quantum dots. Dalton Trans. 2020, 49. [Google Scholar] [CrossRef]
- Gayen, B.; Palchoudhury, S.; Chowdhury, J. Carbon dots: A mystic star in the world of nanoscience. J. Nanomater. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Li, X.-H.; Chen, X.-B.; Wei, J.-S.; Li, X.-B.; Xiong, H.-M. Surface states of carbon dots and their influences on luminescence. J. Appl. Phys. 2020, 231101, 1–21. [Google Scholar] [CrossRef]
- Liu, M. Optical properties of carbon dots: A review. Nanoarchitectonics 2020, 1, 1–12. [Google Scholar] [CrossRef] [Green Version]
- El-Shabasy, R.M.; Elsadek, M.F.; Ahmed, B.M.; Farahat, M.F.; Mosleh, K.M.; Taher, M.M. Recent developments in carbon quantum dots: Properties, fabrication techniques, and bio-applications. Processes 2021, 9, 388. [Google Scholar] [CrossRef]
- Zhi, B.; Yao, X.; Wu, M.; Mensch, A.; Cui, Y.; Deng, J.; Duchimaza-Heredia, J.J.; Trerayapiwat, K.J.; Niehaus, T.; Nishimoto, Y.; et al. Multicolor polymeric carbon dots: Synthesis, separation and polyamide-supported molecular fluorescence. Chem. Sci. 2021, 12, 2441–2455. [Google Scholar] [CrossRef]
- Anwar, S.; Ding, H.; Xu, M.; Hu, X.; Li, Z.; Wang, J.; Liu, L.; Jiang, L.; Wang, D.; Dong, C.; et al. Recent advances in synthesis, optical properties, and biomedical applications of carbon dots. ACS Appl. Bio Mater. 2019, 2, 2317–2338. [Google Scholar] [CrossRef]
- El-Shafey, A.M. Carbon dots: Discovery, structure, fluorescent properties, and applications. Green Process. Synth. 2021, 10, 134–156. [Google Scholar] [CrossRef]
- Jorns, M.; Pappas, D. A review of fluorescent carbon dots, their synthesis, physical and chemical characteristics, and applications. Nanomaterials 2021, 11, 1448. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Damm, C.; Walter, J.; Nacken, T.J.; Peukert, W. Photobleaching and stabilization of carbon nanodots produced by solvothermal synthesis. Phys. Chem. Chem. Phys. 2016, 18, 466–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Gümrükçüoğlu, A.; Başoğlu, A.; Kolayli, S.; DiNç, S.; Kara, M.; Ocak, M.; Ocak, Ü. Highly sensitive fluorometric method based on nitrogen-doped carbon dot clusters for tartrazine determination in cookies samples. Turkish J. Chem. 2020, 44, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Dinc, S.; Kara, M. Synthesis and applications of carbon dots from food and natural products: A mini-review. Gıda ve doğal ürünlerden karbon noktaların sentezi ve uygulamaları: Derleme. J. Apitherapy Nature/Apiterapi ve Doğa Derg. 2018, 1, 33–37. [Google Scholar]
- Sun, X.; Lei, Y. Fluorescent carbon dots and their sensing applications. TrAC - Trends Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Tao, S.; Zhu, S.; Feng, T.; Xia, C.; Song, Y.; Yang, B. The polymeric characteristics and photoluminescence mechanism in polymer carbon dots: A review. Mater. Today Chem. 2017, 6, 13–25. [Google Scholar] [CrossRef]
- Shamsipur, M.; Barati, A.; Karami, S. Long-wavelength, multicolor, and white-light emitting carbon-based dots: Achievements made, challenges remaining, and applications. Carbon N. Y. 2017, 124, 429–472. [Google Scholar] [CrossRef]
- Lin, H.; Huang, J.; Ding, L. Preparation of carbon dots with high-fluorescence quantum yield and their application in dopamine fluorescence probe andcellular imaging. J. Nanomater. 2019, 2019. [Google Scholar] [CrossRef]
- Tong, L.; Wang, X.; Chen, Z.; Liang, Y.; Yang, Y.; Gao, W.; Liu, Z.; Tang, B. One-Step fabrication of functional carbon dots with 90% fluorescence quantum yield for long-term lysosome imaging. Anal. Chem. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Library, F. Green synthesis of high quantum yield carbon dots from phenylalanine and citric acid: Role of stoichiometry and nitrogen doping. ACS Sustain. Chem. Eng. 2020, 1–31. [Google Scholar] [CrossRef]
- Dinç, S.; Kara, M.; Demirel Kars, M.; Aykül, F.; Çiçekci, H.; Akkuş, M. Biocompatible yogurt carbon dots: Evaluation of utilization for medical applications. Appl. Phys. A Mater. Sci. Process. 2017, 123, 1–7. [Google Scholar] [CrossRef]
- Yavuz, E.; Dinc, S.; Kara, M. Effects of endogenous molasses carbon dots on macrophages and their potential utilization as anti-inflammatory agents. Appl. Phys. A Mater. Sci. Process. 2020, 126, 1–10. [Google Scholar] [CrossRef]
- Dinç, S. A simple and green extraction of carbon dots from sugar beet molasses: Biosensor applications. Sugar Ind. 2016, 560–564. [Google Scholar] [CrossRef]
- Kurian, M.; Paul, A. Recent trends in the use of green sources for carbon dot synthesis – A short review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Jamaludin, N.; Rashid, S.A.; Tan, T. Natural Biomass as Carbon Sources for the Synthesis of Photoluminescent Carbon Dots; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128157572. [Google Scholar]
- Kumar, J.V.; Kavitha, G.; Arulmozhi, R.; Arul, V.; Singaravadivel, S. Green sources derived carbon dots for multifaceted applications. J. Fluoresc. 2021, 31, 915–932. [Google Scholar] [CrossRef]
- Wang, S.; Sun, W.; Yang, D.S.; Yang, F. Soybean-derived blue photoluminescent carbon dots. Beilstein J. Nanotechnol. 2020, 11, 606–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhu, Y.; Yu, S.; Jiang, C. Fluorescent carbon dots: Rational synthesis, tunable optical properties and analytical applications. RSC Adv. 2017, 7, 40973–40989. [Google Scholar] [CrossRef] [Green Version]
- Shuaib, E.P.; Shafi, P.M.; Yogesh, G.K.; Chandra Bose, A.; Sastikumar, D. Carbon nanoparticles synthesized by laser ablation of coconut shell charcoal in liquids for glucose sensing applications. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Wang, L.; Bi, Y.; Hou, J.; Li, H.; Xu, Y.; Wang, B.; Ding, H.; Ding, L. Facile, green and clean one-step synthesis of carbon dots from wool: Application as a sensor for glyphosate detection based on the inner filter effect. Talanta 2016, 160, 268–275. [Google Scholar] [CrossRef]
- Majumdar, S.; Bhattacharjee, T.; Thakur, D.; Chowdhury, D. Carbon dot based fluorescence sensor for retinoic acid. ChemistrySelect 2018, 673–677. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; van Le, Q.; Jang, H.W.; Shokouhimehr, M. Carbon and graphene quantum dots: A review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef] [Green Version]
- Roy, P.; Chen, P.C.; Periasamy, A.P.; Chen, Y.N.; Chang, H.T. Photoluminescent carbon nanodots: Synthesis, physicochemical properties and analytical applications. Mater. Today 2015, 18, 447–458. [Google Scholar] [CrossRef]
- Martindale, B.C.M.; Hutton, G.A.M.; Caputo, C.A.; Reisner, E. Solar hydrogen production using carbon quantum dots and a molecular nickel catalyst. J. Am. Chem. Soc. 2015, 137, 6018–6025. [Google Scholar] [CrossRef]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta 2016, 183, 519–542. [Google Scholar] [CrossRef]
- Contreras, D.R.; De Fuentes, O.A.; Prokhorov, E.; Reguera, E. Carbon quantum dots by submerged arc discharge in water: Synthesis, characterization, and mechanism of formation. J. Appl. Phys. 2021, 129, 1–11. [Google Scholar] [CrossRef]
- Essner, J.B.; Baker, G.A. The emerging roles of carbon dots in solar photovoltaics: A critical review. Environ. Sci. Nano 2017, 4, 1216–1263. [Google Scholar] [CrossRef]
- Xie, R.; Wang, Z.; Zhou, W.; Liu, Y.; Fan, L.; Li, Y.; Li, X. Graphene quantum dots as smart probes for biosensing. Anal. Methods 2016, 8, 4001–4006. [Google Scholar] [CrossRef]
- Pan, M.; Xie, X.; Liu, K.; Yang, J.; Hong, L.; Wang, S. Fluorescent carbon quantum dots-synthesis, functionalization and sensing application in food analysis. Nanomaterials 2020, 10, 930. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Bottini, M.; Tautz, L.; Huynh, H.; Monosov, E.; Bottini, N.; Dawson, M.I.; Bellucci, S.; Mustelin, T. Covalent decoration of multi-walled carbon nanotubes with silica nanoparticles. Chem. Commun. 2005, 758–760. [Google Scholar] [CrossRef]
- Arora, N.; Sharma, N.N. Arc discharge synthesis of carbon nanotubes: Comprehensive review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Doñate-Buendia, C.; Torres-Mendieta, R.; Pyatenko, A.; Falomir, E.; Fernández-Alonso, M.; Mínguez-Vega, G. Fabrication by laser irradiation in a continuous flow jet of carbon quantum dots for fluorescence imaging. ACS Omega 2018, 3, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yan, L.; Nguyen, V.; Yu, Y.; Xu, Y. One-step synthesis of nitrogen-doped carbon nanodots for ratiometric pH sensing by femtosecond laser ablation method. Appl. Surf. Sci. 2017, 414, 238–243. [Google Scholar] [CrossRef]
- Cui, L.; Ren, X.; Wang, J.; Sun, M. Synthesis of homogeneous carbon quantum dots by ultrafast dual-beam pulsed laser ablation for bioimaging. Mater. Today Nano 2020, 12, 100091. [Google Scholar] [CrossRef]
- Nguyen, V.; Zhao, N.; Yan, L.; Zhong, P.; Nguyen, V.C.; Le, P.H. Double-pulse femtosecond laser ablation for synthesis of ultrasmall carbon nanodots. Mater. Res. Express 2019, 7. [Google Scholar] [CrossRef]
- Tsai, I.H.; Li, J.T.; Chang, C.W. Effects of sonication and hydrothermal treatments on the optical and chemical properties of carbon dots. ACS Omega 2021, 6, 14174–14181. [Google Scholar] [CrossRef]
- Molaei, M.J. Principles, mechanisms, and application of carbon quantum dots in sensors: A review. Anal. Methods 2020, 12, 1266–1287. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Yin, J.; Li, H.; Huang, J. Facile ultrasonic synthesized NH2-carbon quantum dots for ultrasensitive Co2+ ion detection and cell imaging. Talanta 2019, 205, 120121. [Google Scholar] [CrossRef]
- Huang, H.; Cui, Y.; Liu, M.; Chen, J.; Wan, Q.; Wen, Y.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. A one-step ultrasonic irradiation assisted strategy for the preparation of polymer-functionalized carbon quantum dots and their biological imaging. J. Colloid Interface Sci. 2018, 532, 767–773. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnology 2019, 17, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Stahl, S.S. Electrochemical oxidation of organic molecules at lower overpotential: Accessing broader functional group compatibility with electron-proton transfer mediators. Acc. Chem. Res. 2020, 53, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Shestakova, M.; Sillanpää, M. Electrode materials used for electrochemical oxidation of organic compounds in wastewater. Rev. Environ. Sci. Biotechnol. 2017, 16, 223–238. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Y. A simple and green synthesis of carbon quantum dots from coke for white light-emitting devices. RSC Adv. 2019, 9, 33789–33793. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Xu, Y.; Niu, F.; Gooding, J.J.; Liu, J. Carbon quantum dots directly generated from electrochemical oxidation of graphite electrodes in alkaline alcohols and the applications for specific ferric ion detection and cell imaging. Analyst 2016, 141, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Kalita, H.; Palaparthy, V.S.; Baghini, M.S.; Aslam, M. Electrochemical synthesis of graphene quantum dots from graphene oxide at room temperature and its soil moisture sensing properties. Carbon N. Y. 2020, 165, 9–17. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.; Zuo, S.; Zhao, Y.; Shen, B. Preparation of multicolored carbon quantum dots using HNO3/HClO4 oxidation of graphitized carbon. J. Mater. Res. 2019, 34, 3428–3438. [Google Scholar] [CrossRef]
- Oanh Vuong, T.K.; Le, T.T.; Do, H.D.; Nguyen, X.T.; Nguyen, X.C.; Vu, T.T.; Le, T.L.; Tran, D.L. PMAO-assisted thermal decomposition synthesis of high-stability ferrofluid based on magnetite nanoparticles for hyperthermia and MRI applications. Mater. Chem. Phys. 2020, 245, 122762. [Google Scholar] [CrossRef]
- Dager, A.; Baliyan, A.; Kurosu, S.; Maekawa, T.; Tachibana, M. Ultrafast synthesis of carbon quantum dots from fenugreek seeds using microwave plasma enhanced decomposition: Application of C-QDs to grow fluorescent protein crystals. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Ludmerczki, R.; Mura, S.; Carbonaro, C.M.; Mandity, I.M.; Carraro, M.; Senes, N.; Garroni, S.; Granozzi, G.; Calvillo, L.; Marras, S.; et al. Carbon dots from citric acid and its intermediates formed by thermal decomposition. Chem. - A Eur. J. 2019, 25, 11963–11974. [Google Scholar] [CrossRef]
- Shang, W.; Cai, T.; Zhang, Y.; Liu, D.; Liu, S. Facile one pot pyrolysis synthesis of carbon quantum dots and graphene oxide nanomaterials: All carbon hybrids as eco-environmental lubricants for low friction and remarkable wear-resistance. Tribol. Int. 2018, 118, 373–380. [Google Scholar] [CrossRef]
- Ma, C.; Yin, C.; Fan, Y.; Yang, X.; Zhou, X. Highly efficient synthesis of N-doped carbon dots with excellent stability through pyrolysis method. J. Mater. Sci. 2019, 54, 9372–9384. [Google Scholar] [CrossRef]
- Wang, H.; Ning, G.; He, X.; Ma, X.; Yang, F.; Xu, Z.; Zhao, S.; Xu, C.; Li, Y. Carbon quantum dots derived by direct carbonization of carbonaceous microcrystals in mesophase pitch. Nanoscale 2018, 10, 21492–21498. [Google Scholar] [CrossRef]
- Romero, M.P.; Alves, F.; Stringasci, M.D.; Buzzá, H.H.; Ciol, H.; Inada, N.M.; Bagnato, V.S. One-pot microwave-assisted synthesis of carbon dots and in vivo and in vitro antimicrobial photodynamic applications. Front. Microbiol. 2021, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gul, U.; Kanwal, S.; Tabassum, S.; Gilani, M.A.; Rahim, A. Microwave-assisted synthesis of carbon dots as reductant and stabilizer for silver nanoparticles with enhanced-peroxidase like activity for colorimetric determination of hydrogen peroxide and glucose. Microchim. Acta 2020, 187. [Google Scholar] [CrossRef]
- Yu, T.; Wang, H.; Guo, C.; Zhai, Y.; Yang, J.; Yuan, J. A rapid microwave synthesis of green-emissive carbon dots with solid-state fluorescence and pH-sensitive properties. R. Soc. Open Sci. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Ghanem, A.; Al-Qassar Bani Al-Marjeh, R.; Atassi, Y. Novel nitrogen-doped carbon dots prepared under microwave-irradiation for highly sensitive detection of mercury ions. Heliyon 2020, 6, e03750. [Google Scholar] [CrossRef]
- Chung, S.; Zhang, M. Microwave-assisted synthesis of carbon dot – iron oxide nanoparticles for fluorescence imaging and therapy. Front. Bioeng. Biotechnol. 2021, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cui, J.; Huang, J.; Tian, B.; Jia, F.; Wang, Z. Facile one-pot synthesis of highly fluorescent nitrogen-doped carbon dots by mild hydrothermal method and their applications in detection of Cr(VI) ions. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 2019, 206, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, S.H.; Fang, W.L.; Guo, X.F.; Wang, H. Synthesis of non-doped and non-modified carbon dots with high quantum yield and crystallinity by one-pot hydrothermal method using a single carbon source and used for ClO− detection. Dye. Pigment. 2019, 164, 7–13. [Google Scholar] [CrossRef]
- Wu, P.; Li, W.; Wu, Q.; Liu, Y.; Liu, S. Hydrothermal synthesis of nitrogen-doped carbon quantum dots from microcrystalline cellulose for the detection of Fe3+ ions in an acidic environment. RSC Adv. 2017, 7, 44144–44153. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Cheng, D.; Liu, X.; Han, A. Green hydrothermal synthesis of N-doped carbon dots from biomass highland barley for the detection of Hg2+. Sensors 2019, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, M.R.; Saha, N.; Quaid, T.; Toufiq Reza, M. Formation of carbon quantum dots via hydrothermal carbonization: Investigate the effect of precursors. Energies 2021, 14, 986. [Google Scholar] [CrossRef]
- Luo, J.; Sun, Z.; Zhou, W.; Mo, F.; Wu, Z.C.; Zhang, X. Hydrothermal synthesis of bright blue-emitting carbon dots for bioimaging and fluorescent determination of baicalein. Opt. Mater. 2021, 113, 110796. [Google Scholar] [CrossRef]
- Sun, D.; Liu, T.; Wang, C.; Yang, L.; Yang, S.; Zhuo, K. Hydrothermal synthesis of fluorescent carbon dots from gardenia fruit for sensitive on-off-on detection of Hg2+ and cysteine. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 2020, 240, 118598. [Google Scholar] [CrossRef]
- Lee, H.; Su, Y.C.; Tang, H.H.; Lee, Y.S.; Lee, J.Y.; Hu, C.C.; Chiu, T.C. One-pot hydrothermal synthesis of carbon dots as fluorescent probes for the determination of mercuric and hypochlorite ions. Nanomaterials 2021, 11. [Google Scholar] [CrossRef]
- Kurdyukov, D.A.; Eurov, D.A.; Stovpiaga, E.Y.; Kirilenko, D.A.; Konyakhin, S.V.; Shvidchenko, A.V.; Golubev, V.G. Template synthesis of monodisperse carbon nanodots. Phys. Solid State 2016, 58, 2545–2549. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, D.; Han, S.; Hu, P.; Liu, R. Bottom-up fabrication of photoluminescent carbon dots with uniform morphology via a soft-hard template approach. Chem. Commun. 2013, 49, 4920–4922. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T. Spectroscopic characterization of chemically modified oxide surfaces. Stud. Surf. Sci. Catal. 1999, 120 A, 117–142. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, T.; Pan, Y.; Ciacchi, L.C.; Xu, B.; Wei, G. AFM-based force spectroscopy for bioimaging and biosensing. RSC Adv. 2016, 6, 12893–12912. [Google Scholar] [CrossRef]
- Hu, Q.; Gong, X.; Liu, L.; Choi, M.M.F. Characterization and analytical separation of fluorescent carbon nanodots. J. Nanomater. 2017, 2017, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.J.; Zhong, Z.F.; Rong, M.Z.; Zhou, X.; Chen, X.D.; Zhang, M.Q. An easy approach of preparing strongly luminescent carbon dots and their polymer based composites for enhancing solar cell efficiency. Carbon N. Y. 2014, 70, 190–198. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, S.; Ge, H.; Tian, G.; Cao, N.; Li, Y. A fluorescent turn-off/on method based on carbon dots as fluorescent probes for the sensitive determination of Pb2+ and pyrophosphate in an aqueous solution. Sensors Actuators, B Chem. 2015, 207, 25–33. [Google Scholar] [CrossRef]
- Chen, W.; Hu, C.; Yang, Y.; Cui, J.; Liu, Y. Rapid synthesis of carbon dots by hydrothermal treatment of lignin. Materials 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Voutou, B.; Stefanaki, E.; Giannakopoulos, K. Electron microscopy: The basics. Phys. Adv. Mater. Winter Sch. 2008, 1–11. [Google Scholar]
- Zavagli, G.; Ricci, G. Scanning electron microscopy (SEM) in haemolysis. Ultramicroscopy 1983, 12, 160. [Google Scholar] [CrossRef]
- Hayes, T.L.; Pease, R.F. The Scanning Electron Microscope: Principles and Applications in Biology and Medicine; Academic Press Inc.: Cambridge, MA, USA, 1968; Volume 12. [Google Scholar]
- Hu, Q.; Meng, X.; Chan, W. An investigation on the chemical structure of nitrogen and sulfur codoped carbon nanoparticles by ultra-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 5347–5357. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Meng, X.; Choi, M.M.F.; Gong, X.; Chan, W. Elucidating the structure of carbon nanoparticles by ultra-performance liquid chromatography coupled with electrospray ionisation quadrupole time-of-flight tandem mass spectrometry. Anal. Chim. Acta 2016, 911, 100–107. [Google Scholar] [CrossRef]
- Akimowicz, M.; Bucka-Kolendo, J. MALDI-TOF MS-Application in food microbiology. Acta Biochim. Pol. 2020, 67, 327–332. [Google Scholar] [CrossRef]
- Houdová, D.; Soto, J.; Castro, R.; Rodrigues, J.; Soledad Pino-González, M.; Petković, M.; Bandosz, T.J.; Algarra, M. Chemically heterogeneous carbon dots enhanced cholesterol detection by MALDI TOF mass spectrometry. J. Colloid Interface Sci. 2021, 591, 373–383. [Google Scholar] [CrossRef]
- Hu, Q.; Paau, M.C.; Choi, M.M.F.; Zhang, Y.; Gong, X.; Zhang, L.; Liu, Y.; Yao, J. Better understanding of carbon nanoparticles via high-performance liquid chromatography-fluorescence detection and mass spectrometry. Electrophoresis 2014, 35, 2454–2462. [Google Scholar] [CrossRef]
- Dager, A.; Uchida, T.; Maekawa, T.; Tachibana, M. Synthesis and characterization of mono-disperse carbon quantum dots from fennel seeds: Photoluminescence analysis using machine learning. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Sim, L.C.; Tai, J.Y.; Khor, J.M.; Wong, J.L.; Lee, J.Y.; Leong, K.H.; Saravanan, P.; Aziz, A.A. Carbon dots synthesized from green precursors with an amplified photoluminescence: Synthesis, characterization, and its application. In Plant Nanobionics; Springer: Cham, Switzerland, 2019; ISBN 9783030163792. [Google Scholar]
- Hu, Y.; Yang, J.; Tian, J.; Jia, L.; Yu, J.S. Waste frying oil as a precursor for one-step synthesis of sulfur-doped carbon dots with pH-sensitive photoluminescence. Carbon N. Y. 2014, 77, 775–782. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, R.; Liu, J.; Sun, J.; Wang, Q. Recent progress on the characterization of cellulose nanomaterials by nanoscale infrared spectroscopy. Nanomaterials 2021, 11, 1353. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Demirci, S.; McNally, A.B.; Ayyala, R.S.; Lawson, L.B.; Sahiner, N. Synthesis and characterization of nitrogen-doped carbon dots as fluorescent nanoprobes with antimicrobial properties and skin permeability. J. Drug Deliv. Sci. Technol. 2020, 59, 101889. [Google Scholar] [CrossRef]
- Kazemifard, N.; Ensafi, A.A.; Rezaei, B. Green synthesized carbon dots embedded in silica molecularly imprinted polymers, characterization and application as a rapid and selective fluorimetric sensor for determination of thiabendazole in juices. Food Chem. 2020, 310, 125812. [Google Scholar] [CrossRef]
- Nagarajan, D.; Varada, O.M.; Venkatanarasimhan, S. Carbon dots coated on amine functionalized cellulose sponge for the adsorption of the toxic herbicide atrazine. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Saraswat, V.; Yadav, M. Carbon dots as green corrosion inhibitor for mild steel in HCl solution. ChemistrySelect 2020, 5, 7347–7357. [Google Scholar] [CrossRef]
- Khairol Anuar, N.K.; Tan, H.L.; Lim, Y.P.; So’aib, M.S.; Abu Bakar, N.F. A review on multifunctional carbon-dots synthesized from biomass waste: Design/ fabrication, characterization and applications. Front. Energy Res. 2021, 9, 626549. [Google Scholar] [CrossRef]
- Zaib, M.; Akhtar, A.; Maqsood, F.; Shahzadi, T. Green synthesis of carbon dots and their application as photocatalyst in dye degradation studies. Arab. J. Sci. Eng. 2021, 46, 437–446. [Google Scholar] [CrossRef]
- Carbonaro, C.M.; Corpino, R.; Salis, M.; Mocci, F.; Thakkar, S.V.; Olla, C.; Ricci, P.C. On the emission properties of carbon dots: Reviewing data and discussing models. C. — J. Carbon Res. 2019, 5, 60. [Google Scholar] [CrossRef] [Green Version]
- Edison, T.N.J.I.; Atchudan, R.; Sethuraman, M.G.; Shim, J.J.; Lee, Y.R. Microwave Assisted Green Synthesis of Fluorescent N-Doped Carbon Dots: Cytotoxicity and Bio-Imaging Applications; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 161, ISBN 9145124523. [Google Scholar]
- Vinci, J.C.; Ferrer, I.M.; Guterry, N.W.; Colón, V.M.; Destino, J.F.; Bright, F.V.; Colón, L.A. Spectroscopic characteristics of carbon dots (C-dots) derived from carbon fibers and conversion to sulfur-bridged C-dots nanosheets. Appl. Spectrosc. 2015, 69, 1082–1090. [Google Scholar] [CrossRef] [Green Version]
- Mewada, A.; Pandey, S.; Shinde, S.; Mishra, N.; Oza, G.; Thakur, M.; Sharon, M.; Sharon, M. Green synthesis of biocompatible carbon dots using aqueous extract of Trapa bispinosa peel. Mater. Sci. Eng. C 2013, 33, 2914–2917. [Google Scholar] [CrossRef] [PubMed]
- Dervishi, E.; Ji, Z.; Htoon, H.; Sykora, M.; Doorn, S.K. Raman spectroscopy of bottom-up synthesized graphene quantum dots: Size and structure dependence. Nanoscale 2019, 11, 16571–16581. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Lee, Y.R. In-situ green synthesis of nitrogen-doped carbon dots for bioimaging and TiO2 nanoparticles@nitrogen-doped carbon composite for photocatalytic degradation of organic pollutants. J. Alloys Compd. 2018, 766, 12–24. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Arul, V.; Sethuraman, M.G. Ecofriendly synthesis of fluorescent nitrogen-doped carbon dots from Coccinia grandis and its efficient catalytic application in the reduction of methyl orange. J. Fluoresc. 2020, 30, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Pudza, M.Y.; Abidin, Z.Z.; Rashid, S.A.; Yasin, F.M.; Noor, A.S.M.; Issa, M.A. Eco-friendly sustainable fluorescent carbon dots for the adsorption of heavy metal ions in aqueous environment. Nanomaterials 2020, 10. [Google Scholar] [CrossRef] [Green Version]
- Arul, V.; Sethuraman, M.G. Facile green synthesis of fluorescent N-doped carbon dots from Actinidia deliciosa and their catalytic activity and cytotoxicity applications. Opt. Mater. 2018, 78, 181–190. [Google Scholar] [CrossRef]
- Shamsipur, M.; Molaei, K.; Molaabasi, F.; Alipour, M.; Alizadeh, N.; Hosseinkhani, S.; Hosseini, M. Facile preparation and characterization of new green emitting carbon dots for sensitive and selective off/on detection of Fe3+ ion and ascorbic acid in water and urine samples and intracellular imaging in living cells. Talanta 2018, 183, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wu, H.; Ye, Z.; Li, R.; Xu, T.; Zhang, B. Optical properties of pH-sensitive carbon-dots with different modifications. J. Lumin. 2014, 148, 238–242. [Google Scholar] [CrossRef]
- Talib, A.B.; Mohammed, M.H. Preparation, characterization and preliminary cytotoxic evaluation of 6-mercaptopurine-coated biotinylated carbon dots nanoparticles as a drug delivery system. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Duan, P.; Zhi, B.; Coburn, L.; Haynes, C.L.; Schmidt-Rohr, K. A molecular fluorophore in citric acid/ethylenediamine carbon dots identified and quantified by multinuclear solid-state nuclear magnetic resonance. Magn. Reson. Chem. 2020, 58, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Karak, N. A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Huang, G.; Chen, X.; Wang, C.; Zheng, H.; Huang, Z.; Chen, D.; Xie, H. Photoluminescent carbon dots derived from sugarcane molasses: Synthesis, properties, and applications. RSC Adv. 2017, 7, 47840–47847. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; van der Mei, H.C.; Busscher, H.J.; Ren, Y. Enhanced bacterial killing by vancomycin in staphylococcal biofilms disrupted by novel, DMMA-modified carbon dots depends on EPS production. Colloids Surf. B Biointerfaces 2020, 193, 111114. [Google Scholar] [CrossRef]
- Rojas-Valencia, O.G.; Regules-Carrasco, M.; Hernández-Fuentes, J.; Germán, C.M.R.-S.; Estrada-Flores, M.; Villagarcía-Chávez, E. Synthesis of blue emissive carbon quantum dots from Hibiscus Sabdariffa flower: Surface functionalization analysis by FT-IR spectroscopy. Materialia 2021, 19, 101182. [Google Scholar] [CrossRef]
- Kholiya, F.; Jauhari, S.; Meena, R. Seaweed-derived polymer-based blue-emitting C-dots: Synthesis, characterization and evaluation for iron sensing. Polym. Int. 2021, 1309–1315. [Google Scholar] [CrossRef]
- Demir, B.; Moulahoum, H.; Ghorbanizamani, F.; Barlas, F.B.; Yesiltepe, O.; Gumus, Z.P.; Meral, K.; Odaci Demirkol, D.; Timur, S. Carbon dots and curcumin-loaded CD44-targeted liposomes for imaging and tracking cancer chemotherapy: A multi-purpose tool for theranostics. J. Drug Deliv. Sci. Technol. 2021, 62, 102363. [Google Scholar] [CrossRef]
- Esfandiari, N.; Bagheri, Z.; Ehtesabi, H.; Fatahi, Z.; Tavana, H.; Latifi, H. Effect of carbonization degree of carbon dots on cytotoxicity and photo-induced toxicity to cells. Heliyon 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.N.; Li, Y.; Cao, M.J.; Dai, C.L.; He, L.; Yang, Y.P. Preparation and stabilization mechanism of carbon dots nanofluids for drag reduction. Pet. Sci. 2020, 17, 1717–1725. [Google Scholar] [CrossRef]
- Alhaji Haruna, M.; Hu, Z.; Gao, H.; Gardy, J.; Musa Magami, S.; Wen, D. Influence of carbon quantum dots on the viscosity reduction of polyacrylamide solution. Fuel 2019, 248, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Joseph, J.; Anappara, A.A. White light emission of carbon dots by creating different emissive traps. J. Lumin. 2016, 178, 128–133. [Google Scholar] [CrossRef]
- Jlassi, K.; Eid, K.; Sliem, M.H.; Abdullah, A.M.; Chehimi, M.M.; Krupa, I. Rational synthesis, characterization, and application of environmentally friendly (polymer–carbon dot) hybrid composite film for fast and efficient UV-assisted Cd2+ removal from water. Environ. Sci. Eur. 2020, 32. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Wu, Z.; Wang, L.; Liu, J.; Deng, Y.; Xiao, Z.; Fang, J.; Liang, Y. Green preparation of fluorescent nitrogen-doped carbon quantum dots for sensitive detection of oxytetracycline in environmental samples. Nanomaterials 2020, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ding, L.; Zhang, B.; Huang, J. Detection of nitrite based on fluorescent carbon dots by the hydrothermal method with folic acid. R. Soc. Open Sci. 2018, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; He, J.; Zhang, H.; Liu, Y.; Lei, B. Luminescent carbon dots assembled into mesoporous aluminas for oxygen sensing. Opt. Mater. Express 2017, 7, 945. [Google Scholar] [CrossRef]
- Pal, A.; Sk, M.P.; Chattopadhyay, A. Recent advances in crystalline carbon dots for superior application potential. Mater. Adv. 2020, 1, 525–553. [Google Scholar] [CrossRef]
- Bajpai, S.K.; D’Souza, A.; Suhail, B. Blue light-emitting carbon dots (CDs) from a milk protein and their interaction with Spinacia oleracea leaf cells. Int. Nano Lett. 2019, 9, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.; Hao, X.; Liao, L.; Zhang, K.; Chen, S.; Li, Z.; Ou, J.; Qin, A.; Li, Z. Recent advances of biomass carbon dots on syntheses, characterization, luminescence mechanism, and sensing applications. Nano Sel. 2021, 2, 1117–1145. [Google Scholar] [CrossRef]
- Ramachandran, S.; Sathishkumar, M.; Kothurkar, N.K.; Senthilkumar, R. Synthesis and characterization of graphene quantum dots/cobalt ferrite nanocomposite. IOP Conf. Ser. Mater. Sci. Eng. 2018, 310. [Google Scholar] [CrossRef]
- Shaikh, A.F.; Tamboli, M.S.; Patil, R.H.; Bhan, A.; Ambekar, J.D.; Kale, B.B. Bioinspired carbon quantum dots: An antibiofilm agents. J. Nanosci. Nanotechnol. 2018, 19, 2339–2345. [Google Scholar] [CrossRef]
- Muhammad, A.; Hajian, R.; Yusof, N.A.; Shams, N.; Abdullah, J.; Woi, P.M.; Garmestani, H. A screen printed carbon electrode modified with carbon nanotubes and gold nanoparticles as a sensitive electrochemical sensor for determination of thiamphenicol residue in milk. RSC Adv. 2018, 8, 2714–2722. [Google Scholar] [CrossRef] [Green Version]
- Tufa, L.T.; Oh, S.; Tran, V.T.; Kim, J.; Jeong, K.J.; Park, T.J.; Kim, H.J.; Lee, J. Electrochemical immunosensor using nanotriplex of graphene quantum dots, Fe3O4 and Ag nanoparticles for tuberculosis. Electrochim. Acta 2018, 290, 369–377. [Google Scholar] [CrossRef]
- Majumdar, S.; Thakur, D.; Chowdhury, D. DNA carbon-nanodots based electrochemical biosensor for detection of mutagenic nitrosamines. ACS Appl. Bio Mater. 2020, 3, 1796–1803. [Google Scholar] [CrossRef]
- Altintas, Z. Surface plasmon resonance based sensor for the detection of glycopeptide antibiotics in milk using rationally designed nanoMIPs. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sehit, E.; Drzazgowska, J.; Buchenau, D.; Yesildag, C.; Lensen, M.; Altintas, Z. Ultrasensitive nonenzymatic electrochemical glucose sensor based on gold nanoparticles and molecularly imprinted polymers. Biosens. Bioelectron. 2020, 165, 112432. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; Guerreiro, A.; Piletsky, S.A.; Tothill, I.E. NanoMIP based optical sensor for pharmaceuticals monitoring. Sensors Actuators, B Chem. 2015, 213, 305–313. [Google Scholar] [CrossRef]
- Altintas, Z.; Akgun, M.; Kokturk, G.; Uludag, Y. A fully automated microfluidic-based electrochemical sensor for real-time bacteria detection. Biosens. Bioelectron. 2018, 100, 541–548. [Google Scholar] [CrossRef]
- Altintas, Z.; France, B.; Ortiz, J.O.; Tothill, I.E. Computationally modelled receptors for drug monitoring using an optical based biomimetic SPR sensor. Sensors Actuators, B Chem. 2016, 224, 726–737. [Google Scholar] [CrossRef]
- Ravalli, A.; Marrazza, G. Electrochemical-based biosensor technologies in disease detection and diagnostics. In Biosensors and nanotechnology; Altintas, Z., Ed.; Wiley: Hoboken, NJ, USA, 2018; pp. 95–124. [Google Scholar]
- Rama, E.C.; Costa-García, A. Screen-printed electrochemical immunosensors for the detection of cancer and cardiovascular biomarkers. Electroanalysis 2016, 28, 1700–1715. [Google Scholar] [CrossRef]
- Cho, I.H.; Lee, J.; Kim, J.; Kang, M.S.; Paik, J.K.; Ku, S.; Cho, H.M.; Irudayaraj, J.; Kim, D.H. Current technologies of electrochemical immunosensors: Perspective on signal amplification. Sensors 2018, 18, 207. [Google Scholar] [CrossRef] [Green Version]
- Stradiotto, N.R.; Yamanaka, H.; Zanoni, M.V.B. Electrochemical sensors: A powerful tool in analytical chemistry. J. Braz. Chem. Soc. 2003, 14, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.A.; Ahmed, M.U. Electrochemical immunosensors and their recent nanomaterial-based signal amplification strategies: A review. RCS Adv. 2016. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Prodromidis, M.I. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. Trends Anal. Chem. 2015. [Google Scholar] [CrossRef]
- Faridbod, F.; Gupta, V.K.; Zamani, H.A. Electrochemical sensors and biosensors. Int. J. Electrochem. 2011, 24, 717. [Google Scholar] [CrossRef] [Green Version]
- Dhahi, T.H.S.; Bin Hashim, U.D.A.; Ahmed, N.M.; Mat Taib, A. A review on the electrochemical sensors and biosensors composed of nanogaps as sensing material. J. Optoelectron. Adv. Mater. 2010, 12, 1857–1862. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Han, J.; Ma, Z. Polyamidoamine dendrimers-capped carbon dots/Au nanocrystal nanocomposites and its application for electrochemical immunosensor. Biosens. Bioelectron. 2013, 49, 323–328. [Google Scholar] [CrossRef]
- Karaman, C.; Karaman, O.; Atar, N.; Yola, M.L. Electrochemical immunosensor development based on core-shell high-crystalline graphitic carbon nitride@carbon dots and Cd0.5Zn0.5S/d-Ti3C2Tx MXene composite for heart-type fatty acid–binding protein detection. Microchim. Acta 2021, 188. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.; Bohidar, H.B.; Solanki, P.R. Carbon dots-modified chitosan based electrochemical biosensing platform for detection of vitamin D. Int. J. Biol. Macromol. 2018, 109, 687–697. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Zhu, J.J.; Tong, Q.X. Pd-Au@carbon dots nanocomposite: Facile synthesis and application as an ultrasensitive electrochemical biosensor for determination of colitoxin DNA in human serum. Biosens. Bioelectron. 2017, 94, 507–512. [Google Scholar] [CrossRef]
- Song, Y.; He, L.; Chen, K.; Wang, M.; Yang, L.; He, L.; Guo, C.; Jia, Q.; Zhang, Z. Quantification of EGFR and EGFR-overexpressed cancer cells based on carbon dots@bimetallic CuCo prussian blue analogue. RSC Adv. 2020, 10, 28355–28364. [Google Scholar] [CrossRef]
- Guo, W.; Pi, F.; Zhang, H.; Sun, J.; Zhang, Y.; Sun, X. A novel molecularly imprinted electrochemical sensor modified with carbon dots, chitosan, gold nanoparticles for the determination of patulin. Biosens. Bioelectron. 2017, 98, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wu, H.; Jiang, Y.; Xu, J.; Li, X.; Zhang, W.; Qiu, F. A molecularly-imprinted-electrochemical-sensor modified with nano-carbon-dots with high sensitivity and selectivity for rapid determination of glucose. Anal. Biochem. 2018, 555, 42–49. [Google Scholar] [CrossRef]
- Liu, L.; Anwar, S.; Ding, H.; Xu, M.; Yin, Q.; Xiao, Y.; Yang, X.; Yan, M.; Bi, H. Electrochemical sensor based on F,N-doped carbon dots decorated laccase for detection of catechol. J. Electroanal. Chem. 2019, 840, 84–92. [Google Scholar] [CrossRef]
- Shiri, S.; Pajouheshpoor, N.; Khoshsafar, H.; Amidi, S.; Bagheri, H. An electrochemical sensor for the simultaneous determination of rifampicin and isoniazid using a C-dots@CuFe2O4 nanocomposite modified carbon paste electrode. New J. Chem. 2017, 41, 15564–15573. [Google Scholar] [CrossRef]
- Palakollu, V.N.; Karpoormath, R.; Wang, L.; Tang, J.N.; Liu, C. A versatile and ultrasensitive electrochemical sensing platform for detection of chlorpromazine based on nitrogen-doped carbon dots/cuprous oxide composite. Nanomaterials 2020, 10, 1513. [Google Scholar] [CrossRef]
- Fahimeh, J.; Hassanvand, Y.; Barati, A. Electrochemical sensor based on a nanocomposite of carbon dots, hexadecyltrimethylammonium bromide and chitosan for Mesalazine determination. J. Anal. Chem. 2020, 75, 544–552. [Google Scholar] [CrossRef]
- Fu, L.; Wang, A.; Lai, G.; Lin, C.T.; Yu, J.; Yu, A.; Liu, Z.; Xie, K.; Su, W. A glassy carbon electrode modified with N-doped carbon dots for improved detection of hydrogen peroxide and paracetamol. Microchim. Acta 2018, 185. [Google Scholar] [CrossRef]
- Sri, S.; Lakshmi, G.B.V.S.; Gulati, P.; Chauhan, D.; Thakkar, A.; Solanki, P.R. Simple and facile carbon dots based electrochemical biosensor for TNF-α targeting in cancer patient’s sample. Anal. Chim. Acta 2021, 338909. [Google Scholar] [CrossRef]
- Pangajam, A.; Theyagarajan, K.; Dinakaran, K. Highly sensitive electrochemical detection of E. coli O157:H7 using conductive carbon dot/ZnO nanorod/PANI composite electrode. Sens. Bio-Sensing Res. 2020, 29, 100317. [Google Scholar] [CrossRef]
- García-Mendiola, T.; Bravo, I.; López-Moreno, J.M.; Pariente, F.; Wannemacher, R.; Weber, K.; Popp, J.; Lorenzo, E. Carbon nanodots based biosensors for gene mutation detection. Sensors Actuators, B Chem. 2018, 256, 226–233. [Google Scholar] [CrossRef]
- Zaid, M.H.M.; Abdullah, J.; Rozi, N.; Rozlan, A.A.M.; Hanifah, S.A. A sensitive impedimetric aptasensor based on carbon nanodots modified electrode for detection of 17ß-estradiol. Nanomaterials 2020, 10, 1346. [Google Scholar] [CrossRef]
- Yasa, M.; Deniz, A.; Forough, M.; Yildirim, E.; Persil Cetinkol, O.; Udum, Y.A.; Toppare, L. Construction of amperometric biosensor modified with conducting polymer/carbon dots for the analysis of catechol. J. Polym. Sci. 2020, 58, 3336–3348. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, S.; Li, J.; Wang, M.; Wang, C.; Hu, B.; Zhou, N.; Zhang, Z. 0D/2D heteronanostructure–integrated bimetallic CoCu-ZIF nanosheets and MXene-derived carbon dots for impedimetric cytosensing of melanoma B16-F10 cells. Microchim. Acta 2021, 188. [Google Scholar] [CrossRef] [PubMed]
- Yola, M.L.; Atar, N. Development of molecular imprinted sensor including graphitic carbon nitride/N-doped carbon dots composite for novel recognition of epinephrine. Compos. Part B Eng. 2019, 175, 107113. [Google Scholar] [CrossRef]
- Güney, S. Electrochemical synthesis of molecularly imprinted poly(p-aminobenzene sulphonic acid) on carbon nanodots coated pencil graphite electrode for selective determination of folic acid. J. Electroanal. Chem. 2019, 854, 113518. [Google Scholar] [CrossRef]
- Pirzada, M.; Altintas, Z. Recent progress in optical sensors for biomedical diagnostics. Micromachines 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joe, H.; Yun, H.; Jo, S.; Jun, M.B.G.; Min, B. A review on optical fiber sensors for environmental monitoring. Int. J. Precis. Eng. Manuf. Technol. 2018, 5, 173–191. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R. Recent optical sensing technologies for the detection of various biomolecules: Review. Opt. Laser Technol. 2021, 134, 106620. [Google Scholar] [CrossRef]
- Thi, P.; Hong, K.; Jang, C. Sensitive and label-free liquid crystal-based optical sensor for the detection of malathion. Anal. Biochem. 2020, 113589. [Google Scholar] [CrossRef]
- Ashry, I.; Mao, Y.; Al-fehaid, Y.; Al-shawaf, A. Early detection of red palm weevil using distributed optical sensor. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Altintas, Z.; Fakanya, W.M.; Tothill, I.E. Cardiovascular disease detection using bio-sensing techniques. Talanta 2014, 128, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Peltomaa, R.; Glahn-Martínez, B.; Benito-Peña, E.; Moreno-Bondi, M.C. Optical biosensors for label-free detection of small molecules. Sensors 2018, 18, 4216. [Google Scholar] [CrossRef] [Green Version]
- Altintas, Z.; Uludag, Y.; Gurbuz, Y.; Tothill, I. Development of surface chemistry for surface plasmon resonance based sensors for the detection of proteins and DNA molecules. Anal. Chim. Acta 2012, 712, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Pawula, M.; Altintas, Z.; Tothill, I.E. SPR detection of cardiac troponin T for acute myocardial infarction. Talanta 2016, 146, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Zanchetta, G.; Lanfranco, R.; Giavazzi, F.; Bellini, T.; Buscaglia, M. Emerging applications of label-free optical biosensors. Nanophotonics 2017, 6, 627–645. [Google Scholar] [CrossRef]
- Huertas, C.S.; Calvo-Lozano, O.; Mitchell, A.; Lechuga, L.M. Advanced evanescent-wave optical biosensors for the detection of nucleic acids: An analytic perspective. Front. Chem. 2019, 7, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalili, R.; Khataee, A.; Rashidi, M.R.; Razmjou, A. Detection of penicillin G residues in milk based on dual-emission carbon dots and molecularly imprinted polymers. Food Chem. 2020, 314, 126172. [Google Scholar] [CrossRef]
- Othman, H.O.; Salehnia, F.; Hosseini, M.; Hassan, R.; Faizullah, A.; Ganjali, M.R. Fluorescence immunoassay based on nitrogen doped carbon dots for the detection of human nuclear matrix protein NMP22 as biomarker for early stage diagnosis of bladder cancer. Microchem. J. 2020, 157, 104966. [Google Scholar] [CrossRef]
- Liang, S.S.; Qi, L.; Zhang, R.L.; Jin, M.; Zhang, Z.Q. Ratiometric fluorescence biosensor based on CdTe quantum and carbon dots for double strand DNA detection. Sensors Actuators, B Chem. 2017, 244, 585–590. [Google Scholar] [CrossRef]
- Liao, X.; Chen, C.; Yang, J.; Zhou, R.; Si, L.; Huang, Q.; Huang, Z.; Lv, C. Nitrogen-doped carbon dots for dual-wavelength excitation fluorimetric assay for ratiometric determination of phosalone. Microchim. Acta 2021, 188. [Google Scholar] [CrossRef]
- Kong, W.; Wu, D.; Li, G.; Chen, X.; Gong, P.; Sun, Z.; Chen, G.; Xia, L.; You, J.; Wu, Y. A facile carbon dots based fluorescent probe for ultrasensitive detection of ascorbic acid in biological fluids via non-oxidation reduction strategy. Talanta 2017, 165, 677–684. [Google Scholar] [CrossRef]
- Robby, A.I.; Kim, S.G.; Lee, U.H.; In, I.; Lee, G.; Park, S.Y. Wireless electrochemical and luminescent detection of bacteria based on surface-coated CsWO3-immobilized fluorescent carbon dots with photothermal ablation of bacteria. Chem. Eng. J. 2021, 403, 126351. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Cheng, X.; Geng, J.; Wang, L.; Dong, Q.; Liu, C.; Chi, Z.; Chi, Z. Aptamer-superparamagnetic nanoparticles capture coupling siderophore-Fe3+ scavenging actuated with carbon dots to confer an “off-on” mechanism for the ultrasensitive detection of Helicobacter pylori. Biosens. Bioelectron. 2021, 113551. [Google Scholar] [CrossRef]
- An, J.; Chen, M.; Hu, N.; Hu, Y.; Chen, R.; Lyu, Y.; Guo, W.; Li, L.; Liu, Y. Carbon dots-based dual-emission ratiometric fluorescence sensor for dopamine detection. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 2020, 243, 118804. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sun, S.; Jiang, K.; Wang, Y.; Liu, W.; Lin, H. A highly sensitive and selective fluorimetric probe for intracellular peroxynitrite based on photoinduced electron transfer from ferrocene to carbon dots. Biosens. Bioelectron. 2017, 97, 150–156. [Google Scholar] [CrossRef]
- Yue, X.; Zhou, Z.; Wu, Y.; Jie, M.; Li, Y.; Guo, H.; Bai, Y. A green carbon dots-based fluorescent sensor for selective and visual detection of nitrite triggered by the nitrite-thiol reaction. New J. Chem. 2020, 44, 8503–8511. [Google Scholar] [CrossRef]
- Gao, J.; Wu, M.X.; Dai, D.; Cai, Z.; Wang, Y.; Fang, W.; Wang, Y.; Yang, Y.W. N-doped carbon dots covalently functionalized with pillar [5] arenes for Fe3+ sensing. Beilstein J. Org. Chem. 2019, 15, 1262–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Wang, A.; Wang, R.; Liu, Z.; Sun, Y.; Shan, G.; Chen, Y.; Liu, Y. Carbon dots with molecular fluorescence and their application as a “turn-off” fluorescent probe for ferricyanide detection. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.; Li, Q.; Chen, Y.; Tong, L.; Lin, X.; Zhu, J.; Tong, Q. High quantum yield nitrogen-doped carbon dots: Green synthesis and application as “off-on” fluorescent sensors for the determination of Fe3+ and adenosine triphosphate in biological samples. Sens. Actuators B Chem. 2018, 276, 82–88. [Google Scholar] [CrossRef]
- Tafreshi, F.A.; Fatahi, Z.; Ghasemi, S.F.; Taherian, A.; Esfandiari, N. Ultrasensitive fluorescent detection of pesticides in real sample by using green carbon dots. PLoS ONE 2020, 15, e0230646. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Sun, X.; Pan, W.; Yu, G.; Wang, J. Fe3+-sensitive carbon dots for detection of Fe3+ in aqueous solution and intracellular imaging of Fe3+ inside fungal cells. Front. Chem. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Wang, C.Y.; Shang, S.; Zheng, X.; Lei, P.; Han, J.; Yuan, L.; Li, Z.; Wang, R.; Gong, W.; Tang, J.; et al. Fluorescent sensors based on Cu-doped carbon quantum dots for the detection of rutin. J. Braz. Chem. Soc. 2019, 30, 988–996. [Google Scholar] [CrossRef]
- Ren, G.; Yu, L.; Zhu, B.; Tang, M.; Chai, F.; Wang, C.; Su, Z. Orange emissive carbon dots for colorimetric and fluorescent sensing of 2,4,6-trinitrophenol by fluorescence conversion. RSC Adv. 2018, 8, 16095–16102. [Google Scholar] [CrossRef] [Green Version]
- Deka, M.J.; Dutta, P.; Sarma, S.; Medhi, O.K.; Talukdar, N.C.; Chowdhury, D. Carbon dots derived from water hyacinth and their application as a sensor for pretilachlor. Heliyon 2019, 5, e01985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.S.; Pan, C.G.; Cao, H.X.; Yue, M.Z.; Wang, L.; Liang, G.X. Highly sensitive and selective dual-emission ratiometric fluorescence detection of dopamine based on carbon dots-gold nanoclusters hybrid. Sens. Actuators B Chem. 2018, 265, 371–377. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, W.; Han, Y.; Chen, X.; Zhu, L.; Tang, W.; Wang, J.; Yue, T.; Li, Z. N, S co-doped carbon dots based fluorescent “on-off-on” sensor for determination of ascorbic acid in common fruits. Food Chem. 2018, 258, 214–221. [Google Scholar] [CrossRef]
- Wang, M.; Shi, R.; Gao, M.; Zhang, K.; Deng, L.; Fu, Q.; Wang, L.; Gao, D. Sensitivity fluorescent switching sensor for Cr (VI) and ascorbic acid detection based on orange peels-derived carbon dots modified with EDTA. Food Chem. 2020, 318, 126506. [Google Scholar] [CrossRef]
- Xu, X.; Ren, D.; Chai, Y.; Cheng, X.; Mei, J.; Bao, J.; Wei, F.; Xu, G.; Hu, Q.; Cen, Y. Dual-emission carbon dots-based fluorescent probe for ratiometric sensing of Fe(III) and pyrophosphate in biological samples. Sens. Actuators B Chem. 2019, 298, 126829. [Google Scholar] [CrossRef]
- Lu, W.; Jiao, Y.; Gao, Y.; Qiao, J.; Mozneb, M.; Shuang, S.; Dong, C.; Li, C.Z. Bright yellow fluorescent carbon dots as a multifunctional sensing platform for the label-free detection of Fluoroquinolones and Histidine. ACS Appl. Mater. Interfaces 2018, 10, 42915–42924. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.; Bao, L.; Wu, Y.; Jiang, L.; Zheng, Y.; Wang, Y.; Chen, Y. The application of green-synthesis-derived carbon quantum dots to bioimaging and the analysis of mercury(II). J. Anal. Methods Chem. 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shamy, A.G. New nano-composite based on carbon dots (CDots) decorated magnesium oxide (MgO) nano-particles (CDots@MgO) sensor for high H2S gas sensitivity performance. Sens. Actuators B Chem. 2021, 329, 129154. [Google Scholar] [CrossRef]
- Ramos-Ramón, J.A.; Bogireddy, N.K.R.; Giles Vieyra, J.A.; Karthik, T.V.K.; Agarwal, V. Nitrogen-doped carbon dots induced enhancement in CO2 sensing response from ZnO–porous silicon hybrid structure. Front. Chem. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, G.; Dong, Y.; Chi, Y.; Chen, G. Carbon quantum dot-functionalized aerogels for NO2 gas sensing. Anal. Chem. 2013, 85, 8065–8069. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M. CQDs@NiO: An efficient tool for CH4 sensing. Appl. Sci. 2020, 10. [Google Scholar] [CrossRef]
- Aguilar Cosme, J.R.; Bryant, H.E.; Claeyssens, F. Carbon dot-protoporphyrin IX conjugates for improved drug delivery and bioimaging. PLoS ONE 2019, 14, e0220210. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, T.; Yang, M.; Zhang, W.; Kong, C.; Hao, M.; Wang, Y.; Zhang, H.; Yang, B.; Yang, J.; et al. Synthesis of dual functional procaine-derived carbon dots for bioimaging and anticancer therapy. Nanomedicine 2020, 15, 677–689. [Google Scholar] [CrossRef]
- Li, H.; Yan, X.; Kong, D.; Jin, R.; Sun, C.; Du, D.; Lin, Y.; Lu, G. Recent advances in carbon dots for bioimaging applications. Nanoscale Horizons 2020, 5, 218–234. [Google Scholar] [CrossRef]
- Shakhov, A.M.; Astafiev, A.A.; Osychenko, A.A.; Syrchina, M.S.; Nadtochenko, V.A. Live cell bioimaging with carbon dots produced in situ by femtosecond laser from intracellular material. bioRxiv 2018, d, 1–6. [Google Scholar] [CrossRef]
- Wu, F.; Su, H.; Wang, K.; Wong, W.K.; Zhu, X. Facile synthesis of N-rich carbon quantum dots from porphyrins as efficient probes for bioimaging and biosensing in living cells. Int. J. Nanomed. 2017, 12, 7375–7391. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, M.; Bhatt, S.; Vyas, G.; Raval, I.H.; Haldar, S.; Paul, P. Water-dispersible fluorescent carbon dots as bioimaging agents and probes for Hg2+ and Cu2+ ions. ACS Appl. Nano Mater. 2020, 3, 7096–7104. [Google Scholar] [CrossRef]
- Yue, L.; Li, H.; Sun, Q.; Zhang, J.; Luo, X.; Wu, F.; Zhu, X. Red-emissive ruthenium-containing carbon dots for bioimaging and photodynamic cancer therapy. ACS Appl. Nano Mater. 2020, 3, 869–876. [Google Scholar] [CrossRef] [Green Version]
- V, R.; Gujar, V.; Pathan, H.; Islam, S.; Tawre, M.; Pardesi, K.; Santra, M.K.; Ottoor, D. Bioimaging applications of carbon dots (C. dots) and its cystamine functionalization for the sensitive detection of Cr(VI) in aqueous samples. J. Fluoresc. 2019, 29, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Adrita, S.; Tasnim, K.; Ryu, J.; Sharker, S. Nanotheranostic carbon dots as an emerging platform for cancer therapy. J. Nanotheranostics 2020, 1, 58–77. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Negut, I.; Grumezescu, V.; Grumezescu, A.M.; Teleanu, R.I. Nanomaterials for drug delivery to the central nervous system. Nanomaterials 2019, 9, 371. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Lin, Y.; Han, N.; Li, X.; Geng, H.; Wang, X.; Cui, Y.; Wang, S. Mesoporous carbon nanomaterials in drug delivery and biomedical application. Drug Deliv. 2017, 24, 94–107. [Google Scholar] [CrossRef]
- Yunus, U.; Zulfiqar, M.A.; Ajmal, M.; Bhatti, M.H.; Chaudhry, G.E.S.; Muhammad, T.S.T.; Sung, Y.Y. Targeted drug delivery systems: Synthesis and in vitro bioactivity and apoptosis studies of gemcitabine-carbon dot conjugates. Biomed. Mater. 2020, 15. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, S.; Liu, L.; Kong, Y.; Zhang, A.; Xu, K.; Han, C. The cost-effective preparation of green fluorescent carbon dots for bioimaging and enhanced intracellular drug delivery. Nanoscale Res. Lett. 2020, 15. [Google Scholar] [CrossRef] [Green Version]
- Hettiarachchi, S.D.; Graham, R.M.; Mintz, K.J.; Zhou, Y.; Vanni, S.; Peng, Z.; Leblanc, R.M. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale 2019, 11, 6192–6205. [Google Scholar] [CrossRef]
- Su, W.; Guo, R.; Yuan, F.; Li, Y.; Li, X.; Zhang, Y.; Zhou, S.; Fan, L. Red-emissive carbon quantum dots for nuclear drug delivery in cancer stem cells. J. Phys. Chem. Lett. 2020, 11, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Hao, L.; Wei, Y.; Cai, X.; Zhu, B. Doxorubicin conjugated carbon dots as a drug delivery system for human breast cancer therapy. Cell Prolif. 2018, 51, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, T.; Ai, X.; An, G.; Yang, P.; Zhao, Y. Charge-convertible carbon dots for imaging-guided drug delivery with enhanced in vivo cancer therapeutic efficiency. ACS Nano 2016, 10, 4410–4420. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, A.; Mofazzal Jahromi, M.A.; Abdoli, A.; Mohammad-Beigi, H.; Fatahi, Y.; Nourizadeh, H.; Zare, H.; Kiani, J.; Radmanesh, F.; Rabiee, N.; et al. Photoluminescent carbon quantum dot/poly-L-Lysine core-shell nanoparticles: A novel candidate for gene delivery. J. Drug Deliv. Sci. Technol. 2021, 61, 102118. [Google Scholar] [CrossRef]
- Taghavi, S.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Hybrid carbon-based materials for gene delivery in cancer therapy. J. Control. Release 2020, 318, 158–175. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, W.; Wang, Y.; Cao, X.; Chen, J.; Wang, Q.; Xu, W.; Du, P.; Yu, Q.; Chen, J.; et al. Cationic carbon quantum dots derived from alginate for gene delivery: One-step synthesis and cellular uptake. Acta Biomater. 2016, 42, 209–219. [Google Scholar] [CrossRef]
- Cao, X.; Wang, J.; Deng, W.; Chen, J.; Wang, Y.; Zhou, J.; Du, P.; Xu, W.; Wang, Q.; Wang, Q.; et al. Photoluminescent cationic carbon dots as efficient non-viral delivery of plasmid SOX9 and chondrogenesis of fibroblasts. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Dadashzadeh, A.; Moghassemi, S.; Ashrafizadeh, M.; Dehshahri, A.; Pardakhty, A.; Sassan, H.; Sohrevardi, S.M.; Mandegary, A. Shedding light on gene therapy: Carbon dots for the minimally invasive image-guided delivery of plasmids and noncoding RNAs - A review. J. Adv. Res. 2019, 18, 81–93. [Google Scholar] [CrossRef]
- Yang, L.; Xue, S.; Du, M.; Lian, F. Highly efficient MicroRNA delivery using functionalized carbon dots for enhanced conversion of fibroblasts to cardiomyocytes. Int. J. Nanomedicine 2021, 16, 3741–3754. [Google Scholar] [CrossRef]
- He, X.; Chen, P.; Zhang, J.; Luo, T.Y.; Wang, H.J.; Liu, Y.H.; Yu, X.Q. Cationic polymer-derived carbon dots for enhanced gene delivery and cell imaging. Biomater. Sci. 2019, 7, 1940–1948. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Shen, G.; Liu, H.; Fu, H.; Cui, D. Fluorescent carbon dots as an efficient siRNA nanocarrier for its interference therapy in gastric cancer cells. J. Nanobiotechnol. 2014, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Yue, L.; Su, H.; Wang, K.; Yang, L.; Zhu, X. Carbon dots @ platinum porphyrin composite as theranostic nanoagent for efficient photodynamic cancer therapy. Nanoscale Res. Lett. 2018, 13. [Google Scholar] [CrossRef]
- Felsher, D.W. Cancer revoked: Oncogenes as therapeutic targets. Nat. Rev. Cancer 2003, 3, 375–380. [Google Scholar] [CrossRef]
- He, H.; Zheng, X.; Liu, S.; Zheng, M.; Xie, Z.; Wang, Y.; Yu, M.; Shuai, X. Diketopyrrolopyrrole-based carbon dots for photodynamic therapy. Nanoscale 2018, 10, 10991–10998. [Google Scholar] [CrossRef]
- Zhang, D.; Wen, L.; Huang, R.; Wang, H.; Hu, X.; Xing, D. Mitochondrial specific photodynamic therapy by rare-earth nanoparticles mediated near-infrared graphene quantum dots. Biomaterials 2018, 153, 14–26. [Google Scholar] [CrossRef]
- Fowley, C.; McHale, A.P.; McCaughan, B.; Fraix, A.; Sortino, S.; Callan, J.F. Carbon quantum dot-NO photoreleaser nanohybrids for two-photon phototherapy of hypoxic tumors. Chem. Commun. 2015, 51, 81–84. [Google Scholar] [CrossRef]
- Lovell, J.F.; Liu, T.W.B.; Chen, J.; Zheng, G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z.; Zha, S.; Zhu, Y.; Wu, P.; Ehrenberg, B.; Chen, J.Y. Carbon nanodots featuring efficient FRET for two-photon photodynamic cancer therapy with a low fs laser power density. Biomaterials 2014, 35, 9372–9381. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.B.; Liu, M.L.; Huang, C.Z. Recent advances of carbon dots in imaging-guided theranostics. TrAC - Trends Anal. Chem. 2021, 134, 116116. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S.; Zhang, J.; Zhou, R.; Cai, X. Sulphur doped carbon dots enhance photodynamic therapy via PI3K/Akt signalling pathway. Cell Prolif. 2020, 53, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, D.; Xu, M.; Wang, J.; Hu, X.; Anwar, S.; Tedesco, A.C.; Morais, P.C.; Bi, H. Fluorine-containing graphene quantum dots with a high singlet oxygen generation applied for photodynamic therapy. J. Mater. Chem. B 2020, 8, 2598–2606. [Google Scholar] [CrossRef]
- Chen, S.; Sun, T.; Zheng, M.; Xie, Z. Carbon dots based nanoscale covalent organic frameworks for photodynamic therapy. Adv. Funct. Mater. 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Xu, N.; Gu, Q.; Du, J.; Ge, H.; Long, S.; Sun, W.; Fan, J.; Peng, X. Photodynamic inheritance from methylene blue to carbon dots against reduction, aggregation, and DNA interference. Sci. China Mater. 2021, 64, 2325–2336. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Wang, D.; Li, Z.; Primo, F.L.; Tedesco, A.C.; Bi, H. Copper-doped carbon dots for optical bioimaging and photodynamic therapy. Inorg. Chem. 2019, 58, 13394–13402. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, X.; Zhang, X.; Liu, S.; Pei, Q.; Zheng, M.; Xie, Z. Porphyrin-based carbon dots for photodynamic therapy of hepatoma. Adv. Healthc. Mater. 2017, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Du, J.; Yao, Q.; Ge, H.; Li, H.; Xu, F.; Gao, F.; Xian, L.; Fan, J.; Peng, X. Precise photodynamic therapy: Penetrating the nuclear envelope with photosensitive carbon dots. Carbon N. Y. 2020, 159, 74–82. [Google Scholar] [CrossRef]
- Li, X.; Vinothini, K.; Ramesh, T.; Rajan, M.; Ramu, A. Combined photodynamic-chemotherapy investigation of cancer cells using carbon quantum dot-based drug carrier system. Drug Deliv. 2020, 27, 791–804. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, R.; Xie, Y.; Li, Y.; Chen, Y.; Cai, X. Sulphur-doped carbon dots as a highly efficient nano-photodynamic agent against oral squamous cell carcinoma. Cell Prolif. 2020, 53, 1–11. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, B.; Chen, L.; Lin, Z.; Zhang, X.; Ge, D.; Shi, W.; Sun, Y. Facile one-pot synthesis of polydopamine carbon dots for photothermal therapy. Nanoscale Res. Lett. 2018, 13. [Google Scholar] [CrossRef]
- Zheng, M.; Li, Y.; Liu, S.; Wang, W.; Xie, Z.; Jing, X. One-pot to synthesize multifunctional carbon dots for near infrared fluorescence imaging and photothermal cancer therapy. ACS Appl. Mater. Interfaces 2016, 8, 23533–23541. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, F.; Wang, Q.; Tong, R.; Lin, H.; Qu, F. Near-infrared light-mediated LA-UCNPs@SiO2-C/HA@mSiO2-DOX@NB nanocomposite for chemotherapy/PDT/PTT and imaging. Dalt. Trans. 2017, 46, 14293–14300. [Google Scholar] [CrossRef]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-phase semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.; Chen, S.; Wang, M. Facile preparation of carbon-dot-supported nanoflowers for efficient photothermal therapy of cancer cells. Dalt. Trans. 2018, 47, 1777–1781. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, R.; Wang, T.; Yang, W.; Wang, H.; Gu, W.; Ye, L. Carbon dots/prussian blue satellite/core nanocomposites for optical imaging and photothermal therapy. ACS Appl. Mater. Interfaces 2018, 10, 1084–1092. [Google Scholar] [CrossRef]
- Geng, B.; Shen, W.; Fang, F.; Qin, H.; Li, P.; Wang, X.; Li, X.; Pan, D.; Shen, L. Enriched graphitic N dopants of carbon dots as F cores mediate photothermal conversion in the NIR-II window with high efficiency. Carbon N. Y. 2020, 162, 220–233. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, X.; Liang, L.; Yue, J.; Huang, D.; Xu, W.; Shi, W.; Liang, C.; Xu, S. Mitochondria-targeting supra-carbon dots: Enhanced photothermal therapy selective to cancer cells and their hyperthermia molecular actions. Carbon N. Y. 2020, 156, 558–567. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Q.; Zhang, L.; Zhang, H.; Han, Y.; Sun, Q.; Cheng, Z.; Qin, H.; Dou, S.; Li, Z. Vacancy engineering of Cu2-: XSe nanoparticles with tunable LSPR and magnetism for dual-modal imaging guided photothermal therapy of cancer. Nanoscale 2018, 10, 3130–3143. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shen, J.; Li, Y.; Wei, Z.; Cao, G.; Gai, Z.; Hong, K.; Banerjee, P.; Zhou, S. Magnetic iron oxide-fluorescent carbon dots integrated nanoparticles for dual-modal imaging, near-infrared light-responsive drug carrier and photothermal therapy. Biomater. Sci. 2014, 2, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Yang, D.; Pan, D.; Wang, L.; Zheng, F.; Shen, W.; Zhang, C.; Li, X. NIR-responsive carbon dots for efficient photothermal cancer therapy at low power densities. Carbon N. Y. 2018, 134, 153–162. [Google Scholar] [CrossRef]
- Li, Y.; Bai, G.; Zeng, S.; Hao, J. Theranostic carbon dots with innovative NIR-II emission for in vivo renal-excreted optical imaging and photothermal therapy. ACS Appl. Mater. Interfaces 2019, 11, 4737–4744. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Yuan, Y.; Chen, J.; Zhang, B.; Li, D.; Zhou, D.; Jing, P.; Xu, G.; Wang, Y.; Holá, K.; et al. In vivo theranostics with near-infrared-emitting carbon dots—highly efficient photothermal therapy based on passive targeting after intravenous administration. Light Sci. Appl. 2018, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Permatasari, F.A.; Fukazawa, H.; Ogi, T.; Iskandar, F.; Okuyama, K. Design of pyrrolic-N-rich carbon dots with absorption in the first near-infrared window for photothermal therapy. ACS Appl. Nano Mater. 2018, 1, 2368–2375. [Google Scholar] [CrossRef]
- Lee, D.; Lee, C.; Kwon, W.; Beack, S.; Kim, C. N-doped carbon nanodots for non-invasive photoacoustic imaging and photothermal therapy. Photons Plus Ultrasound Imaging Sens. 2017, 10064, 1006433. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Yingjun, C.; Zhou, N.; Shen, J. photothermal therapy nanoplatform by the assembly of Fe3O4 carbon dots with graphitic black phosphorus quantum dots. Int. J. Nanomedicine 2018, 3, 2803–2819. [Google Scholar] [CrossRef] [Green Version]
- Lan, M.; Guo, L.; Zhao, S.; Zhang, Z.; Jia, Q.; Yan, L.; Xia, J.; Zhang, H.; Wang, P.; Zhang, W. Carbon dots as multifunctional phototheranostic agents for photoacoustic/fluorescence imaging and photothermal/photodynamic synergistic cancer therapy. Adv. Ther. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Huang, G.; Lin, Y.; Zhang, L.; Yan, Z.; Wang, Y.; Liu, Y. Synthesis of sulfur-selenium doped carbon quantum dots for biological imaging and scavenging reactive oxygen species. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.; Wang, Y.; Lin, F.; Liu, Y.; Gu, R.; Liu, W.; Xiao, C. Selenium-doped carbon quantum dots efficiently ameliorate secondary spinal cord injury via scavenging reactive oxygen species. Int. J. Nanomedicine 2020, 15, 10113–10125. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Ma, Y.; Wang, B.; Huang, H.; Liu, Y.; Shao, M.; Kang, Z.; Huang, H.; Liu, Y.; et al. Carbon dots derived from citric acid and glutathione as a highly efficient intracellular reactive oxygen species scavenger for alleviating the lipopolysaccharide-induced inflammation in macrophages. ACS Appl. Mater. Interfaces 2020, 12, 41088–41095. [Google Scholar] [CrossRef]
- Das, B.; Pal, P.; Dadhich, P.; Dutta, J.; Dhara, S. In vivo cell tracking, reactive oxygen species scavenging, and antioxidative gene down regulation by long-term exposure of biomass-derived carbon dots. ACS Biomater. Sci. Eng. 2019, 5, 346–356. [Google Scholar] [CrossRef]







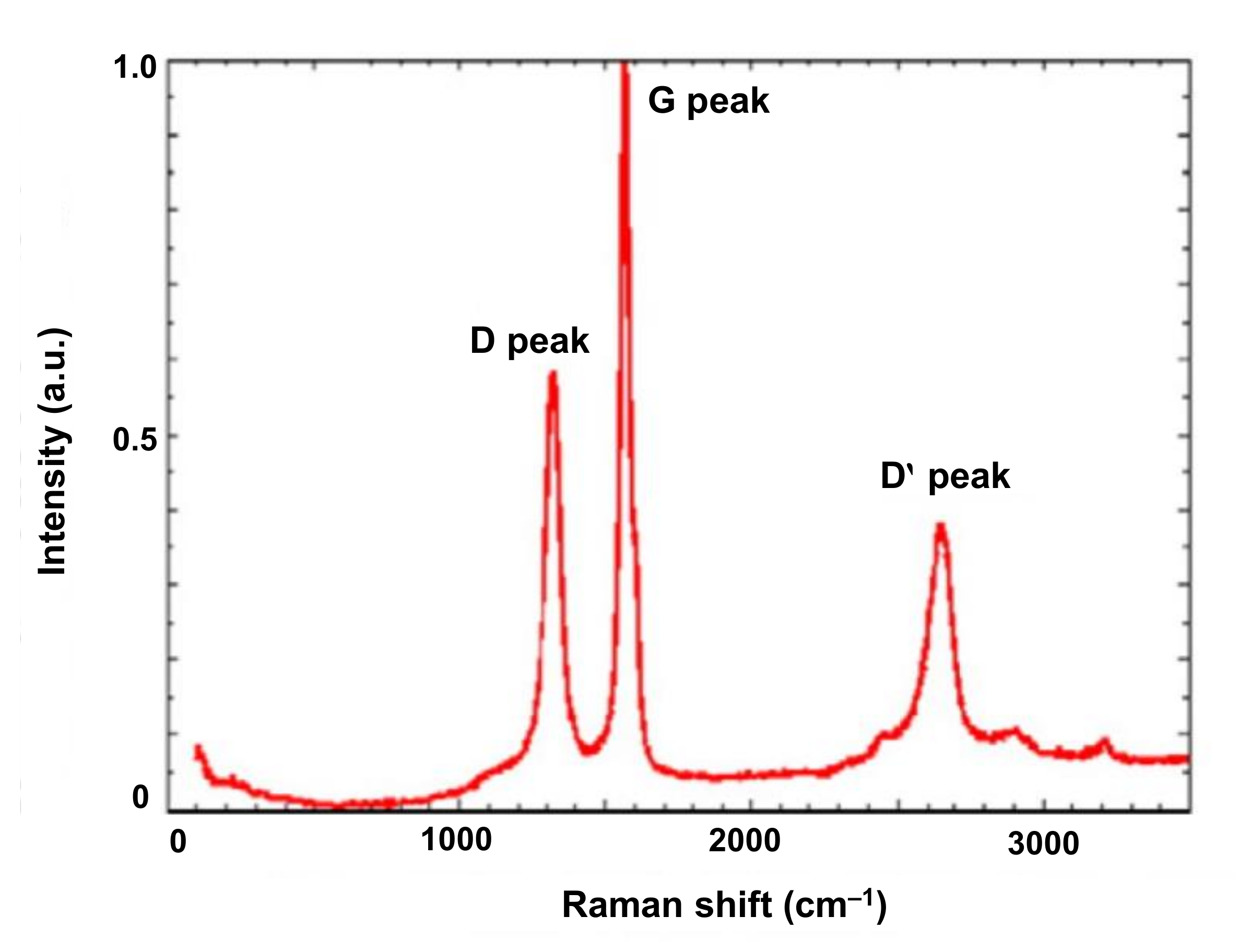




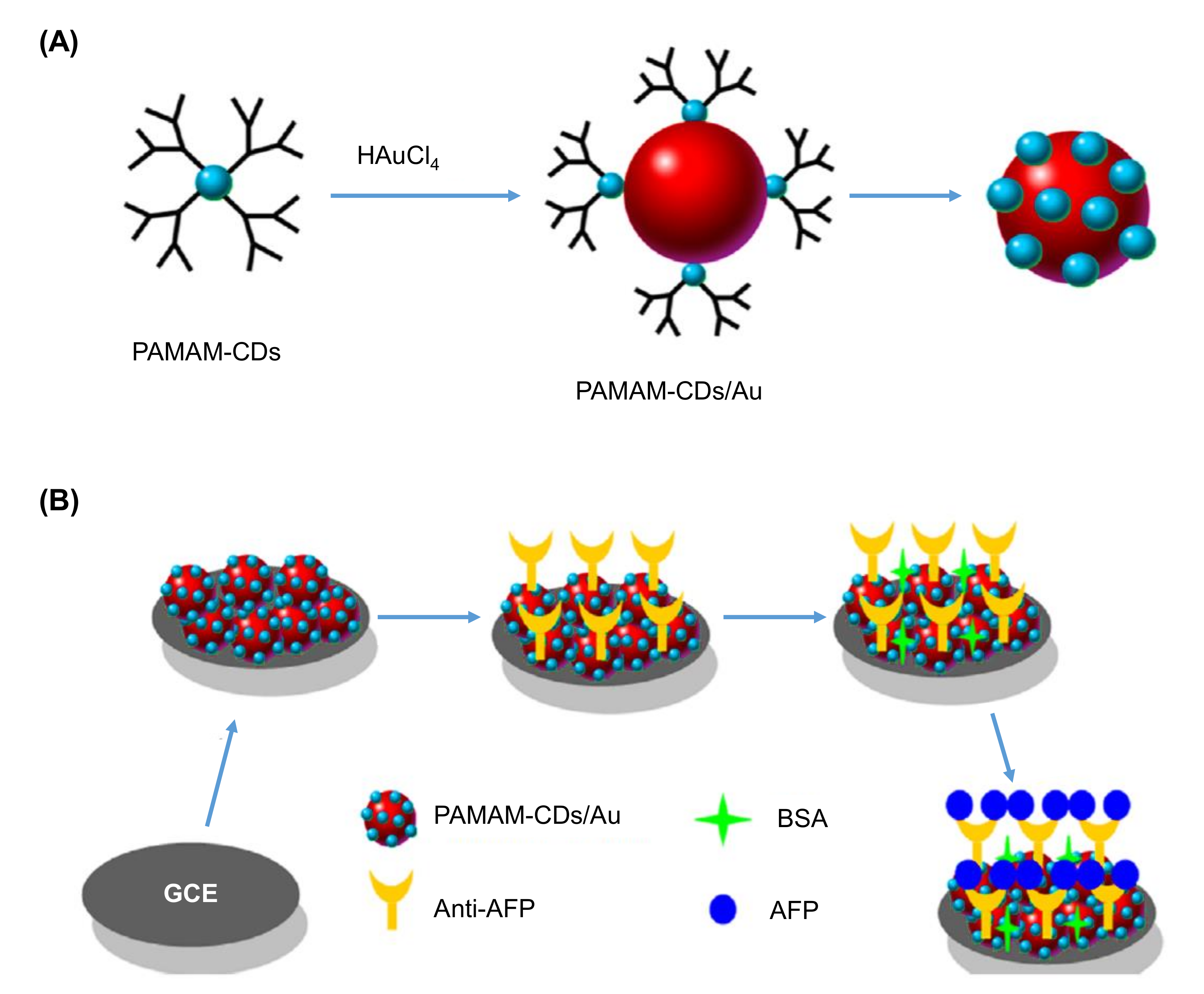



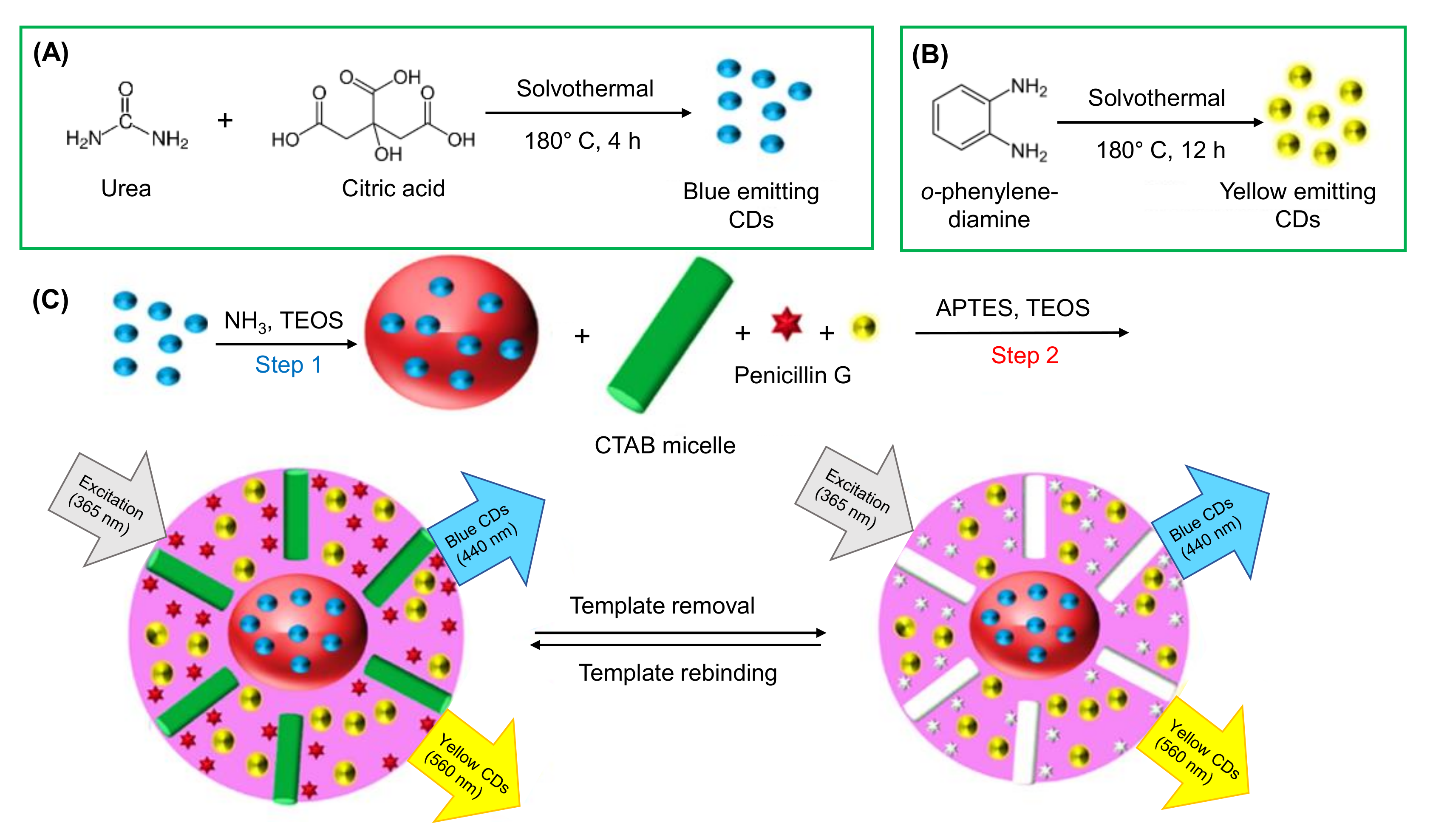














| Electrode | Nanomaterials | Receptor Type | Receptor | Target Analyte | Detection Technique(s) | Specimen | Linear Range | Detection Limit | Reference |
|---|---|---|---|---|---|---|---|---|---|
| ITO | CDs-PMMA | Antibody | Anti-TNFα | TNFα | Amperometry | Buffer; human blood | 0.05–160 pg mL−1 | 0.05 pg mL−1 | [238] |
| SPCE | CDs/ZnO/PANI | Nucleic acid | ss-DNA probe | E. coli O157:H7 | DPV | Water samples | 1.3 × 10−18–10 × 10−12 M | 1.3 × 10−18 M | [239] |
| SPGE | CDs | Nucleic acid | ds-DNA probe | Target DNAs | DPV | DNA isolated from peripheral leucocytes | 0.001–20 µM | 0.16 nM | [240] |
| SPCE | CDs | Nucleic acid | Aptamer probe | 17 β- estradiol | EIS | Water samples | 1 × 10−7–1 × 10−12 M | 0.5 × 10−12 M | [241] |
| Graphite electrode | CDs/PFTBDT | Enzyme | Laccase | Catechol | Amperometry | Water samples | 1.25–175 µM | 1.23 µM | [242] |
| - | CoCu-ZIF@CDs | Cells | B16-F10 cell-targeted aptamer strands | B16-F10 cells | EIS | Human B16-F10 living cells | 1 × 102–1 × 105 cells mL−1 | 33 cells mL−1 | [243] |
| GCE | g-C3N4/N-CDs | MIP | EPI imprinted polymer | EPI | CV; EIS | Human urine samples | 1 pM–1 nM | 0.3 pM | [244] |
| PGE | ABSA/CDs | MIP | FA imprinted polymer | FA | CV; DPCSV | Pharmaceuticals; human urine samples | 2.2–30.8 ng mL−1 | 2.02 ng mL−1 | [245] |
| Nanomaterials | Target Analyte | Specimen | Linear Range | Detection Limit | Reference |
|---|---|---|---|---|---|
| CDs | Ferricyanide | Real water samples | 5–100 µM | 1.7 µM | [269] |
| N-CDs | Fe3+; ATP | Water samples; Human serum | 0–350 µM; 0.01–450 µM | 0.01 µM; 0.005 µM | [270] |
| CDs | Diazinon; Amicarbazone; Glyphosphate | Fruit samples | 0.25–5000 ng mL‒1; 0.5–5000 ng mL‒1; 2–5000 ng mL‒1 | 0.25 ng mL‒1; 0.5 ng mL‒1; 2 ng mL‒1 | [271] |
| CDs | Fe3+ | Real water samples | 8–80 µM | 3.8 µM | [272] |
| Cu-CDs | Rutin | Pharmaceutical samples | 0.1–15 µg mL‒1 | 0.05 µg mL‒1 | [273] |
| CDs | TNP | HeLa cells | 5–1000 µM | 0.5 µM | [274] |
| CDs | Pretilachlor | Soil samples | 5.7–61.5 µM | 2.9 µM | [275] |
| CDs-AuNCs | Dopamine | Human serum samples | 5‒180 nM | 2.9 nM | [276] |
| N,S-CDs | Ascorbic acid | Fruit samples | 10–200 µmol L‒1 | 4.69 µmol L‒1 | [277] |
| CDs@EDTA | Cr(IV); Ascorbic acid | Real water samples | 10 nM–50 µM; 0.1–400 µM | 10 nM; 0.1 µM | [278] |
| CDs | Fe3+; Pyrophosphate | Tap water samples; Human urine; Human serum | 1–60 µM; 0.1–120 µM | 0.28 µM; 0.032 µM | [279] |
| CDs | Norfloxacin; Ciprofloxacin; Ofloxacin; Histidine | Pharmaceutical tablets; Milk samples | 0.05–50 µmol L‒1; 0.2–25 µmol L‒1; 0.4–10 µmol L‒1; 0.05–10 µM | 17 nmol L‒1; 35 nmol L‒1; 65 nmol L‒1; 35 nM | [280] |
| CDs | Hg2+ | Lake water samples; Human serum | 0.50–20 μM | 12.4 nM | [281] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansuriya, B.D.; Altintas, Z. Carbon Dots: Classification, Properties, Synthesis, Characterization, and Applications in Health Care—An Updated Review (2018–2021). Nanomaterials 2021, 11, 2525. https://doi.org/10.3390/nano11102525
Mansuriya BD, Altintas Z. Carbon Dots: Classification, Properties, Synthesis, Characterization, and Applications in Health Care—An Updated Review (2018–2021). Nanomaterials. 2021; 11(10):2525. https://doi.org/10.3390/nano11102525
Chicago/Turabian StyleMansuriya, Bhargav D., and Zeynep Altintas. 2021. "Carbon Dots: Classification, Properties, Synthesis, Characterization, and Applications in Health Care—An Updated Review (2018–2021)" Nanomaterials 11, no. 10: 2525. https://doi.org/10.3390/nano11102525








