One-Pot Synthesis of Glucose-Derived Carbon Coated Ni3S2 Nanowires as a Battery-Type Electrode for High Performance Supercapacitors
Abstract
:1. Introduction
2. Experimental Section
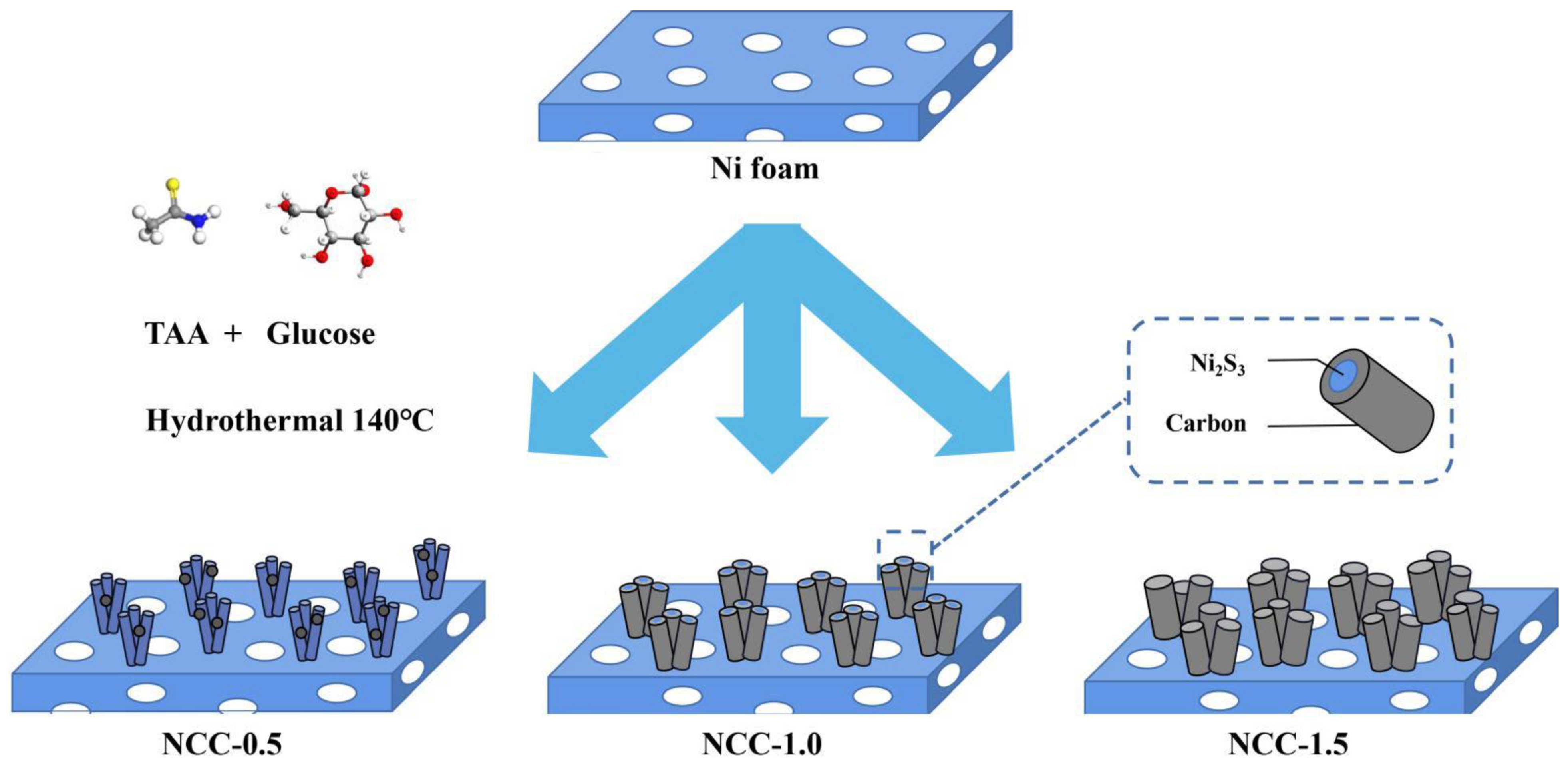
2.1. The Preparation of Bare Ni3S2 Rod-Like Structure
2.2. The Preparation of NCC-0.5/1/1.5
2.3. Characterization of Samples
2.4. Electrochemical Tests
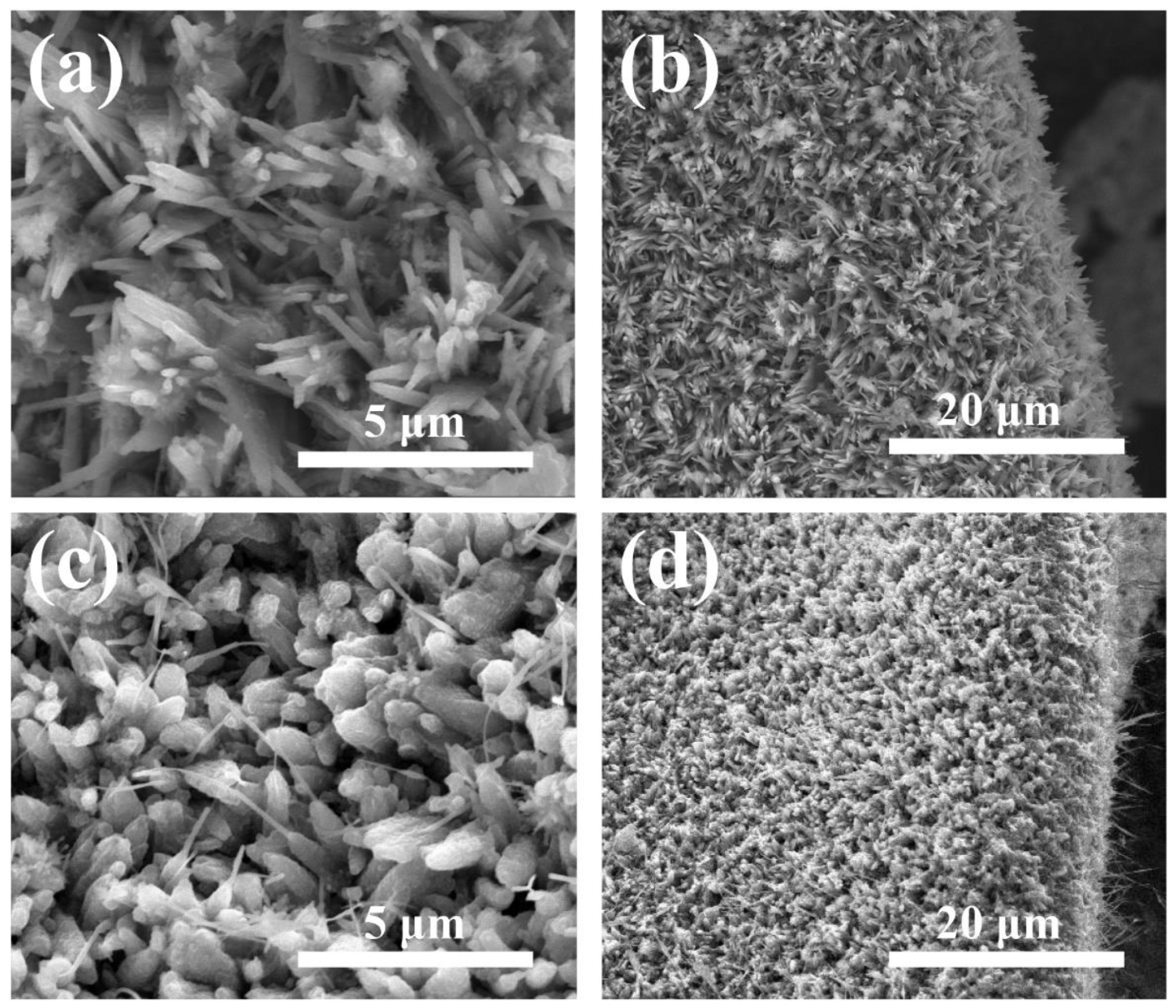
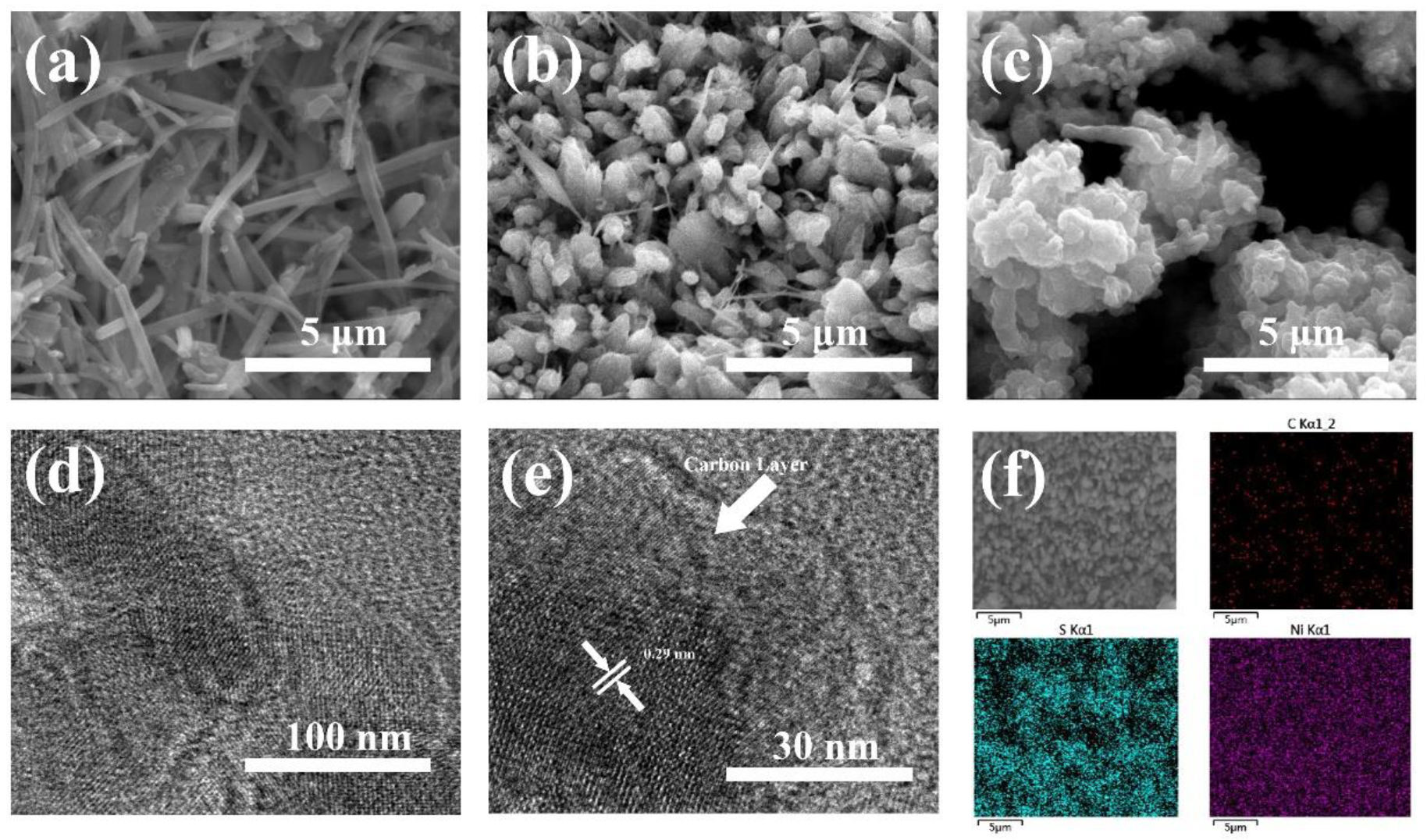
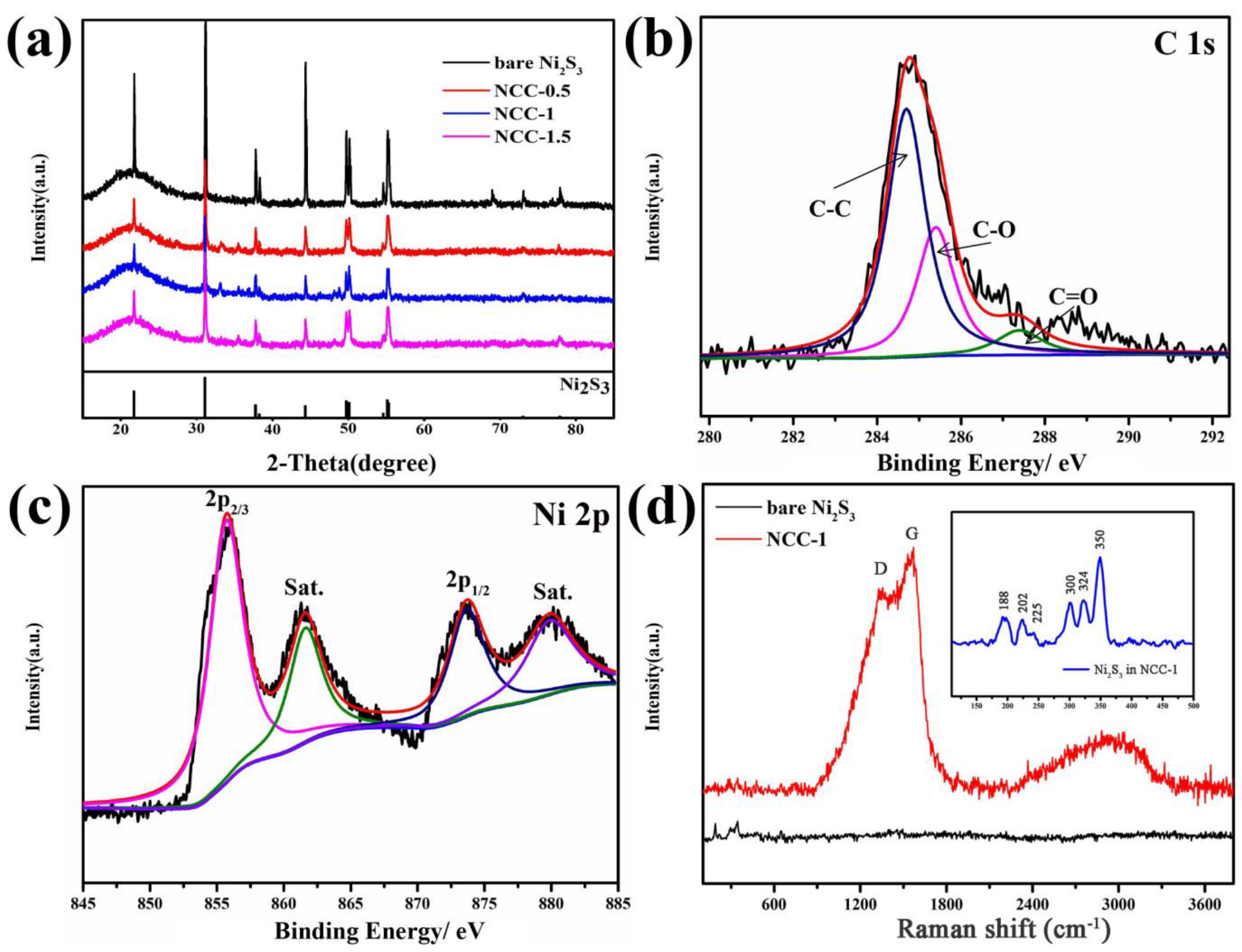
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, Y.; Sui, Y.; Qi, J.; Jiang, L.; He, Y.; Wei, F.; Meng, Q.; Jin, Y. Facile synthesis of Ni3S2 and Co9S8 double-size nanoparticles decorated on rGO for high-performance supercapacitor electrode materials. Electrochim. Acta 2017, 226, 69–78. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 5, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon materials for chemical capacitive energy storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zheng, M.; Xue, H.; Pang, H. High performance electrochemical capacitor materials focusing on nickel based materials. Inorg. Chem. Front. 2016, 3, 175–202. [Google Scholar] [CrossRef]
- Lai, F.; Miao, Y.E.; Huang, Y.; Chung, T.S.; Liu, T. Flexible hybrid hembranes of NiCo2O4-doped carbon nanofiber@mno2 core-sheath nanostructures for high-performance supercapacitors. J. Phys. Chem. C 2015, 119, 13442–13450. [Google Scholar] [CrossRef]
- Yu, X.Y.; Yu, L.; Lou, X.W. Metal sulfide hollow nanostructures for electrochemical energy storage. Adv. Energy Mater. 2016, 6, 1501333. [Google Scholar] [CrossRef]
- Chen, W.; Xia, C.; Alshareef, H.N. One-step electrodeposited nickel cobalt sulfide nanosheet arrays for high-performance asymmetric supercapacitors. ACS Nano 2014, 8, 9531–9541. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Senthil, R.A.; Pan, J.; Osman, S.; Sun, Y.; Shu, X. A new biomass derived rod-like porous carbon from tea-waste as inexpensive and sustainable energy material for advanced supercapacitor application. Electrochim. Acta 2020, 335, 135588. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Yang, V.; Senthil, R.A.; Pan, J.; Kumar, T.R.; Sun, Y.; Liu, X. Hierarchical porous carbon derived from jujube fruits as sustainable and ultrahigh capacitance material for advanced supercapacitors. J. Colloid Interfaces Sci. 2020, 579, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.M.; Sahu, R.P.; Wallar, C.J.; Chen, R.; Zhitomirsky, I.; Puri, I.K. Nickel oxide nanotubes synthesis using multiwalled carbon nanotubes as sacrificial templates for supercapacitor application. Nanotechnology 2016, 28, 075603. [Google Scholar] [CrossRef]
- Xing, Z.; Chu, Q.; Ren, X.; Ge, C.; Qusti, A.H.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Ni3S2 coated ZnO array for high-performance supercapacitors. J. Power Sources 2014, 245, 463–467. [Google Scholar] [CrossRef]
- Zhou, W.; Cao, X.; Zeng, Z.; Shi, W.; Zhu, Y.; Yan, Q.; Liu, H.; Wang, J.; Zhang, H. One-step synthesis of Ni3S2 nanorod@ Ni(OH)2 nanosheet core–shell nanostructures on a three-dimensional graphene network for high-performance supercapacitors. Energy Environ. Sci. 2016, 6, 2216–2221. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.C.; Pereira, M.L.; Sousa, J.P. Evaluation of nickel toxicity on liver, spleen, and kidney of mice after administration of high-dose metal ion. J. Biomed. Mater. Res. 1998, 40, 40–47. [Google Scholar] [CrossRef]
- Gao, M.R.; Xu, Y.F.; Jiang, J.; Yu, S.H. Nanostructured metal chalcogenides: Synthesis, modification, and applications in energy conversion and storage devices. Chem. Soc. Rev. 2013, 42, 2986–3167. [Google Scholar] [CrossRef]
- Zhang, F.; Xiao, F.; Dong, Z.H.; Shi, W. Synthesis of polypyrrole wrapped graphene hydrogels composites as supercapacitor electrodes. Electrochim. Acta 2013, 114, 125–132. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Ma, G.; Wang, Z.; Liu, K.; Liu, H. Ethylene glycol reduced.graphene oxide/polypyrrole composite for supercapacitor. Electrochim. Acta 2013, 88, 519–525. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, W.; Zhang, Y.; Dou, Y.; Jiang, Y.; Dou, S.X. Engineering hierarchical hollow nickel sulfifide spheres for high-performance sodium storage. Adv. Funct. Mater. 2016, 26, 7479–7485. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Li, L.; Tan, H.; Cai, R.; Shi, W.; Li, C.; Mhaisalkar, S.G.; Srinivasan, M.; Ramakrishna, S.; Yan, Q. MS2(M ¼ Co and Ni) hollow spheres with tunable interiors for high-performance supercapacitors and photovoltaics. Adv. Funct. Mater. 2014, 24, 2155–2162. [Google Scholar] [CrossRef]
- Shi, Z.; Xing, L.; Liu, Y.; Gao, Y.; Liu, J. A porous biomass-based sandwich-structured Co3O4@ carbon fiber @Co3O4 composite for high-performance supercapacitors. Carbon 2018, 129, 819–825. [Google Scholar] [CrossRef]
- Lin, H.; Liu, F.; Wang, X.; Ai, Y.; Yao, Z.; Chu, L.; Han, S.; Zhuang, X. Graphene-coupled flower-Like Ni3S2 for a free-standing 3D aerogel with an ultra-high electrochemical capacity. Electrochim. Acta 2016, 191, 705–715. [Google Scholar] [CrossRef]
- Xiao, S.; Huang, J.; Lin, C.; Xie, A.; Lin, B.; He, L.; Sun, D. Porous carbon derived from rice husks as sustainable bioresources: Insights into the role of micro/mesoporous hierarchy in Co3O4/C composite for asymmetric supercapacitors. Microporous Mesoporous Mater. 2020, 291, 1387. [Google Scholar] [CrossRef]
- Yang, B.; Yu, L.; Liu, Q.; Liu, J.; Yang, W.; Zhang, H.; Wang, F.; Hu, S.; Yuan, Y.; Wang, J. The growth and assembly of the multidimensional hierarchical Ni3S2 for aqueous asymmetric supercapacitors. CrystEngComm 2015, 17, 4495–4501. [Google Scholar] [CrossRef]
- Yin, Q.; He, L.; Lian, J.; Sun, J.; Xiao, S.; Luo, J.; Sun, D.; Xie, A.; Lin, B. The synthesis of Co3O4/C composite with aloe juice as the carbon aerogel substrate for asymmetric supercapacitors. Carbon 2019, 155, 147–154. [Google Scholar] [CrossRef]
- Xia, X.; Zhu, C.; Luo, J.; Zeng, Z.; Guan, C.; Ng, C.F.; Zhang, H.; Fan, H.J. Synthesis of free-standing metal sulfide nanoarrays via anion exchange reaction and their electrochemical energy storage application. Small 2014, 10, 766–773. [Google Scholar] [CrossRef]
- Ji, F.; Jiang, D.; Chen, X.; Pan, X.; Kuang, L.; Zhang, Y.; Alameh, K.; Ding, B. Simple in-situ growth of layered Ni3S2 thin film electrode for the development of high-performance supercapacitors. J. Appl. Surf. 2016, 99, 432–439. [Google Scholar]
- Shen, M.; Liu, J.; Liu, T.; Yang, C.; He, Y.; Li, Z.; Li, J.; Qian, D. Oxidant-assisted direct-sulfidization of nickel foam toward a self-supported hierarchical Ni3S2@Ni electrode for asymmetric all-solid-state supercapacitors. J. Power Sources 2019, 448, 227408. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Z.; Ren, L.; Shen, Y.; Qi, X.; Zhong, J. One-pot synthesis of hierarchically nanostructured Ni3S2 dendrites as active materials for supercapacitors. Electrochim. Acta 2014, 149, 316–323. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, X.J.; Cao, X.; Huang, X.; Tan, C.; Tian, J.; Liu, H.; Wang, J.; Zhang, H. Ni3S2 nanorods/Ni foam composite electrode with low overpotential for electrocatalytic oxygen evolution. Energy Environ. Sci. 2013, 6, 2921–2924. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Xiao, T.; Tan, X.; Xiang, P.; Jiang, L.; Deng, C.; Li, W.; Li, M. Controllable preparation of nanoporous Ni3S2 films by sulfuration of nickel foam as promising asymmetric supercapacitor electrodes. Appl. Surf. Sci. 2017, 420, 919–926. [Google Scholar] [CrossRef]
- Zhong, J.H.; Wang, A.L.; Li, G.R.; Wang, J.W.; Ou, Y.N.; Tong, Y.X. Co3O4/Ni(OH)2 composite mesoporous nanosheet networks as a promising electrode for supercapacitor applications. J. Mater. Chem. 2012, 22, 5656–5665. [Google Scholar] [CrossRef]
- Yu, W.; Lin, W.; Shao, X.; Hu, Z.; Li, R.; Yuan, D. High performance supercapacitor based on Ni3S2/carbon nanofibers and carbon nanofibers electrodes derived from bacterial cellulose. J. Power Sources 2014, 272, 137–143. [Google Scholar] [CrossRef]
- Chen, J.S.; Guan, C.; Gui, Y.; Blackwood, D.J. Rational design of self-supported Ni3S2 nanosheets array for advanced asymmetric supercapacitor with a superior energy density. ACS Appl. Mater. Interfaces 2016, 9, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiao, Y.; Li, X.; Cheng, Q.; Wang, G. Bismuth oxide self-standing anodes with concomitant carbon dots welded graphene layer for enhanced performance supercapacitor-battery hybrid devices. J. Chem. Eng. 2019, 371, 327–336. [Google Scholar] [CrossRef]
- Liu, T.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical NiS/N-doped carbon composite hollow spheres with excellent supercapacitor performance. J. Mater. Chem. A 2017, 5, 1039. [Google Scholar] [CrossRef]
- Wen, J.; Li, S.; Zhou, K.; Song, Z.; Li, B.; Chen, Z.; Chen, T.; Guo, Y.; Fang, G. Flexible coaxial-type fiber solid-state asymmetrical supercapacitor based on Ni3S2 nanorod array and pen ink electrodes. J. Power Sources 2016, 324, 325–333. [Google Scholar] [CrossRef]
- Tian, Z.; Yin, J.; Wang, X.; Wang, Y. Construction of Ni3S2 wrapped by rGO on carbon cloth for flexible supercapacitor application. J. Alloys Compd. 2018, 777, 806–811. [Google Scholar] [CrossRef]
- Wu, P.; Wang, D.; Ning, J.; Zhang, J.; Feng, X.; Dong, J.; Hao, Y. Novel 3D porous graphene/Ni3S2 nanostructures for high-performance supercapacitor electrodes. J. Alloys Compd. 2018, 731, 1063–1078. [Google Scholar] [CrossRef]
- Dai, C.S.; Chien, P.Y.; Lin, J.Y.; Chou, S.W.; Wu, W.K.; Li, P.H.; Wu, K.Y.; Lin, T.W. Hierarchically structured Ni3S2/carbon nanotube composites as high performance cathode materials for asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 12168–12174. [Google Scholar] [CrossRef]
- Wang, L.; Bo, M.; Guo, Z.; Li, H.; Huang, Z.; Che, H.; Feng, Z.; Wang, Y.; Mu, J. Construction of ultra-stable trinickel disulphide (Ni3S2)/polyaniline (PANI) electrodes based on carbon fibers for high performance flexible asymmetric supercapacitors. J. Colloid Interface Sci. 2020, 577, 29–37. [Google Scholar] [CrossRef] [PubMed]
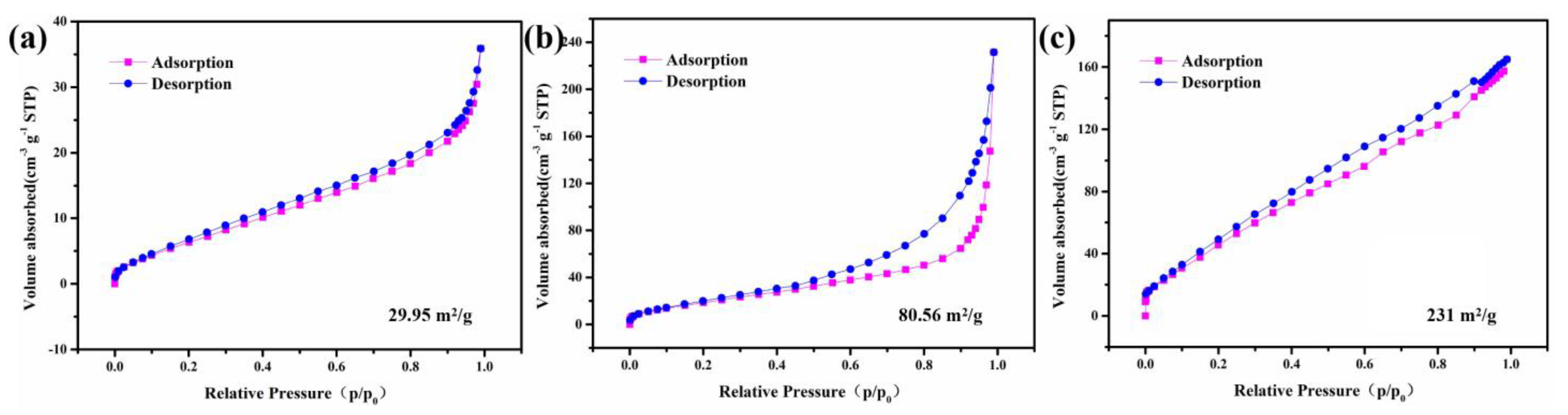
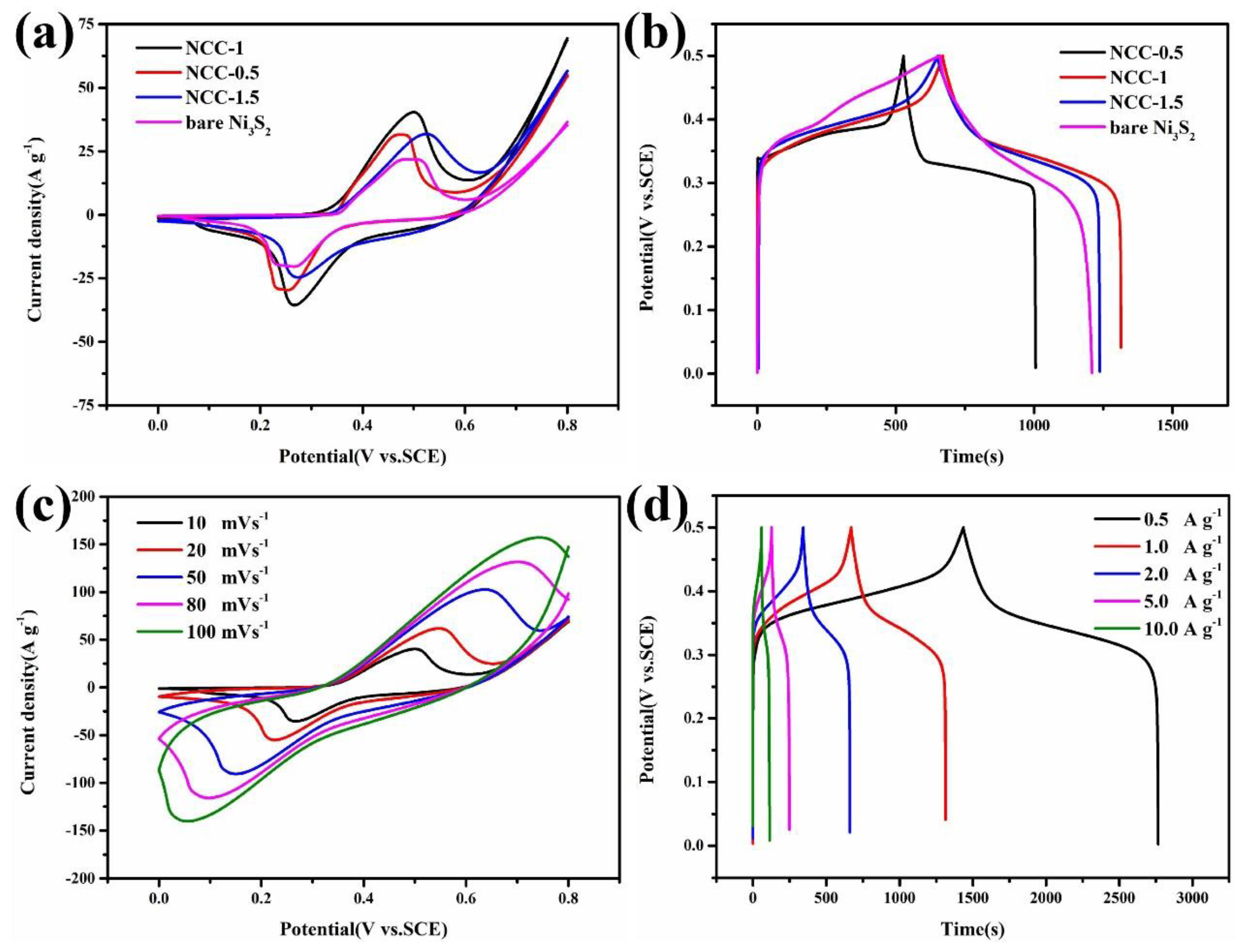

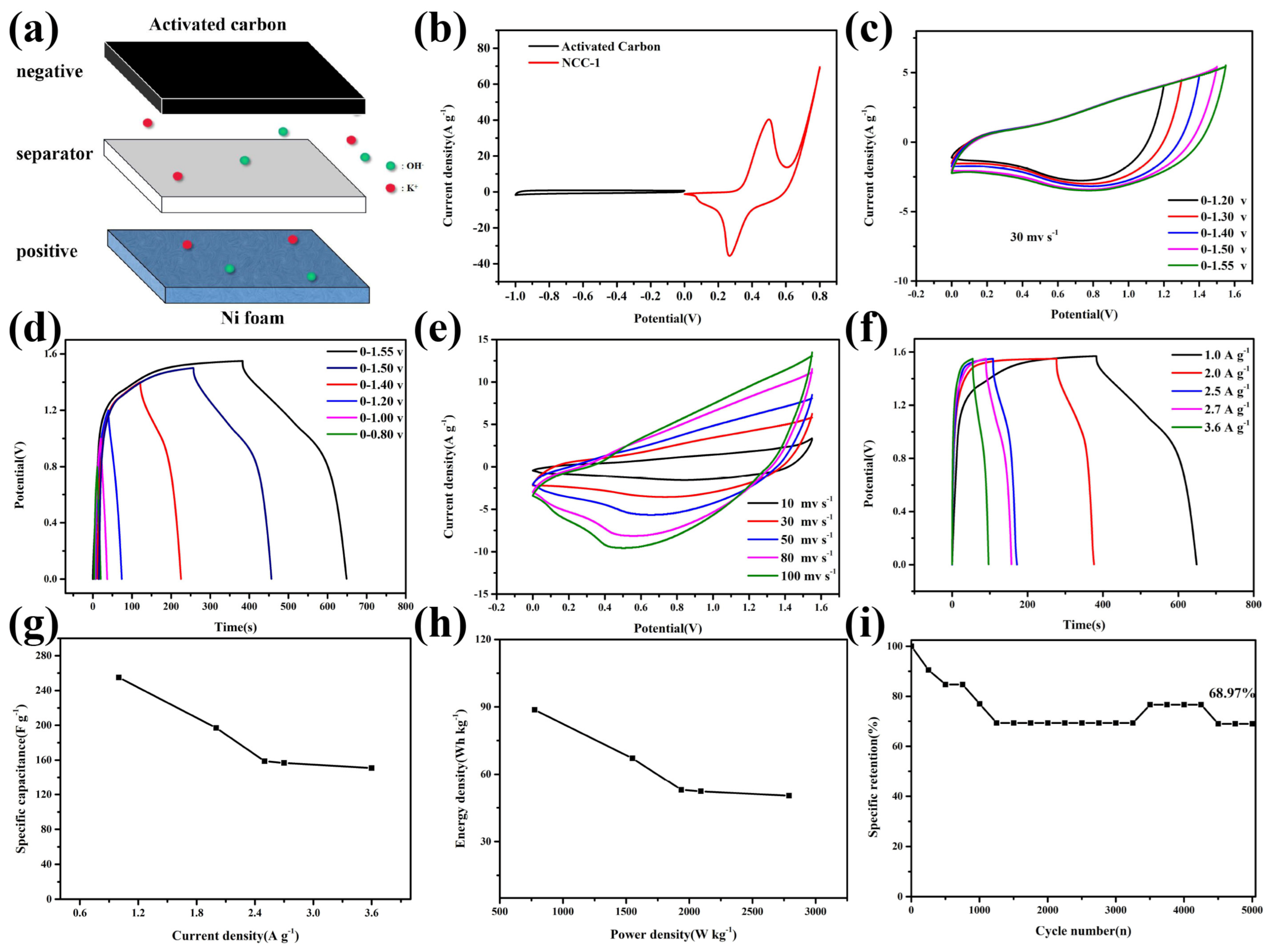
| Materials | Method of Synthesis | Specific Capacity (C g−1)/Current Density (A g−1) | Rate Performance from 1 to 10 A g−1 | Capacity Retention/Cycles |
|---|---|---|---|---|
| NiS@N-doped carbon sphere [36] | Multistep Transformation method | 517.5 C g−1/1 A g−1 | 78% | 76%/4000 |
| Ni3S2@CNF [37] | Hydrothermal | 380.6 C g−1/2 A g−1 | 83% | 88%/1500 |
| Ni3S2@rGO [38] | Vacuum filtration method | 477.37 C g−1/2 A g−1 | 76% | 88%/5000 |
| Ni3S2@Graphene [39] | CVD method | 372 C g−1/1 mv s−1 | 69.3% | 92.3%/5000 |
| Ni3S2@CNT [40] | Hydrothermal | 360 C g−1/3.2 A g−1 | 58.6% | 80%/1000 |
| Ni3S2@PAN [41] | Electrochemical deposition | 440 C g−1/1 A g−1 | 78.73% | 93.14%/2500 |
| NCC-1 (this work) | Hydrothermal | 657 C g−1/1 A g−1 | 87.7% | 76.7%/3500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Huang, H.; Xiong, W.; Yang, S.; Huang, H.; Zou, Y.; Zhou, W.; Cheng, Z.; Wang, J.; Luo, G. One-Pot Synthesis of Glucose-Derived Carbon Coated Ni3S2 Nanowires as a Battery-Type Electrode for High Performance Supercapacitors. Nanomaterials 2021, 11, 678. https://doi.org/10.3390/nano11030678
Wu Z, Huang H, Xiong W, Yang S, Huang H, Zou Y, Zhou W, Cheng Z, Wang J, Luo G. One-Pot Synthesis of Glucose-Derived Carbon Coated Ni3S2 Nanowires as a Battery-Type Electrode for High Performance Supercapacitors. Nanomaterials. 2021; 11(3):678. https://doi.org/10.3390/nano11030678
Chicago/Turabian StyleWu, Zhongkai, Haifu Huang, Wenhui Xiong, Shiming Yang, Huanhuan Huang, Yaohui Zou, Weiping Zhou, Zhenzhi Cheng, Jun Wang, and Guangsheng Luo. 2021. "One-Pot Synthesis of Glucose-Derived Carbon Coated Ni3S2 Nanowires as a Battery-Type Electrode for High Performance Supercapacitors" Nanomaterials 11, no. 3: 678. https://doi.org/10.3390/nano11030678





