Synthesis of Silver and Gold Nanoparticles from Rumex roseus Plant Extract and Their Application in Electrochemical Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Synthesis of Ag and Au NPs
2.3. Characterization
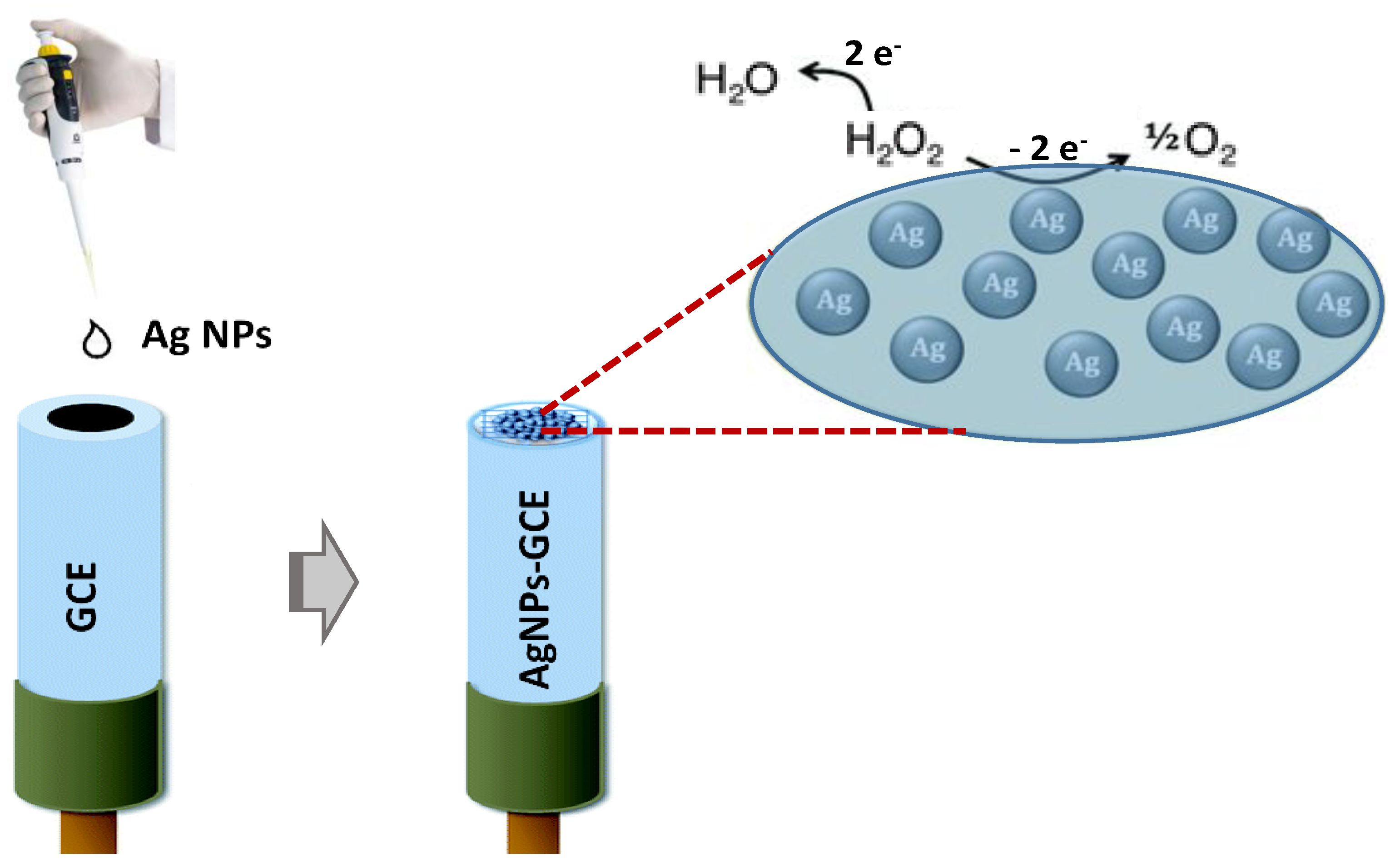
2.4. Modified Electrodes Fabrication
2.5. Electrochemical Tests
3. Results and Discussion
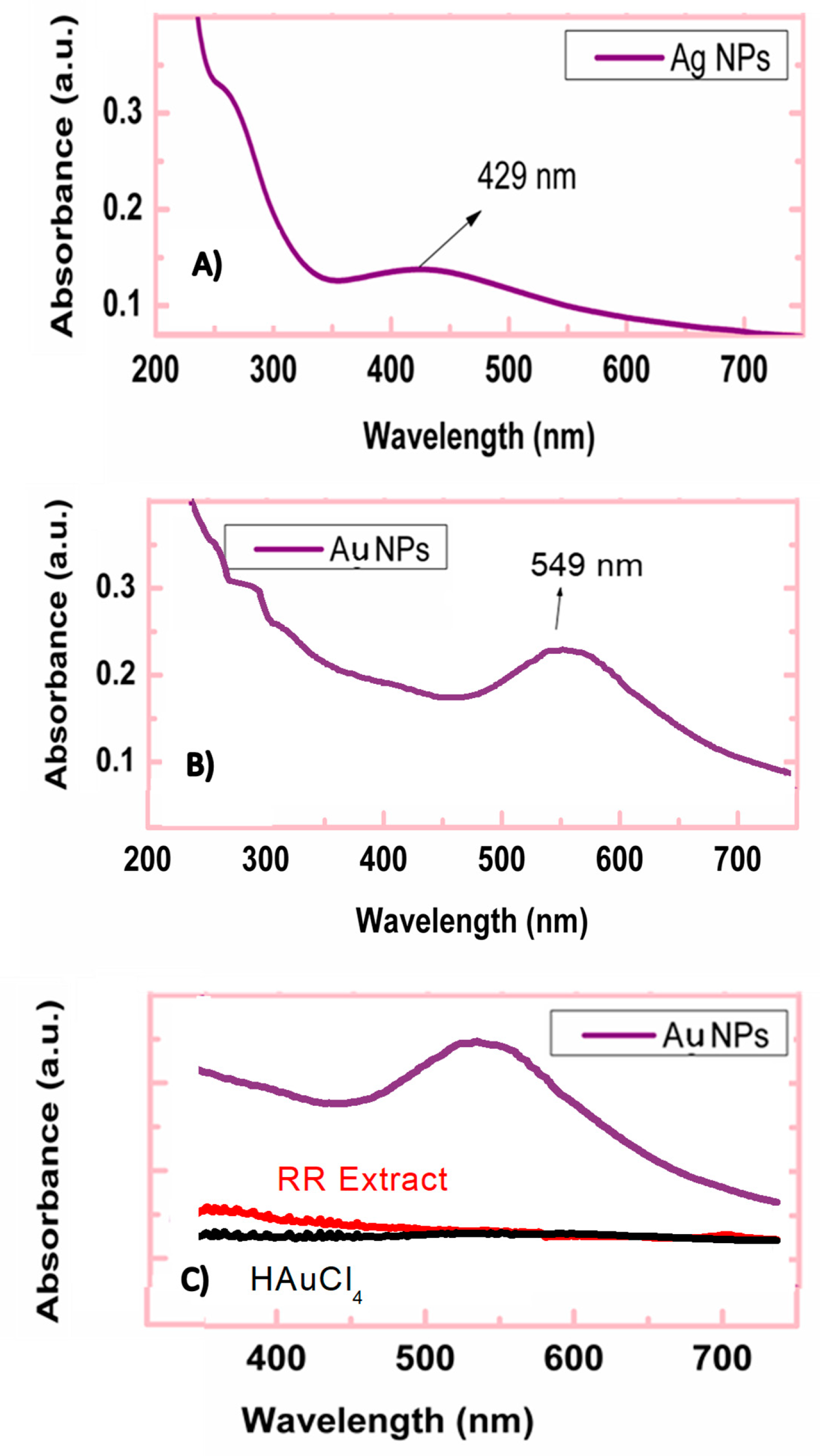
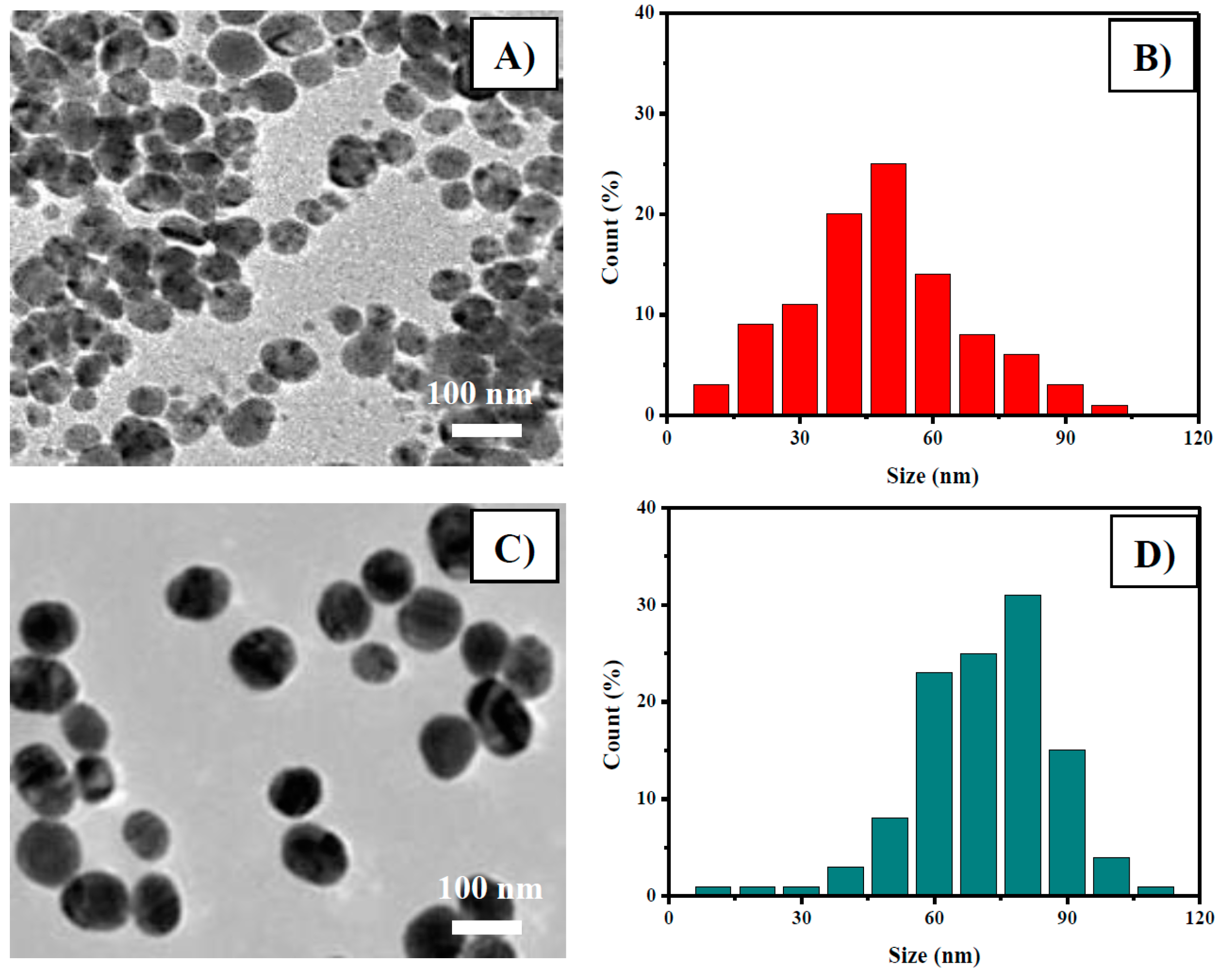
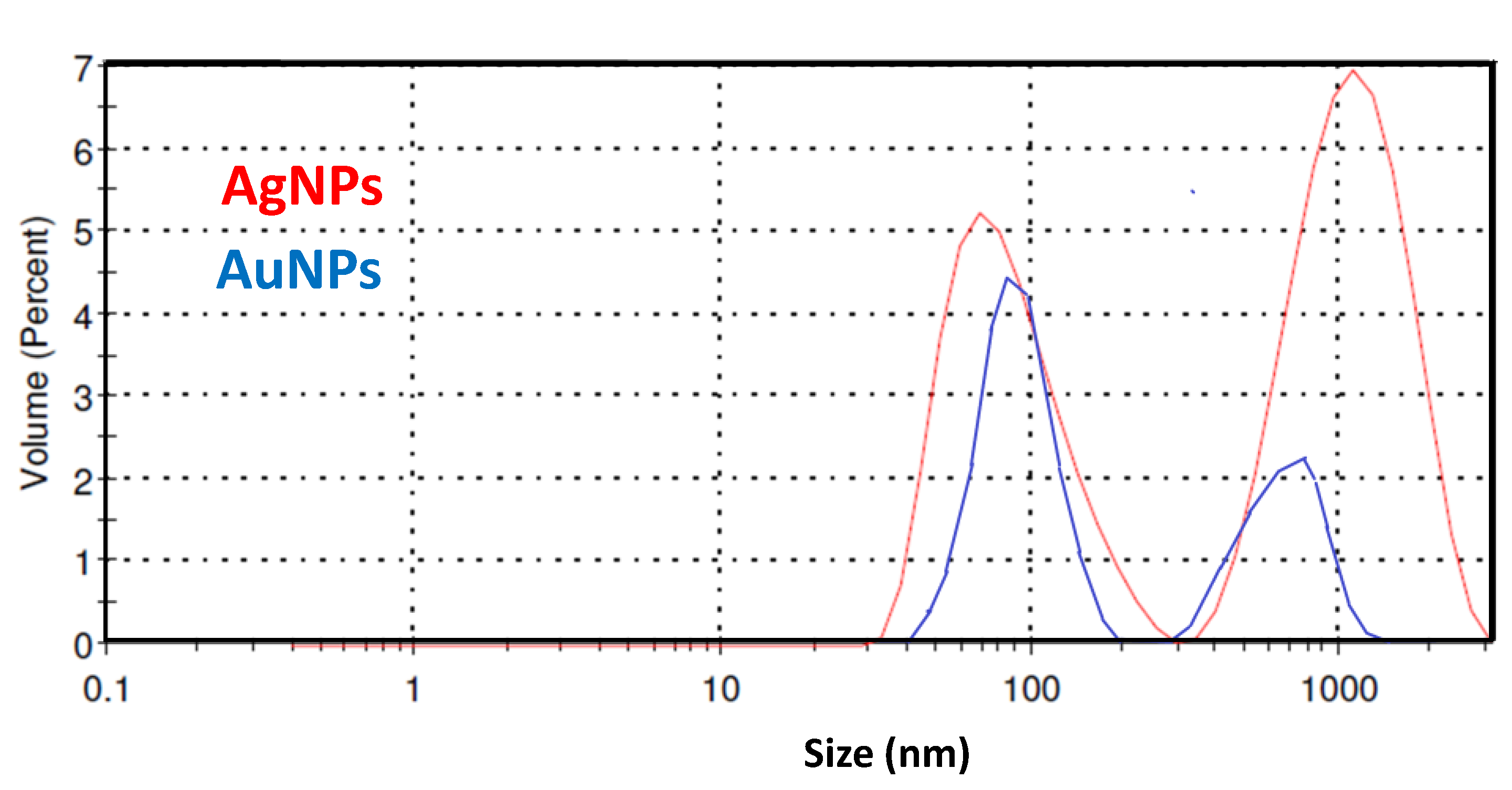
3.1. Ag and Au NPs’ Preparation and Characterization
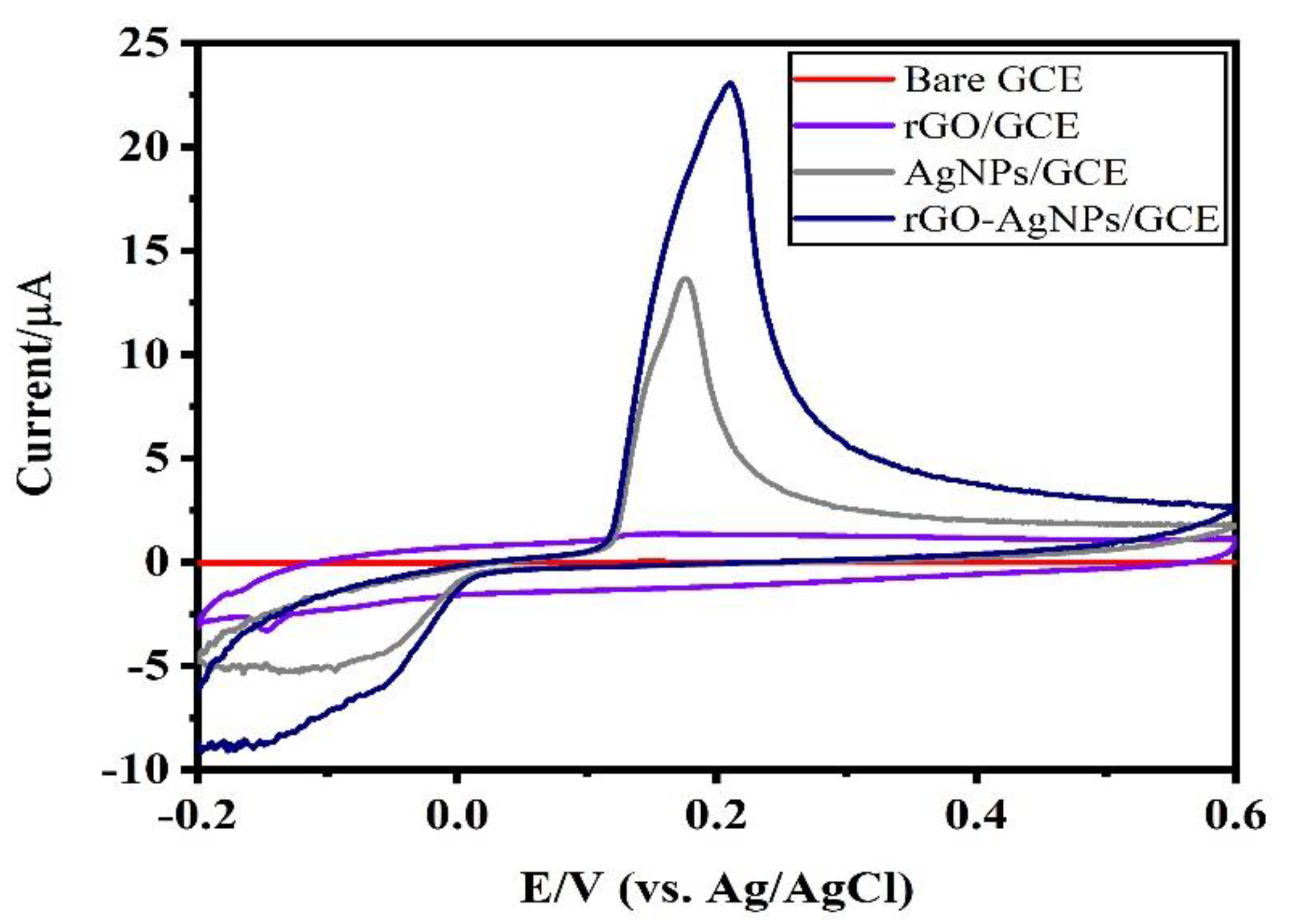
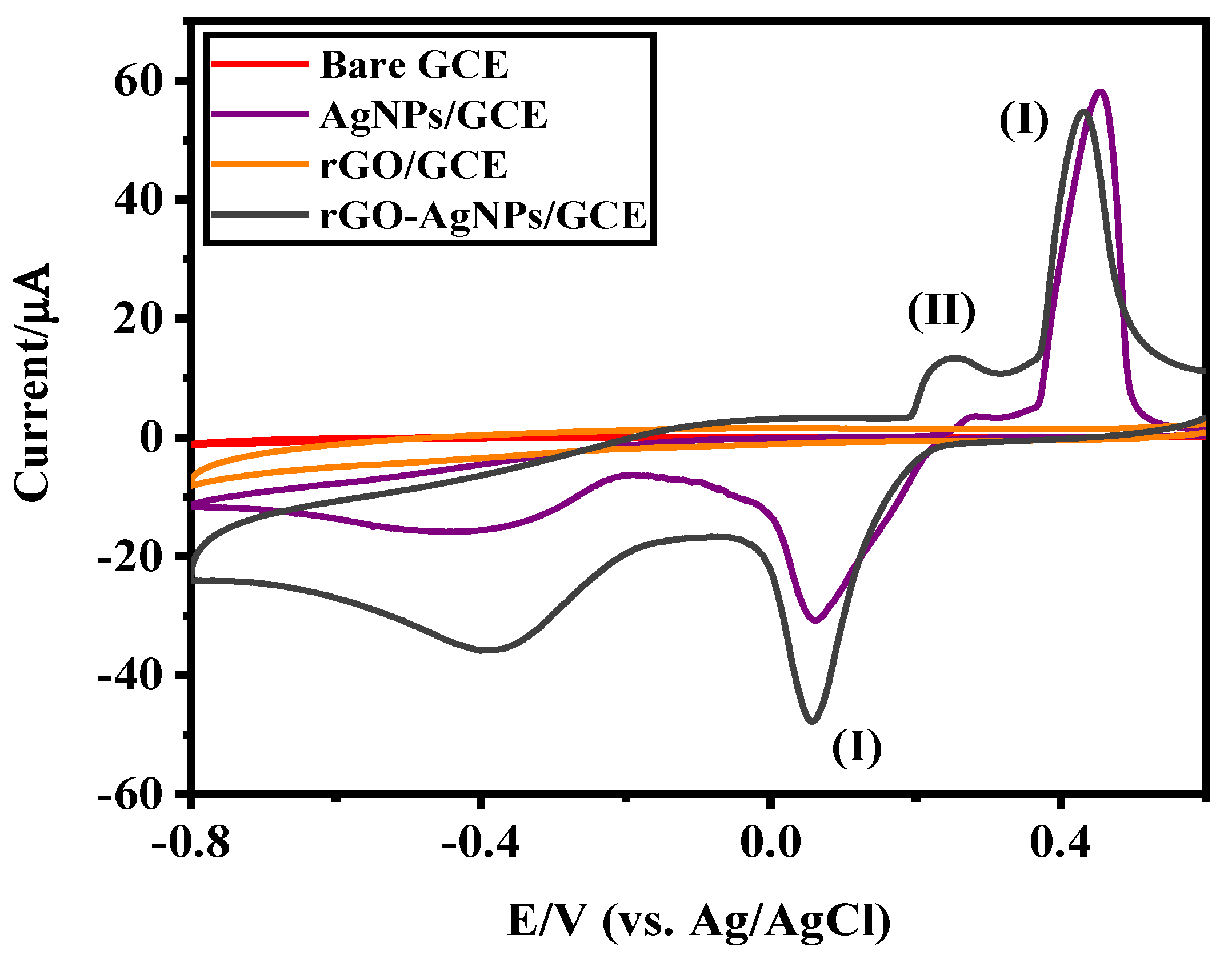
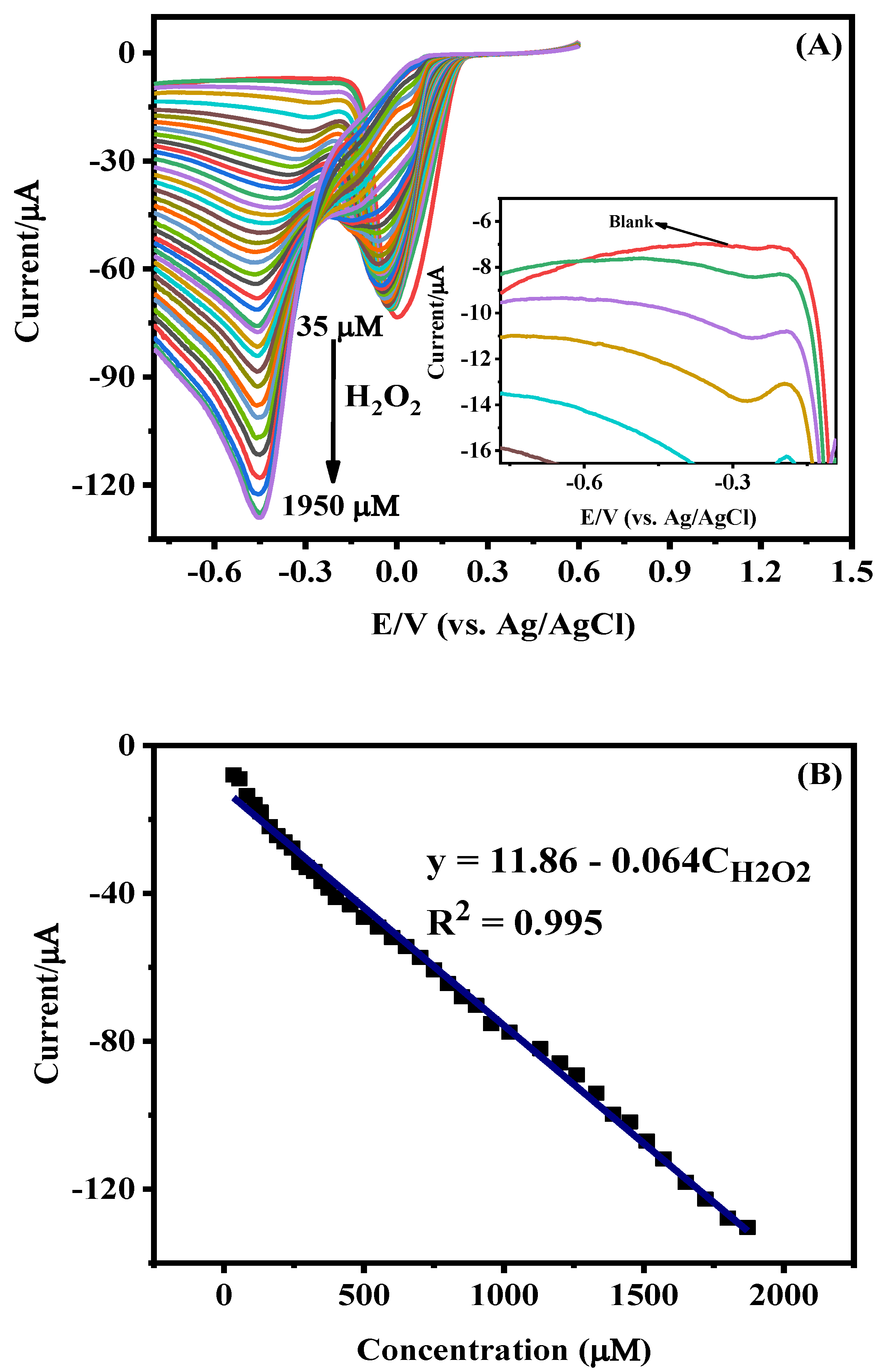
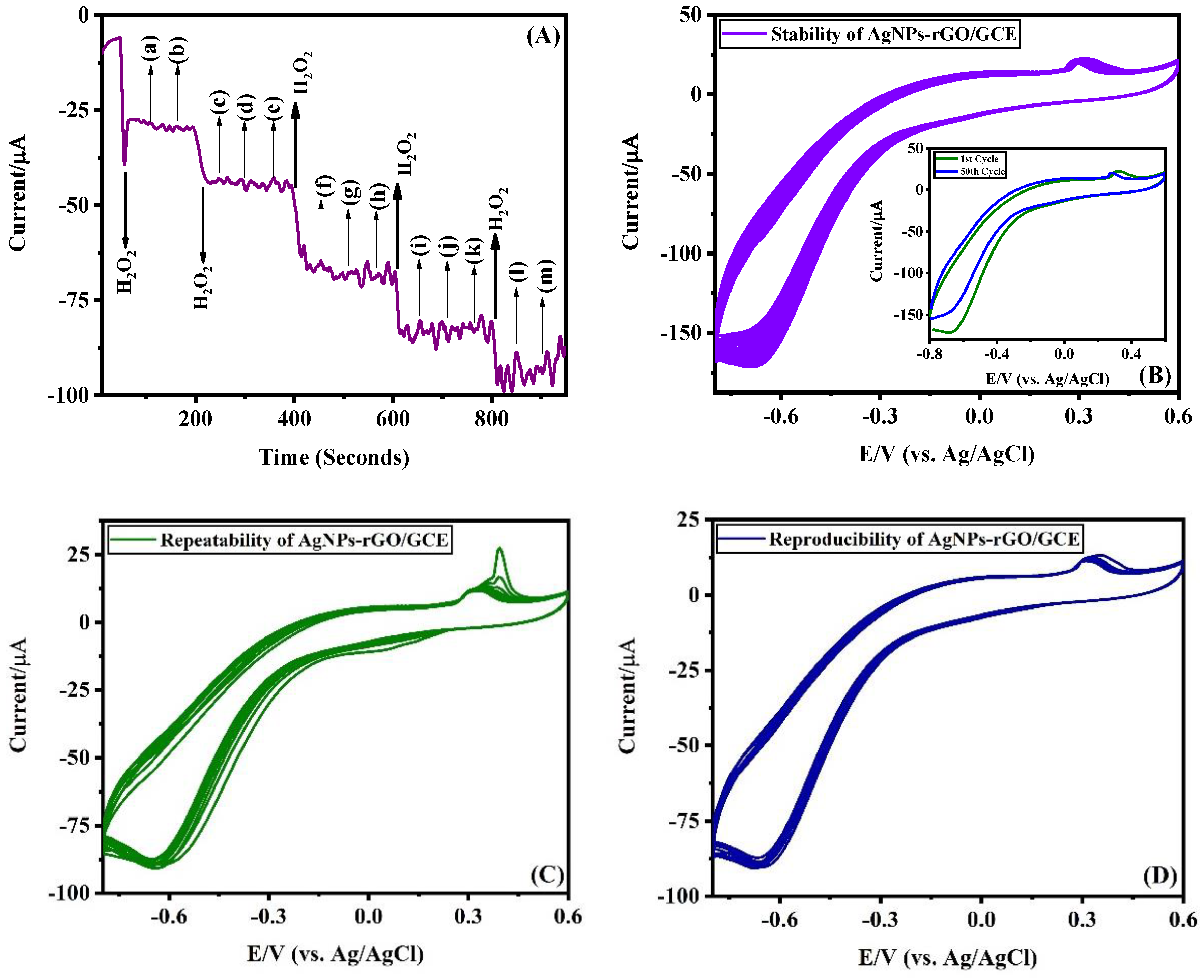
3.2. Electrochemical Characteristics of Ag-NPs/GCE
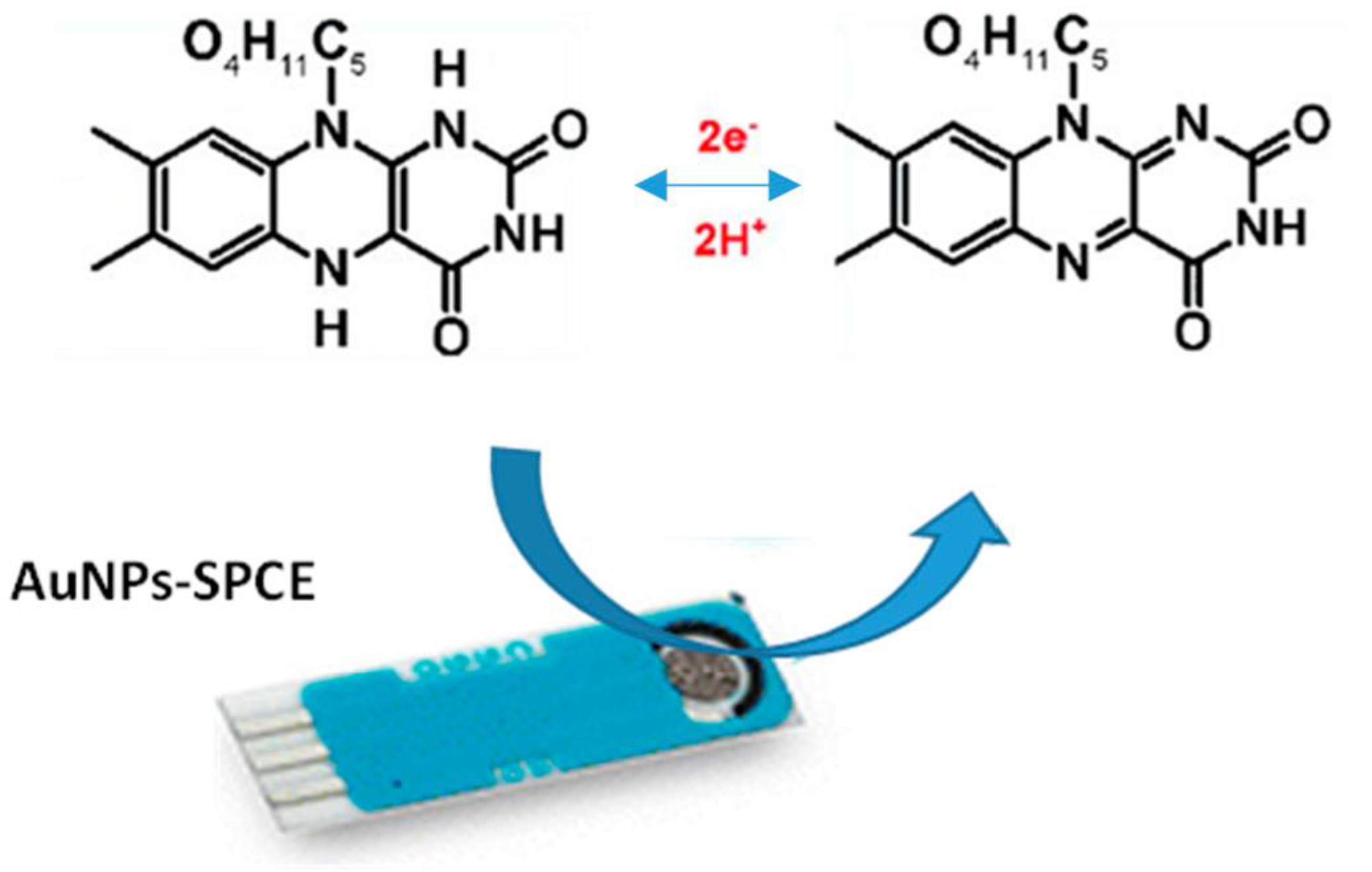
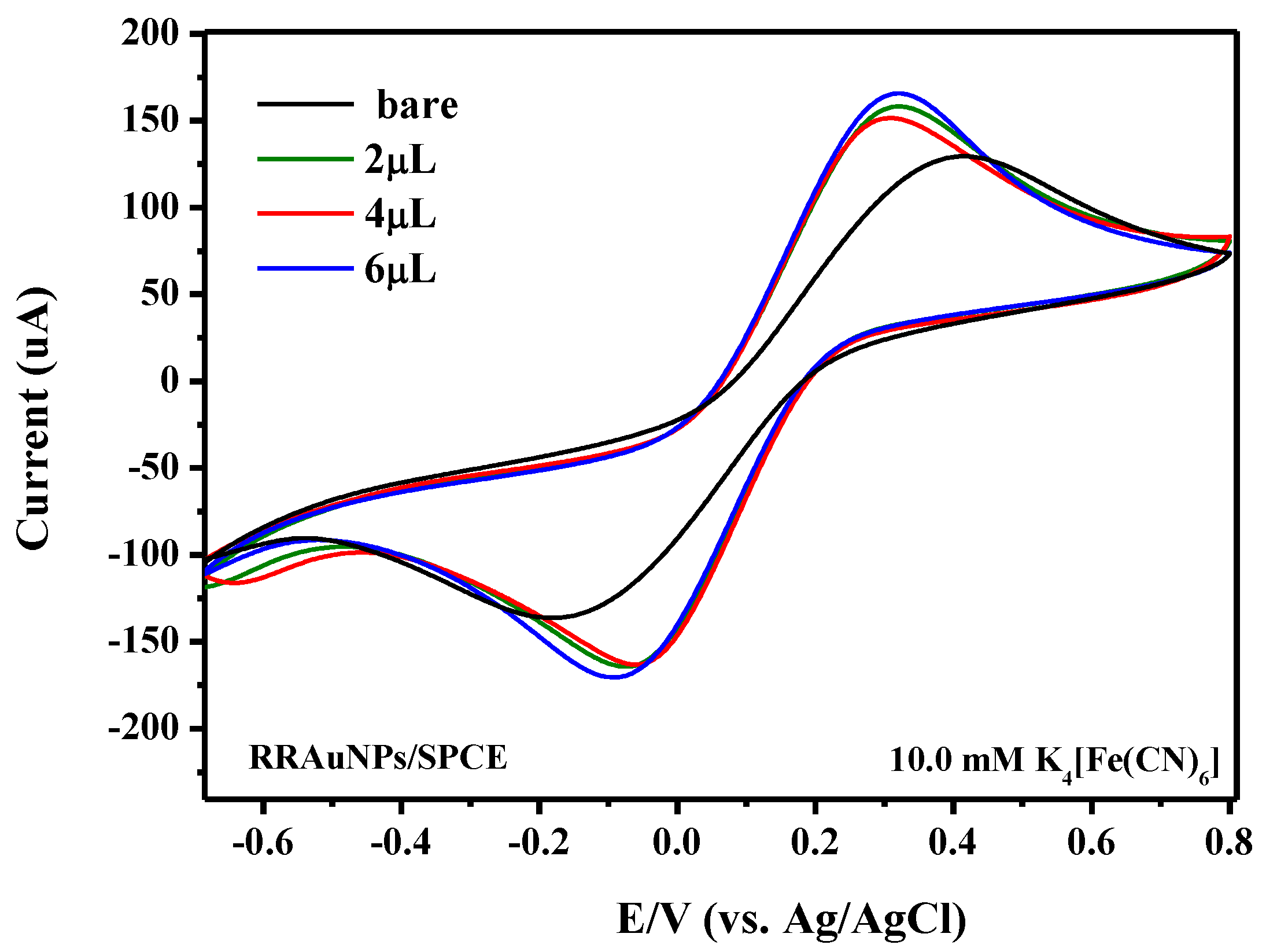
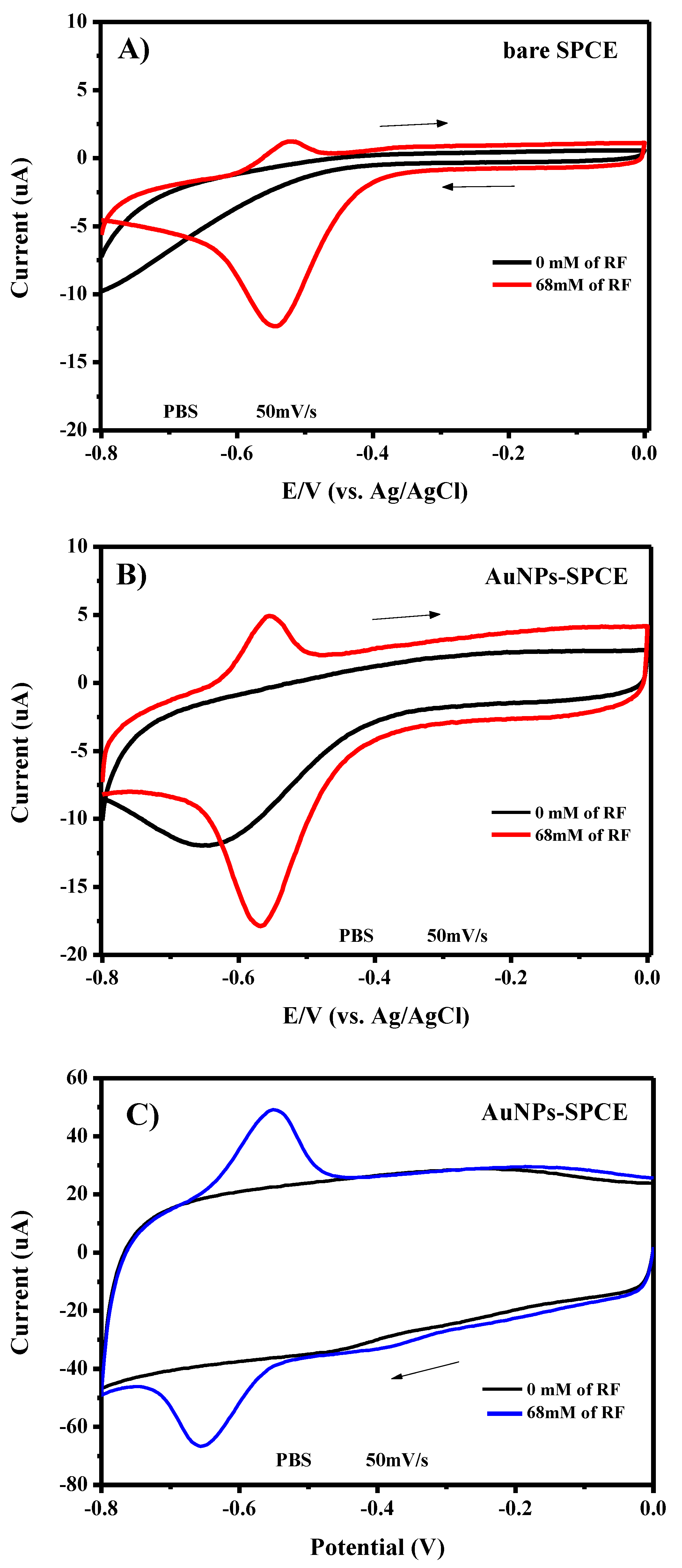
3.3. Electrochemical Characteristics of AuNPs/SPCE
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tonelli, D.; Scavetta, E.; Gualandi, I. Electrochemical Deposition of Nanomaterials for Electrochemical Sensing. Sensors 2019, 19, 1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Zhu, C.; Du, D.; Eychmüller, A.; Lin, Y. Engineering Ordered and Nonordered Porous Noble Metal Nanostructures: Synthesis, Assembly, and Their Applications in Electrochemistry. Chem. Rev. 2015, 115, 8896–8943. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yin, B.; Wang, S.; Jiao, Y.; Pan, W.; Huang, S.; Chen, S.; Meng, F. Synthesis of Silver and Gold Nanoparticles by a Novel Electrochemical Method. ChemPhysChem 2004, 5, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Slepička, P.; Kasálková, N.S.; Siegel, J.; Kolská, Z.; Švorčík, V. Methods of Gold and Silver Nanoparticles Preparation. Materials 2019, 13, 1. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.G.; Xia, Y.N. Shape-controlled synthesis of gold and silver nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.; Liz-Marzán, L.M.; De Abajo, F.J.G. Universal analytical modeling of plasmonic nanoparticles. Chem. Soc. Rev. 2017, 46, 6710–6724. [Google Scholar] [CrossRef] [Green Version]
- Kumar, H.; Bhardwaj, K.; Kuča, K.; Kalia, A.; Nepovimova, E.; Verma, R.; Kumar, D. Flower-Based Green Synthesis of Metallic Nanoparticles: Applications beyond Fragrance. Nanomaterials 2020, 10, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and ap-plications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of Gold Nanotriangles and Silver Nanoparticles Using Aloe vera Plant Extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qui, L.; Shen, Y.; Xie, A.; Yu, X.; Zhang, L.; Zhang, Q. Green synthesis of silver nanoparticles using Capsicum annum L. extract. Green Chem. 2007, 9, 852–858. [Google Scholar] [CrossRef]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010, 8, 1065–1071. [Google Scholar] [CrossRef]
- Naznin, A.B.; Samiran, M.; Saswati, B.; Rajibul, A.L.; Debabrata, M. Biogenic synthesis of Au and Ag nanoparticles using aqueous solutions of Black Tea leaf extracts. Colloids Surf. B Biointerface 2009, 71, 113–118. [Google Scholar]
- Rodríguez-León, E.; Iñiguez-Palomares, R.; Navarro, R.E.; Herrera-Urbina, R.; Tánori, J.; Iñiguez-Palomares, C.; Maldonado, A. Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res. Lett. 2013, 8, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chelly, M.; Chelly, S.; Ben Salah, H.; Athmouni, K.; Bitto, A.; Sellami, H.; Kallel, C.; Allouche, N.; Gdoura, R.; Bouaziz-Ketata, H. Characterization, antioxidant and protective effects of edible Rumex roseus on erythrocyte oxidative damage induced by methomyl. J. Food Meas. Charact. 2019, 14, 229–243. [Google Scholar] [CrossRef]
- Meier, J.; Hofferber, E.M.; Stapleton, J.A.; Iverson, N.M. Hydrogen Peroxide Sensors for Biomedical Applications. Chemosensors 2019, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Powers, H.J. Riboflavin (vitamin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003, 77, 1352. [Google Scholar] [CrossRef]
- Gartner, L.M.; Morton, J.; Lawrence, R.A.; Naylor, A.J.; O’Hare, D.; Schanler, R.J.; Eidelman, A.I. Breastfeeding and the use of human milk. Pediatrics 2005, 115, 496–506. [Google Scholar]
- Kitte, S.A.; Assresahegn, B.D.; Soreta, T.R. Electrochemical determination of hydrogen peroxide at a glassy carbon electrode modified with palladium nanoparticles. J. Serb. Chem. Soc. 2013, 78, 701–711. [Google Scholar] [CrossRef]
- Kadara, R.O.; Haggett, B.G.D.; Birch, B.J. Disposable Sensor for Measurement of Vitamin B2 in Nutritional Premix, Cereal, and Milk Powder. J. Agric. Food Chem. 2006, 54, 4921–4924. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.M.; Compton, R.G. The use of nanoparticles in electroanalysis: A review. Anal. Bioanal. Chem. 2006, 384, 601–619. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, N.; Radhakrishnan, S.; Sekar, C. Fabrication of hydrogen peroxide biosensor based on Ni doped SnO2 nanoparticles. Biosens. Bioelectron. 2012, 36, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, S.G.; Aloisio, D.; Donato, N.; Ferro, M.C.; Pinna, N.; Russo, P.A.; Neri, G. Amperometric Sensing of H2O2 using Pt-TiO2/Reduced Graphene Oxide Nanocomposites. ChemElectroChem 2013, 1, 617–624. [Google Scholar] [CrossRef]
- Lavanya, N.; Radhakrishnan, S.; Sekar, C.; Navaneethan, M.; Hayakawa, Y. Fabrication of Cr doped SnO2 nanoparticles based biosensor for the selective determination of riboflavinin pharmaceuticals. Analyst 2013, 138, 2061–2067. [Google Scholar] [CrossRef] [Green Version]
- Lavanya, N.; Radhakrishnan, S.; Sudhan, N.; Sekar, C.; Leonardi, S.G.; Cannilla, C.; Neri, G. Fabrication of folic acid sensor based on the Cu doped SnO2nanoparticles modified glassy carbon electrode. Nanotechnology 2014, 25, 295501. [Google Scholar] [CrossRef]
- Rowley-Neale, S.J.; Randviir, E.P.; Dena, A.S.A.; Banks, C.E. An overview of recent applications of reduced graphene oxide as a basis of electroanalytical sensing platforms. Appl. Mater. Today 2018, 10, 218–226. [Google Scholar] [CrossRef]
- Sang, S.; Li, D.; Zhang, H.; Sun, Y.; Jian, A.; Zhang, Q.; Zhang, W. Facile synthesis of AgNPs on reduced graphene oxide for highly sensitive simultaneous detection of heavy metal ions. RSC Adv. 2017, 7, 21618–21624. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2014, 87, 230–249. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Krishnamoorthy, K.; Sekar, C.; Wilson, J.; Kim, S.J. A highly sensitive electrochemical sensor for nitrite detection based on Fe2O3 nanoparticles decorated reduced graphene oxide nanosheets. Appl. Catal. B Environ. 2014, 148, 22–28. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibac-terial activity against water borne pathogens. Colloids Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef]
- Papoff, F.; Hourahine, B. Geometrical Mie theory for resonances in nanoparticles of any shape. Opt. Express 2011, 19, 21432–21444. [Google Scholar] [CrossRef] [PubMed]
- Prathna, T.; Chandrasekaran, N.; Raichur, A.M.; Mukherjee, A. Biomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle size. Colloids Surfaces B Biointerfaces 2011, 82, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Nestor, A.R.V.; Mendieta, V.S.; Lopez, M.A.C.; Espinosa, R.M.G.; Lopez, M.A.C.; Alatorr, J.A.A. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camiellia sinensis extract. Mater. Lett. 2008, 62, 3103–3105. [Google Scholar] [CrossRef]
- Chelly, S.; Chelly, M.; Zribi, R.; Gdoura, R.; Bouaziz-Ketata, H.; Neri, G. Electrochemical detection of dopamine and riboflavine on screen printed carbon electrode modified by AuNPs derived from Rhanterium suaveolens plant extract. ACS Omega 2021. submitted. [Google Scholar]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Zheng, T.; Bott, S.; Huo, Q. Techniques for Accurate Sizing of Gold Nanoparticles Using Dynamic Light Scattering with Particular Application to Chemical and Biological Sensing Based on Aggregate Formation. ACS Appl. Mater. Interfaces 2016, 8, 21585–21594. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Ji, Y.; Liu, S. Synthesis of Ag nanoparticle–carbon nanotube–reduced graphene oxide hybrids for highly sensitive non-enzymatic hydrogen peroxide detection. RSC Adv. 2015, 5, 39037–39041. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, R.K.; Gundampati, R.K.; Singh, D.K.; Mohan, S.; Hasan, S.H.; Malviy, M. Enhanced electron transfer mediated detection of hydrogen peroxide using a silver nanoparticle–reduced graphene oxide–polyaniline fabricatedelectro-chemical sensor. RSC Adv. 2018, 8, 619–631. [Google Scholar] [CrossRef] [Green Version]
- Ashok, A.; Anand, K.; Md Abdul, M.; Faris, T. Synthesis of highly efficient bifunctional Ag/Co3O4 catalyst for oxygen reduction and oxygen evolution reactions in alkaline medium. ACS Omega 2018, 3, 7745–7756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Chaubey, A. Biosensors for clinical diagnostics industry. Sens. Actuators B Chem. 2003, 91, 117–127. [Google Scholar] [CrossRef]
- Negut Cioatesz, C. Review—Electrochemical sensors used in the determination of riboflavin. J. Electrochem. Soc. 2020, 167, 037558. [Google Scholar] [CrossRef]
- Revin, S.B.; John, S.A. Simultaneous determination of vitamins B2, B9 and C using a heterocyclic conducting polymer modified electrode. Electrochim. Acta 2012, 75, 35–41. [Google Scholar] [CrossRef]
- Mehmeti, E.; Stanković, D.M.; Chaiyo, S.; Švorc, L’.; Kalcher, K. Manganese dioxide-modified carbon paste electrode for voltammetric determination of riboflavin. Microchim. Acta 2016, 183, 1619–1624. [Google Scholar] [CrossRef] [Green Version]
- Nezamzadeh-Ejhieh, A.; Pouladsaz, P. Voltammetric determination of riboflavin based on electrocatalytic oxidation at zeo-lite-modified carbon paste electrodes. J. Ind. Eng. Chem. 2014, 20, 2146–2152. [Google Scholar] [CrossRef]
- Huang, D.-Q.; Wu, H.; Song, C.; Zhu, Q.; Zhang, H.; Sheng, L.-Q.; Xu, H.-J.; Liu, Z.-D. The determination of Riboflavin (Vitamin B2) using manganese dioxide modified glassy carbon electrode by differential pulse voltammetry. Int. J. Electrochem. Sci. 2018, 13, 8303–8312. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelly, M.; Chelly, S.; Zribi, R.; Bouaziz-Ketata, H.; Gdoura, R.; Lavanya, N.; Veerapandi, G.; Sekar, C.; Neri, G. Synthesis of Silver and Gold Nanoparticles from Rumex roseus Plant Extract and Their Application in Electrochemical Sensors. Nanomaterials 2021, 11, 739. https://doi.org/10.3390/nano11030739
Chelly M, Chelly S, Zribi R, Bouaziz-Ketata H, Gdoura R, Lavanya N, Veerapandi G, Sekar C, Neri G. Synthesis of Silver and Gold Nanoparticles from Rumex roseus Plant Extract and Their Application in Electrochemical Sensors. Nanomaterials. 2021; 11(3):739. https://doi.org/10.3390/nano11030739
Chicago/Turabian StyleChelly, Meryam, Sabrine Chelly, Rayhane Zribi, Hanen Bouaziz-Ketata, Radhouane Gdoura, Nehru Lavanya, Ganesan Veerapandi, Chinnathambi Sekar, and Giovanni Neri. 2021. "Synthesis of Silver and Gold Nanoparticles from Rumex roseus Plant Extract and Their Application in Electrochemical Sensors" Nanomaterials 11, no. 3: 739. https://doi.org/10.3390/nano11030739





