Electrochemical Performance of Titania 3D Nanonetwork Electrodes Induced by Pulse Ionization at Varied Pulse Repetitions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrode Materials and Fabrication
2.2. Supercapacitor Assembly
2.3. Material Characterization
2.4. Electrochemical Characterization
3. Results and Discussion
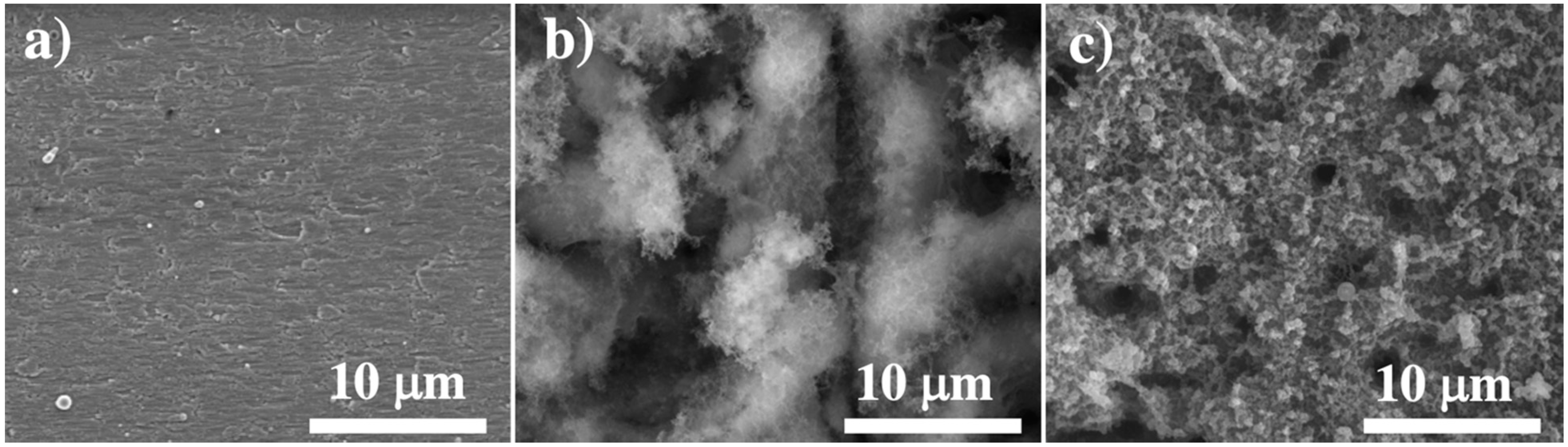
3.1. Surface Morphological and Compositional Analysis
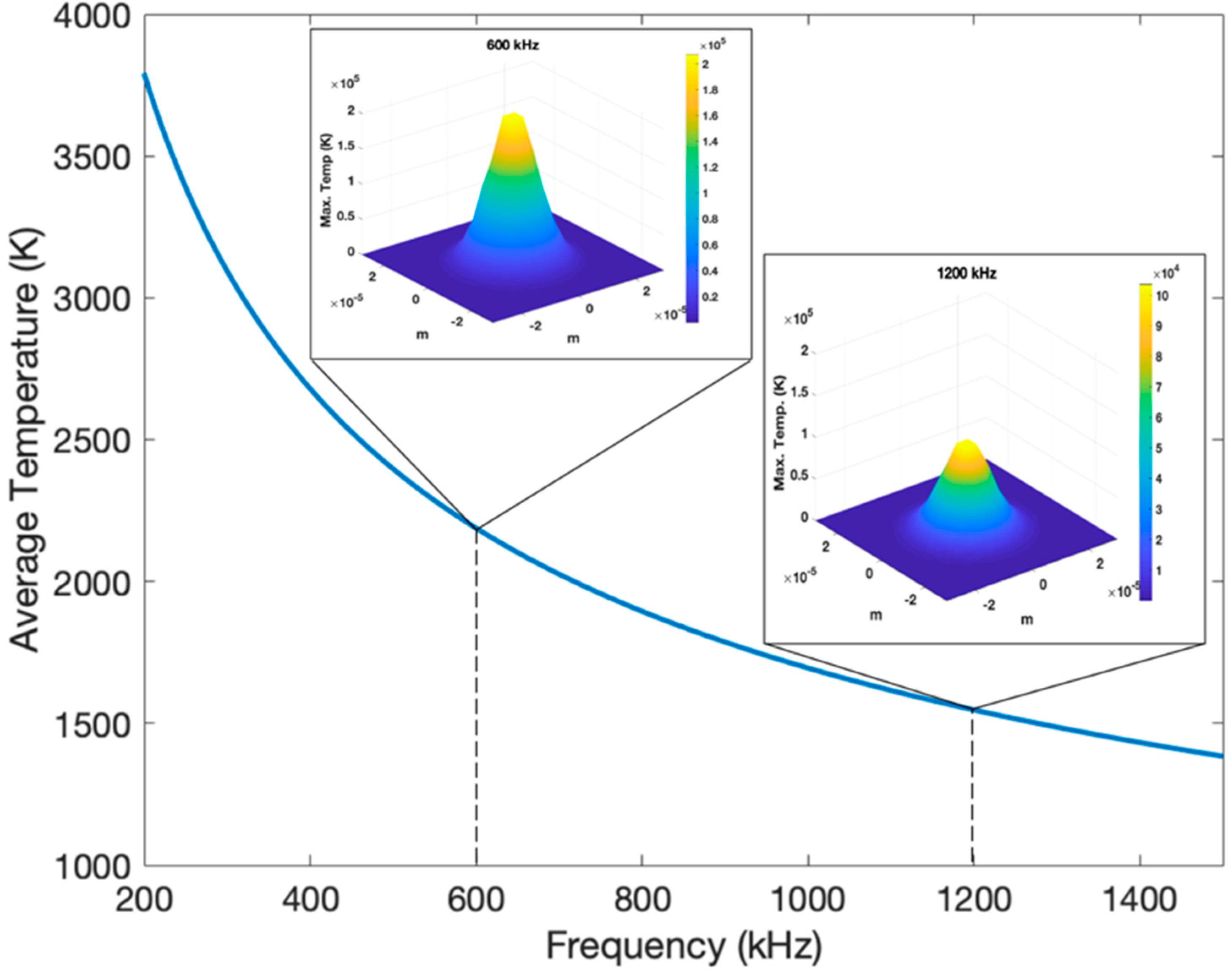
3.2. Theoretical Models for Surface Temperature
3.3. Supercapacitor Application
3.3.1. Cyclic Voltammetry
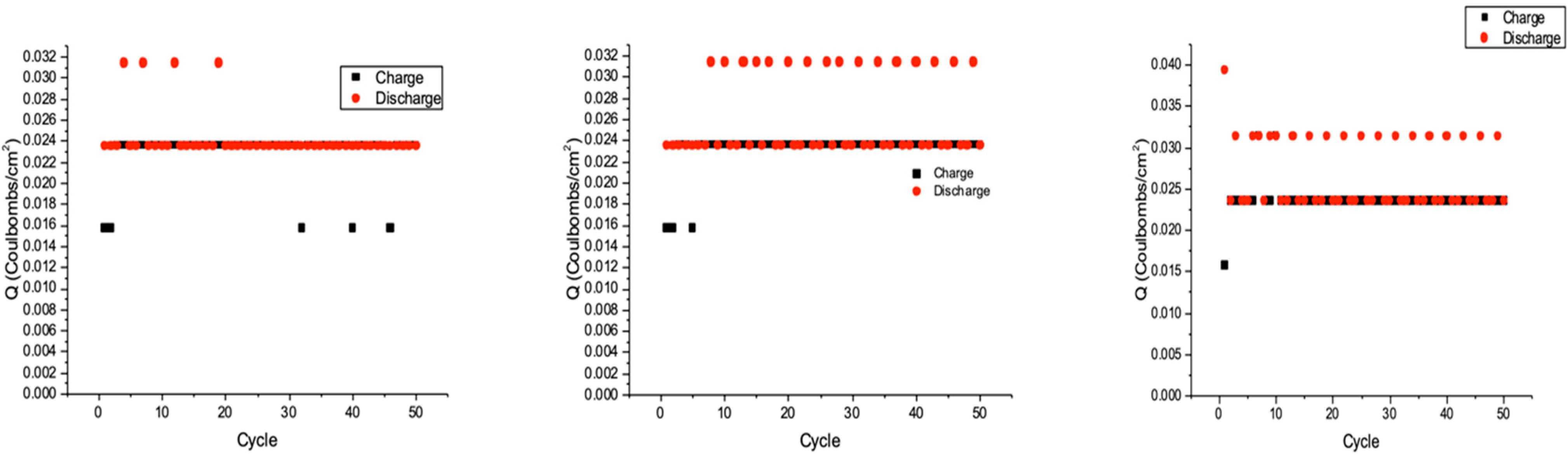
3.3.2. Charge/Discharge
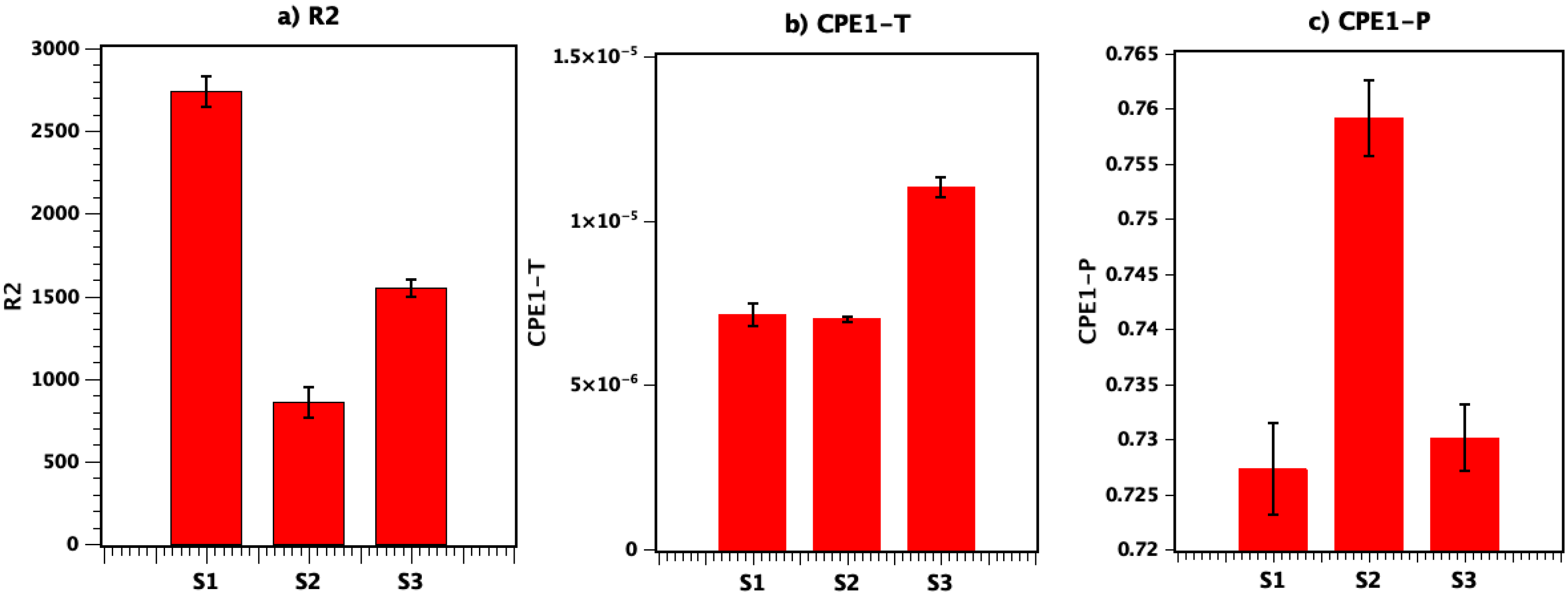
3.3.3. Electrochemical Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, L.; Ning, H.; Song, S.; Xu, C.; Hu, N. Flexible electrochemical energy storage: The role of composite materials. Compos. Sci. Technol. 2020, 192, 108102. [Google Scholar] [CrossRef]
- Zou, C.; Zhang, L.; Hu, X.; Wang, Z.; Wik, T.; Pecht, M. A review of fractional-order techniques applied to lithium-ion batteries, lead-acid batteries, and supercapacitors. J. Power Sources 2018, 390, 286–296. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Appadurai, T.; Subramaniyam, C.M.; Kuppusamy, R.; Karazhanov, S.; Subramanian, B. Electrochemical performance of nitrogen-doped TiO2 nanotubes as electrode material for supercapacitor and Li-ion battery. Molecules 2019, 24, 2952. [Google Scholar] [CrossRef] [Green Version]
- Khaligh, A.; Li, Z. Battery, ultracapacitor, fuel cell, and hybrid energy storage systems for electric, hybrid electric, fuel cell, and plug-in hybrid electric vehicles: State of the art. IEEE Trans. Veh. Technol. 2010, 59, 2806–2814. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Kim, D.K.; Muralidharan, P.; Lee, H.W.; Ruffo, R.; Yang, Y.; Chan, C.K.; Cui, Y. Spinel LiMn2O4 nanorods as lithium ion battery cathodes. Nano Lett. 2008, 8, 3948–3952. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [Green Version]
- Chockla, A.M.; Harris, J.T.; Akhavan, V.A.; Bogart, T.D.; Holmberg, V.C.; Steinhagen, C.; Korgel, B.A. Silicon nanowire fabric as a lithium ion battery electrode material. J. Am. Chem. Soc. 2011, 133, 20914–20921. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Z.; Jiang, R.; Bian, C.; Huang, T.; Yu, A. TiO2 nanotube array film prepared by anodization as anode material for lithium ion batteries. J. Solid State Electrochem. 2010, 14, 1045–1050. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Huang, K.; Fang, D.; Zhuang, S. Electrochemical properties of freestanding TiO2 nanotube membranes annealed in Ar for lithium anode material. J. Solid State Electrochem. 2012, 16, 723–729. [Google Scholar] [CrossRef]
- Pei, Z.; Zhu, M.; Huang, Y.; Huang, Y.; Xue, Q.; Geng, H.; Zhi, C. Dramatically improved energy conversion and storage efficiencies by simultaneously enhancing charge transfer and creating active sites in MnOx/TiO2 nanotube composite electrodes. Nano Energy 2016, 20, 254–263. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Zhu, M.; Huang, Y.; Tang, Z.; Pei, Z.; Zhi, C. Towards wearable electronic devices: A quasi-solid-state aqueous lithium-ion battery with outstanding stability, flexibility, safety and breathability. Nano Energy 2018, 44, 164–173. [Google Scholar] [CrossRef]
- Pan, D.; Huang, H.; Wang, X.; Wang, L.; Liao, H.; Li, Z.; Wu, M. C-axis preferentially oriented and fully activated TiO2 nanotube arrays for lithium ion batteries and supercapacitors. J. Mater. Chem. A 2014, 2, 11454–11464. [Google Scholar] [CrossRef]
- Bresser, D.; Oschmann, B.; Tahir, M.N.; Mueller, F.; Lieberwirth, I.; Tremel, W.; Passerini, S. Carbon-coated anatase TiO2 nanotubes for Li-and Na-ion anodes. J. Electrochem. Soc. 2014, 162, 3013. [Google Scholar] [CrossRef]
- Madian, M.; Giebeler, L.; Klose, M.; Jaumann, T.; Uhlemann, M.; Gebert, A.; Eckert, J. Self-organized TiO2/CoO nanotubes as potential anode materials for lithium ion batteries. ACS Sustain. Chem. Eng. 2015, 3, 909–919. [Google Scholar] [CrossRef]
- Ding, Y.; Sheng, H.; Gong, B.; Tang, P.; Pan, G.; Zeng, Y.; Liu, C. Polyaniline/reduced graphene oxide nanosheets on TiO2 nanotube arrays as a high-performance supercapacitor electrode: Understanding the origin of high rate capability. Electrochim. Acta 2021, 368, 137615. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Dong, W.; Chen, I.W.; Wang, Z.; Xu, J.; Huang, F. Nitrogen-doped black titania for high performance supercapacitors. Sci. China Mater. 2020, 63, 1227–1234. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fu, Q.; Xu, Q.; Yan, X.; Zhang, R.; Guo, Z.; Chen, G. Improved electrochemical performance of nitrogen doped TiO2-B nanowires as anode materials for Li-ion batteries. Nanoscale 2015, 7, 12215–12224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojha, D.P.; Poudel, M.B.; Kim, H.J. Investigation of electrochemical performance of a high surface area mesoporous Mn doped TiO2 nanoparticle for a supercapacitor. Mater. Lett. 2020, 264, 127363. [Google Scholar] [CrossRef]
- Ongaro, M.; Signoretto, M.; Trevisan, V.; Stortini, A.M.; Ugo, P. Arrays of TiO2 nanowires as photoelectrochemical sensors for hydrazine detection. Chemosensors 2015, 3, 146–156. [Google Scholar] [CrossRef] [Green Version]
- Miao, Z.; Xu, D.; Ouyang, J.; Guo, G.; Zhao, X.; Tang, Y. Electrochemically induced sol−gel preparation of single-crystalline TiO2 nanowires. Nano Lett. 2002, 2, 717–720. [Google Scholar] [CrossRef]
- Gholami, A.; Kiani, A. Laser-induced nanofibrous titania film electrode: A new approach for energy storage materials. J. Energy Storage 2020, 31, 101654. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Mao, S.; Sun, Z.; Song, Y.; Zhao, R. Fabrication of porous graphene electrodes via CO2 activation for the enhancement of capacitive deionization. J. Colloid Interface Sci. 2019, 536, 252–260. [Google Scholar] [CrossRef]
- Wang, H.; Wu, X.; Qi, X.; Zhao, W.; Ju, Z. Sb nanoparticles encapsulated in 3D porous carbon as anode material for lithium-ion and potassium-ion batteries. Mater. Res. Bull. 2018, 103, 32–37. [Google Scholar] [CrossRef]
- Hardcastle, F.D. Raman spectroscopy of titania (TiO2) nanotubular water-splitting catalysts. J. Ark. Acad. Sci. 2011, 65, 43–48. [Google Scholar]
- Scarpelli, F.; Mastropietro, T.F.; Poerio, T.; Godbert, N. Mesoporous TiO2 thin films: State of the art. Titan. Dioxide-Mater. Sustain. Environ. 2018, 508. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.J.; Song, H.S.; Im, H.B.; Nam, J.E.; Kang, J.K.; Hwang, T.S.; Yi, K.B. Preparationand Characterization of Rutile-anatase Hybrid TiO2 Thin Film by Hydrothermal Synthesis. Clean Technol. 2014, 20, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Hendow, S.T.; Shakir, S.A. Structuring materials with nanosecond laser pulses. Opt. Express 2010, 18, 10188–10199. [Google Scholar] [CrossRef]
- Colpitts, C.; Kiani, A. Laser processing of silicon for synthesis of better biomaterials. Biomater. Regen. Med. 2018, 4, 229. [Google Scholar]
- Beigi, M.H.; Safaie, N.; Nasr-Esfahani, M.H.; Kiani, A. 3D titania nanofiber-like webs induced by plasma ionization: A new direction for bioreactivity and osteoinductivity enhancement of biomaterials. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Ahmadi, N.; Rezaei, B. Electrochemical preparation and characterization of a polypyrrole/nickel-cobalt hexacyanoferrate nanocomposite for supercapacitor applications. RSC Adv. 2015, 5, 91448–91456. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.; Hou, D.; Yin, J.; Liu, D.; He, Y.; Lin, H. Hierarchical porous carbon prepared from biomass through a facile method for supercapacitor applications. J. Colloid Interface Sci. 2018, 530, 338–344. [Google Scholar] [CrossRef]
- Taer, E.; Apriwandi, A.; Dalimunthe, B.K.L.; Taslim, R. A rod-like mesoporous carbon derived from agro-industrial cassava petiole waste for supercapacitor application. J. Chem. Technol. Biotechnol. 2021, 96, 662–671. [Google Scholar] [CrossRef]
- Choi, J.; Zequine, C.; Bhoyate, S.; Lin, W.; Li, X.; Kahol, P.; Gupta, R. Waste Coffee Management: Deriving High-Performance Supercapacitors Using Nitrogen-Doped Coffee-Derived Carbon. C—J. Carbon Res. 2019, 5, 44. [Google Scholar] [CrossRef] [Green Version]
- Mishra, N.; Shinde, S.; Vishwakarma, R.; Kadam, S.; Sharon, M.; Sharon, M. MWCNTs synthesized from waste polypropylene plastics and its application in super-capacitors. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2013; Volume 1538, pp. 228–236. [Google Scholar]
- Zhu, J.; Jiang, J.; Sun, Z.; Luo, J.; Fan, Z.; Huang, X.; Yu, T. 3D carbon/cobalt-nickel mixed-oxide hybrid nanostructured arrays for asymmetric supercapacitors. Small 2014, 10, 2937–2945. [Google Scholar] [CrossRef]
- Ali, G.A.; Yusoff, M.M.; Shaaban, E.R.; Chong, K.F. High performance MnO2 nanoflower supercapacitor electrode by electrochemical recycling of spent batteries. Ceram. Int. 2017, 43, 8440–8448. [Google Scholar] [CrossRef] [Green Version]
- Sankar, K.V.; Selvan, R.K. Improved electrochemical performances of reduced graphene oxide based supercapacitor using redox additive electrolyte. Carbon 2015, 90, 260–273. [Google Scholar] [CrossRef]
- Xu, R.; Guo, F.; Cui, X.; Zhang, L.; Wang, K.; Wei, J. High performance carbon nanotube based fiber-shaped supercapacitors using redox additives of polypyrrole and hydroquinone. J. Mater. Chem. A 2015, 3, 22353–22360. [Google Scholar] [CrossRef]
- Lyu, J.; Mayyas, M.; Salim, O.; Chu, D.; Joshi, R. Oriented Graphene Based Electrochemical Supercapacitor; UNSW Sydney: Sydney, NSW, Australia, 2019. [Google Scholar]
- Tuncer, M.; Bakan, F.; Gocmez, H.; Erdem, E. Capacitive behaviour of nanocrystalline octacalcium phosphate (OCP) (Ca8H2 (PO4) 6· 5H2O) as an electrode material for supercapacitors: Biosupercaps. Nanoscale 2019, 11, 18375–18381. [Google Scholar] [CrossRef]
- Fletcher, S.; Black, V.J.; Kirkpatrick, I. A universal equivalent circuit for carbon-based supercapacitors. J. Solid State Electrochem. 2014, 18, 1377–1387. [Google Scholar] [CrossRef] [Green Version]
- Gönüllü, Y.; Kelm, K.; Mathur, S.; Saruhan, B. Equivalent circuit models for determination of the relation between the sensing behavior and properties of undoped/cr doped TiO2 NTs. Chemosensors 2014, 2, 69–84. [Google Scholar] [CrossRef]



| Sample | Scan Rate (V/s) | Potential Window (V) | Mass of Active Material (g) | Area (AV) | Csp (F/g) |
|---|---|---|---|---|---|
| S2 | 0.500 | 2.5 | 0.01002 | 0.0014826 | 0.05918563 |
| 0.400 | 0.001469 | 0.07330339 | |||
| 0.300 | 0.001442 | 0.09594145 | |||
| 0.200 | 0.0012018 | 0.11994012 | |||
| 0.020 | 0.0004284 | 0.42754491 | |||
| S3 | 0.500 | 2.5 | 0.00824 | 0.001120548 | 0.05439553 |
| 0.400 | 0.001050816 | 0.06376311 | |||
| 0.300 | 0.000954892 | 0.07644757 | |||
| 0.200 | 0.00089702 | 0.09551214 | |||
| 0.020 | 0.000312004 | 0.37864563 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gholami, A.; Yim, C.-H.; Kiani, A. Electrochemical Performance of Titania 3D Nanonetwork Electrodes Induced by Pulse Ionization at Varied Pulse Repetitions. Nanomaterials 2021, 11, 1062. https://doi.org/10.3390/nano11051062
Gholami A, Yim C-H, Kiani A. Electrochemical Performance of Titania 3D Nanonetwork Electrodes Induced by Pulse Ionization at Varied Pulse Repetitions. Nanomaterials. 2021; 11(5):1062. https://doi.org/10.3390/nano11051062
Chicago/Turabian StyleGholami, Amirhossein, Chae-Ho Yim, and Amirkianoosh Kiani. 2021. "Electrochemical Performance of Titania 3D Nanonetwork Electrodes Induced by Pulse Ionization at Varied Pulse Repetitions" Nanomaterials 11, no. 5: 1062. https://doi.org/10.3390/nano11051062






