Enzyme-Loaded Flower-Shaped Nanomaterials: A Versatile Platform with Biosensing, Biocatalytic, and Environmental Promise
Abstract
:1. Introduction
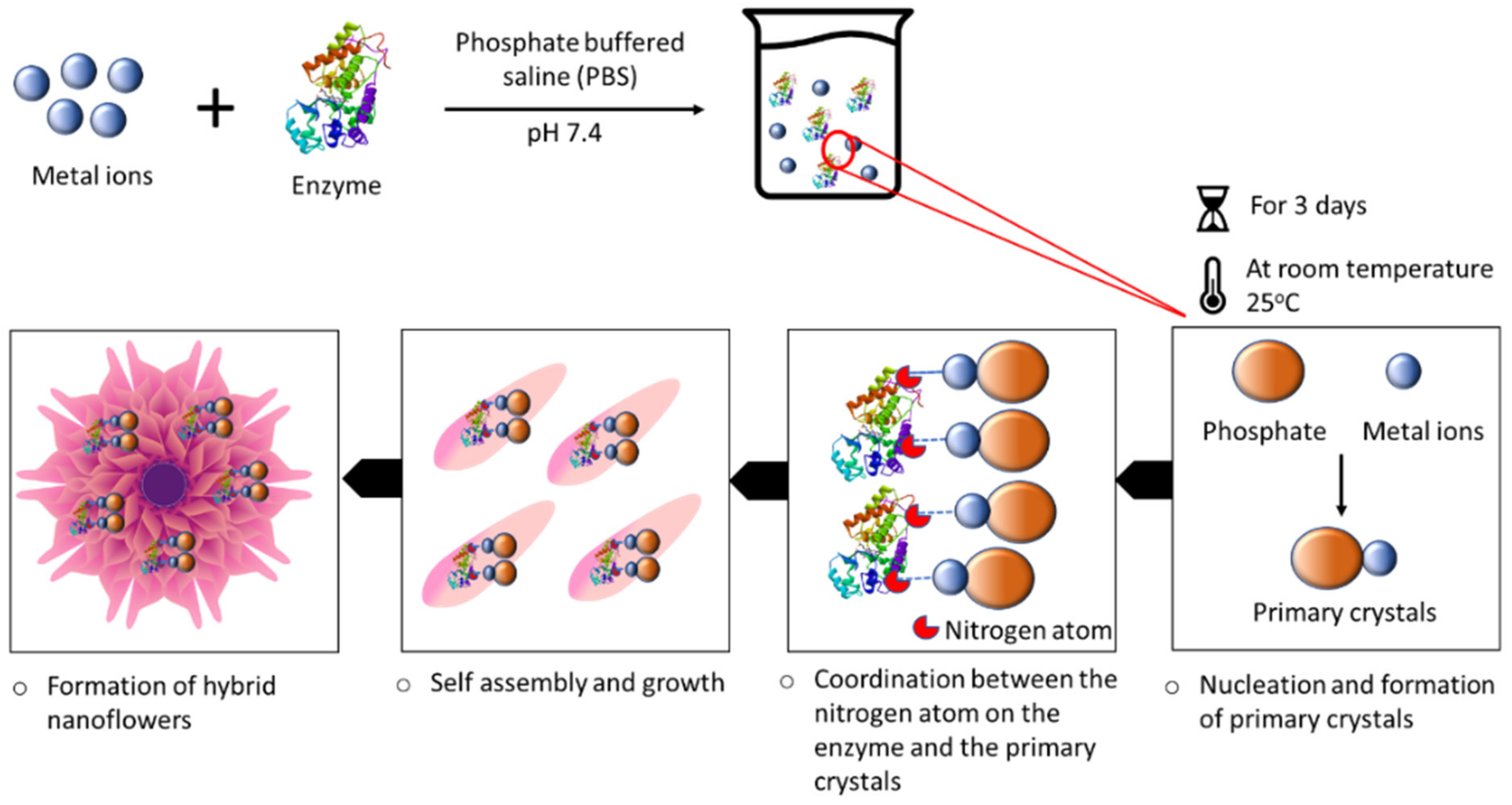
2. Organic–Inorganic Hybrid Nanoflowers
3. Advantages or (Properties) of Hybrid Nanoflowers
3.1. Catalytic Activity
3.2. Stability
3.2.1. Thermal Stability
3.2.2. Storage Stability
3.3. Reusability
4. Types and Synthesis of Organic–Inorganic Hybrid Nanoflowers
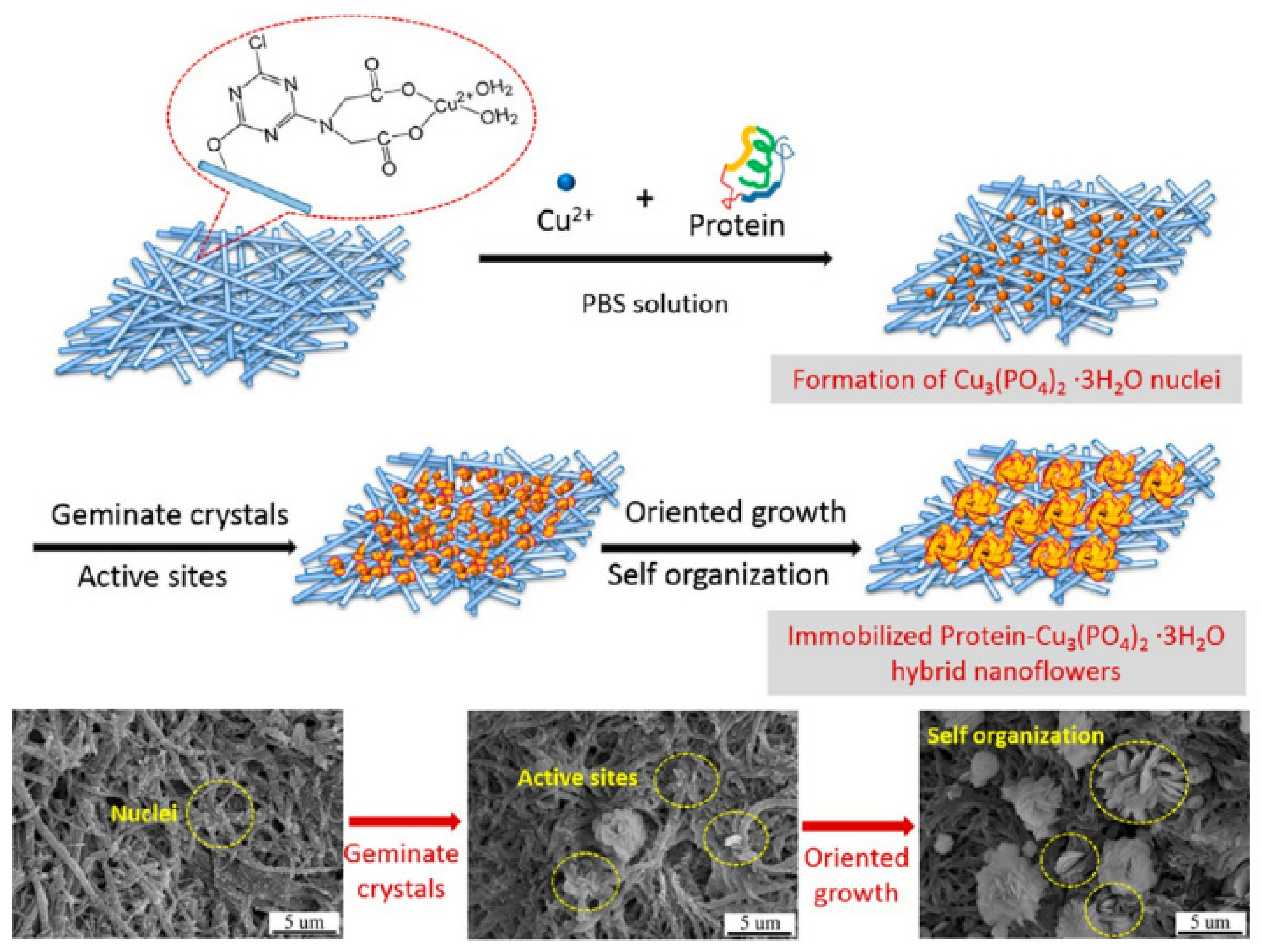
4.1. Copper-Based Hybrid Nanoflowers
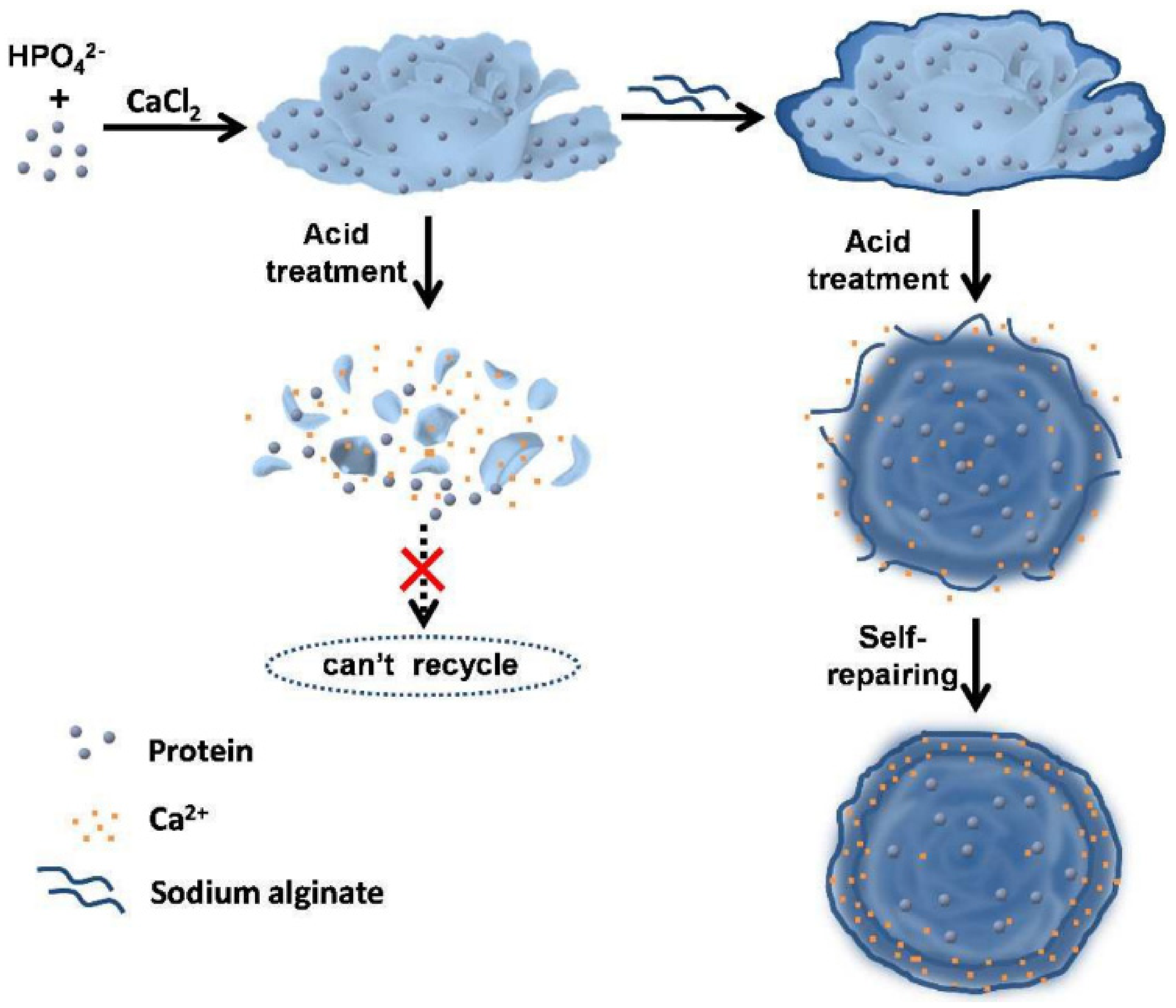
4.2. Calcium-Based Hybrid Nanoflowers
4.3. Manganese-Based Hybrid Nanoflowers
4.4. Zinc-Based Hybrid Nanoflowers
4.5. Cobalt-Based Hybrid Nanoflowers
4.6. Iron-Based Hybrid Nanoflowers
4.7. Multi-Metal-Based Hybrid Nanoflowers
4.8. Non-Metal-Based Hybrid Nanoflowers
4.9. Enhancing Hybrid Nanoflowers Synthesis
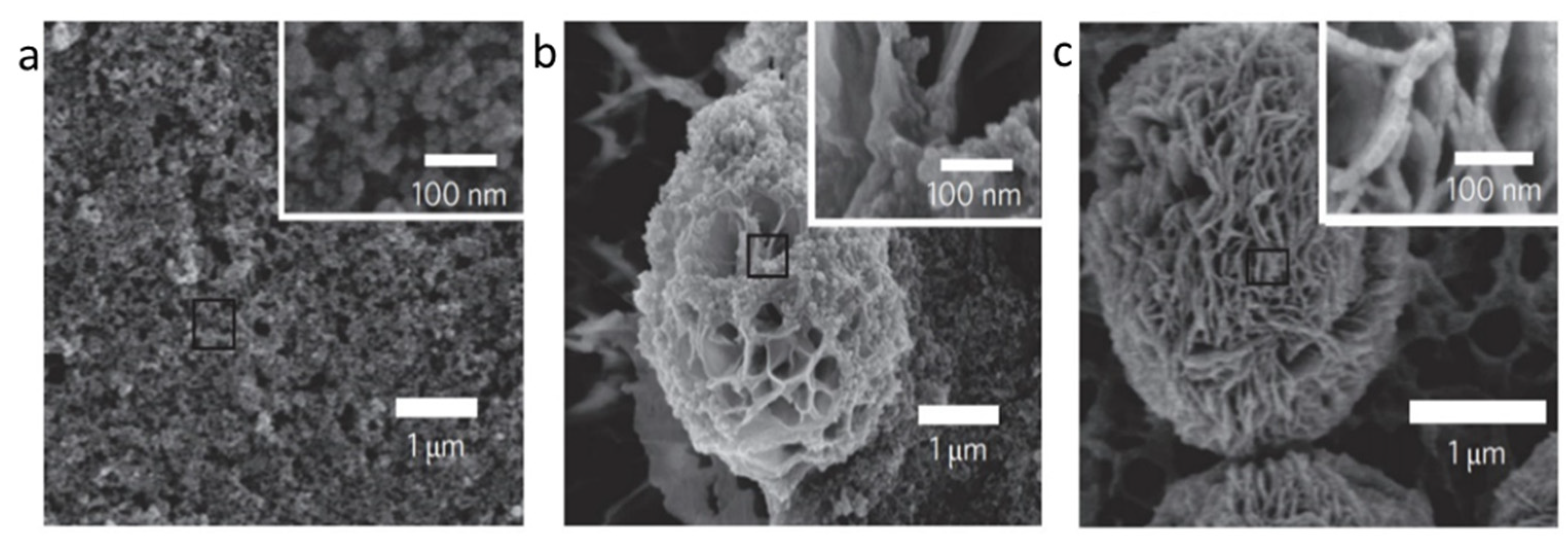
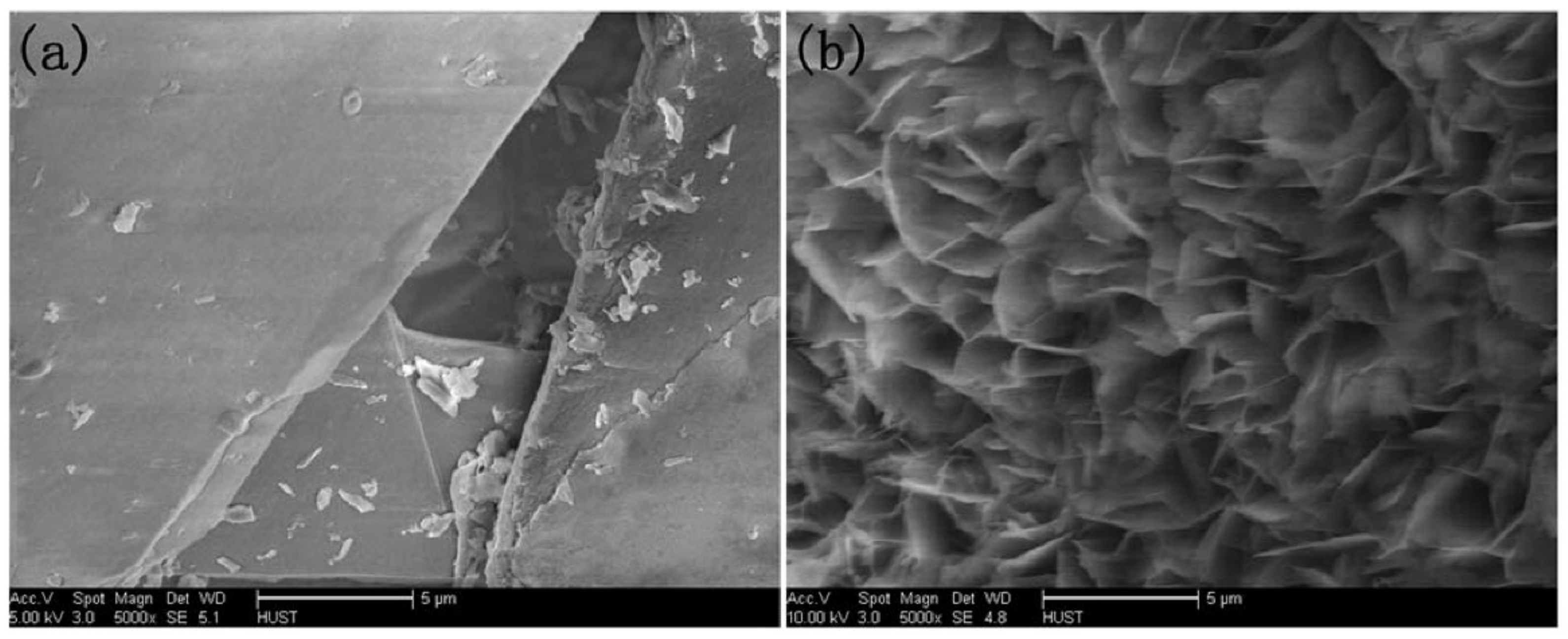
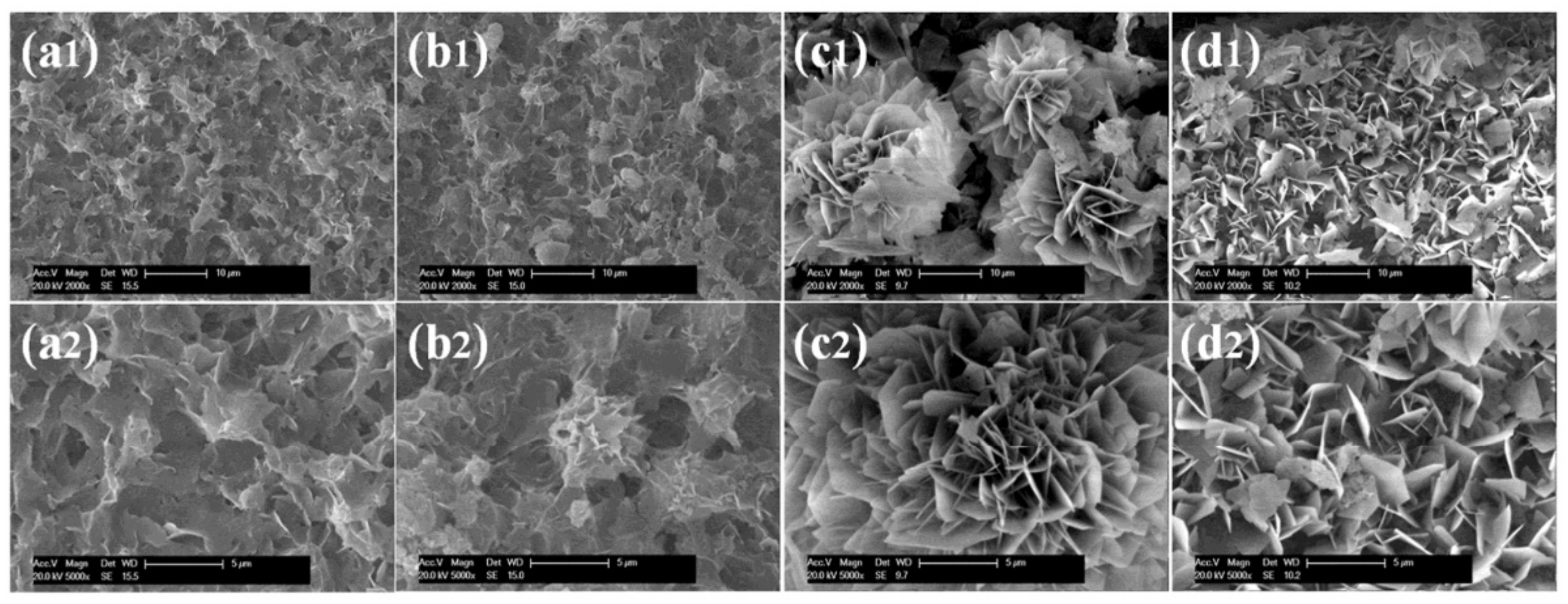
4.10. Morphology of Hybrid Nanoflowers
4.11. The Type of Enzyme Used
4.12. The Type of Metal Ion Used
4.13. Reaction Condition (pH, Temperature, and Time)
5. Applications for Hybrid Nanoflowers
5.1. Biosensors
5.2. Environmental Applications
5.3. Industrial Biocatalytic Applications
6. Cost Assessment and Industrial Integration Aspects
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An Overview of Technologies for Immobilization of Enzymes and Surface Analysis Techniques for Immobilized Enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.; Serban, S. Industrial Applications of Immobilized Enzymes—A Review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial Use of Immobilized Enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Truppo, M.D.; Hughes, G. Development of an Improved Immobilized CAL-B for the Enzymatic Resolution of a Key Intermediate to Odanacatib. Org. Process Res. Dev. 2011, 15, 1033–1035. [Google Scholar] [CrossRef]
- Al-Maqdi, K.A.; Hisaindee, S.; Rauf, M.A.; Ashraf, S.S. Detoxification and Degradation of Sulfamethoxazole by Soybean Peroxidase and UV + H2O2 Remediation Approaches. Chem. Eng. J. 2018, 352, 450–458. [Google Scholar] [CrossRef]
- Almaqdi, K.A.; Morsi, R.; Alhayuti, B.; Alharthi, F.; Ashraf, S.S. LC-MSMS Based Screening of Emerging Pollutant Degradation by Different Peroxidases. BMC Biotechnol. 2019, 19, 83. [Google Scholar] [CrossRef]
- Jun, L.Y.; Yon, L.S.; Mubarak, N.M.; Bing, C.H.; Pan, S.; Danquah, M.K.; Abdullah, E.C.; Khalid, M. An Overview of Immobilized Enzyme Technologies for Dye and Phenolic Removal from Wastewater. J. Environ. Chem. Eng. 2019, 7, 102961. [Google Scholar] [CrossRef]
- Liu, D.-M.; Chen, J.; Shi, Y.-P. Advances on Methods and Easy Separated Support Materials for Enzymes Immobilization. TrAC Trends Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Girelli, A.M.; Astolfi, M.L.; Scuto, F.R. Agro-Industrial Wastes as Potential Carriers for Enzyme Immobilization: A Review. Chemosphere 2020, 244, 125368. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Kostrov, X. Immobilization of Enzymes on Porous Silicas—Benefits and Challenges. Chem. Soc. Rev. 2013, 42, 6277–6289. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme Immobilization: An Overview on Techniques and Support Materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme Immobilization: An Update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucca, P.; Sanjust, E. Inorganic Materials as Supports for Covalent Enzyme Immobilization: Methods and Mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.P.; Nunes, M.R.; Rodrigues, R.C.; Benvenutti, E.V.; Costa, T.M.H.; Hertz, P.F.; Ninow, J.L. Effect of the Support Size on the Properties of β-Galactosidase Immobilized on Chitosan: Advantages and Disadvantages of Macro and Nanoparticles. Biomacromolecules 2012, 13, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.H.; Tan, H.K.; Lau, S.Y.; Yap, P.-S.; Danquah, M.K. Potential and Challenges of Enzyme Incorporated Nanotechnology in Dye Wastewater Treatment: A Review. J. Environ. Chem. Eng. 2019, 7, 103261. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Dasgupta, A.K.; Chakrabarti, K. Enhanced Functionality and Stabilization of a Cold Active Laccase Using Nanotechnology Based Activation-Immobilization. Bioresour. Technol. 2015, 179, 573–584. [Google Scholar] [CrossRef]
- Gupta, M.N.; Kaloti, M.; Kapoor, M.; Solanki, K. Nanomaterials as Matrices for Enzyme Immobilization. Artif. Cells Blood Substit. Biotechnol. 2011, 39, 98–109. [Google Scholar] [CrossRef]
- Liu, W.; Wang, L.; Jiang, R. Specific Enzyme Immobilization Approaches and Their Application with Nanomaterials. Top. Catal. 2012, 55, 1146–1156. [Google Scholar] [CrossRef]
- Ge, J.; Lei, J.; Zare, R.N. Protein–Inorganic Hybrid Nanoflowers. Nat. Nanotechnol. 2012, 7, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Fan, Y.; Chen, Y.; Xu, L.; Yan, Y. A New Lipase–Inorganic Hybrid Nanoflower with Enhanced Enzyme Activity. RSC Adv. 2016, 6, 19413–19416. [Google Scholar] [CrossRef]
- Yin, Y.; Xiao, Y.; Lin, G.; Xiao, Q.; Lin, Z.; Cai, Z. An Enzyme–Inorganic Hybrid Nanoflower Based Immobilized Enzyme Reactor with Enhanced Enzymatic Activity. J. Mater. Chem. B 2015, 3, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Xiao, Y.; Wang, L.; Yin, Y.; Zheng, J.; Yang, H.; Chen, G. Facile Synthesis of Enzyme–Inorganic Hybrid Nanoflowers and Their Application as an Immobilized Trypsin Reactor for Highly Efficient Protein Digestion. RSC Adv. 2014, 4, 13888–13891. [Google Scholar] [CrossRef]
- Yu, Y.; Fei, X.; Tian, J.; Xu, L.; Wang, X.; Wang, Y. Self-Assembled Enzyme–Inorganic Hybrid Nanoflowers and Their Application to Enzyme Purification. Colloids Surf. B Biointerfaces 2015, 130, 299–304. [Google Scholar] [CrossRef]
- Wu, X.; Hou, M.; Ge, J. Metal–Organic Frameworks and Inorganic Nanoflowers: A Type of Emerging Inorganic Crystal Nanocarrier for Enzyme Immobilization. Catal. Sci. Technol. 2015, 5, 5077–5085. [Google Scholar] [CrossRef]
- Duan, L.; Li, H.; Zhang, Y. Synthesis of Hybrid Nanoflower-Based Carbonic Anhydrase for Enhanced Biocatalytic Activity and Stability. ACS Omega 2018. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Zhao, Y.; Liu, R.; Zhong, C.; Jia, S. Surfactant-Activated Lipase Hybrid Nanoflowers with Enhanced Enzymatic Performance. Sci. Rep. 2016, 6, 27928. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Wang, J.; He, Y.; Abdulrazaq, M.A.; Yan, Y. Carbon Nanotube-Lipase Hybrid Nanoflowers with Enhanced Enzyme Activity and Enantioselectivity. J. Biotechnol. 2018, 281, 87–98. [Google Scholar] [CrossRef]
- Wang, S.; Ding, Y.; Chen, R.; Hu, M.; Li, S.; Zhai, Q.; Jiang, Y. Multilayer Petal-like Enzymatic-Inorganic Hybrid Micro-Spheres [CPO-(Cu/Co/Cd)3(PO4)2] with High Bio-Catalytic Activity. Chem. Eng. Res. Des. 2018, 134, 52–61. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Otari, S.V.; Li, J.; Kim, D.R.; Kim, S.C.; Cho, B.-K.; Kalia, V.C.; Kang, Y.C.; Lee, J.-K. Synthesis of Cross-Linked Protein-Metal Hybrid Nanoflowers and Its Application in Repeated Batch Decolorization of Synthetic Dyes. J. Hazard. Mater. 2018, 347, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Altinkaynak, C.; Tavlasoglu, S.; Ÿzdemir, N.; Ocsoy, I. A New Generation Approach in Enzyme Immobilization: Organic-Inorganic Hybrid Nanoflowers with Enhanced Catalytic Activity and Stability. Enzyme Microb. Technol. 2016, 93–94, 105–112. [Google Scholar] [CrossRef]
- Rong, J.; Zhang, T.; Qiu, F.; Zhu, Y. Preparation of Efficient, Stable, and Reusable Laccase–Cu3(PO4)2 Hybrid Microspheres Based on Copper Foil for Decoloration of Congo Red. ACS Sustain. Chem. Eng. 2017, 5, 4468–4477. [Google Scholar] [CrossRef]
- Cui, J.; Jia, S. Organic–Inorganic Hybrid Nanoflowers: A Novel Host Platform for Immobilizing Biomolecules. Coord. Chem. Rev. 2017, 352, 249–263. [Google Scholar] [CrossRef]
- Fu, M.; Xing, J.; Ge, Z. Preparation of Laccase-Loaded Magnetic Nanoflowers and Their Recycling for Efficient Degradation of Bisphenol A. Sci. Total Environ. 2019, 651, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, P.; Zhang, H.; Wang, H.; Li, X.; Tian, L.; Ali, N.; Ali, Z.; Zhang, Q. Preparation of Lipase/Zn3(PO4)2 Hybrid Nanoflower and Its Catalytic Performance as an Immobilized Enzyme. Chem. Eng. J. 2016, 291, 287–297. [Google Scholar] [CrossRef]
- Lin, Z.; Xiao, Y.; Yin, Y.; Hu, W.; Liu, W.; Yang, H. Facile Synthesis of Enzyme-Inorganic Hybrid Nanoflowers and Its Application as a Colorimetric Platform for Visual Detection of Hydrogen Peroxide and Phenol. ACS Appl. Mater. Interfaces 2014, 6, 10775–10782. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Wang, A.; Li, N.; Chen, X.; Pei, X.; Zhang, P.; Gang Wu, S. Dual-Cycle Immobilization to Reuse Both Enzyme and Support by Reblossoming Enzyme–Inorganic Hybrid Nanoflowers. RSC Adv. 2018, 8, 16088–16094. [Google Scholar] [CrossRef] [Green Version]
- Somturk, B.; Yilmaz, I.; Altinkaynak, C.; Karatepe, A.; Özdemir, N.; Ocsoy, I. Synthesis of Urease Hybrid Nanoflowers and Their Enhanced Catalytic Properties. Enzyme Microb. Technol. 2016, 86, 134–142. [Google Scholar] [CrossRef]
- Nadar, S.S.; Gawas, S.D.; Rathod, V.K. Self-Assembled Organic-inorganic Hybrid Glucoamylase Nanoflowers with Enhanced Activity and Stability. Int. J. Biol. Macromol. 2016, 92, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.H.; Ding, R.; Yuan, Q.; Wei, Y.; Liang, H. Facile Synthesis of Alcalase-Inorganic Hybrid Nanoflowers Used for Soy Protein Isolate Hydrolysis to Improve Its Functional Properties. Food Chem. 2019, 289, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, P.; Zhang, H.; Fan, L.; Wang, H.; Li, X.; Tian, L.; Ali, N.; Ali, Z.; Zhang, Q. Papain/Zn3(PO4)2 Hybrid Nanoflower: Preparation, Characterization and Its Enhanced Catalytic Activity as an Immobilized Enzyme. RSC Adv. 2016, 6, 46702–46710. [Google Scholar] [CrossRef]
- Li, M.; Luo, M.; Li, F.; Wang, W.; Liu, K.; Liu, Q.; Wang, Y.; Lu, Z.; Wang, D. Biomimetic Copper-Based Inorganic–Protein Nanoflower Assembly Constructed on the Nanoscale Fibrous Membrane with Enhanced Stability and Durability. J. Phys. Chem. C 2016, 120, 17348–17356. [Google Scholar] [CrossRef]
- Altinkaynak, C.; Tavlasoglu, S.; Kalin, R.; Sadeghian, N.; Ozdemir, H.; Ocsoy, I.; Özdemir, N. A Hierarchical Assembly of Flower-like Hybrid Turkish Black Radish Peroxidase-Cu2+ Nanobiocatalyst and Its Effective Use in Dye Decolorization. Chemosphere 2017, 182, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, M.; Wang, W.; Wang, Y.; Tang, J. UV-Vis Detection of Hydrogen Peroxide Using Horseradish Peroxidase/Copper Phosphate Hybrid Nanoflowers. Enzyme Microb. Technol. 2020, 140, 109620. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Du, D.; Zhu, M.-J.; Lin, Y.; Dai, Z. An Improved Ultrasensitive Enzyme-Linked Immunosorbent Assay Using Hydrangea-Like Antibody–Enzyme–Inorganic Three-in-One Nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 6329–6335. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tan, R.; Wang, Q. One-Step Synthesized Flower-like Materials Used for Sensitively Detecting Amyloid Precursor Protein. Anal. Bioanal. Chem. 2018, 410, 6901–6909. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Hu, W.; Li, C.M. Spontaneous Interfacial Reaction between Metallic Copper and PBS to Form Cupric Phosphate Nanoflower and Its Enzyme Hybrid with Enhanced Activity. Colloids Surf. B Biointerfaces 2015, 135, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Somturk, B.; Hancer, M.; Ocsoy, I.; Özdemir, N. Synthesis of Copper Ion Incorporated Horseradish Peroxidase-Based Hybrid Nanoflowers for Enhanced Catalytic Activity and Stability. Dalton Trans. 2015, 44, 13845–13852. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Du, M.; Wang, X.; Ji, X.; He, Z. The Preparation of Dual-Functional Hybrid Nanoflower and Its Application in the Ultrasensitive Detection of Disease-Related Biomarker. Biosens. Bioelectron. 2017, 92, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Zhu, L.; Gao, Y.; Wheeldon, I. Enhancing Enzyme Activity and Immobilization in Nanostructured Inorganic–Enzyme Complexes. Langmuir 2017, 33, 9073–9080. [Google Scholar] [CrossRef]
- Altinkaynak, C.; Yilmaz, I.; Koksal, Z.; Özdemir, H.; Ocsoy, I.; Özdemir, N. Preparation of Lactoperoxidase Incorporated Hybrid Nanoflower and Its Excellent Activity and Stability. Int. J. Biol. Macromol. 2016, 84, 402–409. [Google Scholar] [CrossRef]
- Chung, M.; Nguyen, T.L.; Tran, T.Q.N.; Yoon, H.H.; Kim, I.T.; Kim, M.I. Ultrarapid Sonochemical Synthesis of Enzyme-Incorporated Copper Nanoflowers and Their Application to Mediatorless Glucose Biofuel Cell. Appl. Surf. Sci. 2018, 429, 203–209. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, N.; Liu, Y.; Tang, J. Synthesis of Catalase-Inorganic Hybrid Nanoflowers via Sonication for Colorimetric Detection of Hydrogen Peroxide. Enzyme Microb. Technol. 2019, 128, 22–25. [Google Scholar] [CrossRef]
- Batule, B.S.; Park, K.S.; Kim, M.I.; Park, H.G. Ultrafast Sonochemical Synthesis of Protein-Inorganic Nanoflowers. Int. J. Nanomed. 2015, 10, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Gong, L.; Zhang, Y.; Wang, R.; Ge, J.; Liu, Z.; Zare, R.N. Rapid Detection of Phenol Using a Membrane Containing Laccase Nanoflowers. Chem. Asian J. 2013, 8, 2358–2360. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Wang, Y.; Li, G.; Liu, K.; Liu, Y.; He, J.; Lei, J. Preparation and Application of a Chemically Modified Laccase and Copper Phosphate Hybrid Flower-like Biocatalyst. Biochem. Eng. J. 2019, 144, 235–243. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Zhu, X.; Li, S.; Wang, Z.; Wang, L.; Li, Z.; Chen, G. Using Laccases in the Nanoflower to Synthesize Viniferin. Catalysts 2017, 7, 188. [Google Scholar] [CrossRef] [Green Version]
- Batule, B.S.; Park, K.S.; Gautam, S.; Cheon, H.J.; Kim, M.I.; Park, H.G. Intrinsic Peroxidase-like Activity of Sonochemically Synthesized Protein Copper Nanoflowers and Its Application for the Sensitive Detection of Glucose. Sens. Actuators B Chem. 2019, 283, 749–754. [Google Scholar] [CrossRef]
- Huang, Y.; Ran, X.; Lin, Y.; Ren, J.; Qu, X. Self-Assembly of an Organic-Inorganic Hybrid Nanoflower as an Efficient Biomimetic Catalyst for Self-Activated Tandem Reactions. Chem. Commun. 2015, 51, 4386–4389. [Google Scholar] [CrossRef]
- Kumar, A.; Patel, S.K.S.; Mardan, B.; Pagolu, R.; Lestari, R.; Jeong, S.-H.; Kim, T.; Haw, J.R.; Kim, S.-Y.; Kim, I.-W.; et al. Immobilization of Xylanase Using a Protein-Inorganic Hybrid System. J. Microbiol. Biotechnol. 2018, 28, 638–644. [Google Scholar] [CrossRef]
- Qian, K.; Wang, H.; Liu, J.; Gao, S.; Liu, W.; Wan, X.; Zhang, Y.; Liu, Q.-S.; Yin, X.-Y. Synthesis of α-Glycosidase Hybrid Nano-Flowers and Their Application for Enriching and Screening α-Glycosidase Inhibitors. New J. Chem. 2018, 42, 429–436. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, R.; Liu, C.; Chi, B.; Gao, J.; Chen, B.; Xu, H. A New l -Arabinose Isomerase with Copper Ion Tolerance Is Suitable for Creating Protein–Inorganic Hybrid Nanoflowers with Enhanced Enzyme Activity and Stability. RSC Adv. 2016, 6, 30791–30794. [Google Scholar] [CrossRef]
- Lee, H.R.; Chung, M.; Kim, M.I.; Ha, S.H. Preparation of Glutaraldehyde-Treated Lipase-Inorganic Hybrid Nanoflowers and Their Catalytic Performance as Immobilized Enzymes. Enzyme Microb. Technol. 2017, 105, 24–29. [Google Scholar] [CrossRef]
- Hua, X.; Xing, Y.; Zhang, X. Enhanced Promiscuity of Lipase-Inorganic Nanocrystal Composites in the Epoxidation of Fatty Acids in Organic Media. ACS Appl. Mater. Interfaces 2016, 8, 16257–16261. [Google Scholar] [CrossRef]
- Ren, W.; Fei, X.; Tian, J.; Li, Y.; Jing, M.; Fang, H.; Xu, L.; Wang, Y. Multiscale Immobilized Lipase for Rapid Separation and Continuous Catalysis. New J. Chem. 2018, 42, 13471–13478. [Google Scholar] [CrossRef]
- Wu, Z.; Li, X.; Li, F.; Yue, H.; He, C.; Xie, F.; Wang, Z. Enantioselective Transesterification of (R,S)-2-Pentanol Catalyzed by a New Flower-like Nanobioreactor. RSC Adv. 2014, 4, 33998–34002. [Google Scholar] [CrossRef]
- Escobar, S.; Velasco-Lozano, S.; Lu, C.-H.; Lin, Y.-F.; Mesa, M.; Bernal, C.; López-Gallego, F. Understanding the Functional Properties of Bio-Inorganic Nanoflowers as Biocatalysts by Deciphering the Metal-Binding Sites of Enzymes. J. Mater. Chem. B 2017, 5, 4478–4486. [Google Scholar] [CrossRef]
- Ren, W.; Li, Y.; Wang, J.; Li, L.; Xu, L.; Wu, Y.; Wang, Y.; Fei, X.; Tian, J. Synthesis of Magnetic Nanoflower Immobilized Lipase and Its Continuous Catalytic Application. New J. Chem. 2019, 43, 11082–11090. [Google Scholar] [CrossRef]
- Li, Y.; Fei, X.; Liang, L.; Tian, J.; Xu, L.; Wang, X.; Wang, Y. The Influence of Synthesis Conditions on Enzymatic Activity of Enzyme-Inorganic Hybrid Nanoflowers. J. Mol. Catal. B Enzym. 2016, 133, 92–97. [Google Scholar] [CrossRef]
- Papadopoulou, A.A.; Tzani, A.; Polydera, A.C.; Katapodis, P.; Voutsas, E.; Detsi, A.; Stamatis, H. Green Biotransformations Catalysed by Enzyme-Inorganic Hybrid Nanoflowers in Environmentally Friendly Ionic Solvents. Environ. Sci. Pollut. Res. 2018, 25, 26707–26714. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, X.; Yang, J.; Han, H.; Li, Q.; Tang, J. Lipase-Inorganic Hybrid Nanoflower Constructed through Biomimetic Mineralization: A New Support for Biodiesel Synthesis. J. Colloid Interface Sci. 2018, 514, 102–107. [Google Scholar] [CrossRef]
- Gulmez, C.; Altinkaynak, C.; Özdemir, N.; Atakisi, O. Proteinase K Hybrid Nanoflowers (P-HNFs) as a Novel Nanobiocatalytic Detergent Additive. Int. J. Biol. Macromol. 2018, 119, 803–810. [Google Scholar] [CrossRef]
- Zhang, H.; Fei, X.; Tian, J.; Li, Y.; Zhi, H.; Wang, K.; Xu, L.; Wang, Y. Synthesis and Continuous Catalytic Application of Alkaline Protease Nanoflowers–PVA Composite Hydrogel. Catal. Commun. 2018, 116, 5–9. [Google Scholar] [CrossRef]
- Yu, J.; Chen, X.; Jiang, M.; Wang, A.; Yang, L.; Pei, X.; Zhang, P.; Wu, S.G. Efficient Promiscuous Knoevenagel Condensation Catalyzed by Papain Confined in Cu3(PO4)2 Nanoflowers. RSC Adv. 2018, 8, 2357–2364. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Fei, X.; Li, Y.; Tian, J.; Xu, L.; Wang, X.; Wang, Y. Hierarchical Assembly of Enzyme-Inorganic Composite Materials with Extremely High Enzyme Activity. RSC Adv. 2015, 5, 96997–97002. [Google Scholar] [CrossRef]
- Chung, M.; Jang, Y.J.; Kim, M.I. Convenient Colorimetric Detection of Cholesterol Using Multi-Enzyme Co-Incorporated Organic–Inorganic Hybrid Nanoflowers. J. Nanosci. Nanotechnol. 2018, 18, 6555–6561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, Y.; Wang, C.; Wang, Z.; Chen, X.; Li, M.; Zhao, R.; Wang, L. Application of Dual-Enzyme Nanoflower in the Epoxidation of Alkenes. Process Biochem. 2018, 74, 103–107. [Google Scholar] [CrossRef]
- Jin, R.; Kong, D.; Zhao, X.; Li, H.; Yan, X.; Liu, F.; Sun, P.; Du, D.; Lin, Y.; Lu, G. Tandem Catalysis Driven by Enzymes Directed Hybrid Nanoflowers for On-Site Ultrasensitive Detection of Organophosphorus Pesticide. Biosens. Bioelectron. 2019, 141, 111473. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, G.; Qiu, J.; Zhou, D.; Gou, D.; Tao, Y.; Li, Y.; Chen, H. A New Biosensor Based on the Recognition of Phages and the Signal Amplification of Organic-Inorganic Hybrid Nanoflowers for Discriminating and Quantitating Live Pathogenic Bacteria in Urine. Sens. Actuators B Chem. 2018, 258, 803–812. [Google Scholar] [CrossRef]
- Sun, J.; Ge, J.; Liu, W.; Lan, M.; Zhang, H.; Wang, P.; Wang, Y.; Niu, Z. Multi-Enzyme Co-Embedded Organic–Inorganic Hybrid Nanoflowers: Synthesis and Application as a Colorimetric Sensor. Nanoscale 2014, 6, 255–262. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, J.; Liu, J.; Zhang, H.; Jiang, J.; Yu, R. A Dual Enzyme–Inorganic Hybrid Nanoflower Incorporated Microfluidic Paper-Based Analytic Device (ΜPAD) Biosensor for Sensitive Visualized Detection of Glucose. Nanoscale 2017, 9, 5658–5663. [Google Scholar] [CrossRef]
- Ariza-Avidad, M.; Salinas-Castillo, A.; Capitán-Vallvey, L.F. A 3D ΜPAD Based on a Multi-Enzyme Organic–Inorganic Hybrid Nanoflower Reactor. Biosens. Bioelectron. 2016, 77, 51–55. [Google Scholar] [CrossRef]
- He, X.; Chen, L.; He, Q.; Xiao, H.; Zhou, X.; Ji, H. Cytochrome P450 Enzyme-Copper Phosphate Hybrid Nano-Flowers with Superior Catalytic Performances for Selective Oxidation of Sulfides. Chin. J. Chem. 2017, 35, 693–698. [Google Scholar] [CrossRef]
- Patel, S.S.K.; Otari, S.V.; Kang, Y.C.; Lee, J.-K. Protein–Inorganic Hybrid System for Efficient His-Tagged Enzymes Immobilization and Its Application in l -Xylulose Production. RSC Adv. 2017, 7, 3488–3494. [Google Scholar] [CrossRef] [Green Version]
- Hao, M.; Fan, G.; Zhang, Y.; Xin, Y.; Zhang, L. Preparation and Characterization of Copper-Brevibacterium Cholesterol Oxidase Hybrid Nanoflowers. Int. J. Biol. Macromol. 2019, 126, 539–548. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, C.; Qian, X.; Yu, D. Self-Assembled 2,4-Dichlorophenol Hydroxylase-Inorganic Hybrid Nanoflowers with Enhanced Activity and Stability. RSC Adv. 2018, 8, 20976–20981. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, Y.; Li, X.; Yuan, Q.; Liang, H. Self-Repairing Metal–Organic Hybrid Complexes for Reinforcing Immobilized Chloroperoxidase Reusability. Chem. Commun. 2017, 53, 3216–3219. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, J.; Li, Z.; Zhang, S.; Wu, H.; Jiang, Z.; Yang, C.; Tian, C. Facile One-Pot Preparation of Chitosan/Calcium Pyrophosphate Hybrid Microflowers. ACS Appl. Mater. Interfaces 2014, 6, 14522–14532. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-B.; Wang, Y.-C.; He, R.; Zhuang, A.; Wang, X.; Zeng, J.; Hou, J.G. A New Nanobiocatalytic System Based on Allosteric Effect with Dramatically Enhanced Enzymatic Performance. J. Am. Chem. Soc. 2013, 135, 1272–1275. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, B.; Ji, X.; He, Z. Self-Assembled Protein-Enzyme Nanoflower-Based Fluorescent Sensing for Protein Biomarker. Anal. Bioanal. Chem. 2018, 410, 7591–7598. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, L.; Wang, A.; Li, H.; Wang, C.; Pei, X.; Zhang, P.; Wu, S.G. Efficient Synthesis of the Key Chiral Alcohol Intermediate of Crizotinib Using Dual-Enzyme@CaHPO4 Hybrid Nanoflowers Assembled by Mimetic Biomineralization. J. Chem. Technol. Biotechnol. 2019, 94, 236–243. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Q.; Dong, J.; Xian, M.; Yu, J.; Yin, H.; Chang, Z.; Mu, X.; Hou, T.; Wang, J. Enzyme-Inorganic Nanoflowers/Alginate Microbeads: An Enzyme Immobilization System and Its Potential Application. Process Biochem. 2017, 57, 87–94. [Google Scholar] [CrossRef]
- Ghosh, K.; Balog, E.R.M.; Sista, P.; Williams, D.J.; Kelly, D.; Martinez, J.S.; Rocha, R.C. Temperature-Dependent Morphology of Hybrid Nanoflowers from Elastin-like Polypeptides. APL Mater. 2014, 2, 021101. [Google Scholar] [CrossRef]
- Ye, R.; Zhu, C.; Song, Y.; Song, J.; Fu, S.; Lu, Q.; Yang, X.; Zhu, M.-J.; Du, D.; Li, H.; et al. One-Pot Bioinspired Synthesis of All-Inclusive Protein–Protein Nanoflowers for Point-of-Care Bioassay: Detection of E. Coli O157:H7 from Milk. Nanoscale 2016, 8, 18980–18986. [Google Scholar] [CrossRef]
- Rai, S.K.; Narnoliya, L.K.; Sangwan, R.S.; Yadav, S.K. Self-Assembled Hybrid Nanoflowers of Manganese Phosphate and l-Arabinose Isomerase: A Stable and Recyclable Nanobiocatalyst for Equilibrium Level Conversion of d-Galactose to d-Tagatose. ACS Sustain. Chem. Eng. 2018, 6, 6296–6304. [Google Scholar] [CrossRef]
- Claude Munyemana, J.; He, H.; Ding, S.; Yin, J.; Xi, P.; Xiao, J. Synthesis of Manganese Phosphate Hybrid Nanoflowers by Collagen-Templated Biomineralization. RSC Adv. 2018, 8, 2708–2713. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, Y.; He, L.; Yang, Y.; Liu, S.; Wang, M.; Fang, S.; Fu, G. A Feasible Synthesis of Mn3(PO4)2@BSA Nanoflowers and Its Application as the Support Nanomaterial for Pt Catalyst. J. Power Sources 2015, 284, 170–177. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Song, R.; Wang, M.; Yan, F.; He, L.; Feng, X.; Fang, S.; Zhao, J.; Zhang, H. Manganese(II) Phosphate Nanoflowers as Electrochemical Biosensors for the High-Sensitivity Detection of Ractopamine. Sens. Actuators B Chem. 2015, 211, 310–317. [Google Scholar] [CrossRef]
- Zheng, L.; Xie, X.; Wang, Z.; Zhang, Y.; Wang, L.; Cui, X.; Huang, H.; Zhuang, H. Fabrication of a Nano-Biocatalyst for Regioselective Acylation of Arbutin. Green Chem. Lett. Rev. 2018, 11, 55–61. [Google Scholar] [CrossRef]
- Zhang, B.; Li, P.; Zhang, H.; Li, X.; Tian, L.; Wang, H.; Chen, X.; Ali, N.; Ali, Z.; Zhang, Q. Red-Blood-Cell-like BSA/Zn3(PO4)2 Hybrid Particles: Preparation and Application to Adsorption of Heavy Metal Ions. Appl. Surf. Sci. 2016, 366, 328–338. [Google Scholar] [CrossRef]
- Zheng, L.; Sun, Y.; Wang, J.; Huang, H.; Geng, X.; Tong, Y.; Wang, Z. Preparation of a Flower-Like Immobilized D-Psicose 3-Epimerase with Enhanced Catalytic Performance. Catalysts 2018, 8, 468. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Kim, I.-W.; Patel, S.K.S.; Lee, J.-K. Synthesis of Protein-Inorganic Nanohybrids with Improved Catalytic Properties Using Co3(PO4)2. Indian J. Microbiol. 2018, 58, 100–104. [Google Scholar] [CrossRef]
- Cao, G.; Gao, J.; Zhou, L.; He, Y.; Li, J.; Jiang, Y. Enrichment and Coimmobilization of Cofactors and His-Tagged ω-Transaminase into Nanoflowers: A Facile Approach to Constructing Self-Sufficient Biocatalysts. ACS Appl. Nano Mater. 2018, 1, 3417–3425. [Google Scholar] [CrossRef]
- Kim, K.H.; Jeong, J.-M.; Lee, S.J.; Choi, B.G.; Lee, K.G. Protein-Directed Assembly of Cobalt Phosphate Hybrid Nanoflowers. J. Colloid Interface Sci. 2016, 484, 44–50. [Google Scholar] [CrossRef]
- He, L.; Zhang, S.; Ji, H.; Wang, M.; Peng, D.; Yan, F.; Fang, S.; Zhang, H.; Jia, C.; Zhang, Z. Protein-Templated Cobaltous Phosphate Nanocomposites for the Highly Sensitive and Selective Detection of Platelet-Derived Growth Factor-BB. Biosens. Bioelectron. 2016, 79, 553–560. [Google Scholar] [CrossRef] [PubMed]
- López-Gallego, F.; Yate, L. Selective Biomineralization of Co3(PO4)2-Sponges Triggered by His-Tagged Proteins: Efficient Heterogeneous Biocatalysts for Redox Processes. Chem. Commun. 2015, 51, 8753–8756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocsoy, I.; Dogru, E.; Usta, S. A New Generation of Flowerlike Horseradish Peroxides as a Nanobiocatalyst for Superior Enzymatic Activity. Enzyme Microb. Technol. 2015, 75–76, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Y.; Zhao, M. A Self-Activated Nanobiocatalytic Cascade System Based on an Enzyme-Inorganic Hybrid Nanoflower for Colorimetric and Visual Detection of Glucose in Human Serum. Sens. Actuators B Chem. 2019, 284, 45–54. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Choi, H.; Lee, J.-K. Multimetal-Based Inorganic–Protein Hybrid System for Enzyme Immobilization. ACS Sustain. Chem. Eng. 2019, 7, 13633–13638. [Google Scholar] [CrossRef]
- Nonsuwan, P.; Puthong, S.; Palaga, T.; Muangsin, N. Novel Organic/Inorganic Hybrid Flower-like Structure of Selenium Nanoparticles Stabilized by Pullulan Derivatives. Carbohydr. Polym. 2018, 184, 9–19. [Google Scholar] [CrossRef]
- Lee, I.; Cheon, H.J.; Adhikari, M.D.; Tran, T.D.; Yeon, K.-M.; Kim, M.I.; Kim, J. Glucose Oxidase-Copper Hybrid Nanoflowers Embedded with Magnetic Nanoparticles as an Effective Antibacterial Agent. Int. J. Biol. Macromol. 2020, 155, 1520–1531. [Google Scholar] [CrossRef]
- Sun, X.; Niu, H.; Song, J.; Jiang, D.; Leng, J.; Zhuang, W.; Chen, Y.; Liu, D.; Ying, H. Preparation of a Copper Polyphosphate Kinase Hybrid Nanoflower and Its Application in ADP Regeneration from AMP. ACS Omega 2020, 5, 9991–9998. [Google Scholar] [CrossRef]
- Ben Brahim, F.; Boughzala, H. Crystal Structure, Vibrational Spectra and Thermal Analysis of a New Centrosymmetric Transition Metal Phosphate Compound, Mn(H2PO4)2⋅4H2O. J. Mol. Struct. 2013, 1034, 336–345. [Google Scholar] [CrossRef]
- Ferdov, S.; Lopes, A.M.L.; Lin, Z.; Ferreira, R.A.S. New Template-Free Layered Manganese(III) Phosphate: Hydrothermal Synthesis, Ab Initio Structural Determination, and Magnetic Properties. Chem. Mater. 2007, 19, 6025–6029. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, J.; Liu, Z. Enhanced Activity of Immobilized or Chemically Modified Enzymes. ACS Catal. 2015, 5, 4503–4513. [Google Scholar] [CrossRef]
- Yu, B.; Zhou, Y.; Song, M.; Xue, Y.; Cai, N.; Luo, X.; Long, S.; Zhang, H.; Yu, F. Synthesis of Selenium Nanoparticles with Mesoporous Silica Drug-Carrier Shell for Programmed Responsive Tumor Targeted Synergistic Therapy. RSC Adv. 2015, 6, 2171–2175. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.; Liu, W.; Chen, T.; Li, Y.; Zheng, W.; Man, C.W.-Y.; Wong, M.-K.; Wong, K.-H. Surface Decoration of Selenium Nanoparticles by Mushroom Polysaccharides–Protein Complexes to Achieve Enhanced Cellular Uptake and Antiproliferative Activity. J. Mater. Chem. 2012, 22, 9602–9610. [Google Scholar] [CrossRef]
- Yang, F.; Tang, Q.; Zhong, X.; Bai, Y.; Chen, T.; Zhang, Y.; Li, Y.; Zheng, W. Surface Decoration by Spirulina Polysaccharide Enhances the Cellular Uptake and Anticancer Efficacy of Selenium Nanoparticles. Int. J. Nanomed. 2012, 7, 835–844. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Sevonkaev, I.; Goia, D.V. Synthesis of Selenium Particles with Various Morphologies. J. Colloid Interface Sci. 2014, 416, 119–123. [Google Scholar] [CrossRef]
- Luesakul, U.; Komenek, S.; Puthong, S.; Muangsin, N. Shape-Controlled Synthesis of Cubic-like Selenium Nanoparticles via the Self-Assembly Method. Carbohydr. Polym. 2016, 153, 435–444. [Google Scholar] [CrossRef]
- Yin, H.; Xu, Z.; Bao, H.; Bai, J.; Zheng, Y. Single Crystal Trigonal Selenium Nanoplates Converted from Selenium Nanoparticles. Chem. Lett. 2004, 34, 122–123. [Google Scholar] [CrossRef]
- Song, C.; Li, X.; Wang, S.; Meng, Q. Enhanced Conversion and Stability of Biosynthetic Selenium Nanoparticles Using Fetal Bovine Serum. RSC Adv. 2016, 6, 103948–103954. [Google Scholar] [CrossRef]
- Xia, Y.; You, P.; Xu, F.; Liu, J.; Xing, F. Novel Functionalized Selenium Nanoparticles for Enhanced Anti-Hepatocarcinoma Activity In Vitro. Nanoscale Res. Lett. 2015, 10, 349. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Yang, D.-P.; Ye, D.; Zhang, X.; Fang, X.; Zhang, S.; Liu, B.; Kong, J. Protein-Inorganic Hybrid Nanoflowers as Ultrasensitive Electrochemical Cytosensing Interfaces for Evaluation of Cell Surface Sialic Acid. Biosens. Bioelectron. 2015, 68, 329–335. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Su, Z. Enzyme-Based Hybrid Nanoflowers with High Performances for Biocatalytic, Biomedical, and Environmental Applications. Coord. Chem. Rev. 2020, 416, 213342. [Google Scholar] [CrossRef]
- Lee, S.W.; Cheon, S.A.; Kim, M.I.; Park, T.J. Organic–Inorganic Hybrid Nanoflowers: Types, Characteristics, and Future Prospects. J. Nanobiotechnol. 2015, 13, 54. [Google Scholar] [CrossRef] [Green Version]
- Bilal, M.; Asgher, M.; Shah, S.Z.H.; Iqbal, H.M.N. Engineering Enzyme-Coupled Hybrid Nanoflowers: The Quest for Optimum Performance to Meet Biocatalytic Challenges and Opportunities. Int. J. Biol. Macromol. 2019, 135, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, X.; Li, Y.; Su, Z.; Jandt, K.D.; Wei, G. Protein-Mimetic Peptide Nanofibers: Motif Design, Self-Assembly Synthesis, and Sequence-Specific Biomedical Applications. Prog. Polym. Sci. 2018, 80, 94–124. [Google Scholar] [CrossRef]
- Gong, C.; Sun, S.; Zhang, Y.; Sun, L.; Su, Z.; Wu, A.; Wei, G. Hierarchical Nanomaterials: Via Biomolecular Self-Assembly and Bioinspiration for Energy and Environmental Applications. Nanoscale 2019, 11, 4147–4182. [Google Scholar] [CrossRef]
- Celik, C.; Tasdemir, D.; Demirbas, A.; Katı, A.; Tolga Gul, O.; Cimen, B.; Ocsoy, I. Formation of Functional Nanobiocatalysts with a Novel and Encouraging Immobilization Approach and Their Versatile Bioanalytical Applications. RSC Adv. 2018, 8, 25298–25303. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, E.; Ocsoy, I.; Ozdemir, N.; Soylak, M. Bovine Serum Albumin-Cu(II) Hybrid Nanoflowers: An Effective Adsorbent for Solid Phase Extraction and Slurry Sampling Flame Atomic Absorption Spectrometric Analysis of Cadmium and Lead in Water, Hair, Food and Cigarette Samples. Anal. Chim. Acta 2016, 906, 110–117. [Google Scholar] [CrossRef]
- Songa, E.A.; Okonkwo, J.O. Recent Approaches to Improving Selectivity and Sensitivity of Enzyme-Based Biosensors for Organophosphorus Pesticides: A Review. Talanta 2016, 155, 289–304. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and Their Applications–A Review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Photoelectrochemical Enzymatic Biosensors. Biosens. Bioelectron. 2017, 92, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, X.; Zhang, G.; Wei, G.; Su, Z. Electrospinning Design of Functional Nanostructures for Biosensor Applications. J. Mater. Chem. B 2017, 5, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Thatoi, H.; Das, S.; Mishra, J.; Rath, B.P.; Das, N. Bacterial Chromate Reductase, a Potential Enzyme for Bioremediation of Hexavalent Chromium: A Review. J. Environ. Manag. 2014, 146, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, D.; Pages, G.S.; Catalan, V.; Loret, J.-F.; Greub, G. Biodiversity of Amoebae and Amoeba-Associated Bacteria in Water Treatment Plants. Int. J. Hyg. Environ. Health 2010, 213, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Al-Maqdi, K.A.; Hisaindee, S.M.; Rauf, M.A.; Ashraf, S.S. Comparative Degradation of a Thiazole Pollutant by an Advanced Oxidation Process and an Enzymatic Approach. Biomolecules 2017, 7, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Mohanty, S.K.; Mahendra, S. Nanomaterial-Supported Enzymes for Water Purification and Monitoring in Point-of-Use Water Supply Systems. Acc. Chem. Res. 2019, 52, 876–885. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, H.M.N.; Hu, H.; Zhang, X. Bio-Based Degradation of Emerging Endocrine-Disrupting and Dye-Based Pollutants Using Cross-Linked Enzyme Aggregates. Environ. Sci. Pollut. Res. 2017, 24, 7035–7041. [Google Scholar] [CrossRef]
- Sun, H.; Yang, H.; Huang, W.; Zhang, S. Immobilization of Laccase in a Sponge-like Hydrogel for Enhanced Durability in Enzymatic Degradation of Dye Pollutants. J. Colloid Interface Sci. 2015, 450, 353–360. [Google Scholar] [CrossRef]
- Ildiz, N.; Baldemir, A.; Altinkaynak, C.; Özdemir, N.; Yilmaz, V.; Ocsoy, I. Self Assembled Snowball-like Hybrid Nanostructures Comprising Viburnum Opulus L. Extract and Metal Ions for Antimicrobial and Catalytic Applications. Enzyme Microb. Technol. 2017, 102, 60–66. [Google Scholar] [CrossRef]
- Olofsson, J.; Barta, Z.; Börjesson, P.; Wallberg, O. Integrating enzyme fermentation in lignocellulosic ethanol production: Life-cycle assessment and techno-economic analysis. Biotechnol. Biofuels 2017, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bilal, M.; Iqbal, H.M. Sustainable bioconversion of food waste into high-value products by immobilized enzymes to meet bio-economy challenges and opportunities—A review. Food Res. Int. 2019, 123, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.K.; Ting, V.F.W.; Pogaku, R. Life cycle assessment of biodiesel production using alkali, soluble and immobilized enzyme catalyst processes. Biomass Bioenerg. 2011, 35, 4221–4229. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- Manjrekar, S.; Wadekar, T.; Sumant, O. Enzymes Market Type (Protease, Carbohydrase, Lipase, Polymerase and Nuclease, and Other Types), Source (Microorganisms, Plants, and Animals), Reaction Type (Hydrolase, Oxidoreductase, Transferase, Lyase, and Other Reaction Types), and Application (Food and Beverages, Household Care, Bioenergy, Pharmaceutical and Biotechnology, Feed, and Other Applications)—Global Opportunity Analysis and Industry Forecast, 2020–2027. 2021. Available online: https://www.alliedmarketresearch.com/enzymes-market (accessed on 26 May 2021).
- Li, C.; Zhao, J.; Zhang, Z.; Jiang, Y.; Bilal, M.; Jiang, Y.; Jia, S.; Cui, J. Self-Assembly of Activated Lipase Hybrid Nanoflowers with Superior Activity and Enhanced Stability. Biochem. Eng. J. 2020, 158, 107582. [Google Scholar] [CrossRef]
- Xie, W.; Huang, M. Enzymatic Production of Biodiesel Using Immobilized Lipase on Core-Shell Structured Fe3O4@MIL-100(Fe) Composites. Catalysts 2019, 9, 850. [Google Scholar] [CrossRef] [Green Version]
- Hussain, F.; Arana-Peña, S.; Morellon-Sterling, R.; Barbosa, O.; Ait Braham, S.; Kamal, S.; Fernandez-Lafuente, R. Further Stabilization of Alcalase Immobilized on Glyoxyl Supports: Amination Plus Modification with Glutaraldehyde. Molecules 2018, 23, 3188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Q.; Tang, H. Immobilization of Horseradish Peroxidase on NH2-Modified Magnetic Fe3O4/SiO2 Particles and Its Application in Removal of 2,4-Dichlorophenol. Molecules 2014, 19, 15768–15782. [Google Scholar] [CrossRef] [PubMed]



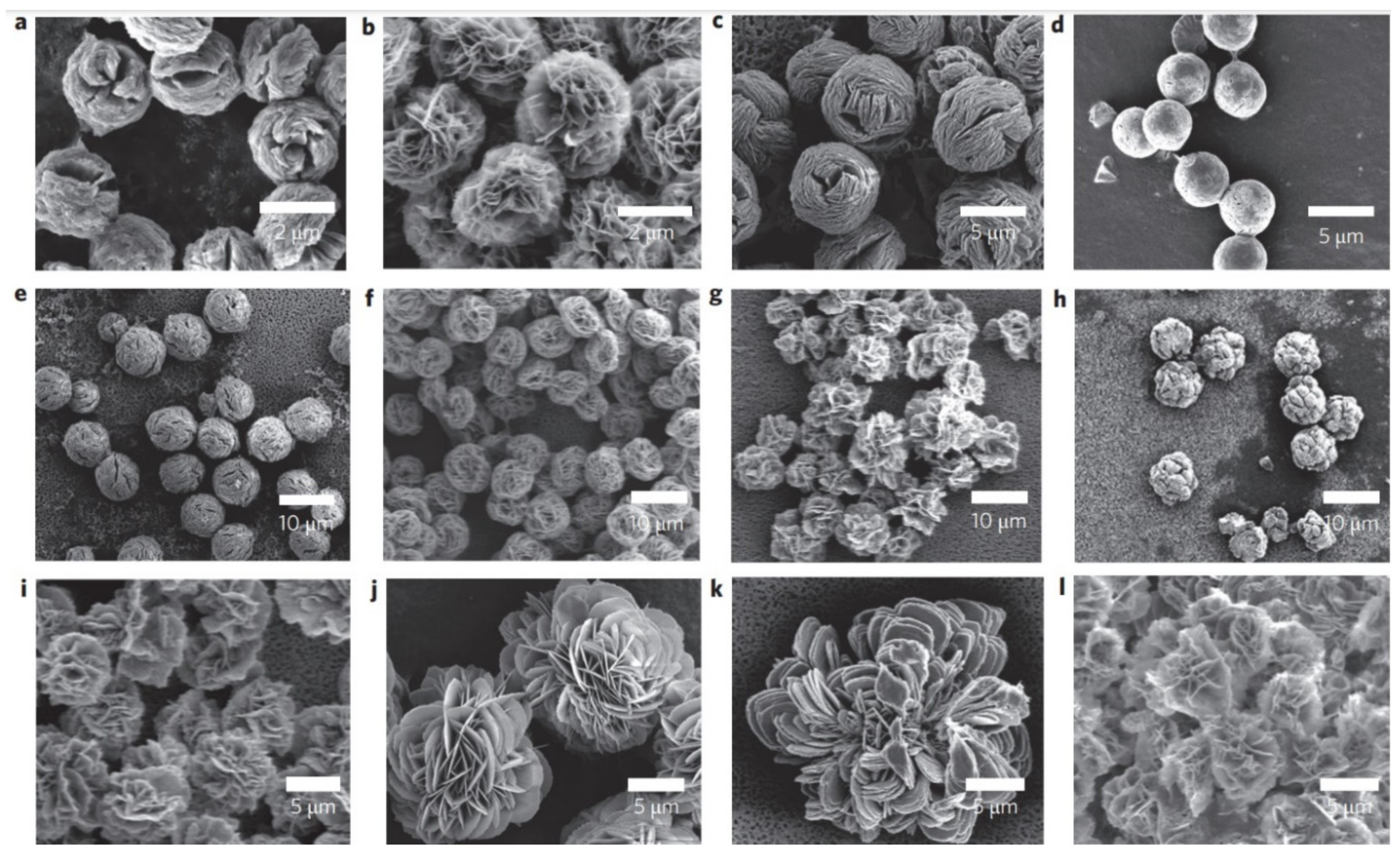




| Metal Ion | Enzyme | Class of Enzyme | Application | Reference |
|---|---|---|---|---|
| Copper (II) ions | Turkish black radish | Peroxidase | Dye decolorization | [43] |
| Horseradish peroxidase | Peroxidase | Detection of hydrogen peroxide | [44] | |
| Horseradish peroxidase | Peroxidase | Detection of E. coli | [45] | |
| Horseradish peroxidase | Peroxidase | Detection of hydrogen peroxide and phenol | [36] | |
| Horseradish peroxidase | Peroxidase | Detection of amyloid | [46] | |
| Horseradish peroxidase | Peroxidase | - | [47] | |
| Horseradish peroxidase | Peroxidase | - | [42] | |
| Horseradish peroxidase | Peroxidase | - | [48] | |
| Horseradish peroxidase | Peroxidase | - | [49] | |
| Horseradish peroxidase | Peroxidase | - | [50] | |
| Chloroperoxidase (CPO) | Peroxidase | Dye decolorization | [29] | |
| Soybean peroxidase (SBP) | Peroxidase | - | [24] | |
| Lactoperoxidase (LPO) | Peroxidase | - | [51] | |
| Catalase | Peroxidase | Glucose biofuel cell | [52] | |
| Catalase | Peroxidase | Detection of hydrogen peroxide | [53] | |
| Catalase | Peroxidase | - | [50] | |
| Laccase | Laccase | [42] | ||
| Laccase | Laccase | Degradation of the pollutant bisphenol A | [34] | |
| Laccase | Laccase | Decolorization of Congo Red (CR) | [32] | |
| Laccase | Laccase | Dye decolorization | [30] | |
| Laccase | Laccase | - | [54] | |
| Laccase | Laccase | Detection of phenol | [55] | |
| Laccase | Laccase | Synthesis of viniferin | [56] | |
| Laccase | Laccase | Synthesis of viniferin | [57] | |
| Laccase | Laccase | Glucose biofuel cell | [52] | |
| Glucose oxidase (GOx) | Carbohydrase | Detection of glucose | [58] | |
| Glucose oxidase (GOx) | Carbohydrase | - | [50] | |
| Glucose oxidase (GOx) | Carbohydrase | Glucose biofuel cell | [52] | |
| Glucose oxidase (GOx) | Carbohydrase | - | [59] | |
| L-Xylanase | Carbohydrase | - | [60] | |
| Glucoamylase | Carbohydrase | - | [39] | |
| α-Glycosidase | Carbohydrase | Testing for α-glycosidase inhibitors | [61] | |
| L-Arabinose Isomerase | Carbohydrase | Preparation of two expensive rare sugar L-ribulose and D-tagatose | [62] | |
| Candida rugosa lipase | Lipase | - | [63] | |
| Candida antarctica lipase | Lipase | Epoxidation of fatty acids | [64] | |
| pseudomonas cepacia lipase | Lipase | - | [65] | |
| Bacillus subtilis lipase | Lipase | Transesterification of (R,S)-2-pentanol | [66] | |
| Lipase | Lipase | p-nitrophenol butyrate hydrolysis | [67] | |
| Lipase | Lipase | - | [27] | |
| Lipase | Lipase | - | [68] | |
| Lipase | Lipase | - | [69] | |
| Lipase | Lipase | Uses as green media solvent | [70] | |
| Lipase | Lipase | Biodiesel synthesis | [71] | |
| proteinase K | Protease | Detergent additive | [72] | |
| Alkaline protease | Protease | - | [73] | |
| Trypsin | Protease | Protein digestion | [23] | |
| Papain | Protease | - | [42] | |
| Papain | Protease | - | [74] | |
| Papain | Protease | - | [69] | |
| Papain | Protease | - | [75] | |
| Cholesterol oxidase (ChOx) and horseradish peroxidase | Dual enzyme | Detection of cholesterol | [76] | |
| Glucose oxidase and lipase | Dual enzyme | Epoxidation of alkenes | [77] | |
| Acetylcholinesterase and choline oxidase | Dual enzyme | On-site detection of the pesticide organophosphorus | [78] | |
| Glucose oxidase and horseradish peroxidase | Dual enzyme | Monitoring urinary tract infection (UTI) in clinical practice | [79] | |
| Glucose oxidase and horseradish peroxidase | Dual enzyme | Glucose sensor | [80] | |
| Glucose oxidase and horseradish peroxidase | Dual enzyme | Detection of glucose | [81] | |
| Glucose oxidase and horseradish peroxidase | Dual enzyme | Detection of glucose | [82] | |
| Cytochrome P450 | Others | Oxidation of sulfides | [83] | |
| L-Arabinitol 4-dehydrogenase | Others | L-xylulose production | [84] | |
| Urease | Others | - | [38] | |
| Brevibacterium cholesterol oxidase (COD) | Others | - | [85] | |
| Carbonic anhydrase | Others | - | [26] | |
| 2,4-dichlorophenol hydroxylase | Others | - | [86] | |
| Calcium (II) ions | chloroperoxidase (CPO) | Peroxidase | - | [87] |
| Chitosan and Catalase | Peroxidase | - | [88] | |
| α-amylase | Carbohydrase | - | [89] | |
| β-Galactosidase | Carbohydrase | Protein biomarker | [90] | |
| Candida antarctica lipase | Lipase | - | [37] | |
| Porcine pancreas lipase | Lipase | - | [37] | |
| Thermomyces lanuginosus lipase | Lipase | - | [37] | |
| Burkholderia cepacia lipase (BCL) | Lipase | - | [21] | |
| Alcalase | Protease | - | [40] | |
| Bromelain | Protease | - | [37] | |
| Trypsin | Protease | - | [37] | |
| Papain | Protease | - | [37] | |
| α-chymotrypsin | Protease | Digestion of bovine serum albumin (BSA) and human serum albumin (HSA) | [22] | |
| Dual enzyme: Aldehyde ketone reductase and alcohol dehydrogenase | Dual enzyme | Production of (S)-1-(2, 6-dichloro-3-fluorophenyl) ethyl alcohol, a key chiral alcohol that is an intermediate of Crizotinib, an anti-cancer drug | [91] | |
| α-Acetolactate decarboxylase (ALDC) | Others | Inhibition of diacetyl formation in beer | [92] | |
| Elastin-like polypeptide (ELPs) | Others | Detection of H2O2 | [93] | |
| Invertase | Others | E. coli detection from milk | [94] | |
| Carbonic Anhydrase | Others | - | [26] | |
| Manganese (II) ions | L-Arabinose Isomerase | Carbohydrase | Transformation of D-Galactose to D-Tagatose | [95] |
| Collagen | Others | Water oxidation | [96] | |
| Bovine serum albumin (BSA) | Others | Catalysis in fuel cells | [97] | |
| Carbonic Anhydrase | Others | - | [26] | |
| Ractopamine antibody | Others | Electrochemical biosensors ractopamine detection | [98] | |
| Zinc (II) ions | Lipase | Lipase | Regioselective acylation of arbutin | [99] |
| Lipase | Lipase | - | [35] | |
| Papain | Protease | - | [41] | |
| Bovine serum albumin (BSA) | Others | Adsorption of heavy metal ions | [100] | |
| Cobalt (II) ions | Chloroperoxidase (CPO) | Peroxidase | Dye decolorization | [29] |
| D-Psicose 3-Epimerase (DPEase) | Carbohydrase | - | [101] | |
| Lipase | Lipase | - | [102] | |
| ω-Transaminase | Others | - | [103] | |
| Bovine serum albumin (BSA) | Others | - | [104] | |
| Bovine serum albumin (BSA) | Others | - | [105] | |
| His-tagged enzyme | Others | Redox reaction cycles | [106] | |
| Iron (II) ions | Horseradish peroxidase | Peroxidase | - | [107] |
| Glucose oxidase (GOx) | Carbohydrase | - | [108] | |
| Multi-metal (Copper+ Zinc) | Laccase | Laccase | Degradation of the pollutant bisphenol A | [109] |
| Non-metal (Selenium) | Pullulan (polysaccharide polymer) | Others | - | [110] |
| Nature Flower | Shape | Example | SEM Image | Size | Reference | |
|---|---|---|---|---|---|---|
 | Spherical | The enzyme: Brevibacterium cholesterol oxidase (COD) | The metal: copper |  | 5 μm | [85] |
| The enzyme: laccase | The metal: copper |  | 1 min sonication: ~2 µm 5 min sonication: ~8 µm 7 min sonication: No additional increase in the size | [54] | ||
| The enzyme: glucose oxidase (GOx) + horseradish peroxidase (HRP) | The metal: copper |  | 30 μm | [80] | ||
 | Rosette | The enzyme: α-glycosidase | The metal: copper | 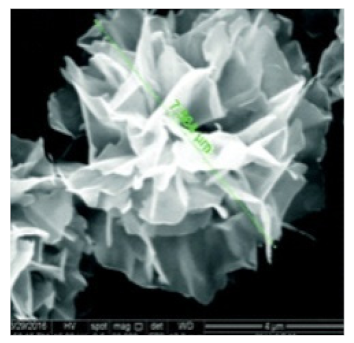 | 7.564 μm | [61] |
| The enzyme: catalase | The metal: copper | 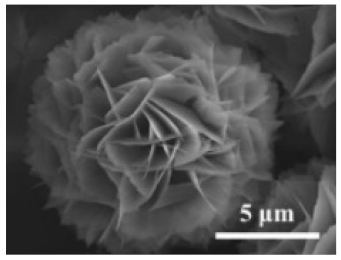 | 10 to 20 μm | [53] | ||
| The enzyme: streptavidin + horseradish peroxidase (HRP) | The metal: copper |  | 5 μm | [49] | ||
 | Rhombic | The enzyme: D-psicose 3-epimerase (DPEase) | The metal: cobalt |  | 7 µm | [101] |
| The enzyme: papain | The metal: zinc |  | - | [41] | ||
| The enzyme: laccase | The metal: copper |  | - | [20] | ||
| Enzyme | Morphology | Reference | |||
|---|---|---|---|---|---|
| Type of enzyme used |
Glucose oxidase (GOx)  |
Laccase 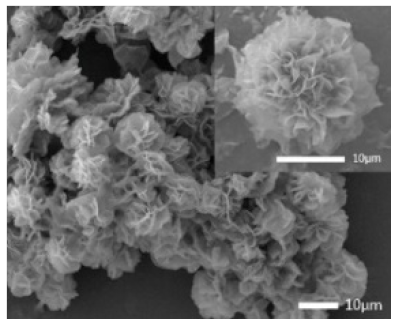 |
Catalase  |
Lipase 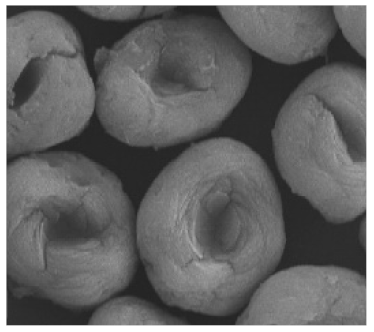 | [35,52] |
| Different amount of the enzyme # Example 1 |
 |
 |
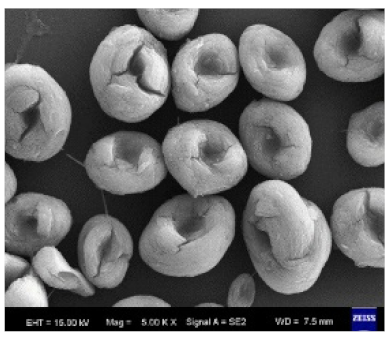 |
 | [35] |
| Different amount of the enzyme # Example 2 |
 |
 |
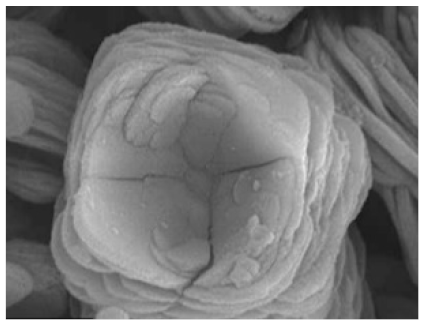 |
 | [41] |
| Metal ion | Morphology | ||||
| Type of metal ion used |
 |
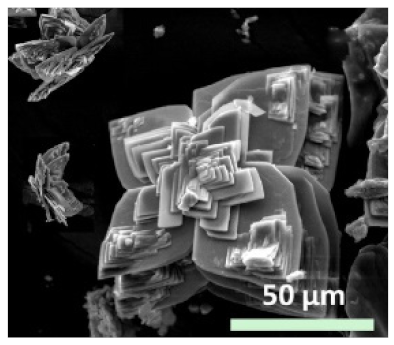 |
 | - | [29] |
| Reaction pH | Morphology | ||||
| Different pH values |
 |
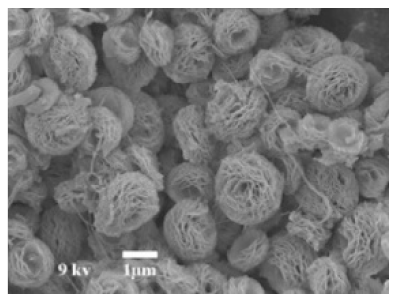 |
 |
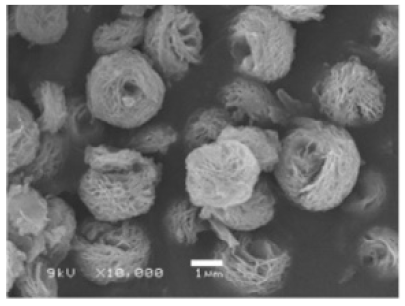 | [69] |
| Reaction temperature | Morphology | ||||
| Different reaction temperature # Example 1 |
Below Tt, at 4 °C ELP/Ca hNFs 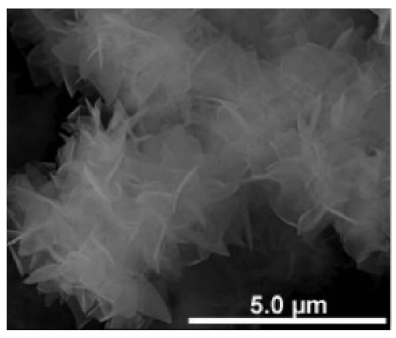 |
Above Tt, at 37 °C ELP/Ca hNFs  |
Below Tt, at 4 °C ELP/Cu hNFs 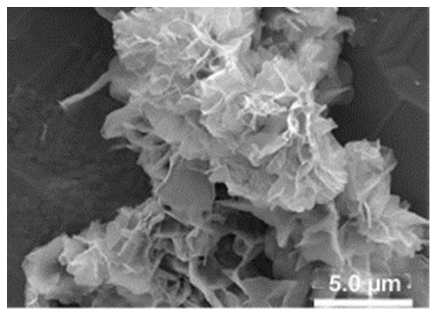 |
Above Tt, at 37 °C ELP/Cu hNFs 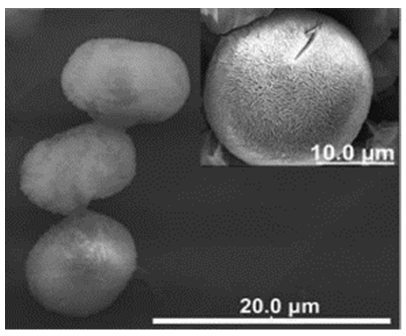 | [93] |
| Different reaction temperature # Example 2 |
20 °C  |
30 °C  |
40 °C  |
60 °C  | [35] |
| Different reaction temperature # Example 3 |
4 °C  |
20 °C  | - | - | [51] |
| Reaction time | Morphology | ||||
| Different reaction time |
 |
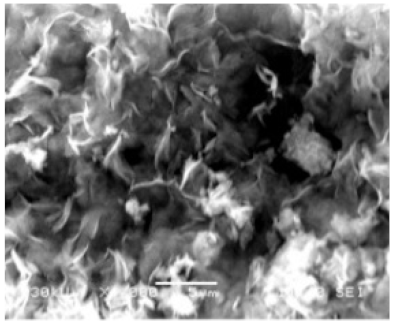 |
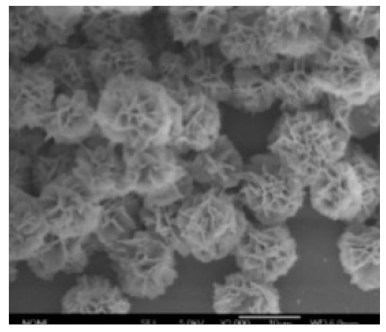 | - | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Maqdi, K.A.; Bilal, M.; Alzamly, A.; Iqbal, H.M.N.; Shah, I.; Ashraf, S.S. Enzyme-Loaded Flower-Shaped Nanomaterials: A Versatile Platform with Biosensing, Biocatalytic, and Environmental Promise. Nanomaterials 2021, 11, 1460. https://doi.org/10.3390/nano11061460
Al-Maqdi KA, Bilal M, Alzamly A, Iqbal HMN, Shah I, Ashraf SS. Enzyme-Loaded Flower-Shaped Nanomaterials: A Versatile Platform with Biosensing, Biocatalytic, and Environmental Promise. Nanomaterials. 2021; 11(6):1460. https://doi.org/10.3390/nano11061460
Chicago/Turabian StyleAl-Maqdi, Khadega A., Muhammad Bilal, Ahmed Alzamly, Hafiz M. N. Iqbal, Iltaf Shah, and Syed Salman Ashraf. 2021. "Enzyme-Loaded Flower-Shaped Nanomaterials: A Versatile Platform with Biosensing, Biocatalytic, and Environmental Promise" Nanomaterials 11, no. 6: 1460. https://doi.org/10.3390/nano11061460
APA StyleAl-Maqdi, K. A., Bilal, M., Alzamly, A., Iqbal, H. M. N., Shah, I., & Ashraf, S. S. (2021). Enzyme-Loaded Flower-Shaped Nanomaterials: A Versatile Platform with Biosensing, Biocatalytic, and Environmental Promise. Nanomaterials, 11(6), 1460. https://doi.org/10.3390/nano11061460









