Removal of Ampicillin by Heterogeneous Photocatalysis: Combined Experimental and DFT Study
Abstract
:1. Introduction
- Determining the correlations between the properties of TiO2 photocatalysts (particle size, degree of agglomeration, crystallinity, phase composition) and their photocatalytic performance, based on the use of extensive sample bank,
- Not only the photocatalysis itself but also with accompanying processes, namely sorption on the photocatalytic surface and photolysis,
- The coupling of the experimental research and DFT calculations to obtain a deep insight into the mechanism of the whole process, which is of major importance for the assessment of the effect of other components present simultaneously in the water,
- The achievement of practically complete mineralization to minimize the risk of potentially toxic organic products.
2. Materials and Methods
2.1. Characterization of Photocatalytic Powders
2.2. Measurement of Degradation Kinetics, Sorption, and Photolysis
2.3. Experimental Detection of Photocatalytic Products by UPLC/MS/MS
2.4. Quantum-Chemical Calculations
3. Results and Discussion
3.1. Characterization of Photocatalysts
3.2. Sorption, Photolysis, and Photocatalysis of Ampicillin
3.2.1. Sorption
3.2.2. Photolysis
3.2.3. Photocatalysis
3.2.4. Mineralization
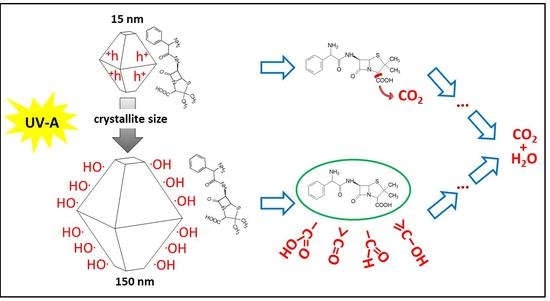
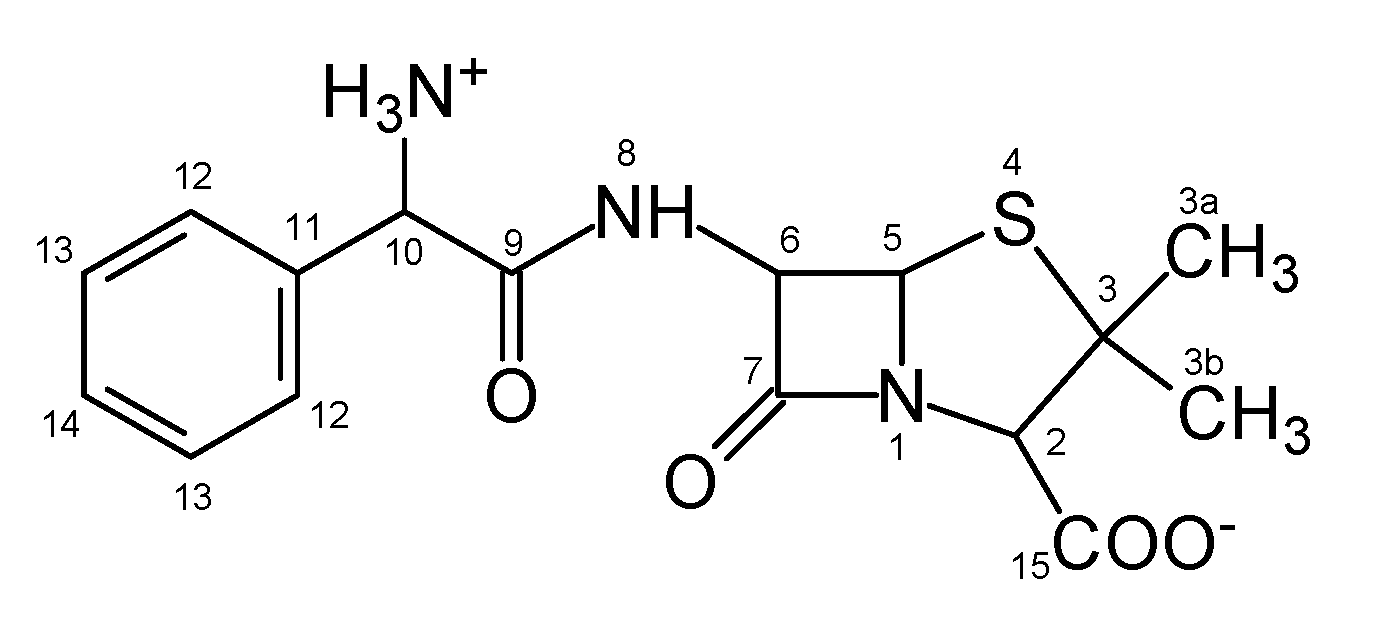
3.3. Mechanism of Photocatalytic Degradation of Ampicillin
3.3.1. Experimental Study
3.3.2. Photocatalytic Degradation with OH Radical Scavenger
3.3.3. DFT Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Aga, D.S.; Lenczewski, M.; Snow, D.; Muurinen, J.; Sallach, J.B.; Wallace, J.S. Challenges in the Measurement of Antibiotics and in Evaluating Their Impacts in Agroecosystems: A Critical Review. J. Environ. Qual. 2016, 45, 407–419. [Google Scholar] [CrossRef] [Green Version]
- Bouki, C.; Venieri, D.; Diamadopoulos, E. Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: A review. Ecotoxicol. Environ. Saf. 2013, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [Green Version]
- Auerbach, E.A.; Seyfried, E.E.; McMahon, K.D. Tetracycline resistance genes in activated sludge wastewater treatment plants. Water Res. 2007, 41, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Wong, K.; Xagoraraki, I. Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan. Water Res. 2011, 45, 681–693. [Google Scholar] [CrossRef]
- Abbassi, B.E.; Saleem, M.A.; Zytner, R.G.; Gharabaghi, B.; Rudra, R. Antibiotics in wastewater: Their degradation and effect on wastewater treatment efficiency. J. Food Agric. Environ. 2016, 14, 95–99. [Google Scholar] [CrossRef]
- Batt, A.L.; Kim, S.; Aga, D.S. Comparison of the occurrence of antibiotics in four full-scale wastewater treatment plants with varying designs and operations. Chemosphere 2007, 68, 428–435. [Google Scholar] [CrossRef]
- Ioannou-Ttofa, L.; Raj, S.; Prakash, H.; Fatta-Kassinos, D. Solar photo-Fenton oxidation form the removal of ampicillin, total cultivable and resistant E. coli and ecotoxicity from secondary-treated wastewater effluents. Chem. Eng. J. 2019, 355, 91–102. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Khan, M.; He, J.; Lo, I.M.C. Recent developments and challenges in practical application of visible–light–driven TiO2–based heterojunctions for PPCP degradation: A critical review. Water Res. 2020, 170, 115356. [Google Scholar] [CrossRef]
- Abramović, B.; Kler, S.; Šojić, D.; Laušević, M.; Radović, T.; Vione, D. Photocatalytic degradation of metoprolol tartrate in suspensions of two TiO 2-based photocatalysts with different surface area. Identification of intermediates and proposal of degradation pathways. J. Hazard. Mater. 2011, 198, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.M.; Aziz, N.A.B.A.; Palaniandy, P.; Abu Amr, S.S. Performance of natural sunlight on paracetamol removal from synthetic pharmaceutical wastewater using heterogeneous TiO2 photocatalyst. Desalin. Water Treat. 2017, 78, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Fawzi Suleiman Khasawneh, O.; Palaniandy, P. Photocatalytic Degradation of Pharmaceuticals Using TiO2 Based Nanocomposite Catalyst-Review. Civ. Environ. Eng. Rep. 2019, 29, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.M.; Palaniandy, P.; Dahlan, I. Pharmaceutical residues in aquatic environment and water remediation by TiO2 heterogeneous photocatalysis: A review. Environ. Earth Sci. 2017, 76. [Google Scholar] [CrossRef]
- Vasiliu, F.; Diamandescu, L.; Macovei, D.; Teodorescu, C.M.; Tarabasanu-Mihaila, D.; Vlaicu, A.M.; Parvulescu, V. Fe- and Eu-doped TiO2 photocatalytical materials prepared by high energy ball milling. Top. Catal. 2009, 52, 544–556. [Google Scholar] [CrossRef]
- Szabó, L.; Tóth, T.; Engelhardt, T.; Rácz, G.; Mohácsi-Farkas, S.; Takács, E.; Wojnárovits, L. Change in hydrophilicity of penicillins during advanced oxidation by radiolytically generated OH compromises the elimination of selective pressure on bacterial strains. Sci. Total Environ. 2016, 551–552, 393–403. [Google Scholar] [CrossRef]
- Andreozzi, R.; Canterino, M.; Marotta, R.; Paxeus, N. Antibiotic removal from wastewaters: The ozonation of amoxicillin. J. Hazard. Mater. 2005, 122, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Rozas, O.; Contreras, D.; Mondaca, M.A.; Pérez-Moya, M.; Mansilla, H.D. Experimental design of Fenton and photo-Fenton reactions for the treatment of ampicillin solutions. J. Haz. Mat. 2010, 177, 1025–1030. [Google Scholar] [CrossRef]
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, mineralization and antibiotic inactivation of AMX by UV-A/TiO2 photocatalysis. J. Environ. Manag. 2012, 98, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solutions using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 2010, 252, 46–52. [Google Scholar] [CrossRef]
- Klauson, D.; Babkina, J.; Stepanova, K.; Krichevskaya, M.; Preis, S. Aqueous photocatalytic oxidation of amoxicillin. Cat. Today 2010, 151, 39–45. [Google Scholar] [CrossRef]
- Shaykhi, Z.M.; Zinatizadeh, A.A.L. Statistical modeling of photocatalytic degradation of synthetic AMX wastewater in an immobilized TiO2 photocatalytic reactor using response surface methodology. J. Taiwan Inst. Chem. Eng. 2014, 45, 1717–1726. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Z.; Gao, J.; Xie, Y.; Li, L.; Qin, S.; Wang, Q.; Mao, D.; Luo, Y. Simultaneous removal of antibiotics and antibiotic resistance genes from pharmaceutical wastewater using the combinations of up-flow anaerobic sludge bed, anoxic-oxic tank, and advanced oxidation technologies. Water Res. 2019, 159, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.W.; Petrasky, L.J.; Seymour, M.D.; Burkhart, R.S.; Schuiling, A.B. Adsorption and breakdown of penicillin antibiotic in the presence of titanium oxide nanoparticles in water. Chemosphere 2012, 87, 911–917. [Google Scholar] [CrossRef]
- Zouzelka, R.; Remzova, M.; Brabec, L.; Rathousky, J. Photocatalytic performance of porous TiO2 layers prepared by quantitative electrophoretic deposition from organic solvents. Appl. Catal. B Environ. 2020, 227, 70–78. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J. Haz. Mater. 2010, 173, 445–449. [Google Scholar] [CrossRef]
- Kim, W.; Tachikawa, T.; Moon, G.; Majima, T.; Choi, W. Molecular-Level Understanding of the Photocatalytic Activity Difference between Anatase and Rutile Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 14036–14041. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Moon, G.; Kim, B.; Tachikawa, T.; Majima, T.; Hong, S.; Cho, K.; Kim, W.; Choi, W. Crystal phase-dependent generation of mobile OH radicals on TiO2: Revisiting the photocatalytic oxidation mechanism of anatase and rutile. Appl. Catal. B Environ. 2021, 286, 119905. [Google Scholar] [CrossRef]
- Ranjit, K.T.; Willner, I.; Bossmann, S.H.; Braun, A.M. Lanthanide Oxide-Doped Titanium Dioxide Photocatalysts: Novel Photocatalysts for the Enhanced Degradation of p-Chlorophenoxyacetic Acid. Environ. Sci. Technol. 2001, 35, 1544–1549. [Google Scholar] [CrossRef] [PubMed]
- Romeiro, A.; Azenha, M.E.; Canle, M.; Rodrigues, V.H.N.; Da Silva, J.P.; Burrows, H.D. Titanium Dioxide Nanoparticle Photocatalysed Degradation of Ibuprofen and Naproxen in Water: Competing Hydroxyl Radical Attack and Oxidative Decarboxylation by Semiconductor Holes. ChemistrySelect 2018, 3, 10915–10924. [Google Scholar] [CrossRef]
- Ojobe, B.; Zouzelka, R.; Satkova, B.; Kuchar, M.; Bartacek, J.; Rathousky, J. Photocatalytic removal of pharmaceuticals from greywater. Catalysts 2021. submitted. [Google Scholar]
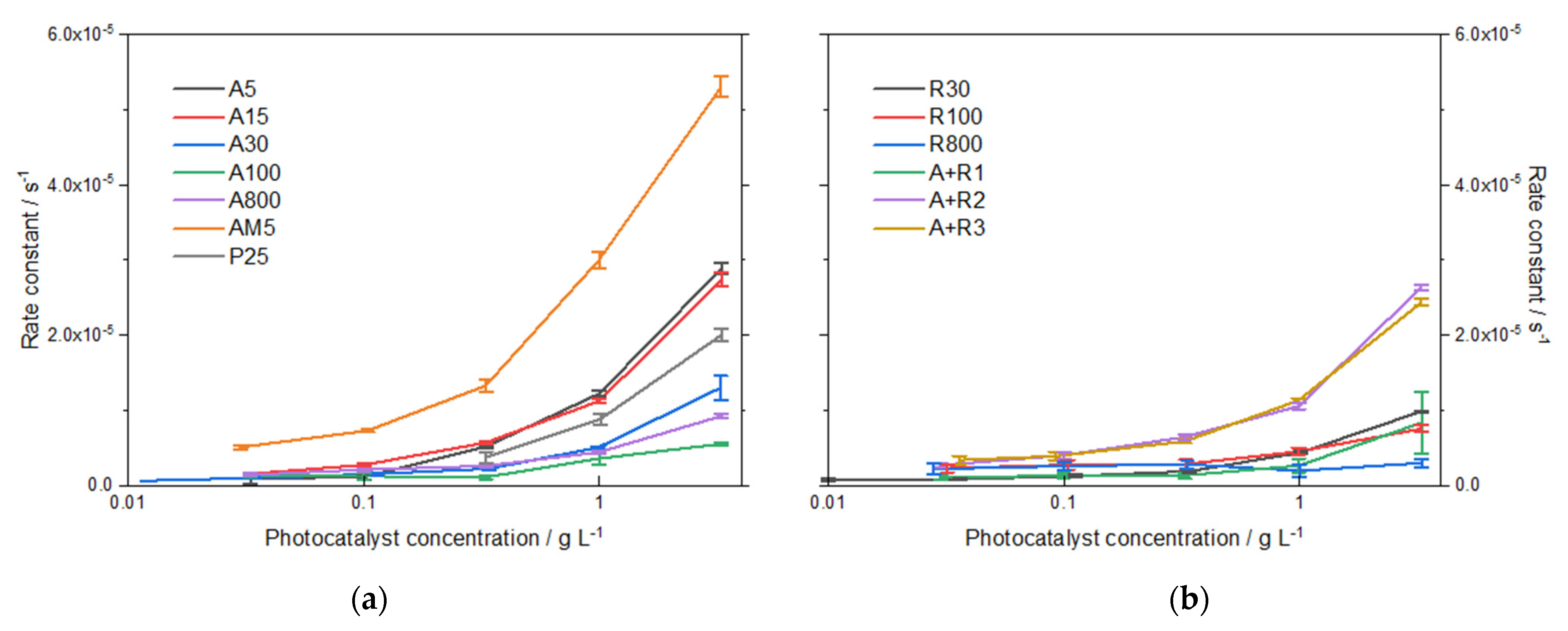
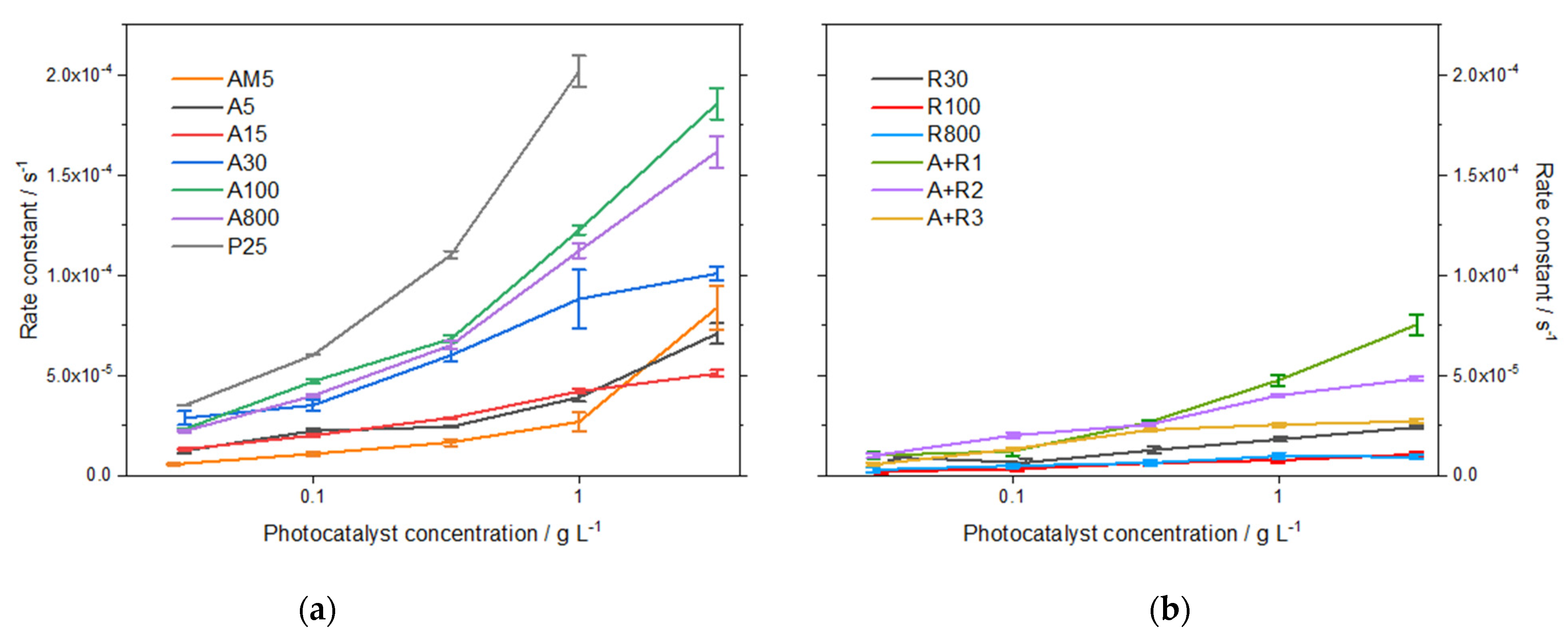


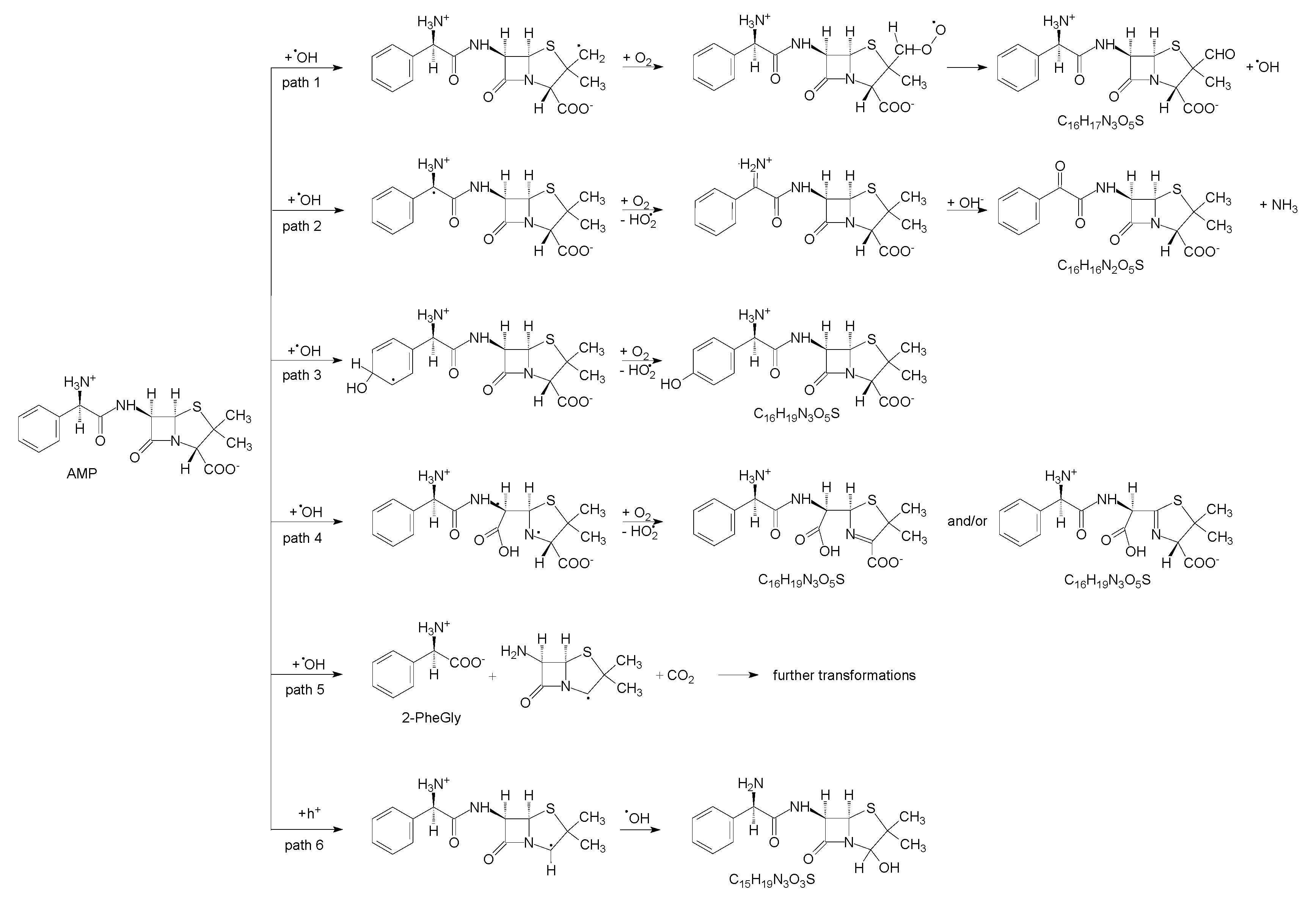

| Photocatalyst Designation | Phase Content a (%) | Crystallite Size a (nm) | Particle Size b (nm) | Particle Size c (nm) | ||
|---|---|---|---|---|---|---|
| Anatase | Rutile | Anatase | Rutile | |||
| A5 | 100 | 0 | 13 | - | 13 | 13–30 |
| A15 | 100 | 0 | 15 | - | 18 | 20–40 |
| A30 | 100 | 0 | 16 | - | 31 | 20–40 |
| A100 | 100 | 0 | 70 | - | 174 | 70–300 |
| A800 | 100 | 0 | 58 | - | 156 | 50–300 |
| R30 | 0 | 100 | - | 29 | 100 | 30–100 |
| R100 | 0 | 100 | - | nd | 290 | 100–400 |
| R800 | 0 | 100 | - | nd | 220 | 100–300 |
| A+R1 | 55 | 45 | 32 | 62 | 92 | 30–150 |
| A+R2 | 64 | 36 | 15 | 18 | 25 | 15–30 |
| A+R3 | 31 | 69 | 16 | 18 | 36 | 15–30 |
| AM5 | 100 | 0 | 5 | - | 6 | 5–10 |
| P25 | 80 | 20 | 27 | nd | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belhacova, L.; Bibova, H.; Marikova, T.; Kuchar, M.; Zouzelka, R.; Rathousky, J. Removal of Ampicillin by Heterogeneous Photocatalysis: Combined Experimental and DFT Study. Nanomaterials 2021, 11, 1992. https://doi.org/10.3390/nano11081992
Belhacova L, Bibova H, Marikova T, Kuchar M, Zouzelka R, Rathousky J. Removal of Ampicillin by Heterogeneous Photocatalysis: Combined Experimental and DFT Study. Nanomaterials. 2021; 11(8):1992. https://doi.org/10.3390/nano11081992
Chicago/Turabian StyleBelhacova, Lenka, Hana Bibova, Tereza Marikova, Martin Kuchar, Radek Zouzelka, and Jiri Rathousky. 2021. "Removal of Ampicillin by Heterogeneous Photocatalysis: Combined Experimental and DFT Study" Nanomaterials 11, no. 8: 1992. https://doi.org/10.3390/nano11081992