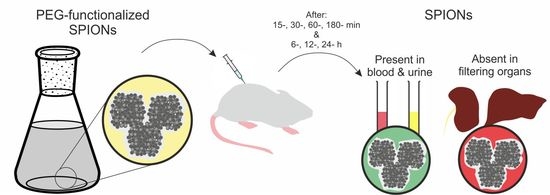
In Vivo Distribution of Poly(ethylene glycol) Functionalized Iron Oxide Nanoclusters: An Ultrastructural Study
Abstract
:1. Introduction
2. Materials and Methods
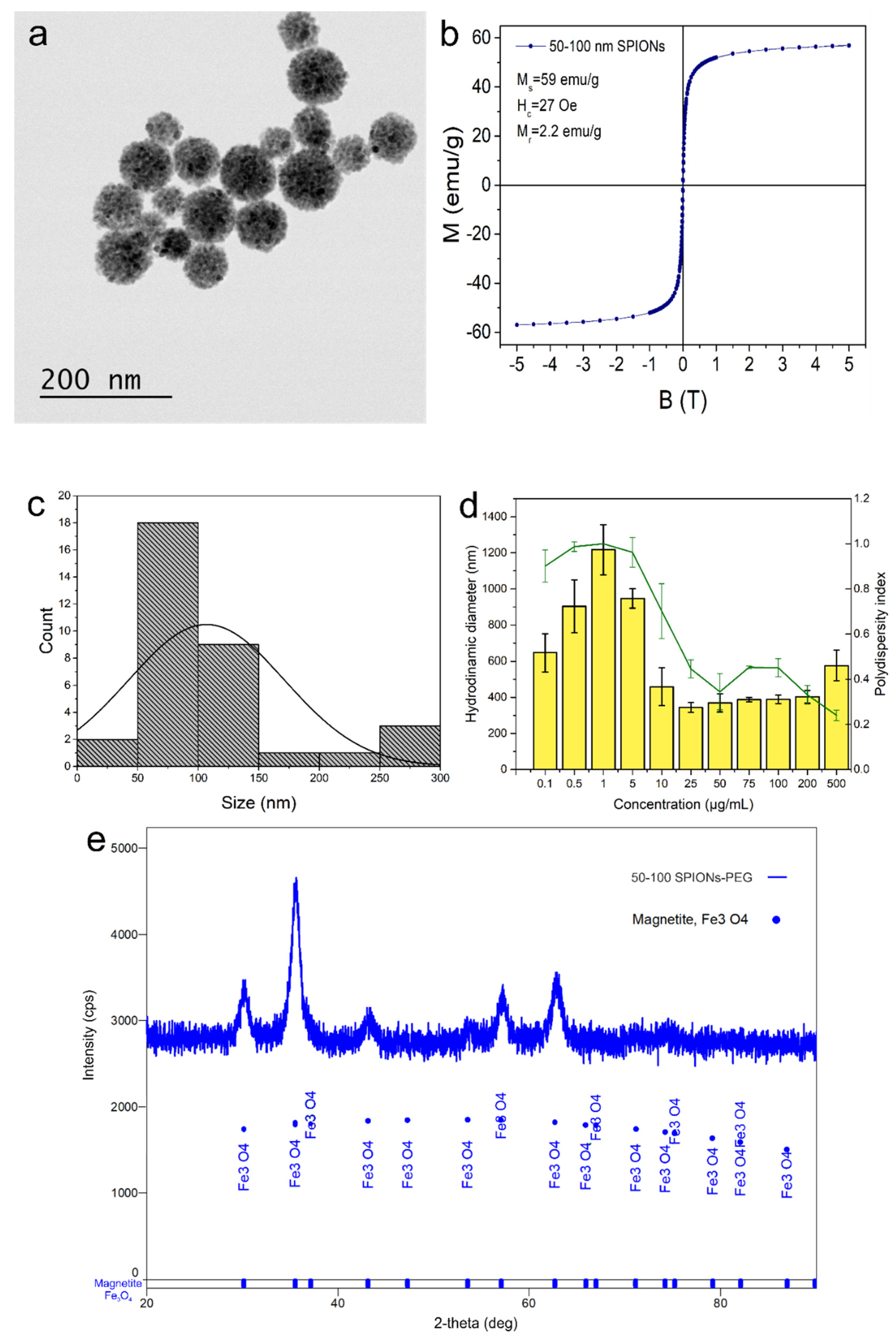
2.1. SPION-PEG Clusters Synthesis and Characterisation
2.2. In Vivo Distribution and Clearance Analyses
2.2.1. Animal Model and SPION-PEG Clusters Administration
2.2.2. Identification and Distribution of SPIONs-PEG in Blood and Urine by Fluorescence Spectroscopy
2.2.3. In-Tissue Identification and Distribution of SPION-PEG Clusters and Histopathological Analyses Using Light and Electron Microscopy
3. Results
3.1. SPION-PEG Clusters Characteristics
3.2. SPION-PEG Cluster Distribution and Clearance
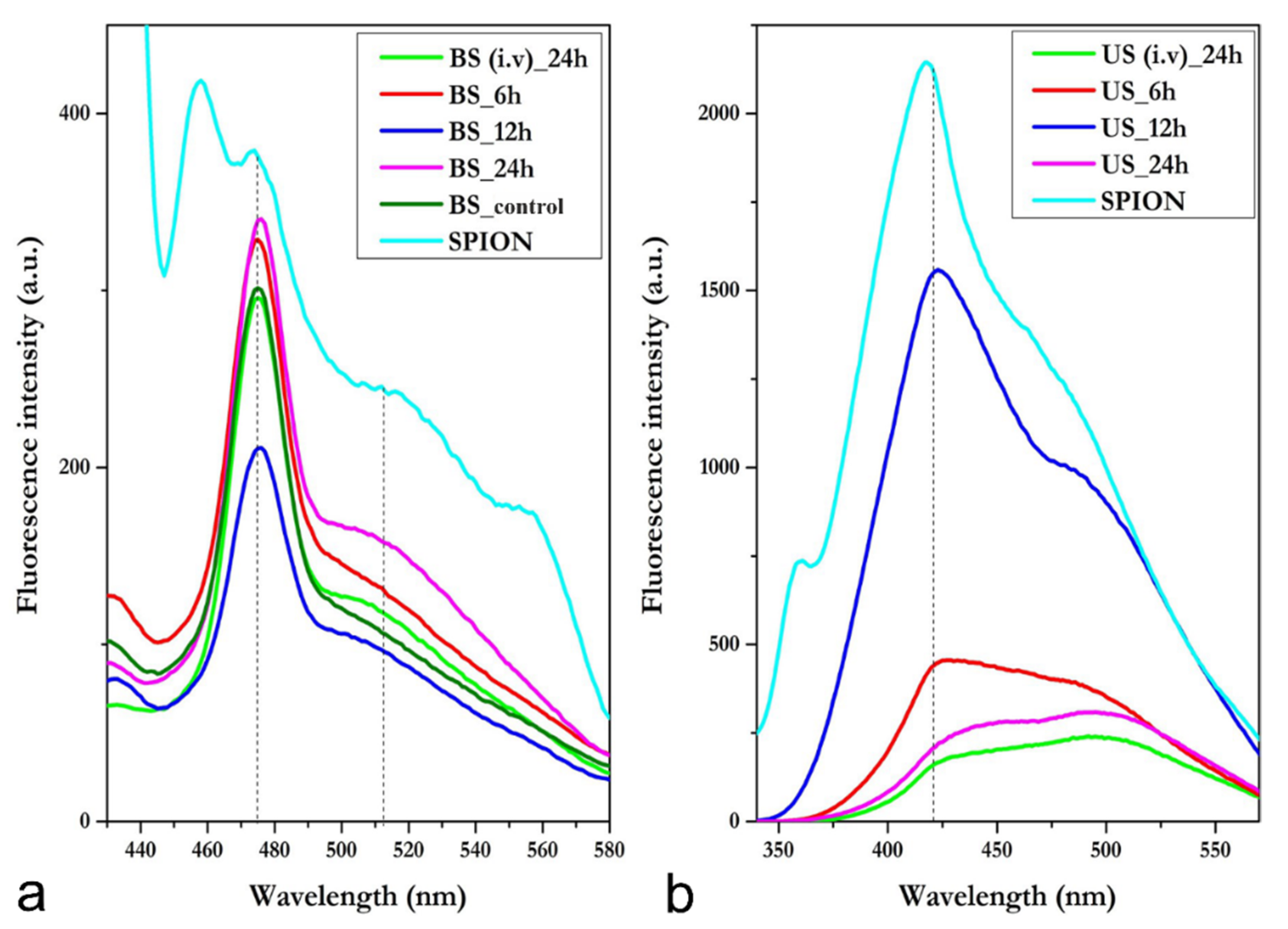
3.3. Blood Serum and Urine Spectrofluorimetric Studies
3.3.1. Blood Serum—i.p.
3.3.2. Blood Serum—i.v.
3.3.3. Urine—i.p.
3.3.4. Urine—i.v.
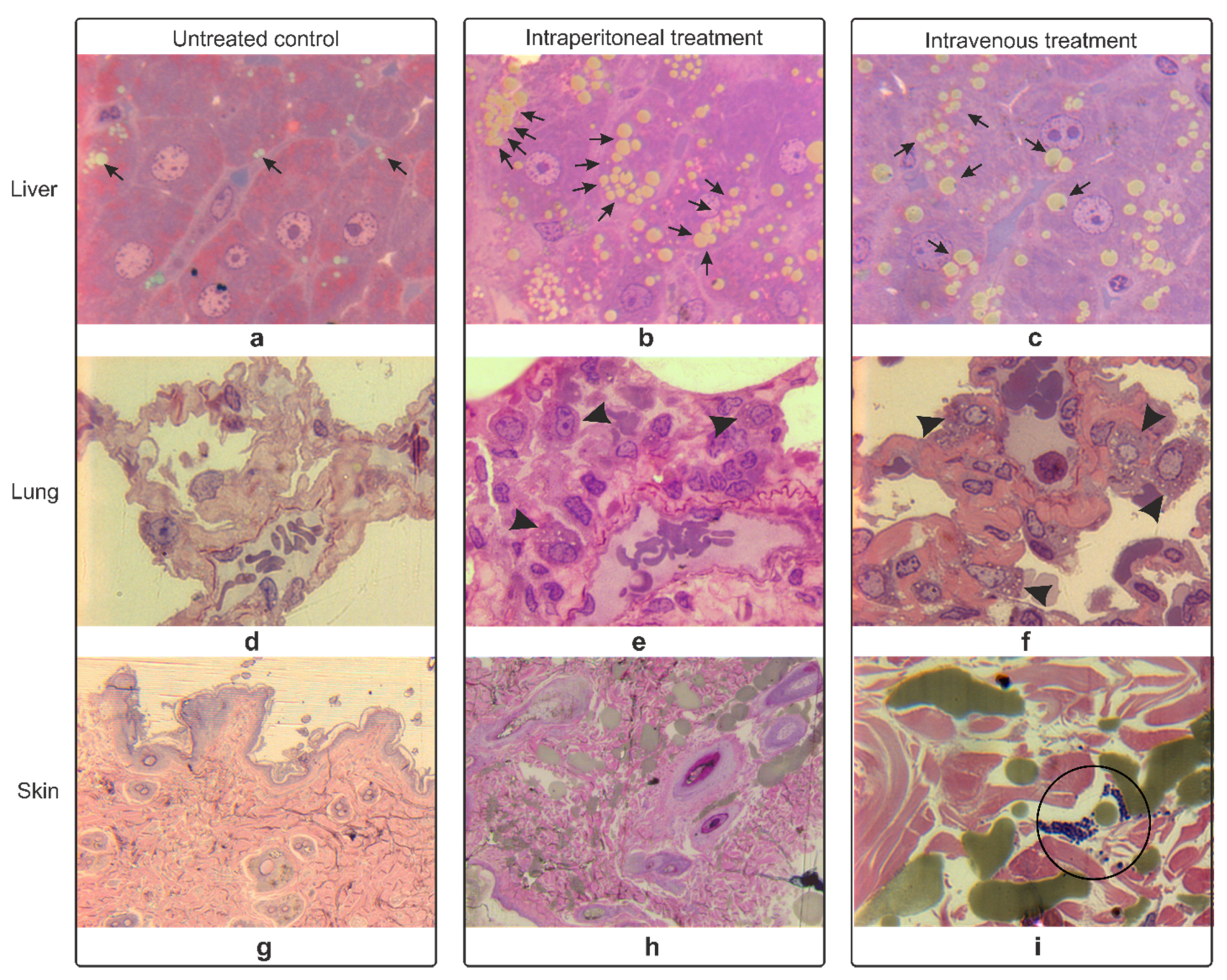
3.4. Histopathology Studies of Rat Organs
3.4.1. Hematoxylin-Eosin Staining
3.4.2. Epoxy Tissue Staining
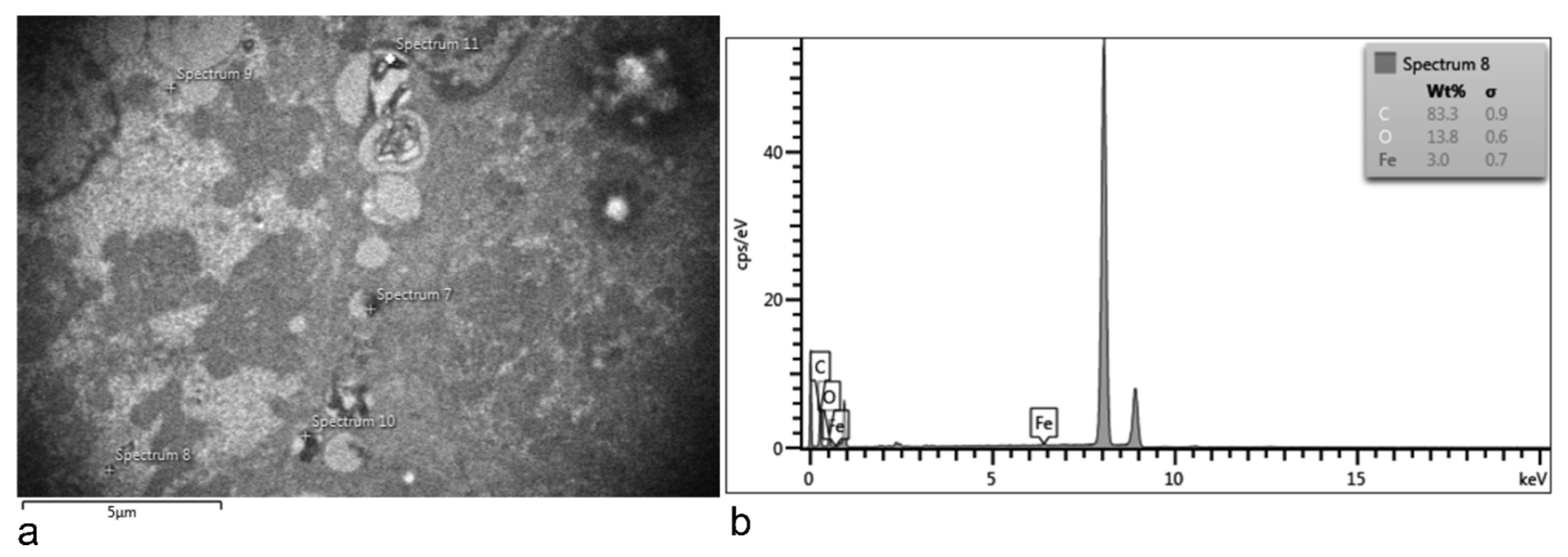
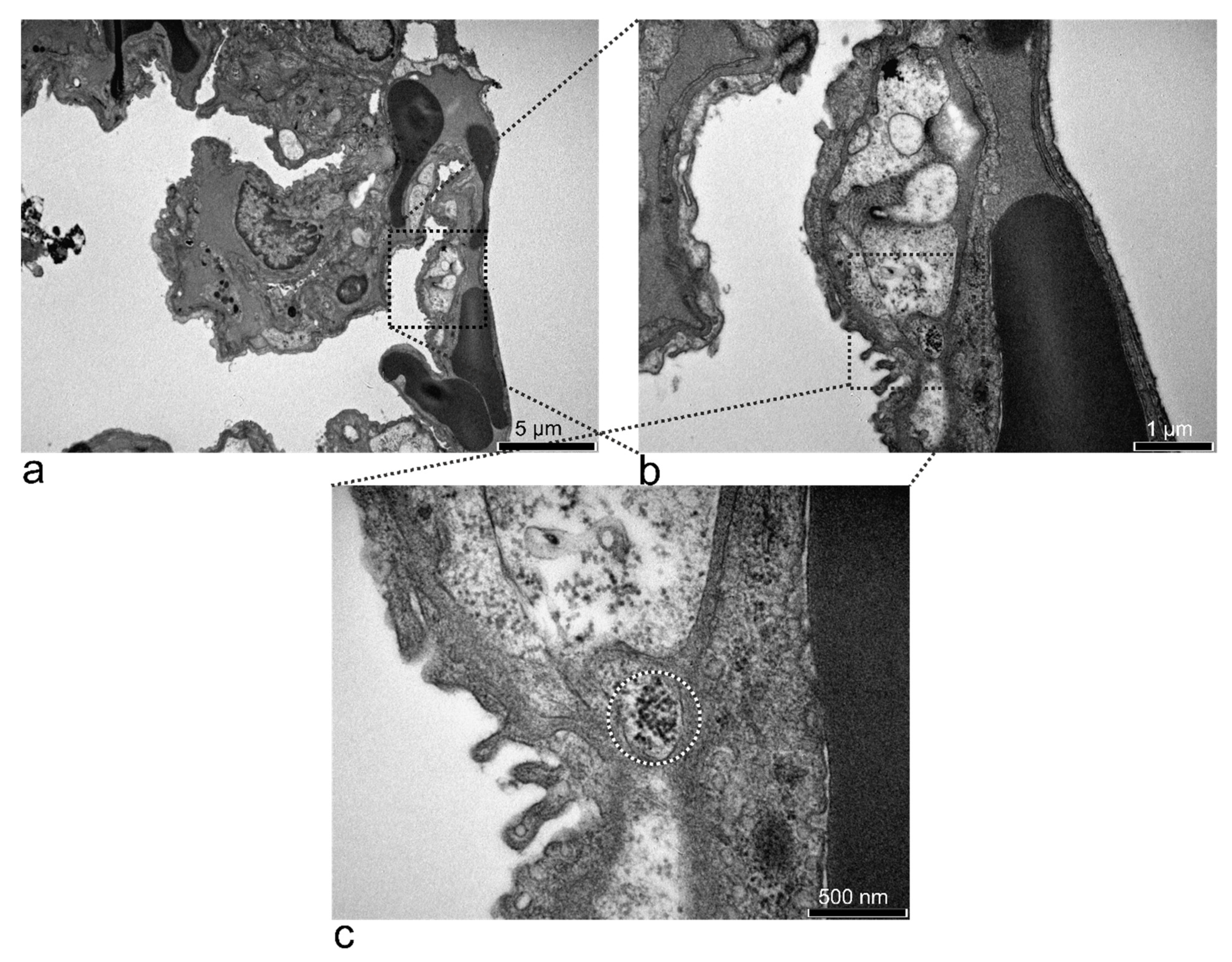
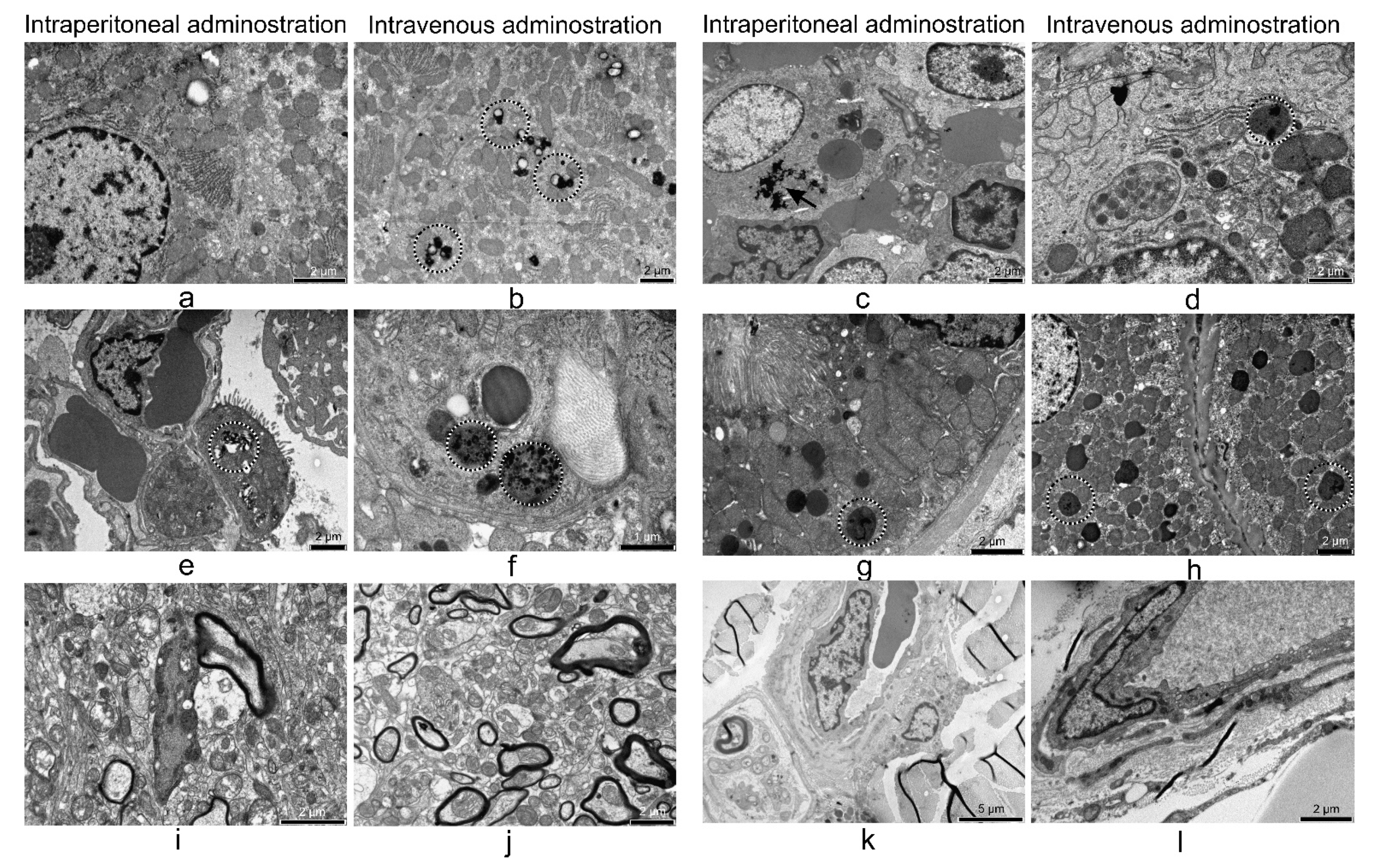
3.5. Electron Microscopy Analyses of Rat Organs
3.5.1. Liver
3.5.2. Lung
3.5.3. Kidney
3.5.4. 24 h after i.p. and i.v. Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Available online: https://ec.europa.eu (accessed on 16 January 2020).
- National Nanotechnology Initiative. Available online: https://www.nano.gov (accessed on 16 January 2020).
- Dulinska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafranski, O.; Karnas, K.; Karewicz, A. Superparamagnetic Iron Oxide Nanoparticles—Current and Prospective Medical Applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef] [Green Version]
- Suciu, M.; Ionescu, C.M.; Ciorita, A.; Tripon, S.C.; Nica, D.; Al-Salami, H.; Barbu-Tudoran, L. Applications of superparamagnetic iron oxide nanoparticles in drug and therapeutic delivery, and biotechnological advancements. Beilstein J. Nanotech. 2020, 11, 1092–1109. [Google Scholar] [CrossRef]
- Kaushik, S.; Thomas, J.; Panwar, V.; Ali, H.; Chopra, V.; Sharma, A.; Tomar, R.; Ghosh, D. In Situ Biosynthesized Superparamagnetic Iron Oxide Nanoparticles (SPIONS) Induce Efficient Hyperthermia in Cancer Cells. ACS Appl. Bio Mater. 2020, 3, 779–788. [Google Scholar] [CrossRef]
- Khan, S.; Setua, S.; Kumari, S.; Dan, N.; Massey, A.; Hafeez, B.B.; Yallapu, M.M.; Stiles, Z.E.; Alabkaa, A.; Yue, J.; et al. Superparamagnetic iron oxide nanoparticles of curcumin enhance gemcitabine therapeutic response in pancreatic cancer. Biomaterials 2019, 208, 83–97. [Google Scholar] [CrossRef]
- Patil, R.M.; Thorat, N.D.; Shete, P.B.; Bedge, P.A.; Gavde, S.; Joshi, M.G.; Tofail, S.A.M.; Bohara, R.A. Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles. Biochem. Biophys. Rep. 2018, 13, 63–72. [Google Scholar] [CrossRef]
- Macavei, S.G.; Suciu, M.; Craciunescu, I.; Barbu-Tudoran, L.; Tripon, S.C.; Balan, R. Hyperthermia effects on normal and tumor skin cells. Ann. RSCB 2016, 21, 11–21. [Google Scholar]
- Mühlberger, M.; Unterweger, H.; Band, J.; Lehmann, C.; Heger, L.; Dudziak, D.; Alexiou, C.; Lee, G.; Janko, C. Loading of Primary Human T Lymphocytes with Citrate-Coated Superparamagnetic Iron Oxide Nanoparticles Does Not Impair Their Activation after Polyclonal Stimulation. Cells 2020, 9, 342. [Google Scholar] [CrossRef] [Green Version]
- Di Bona, K.R.; Xu, Y.; Gray, M.; Fair, D.; Hayles, H.; Milad, L.; Montes, A.; Sherwood, J.; Bao, Y.; Rasco, J.F. Short- and Long-Term Effects of Prenatal Exposure to Iron Oxide Nanoparticles: Influence of Surface Charge and Dose on Developmental and Reproductive Toxicity. Int. J. Molec. Sci. 2015, 16, 30251–30268. [Google Scholar] [CrossRef] [PubMed]
- Geilich, B.M.; Gelfat, I.; Sridhar, S.; van de Ven, A.L.; Webster, T.J. Superparamagnetic iron oxide-encapsulating polymersome nanocarriers for biofilm eradication. Biomaterials 2017, 119, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Jabir, M.S.; Nayef, U.M.; Kadhim, W.K.A. Polyethylene Glycol-Functionalized Magnetic (Fe3O4) Nanoparticles: A Novel DNA-Mediated Antibacterial Agent. Nano Biomed. Eng. 2019, 11, 18–27. [Google Scholar] [CrossRef]
- Chelluri, L.K.; Mohanram, Y.; Jain, R.; Mallarpu, C.S.; Ponnana, M.; Kumar, D.; Venuganti, V.V.K.; Kancherla, R.; Papineni, R.V.; Towner, R.; et al. Effect of engineered superparamagnetic iron oxide nanoparticles in targeted cardiac precursor cell delivery by MRI. Biochem. Biophys. Res. Commun. 2021, 541, 15–21. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Camozzi, D.; Darguzyte, M.; Roemhild, K.; Varvarà, P.; Metselaar, J.; Banala, S.; Straub, M.; Güvener, N.; Engelmann, U.; et al. Size-isolation of superparamagnetic iron oxide nanoparticles improves MRI, MPI and hyperthermia performance. J. Nanobiotech. 2020, 18, 22. [Google Scholar] [CrossRef]
- Deng, L.-H.; Jiang, H.; Lu, F.-L.; Wang, H.-W.; Pu, Y.; Wu, C.-Q.; Tang, H.-J.; Xu, Y.; Chen, T.-W.; Zhu, J.; et al. Size and PEG Length-Controlled PEGylated Monocrystalline Superparamagnetic Iron Oxide Nanocomposite for MRI Contrast Agent. Int. J. Nanomed. 2021, 2021, 201–211. [Google Scholar] [CrossRef]
- Deh, K.; Zaman, M.; Vedvyas, Y.; Liu, Z.; McCabe Gillen, K.; O’Malley, P.; Bedretdinova, D.; Nguyen, T.; Lee, R.; Spincemaille, P.; et al. Validation of MRi quantitative susceptibility mapping of superparamagnetic iron oxide nanoparticles for hyperthermia applications in live subjects. Sci. Rep. 2020, 10, 1171. [Google Scholar] [CrossRef]
- Janko, C.; Ratschker, T.; Tietze, R.; Lyer, S.; Nguyen, K.; Alexiou, C. Functionalized Superparamagnetic Iron Oxide Nanoparticles (SPIONs) as Platform for the Targeted Multimodal Tumor Therapy. Front. Oncol. 2019, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Tietze, R.; Zaloga, J.; Unterweger, H.; Lyer, S.; Friedrich, R.P.; Janko, C.; Pöttler, M.; Dürr, S.; Alexiou, C. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem. Biophys. Res. Commun. 2015, 468, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Thaweesest, W.; Na Bhuket, P.R.; Jantaratana, P.; Rojsitthisak, P.; Rojsitthisak, P. Polyethylene Glycol-Chitosan Oligosaccharide-Coated Superparamagnetic Iron Oxide Nanoparticles: A Novel Drug Delivery System for Curcumin Diglutaric Acid. Biomolecules 2020, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Peng, X.; Hou, J.; Wu, S.; Shen, J.; Wang, L. Folic acid-functionalized polyethylenimine superparamagnetic iron oxide nanoparticles as theranostic agents for magnetic resonance imaging and PD-l1 sirNa delivery for gastric cancer. Int. J. Nanomed. 2017, 2017, 5331–5343. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, F.; Moeeni, M.; Li, C.; Boukherroub, R.; Szunerits, S. Interaction of cellulose and nitrodopamine coated superparamagnetic iron oxide nanoparticles with alpha-lactalbumin. RSC Adv. 2020, 10, 9704–9716. [Google Scholar] [CrossRef]
- Li, K.; Nejadnik, H.; Daldrup-Link, H.E. Next-generation superparamagnetic iron oxide nanoparticles for cancer theranostics. Drug. Dis. Today 2017, 22, 1421–1429. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Conte, M.; Pontico, M.; Pani, A.; Pani, R.; De Vincentis, G. New Frontiers in Molecular Imaging with Superparamagnetic Iron Oxide Nanoparticles (SPIONs): Efficacy, Toxicity, and Future Applications. Nuc. Med. Molec. Imag. 2020, 54, 65–80. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Ma, M.; Chen, Z.; Tang, X.-l.; Wang, Z. Self-Assembly Iron Oxide Nanoclusters for Photothermal-Mediated Synergistic Chemo/Chemodynamic Therapy. J. Immunol. Res. 2021, 2021, 9958239. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, S.; Hu, X.; Li, F.; Ling, D. Controlled synthesis and assembly of ultra-small nanoclusters for biomedical applications. Biomat. Sci. 2019, 7, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zare, I.; Yan, X.; Fan, K. Protein-protected metal nanoclusters: An emerging ultra-small nanozyme. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1602. [Google Scholar] [CrossRef]
- Lai, S.-M.; Hsiao, J.-K.; Yu, H.-P.; Lu, C.-W.; Huang, C.-C.; Shieh, M.-J.; Lai, P.-S. Polyethylene glycol-based biocompatible and highly stable superparamagnetic iron oxide nanoclusters for magnetic resonance imaging. J. Mat. Chem. 2012, 22, 15160–15167. [Google Scholar] [CrossRef]
- Antone, A.J.; Sun, Z.; Bao, Y. Preparation and Application of Iron Oxide Nanoclusters. Magnetochemistry 2019, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Van Landeghem, F.K.H.; Maier-Hauff, K.; Jordan, A.; Hoffmann, K.-T.; Gneveckow, U.; Scholz, R.; Thiesen, B.; Bruck, W.; von Deimling, A. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials 2009, 30, 52–57. [Google Scholar] [CrossRef]
- Doan, L.; Lu, Y.; Karatela, M.; Phan, V.; Jeffryes, C.; Benson, T.; Wujcik, E.K. Surface Modifications of Superparamagnetic Iron Oxide Nanoparticles with Polylactic Acid-Polyethylene Glycol Diblock Copolymer and Graphene Oxide for a Protein Delivery Vehicle. Eng. Sci. 2018, 7, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Butoescu, N.; Jordan, O.; Burdet, P.; Stadelmann, P.; Petri-Fink, A.; Hofmann, H.; Doelker, E. Dexamethasone-containing biodegradable superparamagnetic microparticles for intra-articular administration: Physicochemical and magnetic properties, in vitro and in vivo drug release. Europ. J. Pharma. Biopharma. 2009, 72, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Hoff, D.; Sheikh, L.; Bhattacharya, S.; Nayar, S.; Webster, T.J. Comparison study of ferrofluid and powder iron oxide nanoparticle permeability across the blood–brain barrier. Int. J. Nanomed. 2013, 2013, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, G.; Sudame, A.; Luthra, T.; Saini, K.; Maity, D. Functionalized Hydrophilic Superparamagnetic Iron Oxide Nanoparticles for Magnetic Fluid Hyperthermia Application in Liver Cancer Treatment. ACS Omega 2018, 3, 3991–4005. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia—Biophysical limitations of a visionary tumour therapy. J. Magnet. Magnet. Mat. 2006, 311, 187–192. [Google Scholar] [CrossRef]
- Vakili-Ghartavol, R.; Momtazi-Borojeni, A.A.; Vakili Ghartavol, Z.; Aiyelabegan, H.T.; Jaafari, M.R.; Rezayat, S.M.; Bidgoli, S.A. Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Art. Cell. Nanomed. Biotechnol. 2020, 48, 443–451. [Google Scholar] [CrossRef]
- Enteshari Najafabadi, R.; Kazemipour, N.; Esmaeili, A.; Beheshti, S.; Nazifi, S. Using superparamagnetic iron oxide nanoparticles to enhance bioavailability of quercetin in the intact rat brain. BMC Pharmacol. Toxicol. 2018, 19, 59. [Google Scholar] [CrossRef]
- Vermeij, E.A.; Koenders, M.I.; Bennink, M.B.; Crowe, L.A.; Maurizi, L.; Vallée, J.-P.; Hofmann, H.; van den Berg, W.B.; van Lent, P.L.E.M.; van de Loo, F.A.J. The In-Vivo Use of Superparamagnetic Iron Oxide Nanoparticles to Detect Inflammation Elicits a Cytokine Response but Does Not Aggravate Experimental Arthritis. PLoS ONE 2015, 10, e0126687. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.E.; Jones, N. Nephrin Signaling in the Podocyte: An Updated View of Signal Regulation at the Slit Diaphragm and Beyond. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef]
- Poon, W.; Zhang, Y.-N.; Ouyang, B.; Kingston, B.R.; Wu, J.L.Y.; Wilhelm, S.; Chan, W.C.W. Elimination Pathways of Nanoparticles. ACS Nano 2019, 13, 5785–5798. [Google Scholar] [CrossRef]
- Craciunescu, I.; Petran, A.; Liebscher, J.; Vekas, L.; Turcu, R. Synthesis and characterization of size-controlled magnetic clusters functionalized with polymer layer for wastewater depollution. Mat. Chem. Phys. 2017, 185, 91–97. [Google Scholar] [CrossRef]
- Crăciunescu, I.; Palade, P.; Iacob, N.; Ispas, G.M.; Stanciu, A.E.; Kuncser, V.; Turcu, R.P. High-Performance Functionalized Magnetic Nanoparticles with Tailored Sizes and Shapes for Localized Hyperthermia Applications. J. Phys. Chem. C 2021, 125, 11132–11146. [Google Scholar] [CrossRef]
- ISO10993-2:2006. International Organization for Standardization. In Biological Evaluation of Medical Devices—Part 2: Animal Welfare Requirements; British Standards Institution: London, UK, 2006. [CrossRef]
- Mirescu, C.; Zamfirescu, D.M.; Bungărdean, C. Histopatology Essentials [Esențialul în Histopatologie]; Presa Universitară Clujană: Cluj-Napoca, Romania, 2017. [Google Scholar]
- Hayat, M.A. Principles and Techniques of Electron Microscopy. Biological Applications, 4th ed.; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Craciun, C.; Barbu-Tudoran, L. Identification of new structural elements within ‘porosomes’ of the exocrine pancreas: A detailed study using high-resolution electron microscopy. Micron 2013, 44, 137–142. [Google Scholar] [CrossRef]
- Paris, J.L.; Baeza, A.; Vallet-Regí, M. Overcoming the stability, toxicity, and biodegradation challenges of tumor stimuli-responsive inorganic nanoparticles for delivery of cancer therapeutics. Expert Opin. Drug Deliv. 2019, 16, 1095–1112. [Google Scholar] [CrossRef]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies? Pharm. Res. 2019, 37, 12. [Google Scholar] [CrossRef]
- Jung, C.; Kaul, M.G.; Bruns, O.T.; Dučić, T.; Freund, B.; Heine, M.; Reimer, R.; Meents, A.; Salmen, S.C.; Weller, H.; et al. Intraperitoneal Injection Improves the Uptake of Nanoparticle-Labeled High-Density Lipoprotein to Atherosclerotic Plaques Compared With Intravenous Injection. Circ. Cardiovasc. Imaging 2014, 7, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Tsuchiya, K.; Nitta, N.; Sonoda, A.; Nitta-Seko, A.; Ohta, S.; Otani, H.; Takahashi, M.; Murata, K.; Murase, K.; Nohara, S.; et al. Histological study of the biodynamics of iron oxide nanoparticles with different diameters. Int. J. Nanomed. 2011, 6, 1587–1594. [Google Scholar] [CrossRef] [Green Version]
- Ganea, I.-V.; Nan, A.; Baciu, C.; Turcu, R. Effective Removal of Crystal Violet Dye Using Neoteric Magnetic Nanostructures Based on Functionalized Poly(Benzofuran-co-Arylacetic Acid): Investigation of the Adsorption Behaviour and Reusability. Nanomaterials 2021, 11, 679. [Google Scholar] [CrossRef]
- Cîrcu, M.; Nan, A.; Borodi, G.; Liebscher, J.; Turcu, R. Refinement of Magnetite Nanoparticles by Coating with Organic Stabilizers. Nanomaterials 2016, 6, 228. [Google Scholar] [CrossRef] [Green Version]
- Greish, K. Enhanced Permeability and Retention (EPR) Effect for Anticancer Nanomedicine Drug Targeting. Cancer Nanotechnol. 2010, 624, 25–37. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef]
- Nosrati, H.; Salehiabar, M.; Fridoni, M.; Abdollahifar, M.-A.; Kheiri Manjili, H.; Davaran, S.; Danafar, H. New Insight about Biocompatibility and Biodegradability of Iron Oxide Magnetic Nanoparticles: Stereological and In Vivo MRI Monitor. Sci. Rep. 2019, 9, 7173. [Google Scholar] [CrossRef] [Green Version]
- Belanova, A.A.; Gavalas, N.; Makarenko, Y.M.; Belousova, M.M.; Soldatov, A.V.; Zolotukhin, P.V. Physicochemical Properties of Magnetic Nanoparticles: Implications for Biomedical Applications In Vitro and In Vivo. Oncol. Res. Treat. 2018, 41, 139–143. [Google Scholar] [CrossRef]
- Serkova, N.J. Nanoparticle-Based Magnetic Resonance Imaging on Tumor-Associated Macrophages and Inflammation. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Lukas, G.; Brindle, S.D.; Greengard, P. The Route of Absorption of Intraperitoneally Administered Compounds. J. Pharmacol. Exp. Ther. 1971, 178, 562. [Google Scholar] [PubMed]
- Yao, Y.; Zang, Y.; Qu, J.; Tang, M.; Zhang, T. The Toxicity Of Metallic Nanoparticles On Liver: The Subcellular Damages, Mechanisms, And Outcomes. Int. J. Nanomed. 2019, 14, 8787–8804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boey, A.; Ho, H.K. All Roads Lead to the Liver: Metal Nanoparticles and Their Implications for Liver Health. Small 2020, 16, 2000153. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, H.; Zhou, S.; Zheng, L.; Li, X.; Chu, R.; Chen, W.; Wang, B.; Wang, M.; Chai, Z.; et al. Iron oxide nanoparticles aggravate hepatic steatosis and liver injury in nonalcoholic fatty liver disease through BMP-SMAD-mediated hepatic iron overload. Nanotoxicology 2021, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Molec. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [Green Version]
- Ghasempour, S.; Shokrgozar, M.A.; Ghasempour, R.; Alipour, M. Investigating the cytotoxicity of iron oxide nanoparticles in in vivo and in vitro studies. Exp. Toxicol. Path. 2015, 67, 509–515. [Google Scholar] [CrossRef]
- Huang, Y.; Mao, K.; Zhang, B.; Zhao, Y. Superparamagnetic iron oxide nanoparticles conjugated with folic acid for dual target-specific drug delivery and MRI in cancer theranostics. Mat. Sci. Eng. C 2017, 70, 763–771. [Google Scholar] [CrossRef]
- Jarockyte, G.; Daugelaite, E.; Stasys, M.; Statkute, U.; Poderys, V.; Tseng, T.-C.; Hsu, S.-H.; Karabanovas, V.; Rotomskis, R. Accumulation and Toxicity of Superparamagnetic Iron Oxide Nanoparticles in Cells and Experimental Animals. Int. J. Molec. Sci. 2016, 17, 1193. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.; Zhou, H.; Wang, Y.; Liu, D.; Li, J.; Deng, H.; Qi, X.; Chen, T.; Zhang, L.; Li, G. One-pot synthesis of dextran-coated iron oxide nanoclusters for real-time regional lymph node mapping. Int. J. Nanomed. 2017, 2017, 3365–3374. [Google Scholar] [CrossRef] [Green Version]
- Briley-Saebo, K.; Bjørnerud, A.; Grant, D.; Ahlstrom, H.; Berg, T.; Kindberg, G.M. Hepatic cellular distribution and degradation of iron oxide nanoparticles following single intravenous injection in rats: Implications for magnetic resonance imaging. Cell Tissue Res. 2004, 316, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.M.A.; Shaker, S.A.; Pinol, R.; Millan, A.; Hanafy, M.Y.; Helmy, M.H.; Kamel, M.A.; Mahmoud, S.A. Effect of superparamagnetic iron oxide nanoparticles on glucose homeostasis on type 2 diabetes experimental model. Life Sci. 2020, 245, 117361. [Google Scholar] [CrossRef]
- Asem, H.; Zhao, Y.; Ye, F.; Barrefelt, Å.; Abedi-Valugerdi, M.; El-Sayed, R.; El-Serafi, I.; Abu-Salah, K.M.; Hamm, J.; Muhammed, M.; et al. Biodistribution of biodegradable polymeric nano-carriers loaded with busulphan and designed for multimodal imaging. J. Nanobiotech. 2016, 14, 82. [Google Scholar] [CrossRef] [Green Version]
- Radu, M.; Din, I.M.; Hermenean, A.; Cinteză, O.L.; Burlacu, R.; Ardelean, A.; Dinischiotu, A. Exposure to Iron Oxide Nanoparticles Coated with Phospholipid-Based Polymeric Micelles Induces Biochemical and Histopathological Pulmonary Changes in Mice. Int. J. Molec. Sci. 2015, 16, 29417–29435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsnelson, B.A.; Privalova, L.I.; Sutunkova, M.P.; Gurvich, V.B.; Loginova, N.V.; Minigalieva, I.A.; Kireyeva, E.P.; Shur, V.Y.; Shishkina, E.V.; Beikin, Y.B.; et al. Some inferences from in vivo experiments with metal and metal oxide nanoparticles: The pulmonary phagocytosis response, subchronic systemic toxicity and genotoxicity, regulatory proposals, searching for bioprotectors (a self-overview). Int. J. Nanomed. 2015, 10, 3013–3029. [Google Scholar] [CrossRef] [Green Version]
- Roumy, A.; Liaudet, L.; Rusca, M.; Marcucci, C.; Kirsch, M. Pulmonary complications associated with veno-arterial extra-corporeal membrane oxygenation: A comprehensive review. Crit. Care 2020, 24, 212. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, E.; Langer, T.; Bottino, N.; Brusatori, S.; Carlesso, E.; Colombo, S.M.; Zanella, A.; Pesenti, A.; Grasselli, G. Key Role of Respiratory Quotient to Reduce the Occurrence of Hypoxemia During Extracorporeal Gas Exchange: A Theoretical Analysis*. Crit. Care Med. 2020, 48, e1327–e1331. [Google Scholar] [CrossRef]
- Kodali, V.; Littke, M.H.; Tilton, S.C.; Teeguarden, J.G.; Shi, L.; Frevert, C.W.; Wang, W.; Pounds, J.G.; Thrall, B.D. Dysregulation of Macrophage Activation Profiles by Engineered Nanoparticles. ACS Nano 2013, 7, 6997–7010. [Google Scholar] [CrossRef] [PubMed]
- Blank, F.; Gerber, P.; Rothen-Rutishauser, B.; Sakulkhu, U.; Salaklang, J.; De Peyer, K.; Gehr, P.; Nicod, L.P.; Hofmann, H.; Geiser, T.; et al. Biomedical nanoparticles modulate specific CD4+ T cell stimulation by inhibition of antigen processing in dendritic cells. Nanotoxicology 2011, 5, 606–621. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Fang, R.H.; Sailor, M.J.; Park, J.-H. In vivo clearance and toxicity of monodisperse iron oxide nanocrystals. ACS Nano 2012, 6, 4947–4954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, S.; Yamaguchi, S.; Kaseda, M.; Ichihara, N.; Hayakawa, T.; Asari, M. The time course of lymphatic routes emanating from the peritoneal cavity in rats. Anat. Histol. Embryol. 2007, 36, 78–82. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconj. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Branca, R.T.; Cleveland, Z.I.; Fubara, B.; Kumar, C.S.S.R.; Maronpot, R.R.; Leuschner, C.; Warren, W.S.; Driehuys, B. Molecular MRI for sensitive and specific detection of lung metastases. Proc. Natl. Acad. Sci. USA 2010, 107, 3693–3697. [Google Scholar] [CrossRef] [Green Version]
- Barrefelt, Å.; Saghafian, M.; Kuiper, R.; Ye, F.; Egri, G.; Klickermann, M.; Brismar, T.; Aspelin, P.; Muhammed, M.; Dähne, L.; et al. Biodistribution, kinetics, and biological fate of SPION microbubbles in the rat. Int. J. Nanomed. 2013, 8, 3241–3254. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.-S.; Cho, M.; Kim, S.R.; Choi, M.; Lee, J.Y.; Han, B.S.; Park, S.N.; Yu, M.K.; Jon, S.; Jeong, J. Pulmonary toxicity and kinetic study of Cy5.5-conjugated superparamagnetic iron oxide nanoparticles by optical imaging. Toxicol. Appl. Pharmacol. 2009, 239, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Le, W.; Mei, T.; Wang, T.; Chen, L.; Lei, Y.; Cui, S.; Chen, B.; Cui, Z.; Shao, C. In vitro and in vivo targeting imaging of pancreatic cancer using a Fe3O4@SiO2 nanoprobe modified with anti-mesothelin antibody. Int. J. Nanomed. 2016, 11, 2195–2207. [Google Scholar] [CrossRef] [Green Version]
- Balas, M.; Popescu Din, I.M.; Hermenean, A.; Cinteza, L.O.; Dinischiotu, A. Exposure to Iron Oxide Nanoparticles Coated with Phospholipid-Based Polymeric Micelles Induces Renal Transitory Biochemical and Histopathological Changes in Mice. Materials 2021, 14, 2605. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Vallejo, V.; Puigivila, M.; Plaza-García, S.; Szczupak, B.; Piñol, R.; Murillo, J.L.; Sorribas, V.; Lou, G.; Veintemillas, S.; Ramos-Cabrer, P.; et al. PEG-copolymer-coated iron oxide nanoparticles that avoid the reticuloendothelial system and act as kidney MRI contrast agents. Nanoscale 2018, 10, 14153–14164. [Google Scholar] [CrossRef] [Green Version]
- Imam, S.Z.; Lantz-McPeak, S.M.; Cuevas, E.; Rosas-Hernandez, H.; Liachenko, S.; Zhang, Y.; Sarkar, S.; Ramu, J.; Robinson, B.L.; Jones, Y.; et al. Iron Oxide Nanoparticles Induce Dopaminergic Damage: In vitro Pathways and In Vivo Imaging Reveals Mechanism of Neuronal Damage. Mol. Neurobiol 2015, 52, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Han, G.; Wang, S.; Chong, C.; Han, D.; Tan, J.; Zhang, B. The distribution of the iron oxide nanoparticles modified with polyethylene glycol in rat brains. Mat. Chem. Phys. 2021, 260, 124108. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Santini, B.; Selmin, F.; Marini, V.; Corsi, F.; Allevi, R.; Ferretti, A.M.; Prosperi, D.; Cilurzo, F.; Colombo, M.; et al. Impact of semi-solid formulations on skin penetration of iron oxide nanoparticles. J. Nanobiotech. 2017, 15, 14. [Google Scholar] [CrossRef] [Green Version]
- Coricovac, D.-E.; Moacă, E.-A.; Pinzaru, I.; Cîtu, C.; Soica, C.; Mihali, C.-V.; Păcurariu, C.; Tutelyan, V.A.; Tsatsakis, A.; Dehelean, C.-A. Biocompatible Colloidal Suspensions Based on Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Toxicological Profile. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, Y.-f.; Chen, W.; Liang, X.-G.; Huang, Y.-z.; Miao, J.; Liu, L.; Lou, Y.; Zhang, X.-g.; Wang, B.; Tang, R.-k.; et al. Epirubicin-Loaded Superparamagnetic Iron-Oxide Nanoparticles for Transdermal Delivery: Cancer Therapy by Circumventing the Skin Barrier. Small 2015, 11, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Murros, K.; Wasiljeff, J.; Macías-Sánchez, E.; Faivre, D.; Soinne, L.; Valtonen, J.; Pohja, M.; Saari, P.; Pesonen, L.J.; Salminen, J.M. Magnetic Nanoparticles in Human Cervical Skin. Front. Med. 2019, 6. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®-the first FDA-approved nano-drug: Lessons learned. J. Control. Release Off. J. Control. Release Soc. 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E. Ten Things You Might Not Know about Iron Oxide Nanoparticles. Radiology 2017, 284, 616–629. [Google Scholar] [CrossRef]
- Van de Walle, A.; Sangnier, A.P.; Abou-Hassan, A.; Curcio, A.; Hémadi, M.; Menguy, N.; Lalatonne, Y.; Luciani, N.; Wilhelm, C. Biosynthesis of magnetic nanoparticles from nano-degradation products revealed in human stem cells. Proc. Natl. Acad. Sci. USA 2019, 116, 4044–4053. [Google Scholar] [CrossRef] [Green Version]
- Hobson, N.J.; Weng, X.; Siow, B.; Veiga, C.; Ashford, M.; Thanh, N.T.K.; Schätzlein, A.G.; Uchegbu, I.F. Clustering superparamagnetic iron oxide nanoparticles produces organ-targeted high-contrast magnetic resonance images. Nanomedicine 2019, 14, 1135–1152. [Google Scholar] [CrossRef]
- Feliu, N.; Fadeel, B. Nanotoxicology: No small matter. Nanoscale 2010, 2, 2514–2520. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.S.; Adewumi, I. Biochemical Evaluation of Silver Nanoparticles in Wistar Rats. Int. Sch. Res. Not. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, H.; Kim, J.; Lee, Y.M.; Kim, J.; Woo Choi, H.; Lee, J.; Park, H.; Kang, Y.; Kim, I.-S.; Lee, B.-H.; et al. Poly-paclitaxel/cyclodextrin-SPION nano-assembly for magnetically guided drug delivery system. J. Contrl. Rel. 2016, 231, 68–76. [Google Scholar] [CrossRef] [PubMed]



| Organ/Tissue SPION Concentration/Effects | i.p. | i.v. | ||||||
|---|---|---|---|---|---|---|---|---|
| 15 min | 30 min | 1 h | 3 h | 6 h | 12 h | 24 h | 24 h | |
| blood | high | high | high | high | high | medium | high | high |
| urine | - | - | - | low | low | high | low | low |
| liver | u.u. SPION ex | n.p.m. | n.p.m. | n.p.m. | n.p.m. | n.p.m. | inflammatory infiltrates, high density of small lipid droplets, high number of electron-dense particles in the macrophages and parenchymal cells | inflammatory infiltrates, a few large lipid droplets, high number of electron-dense particles in the macrophages and parenchymal cells |
| spleen | n.p.m. | n.p.m. | n.p.m. | n.p.m. | n.p.m. | n.p.m. | n.p.m. high number of electron-dense particles in the macrophages and parenchymal cells | n.p.m. high number of electron-dense particles in the macrophages and parenchymal cells |
| lung | u.u. SPION in | n.p.m. | n.p.m. | n.p.m. | n.p.m. | n.p.m. | SPION-loaded and activated macrophages, extended areas of emphysema, local hypoxia, high number of electron-dense particles in the macrophages and parenchymal cells | SPION-loaded and activated macrophages, extended areas of emphysema, local hypoxia, high number of electron-dense particles in the macrophages and parenchymal cells |
| kidney | u.u. | n.p.m. | u.u. SPION in | n.p.m. | n.p.m. | n.p.m. | n.p.m. high number of electron-dense particles in the macrophages and parenchymal cells | n.p.m. high number of electron-dense particles in the macrophages and parenchymal cells |
| brain | n.p.m. | n.p.m. | n.p.m. | n.p.m. | n.p.m. | n.p.m. | shriveled pyramidal neurons, hypochromic nuclei, local hypoxia | shriveled pyramidal neurons, hypochromic nuclei, local hypoxia |
| skin | n.p.m. | n.p.m. | n.p.m. | n.p.m. | n.p.m. | n.p.m. | n.p.m. | d.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suciu, M.; Mirescu, C.; Crăciunescu, I.; Macavei, S.G.; Leoștean, C.; Ştefan, R.; Olar, L.E.; Tripon, S.-C.; Ciorîță, A.; Barbu-Tudoran, L. In Vivo Distribution of Poly(ethylene glycol) Functionalized Iron Oxide Nanoclusters: An Ultrastructural Study. Nanomaterials 2021, 11, 2184. https://doi.org/10.3390/nano11092184
Suciu M, Mirescu C, Crăciunescu I, Macavei SG, Leoștean C, Ştefan R, Olar LE, Tripon S-C, Ciorîță A, Barbu-Tudoran L. In Vivo Distribution of Poly(ethylene glycol) Functionalized Iron Oxide Nanoclusters: An Ultrastructural Study. Nanomaterials. 2021; 11(9):2184. https://doi.org/10.3390/nano11092184
Chicago/Turabian StyleSuciu, Maria, Claudiu Mirescu, Izabell Crăciunescu, Sergiu Gabriel Macavei, Cristian Leoștean, Rǎzvan Ştefan, Loredana E. Olar, Septimiu-Cassian Tripon, Alexandra Ciorîță, and Lucian Barbu-Tudoran. 2021. "In Vivo Distribution of Poly(ethylene glycol) Functionalized Iron Oxide Nanoclusters: An Ultrastructural Study" Nanomaterials 11, no. 9: 2184. https://doi.org/10.3390/nano11092184