Controlled Lattice Thermal Conductivity of Transparent Conductive Oxide Thin Film via Localized Vibration of Doping Atoms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thin-Film Fabrication
2.2. Thin-Film Characterization
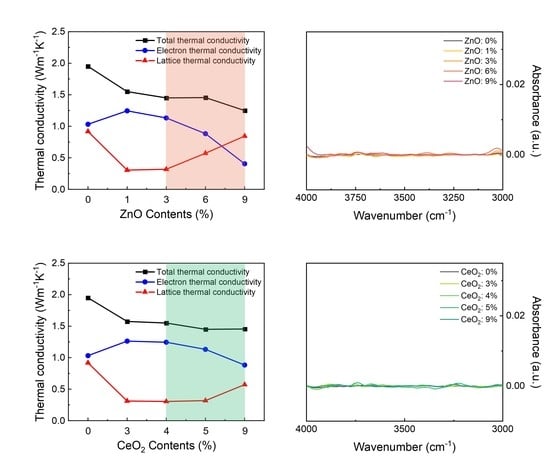
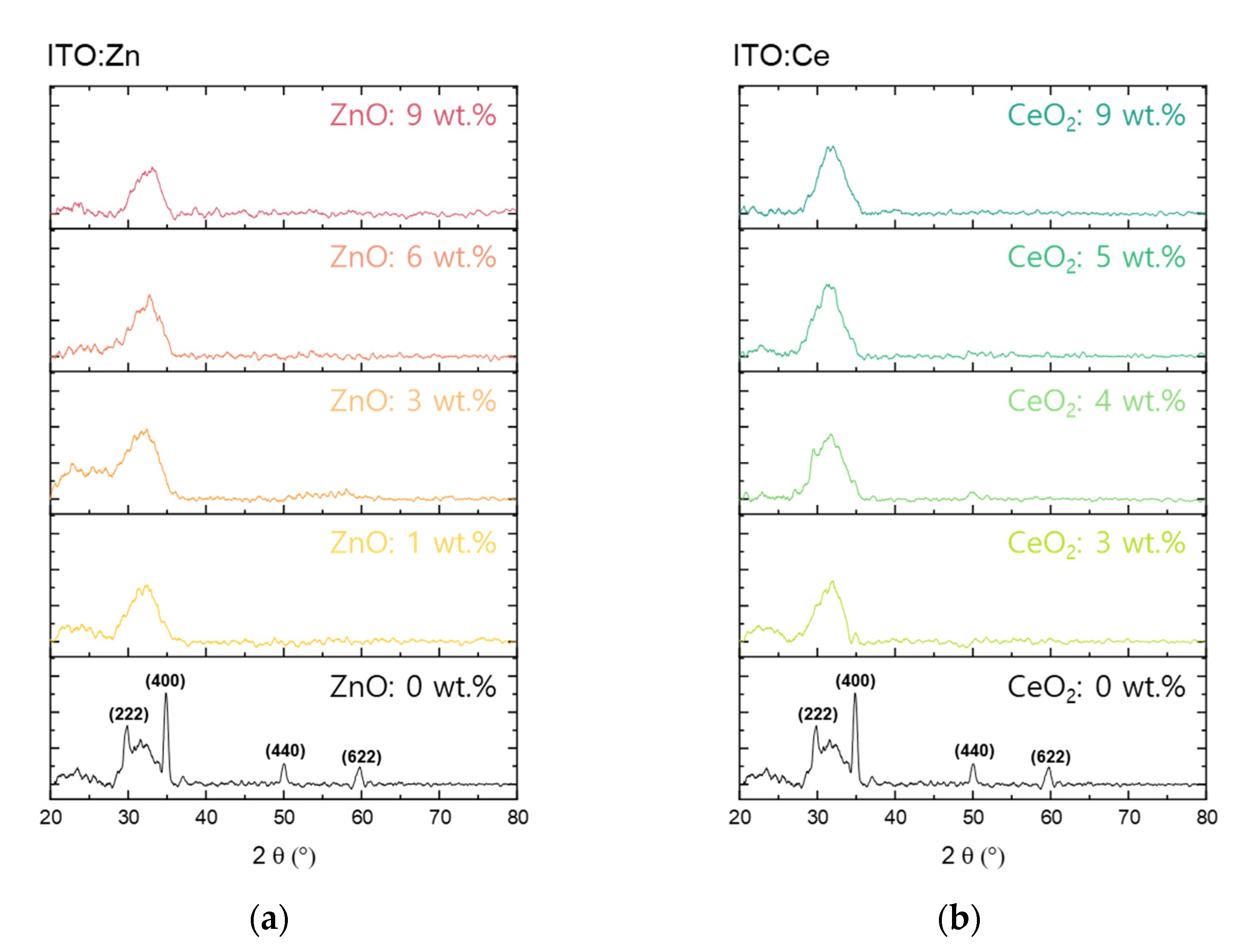
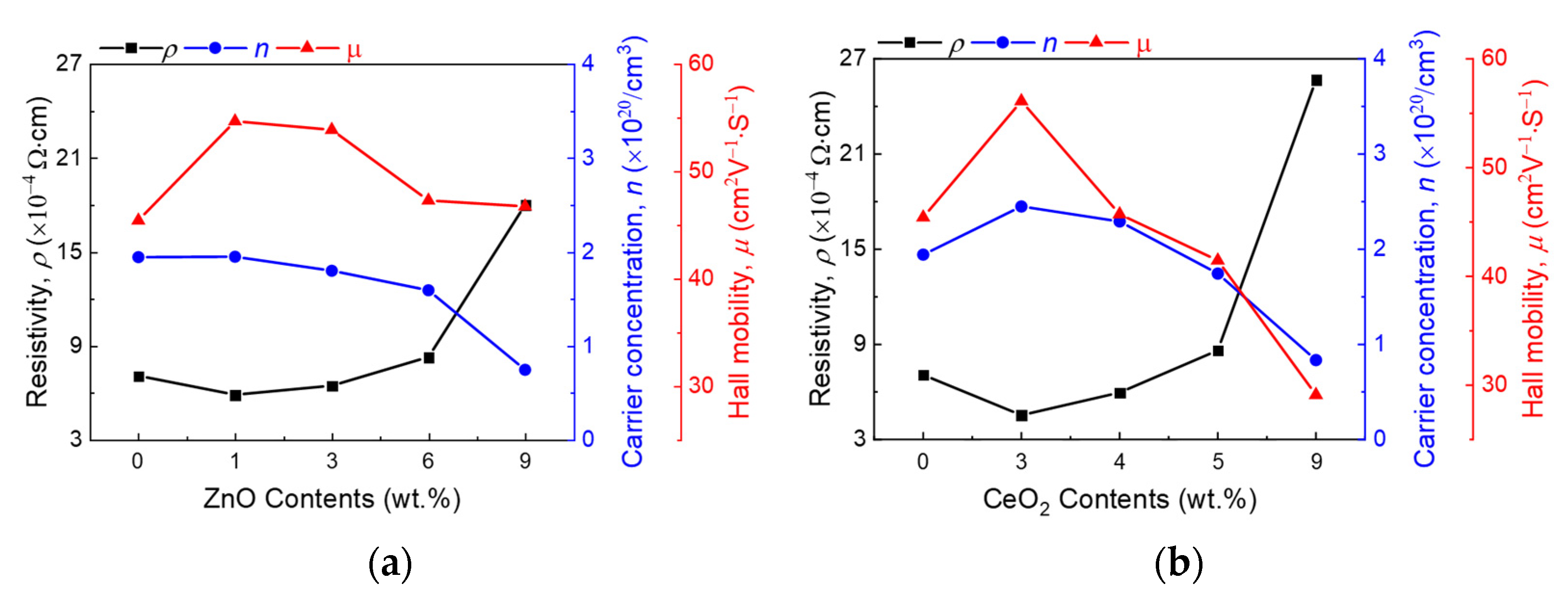
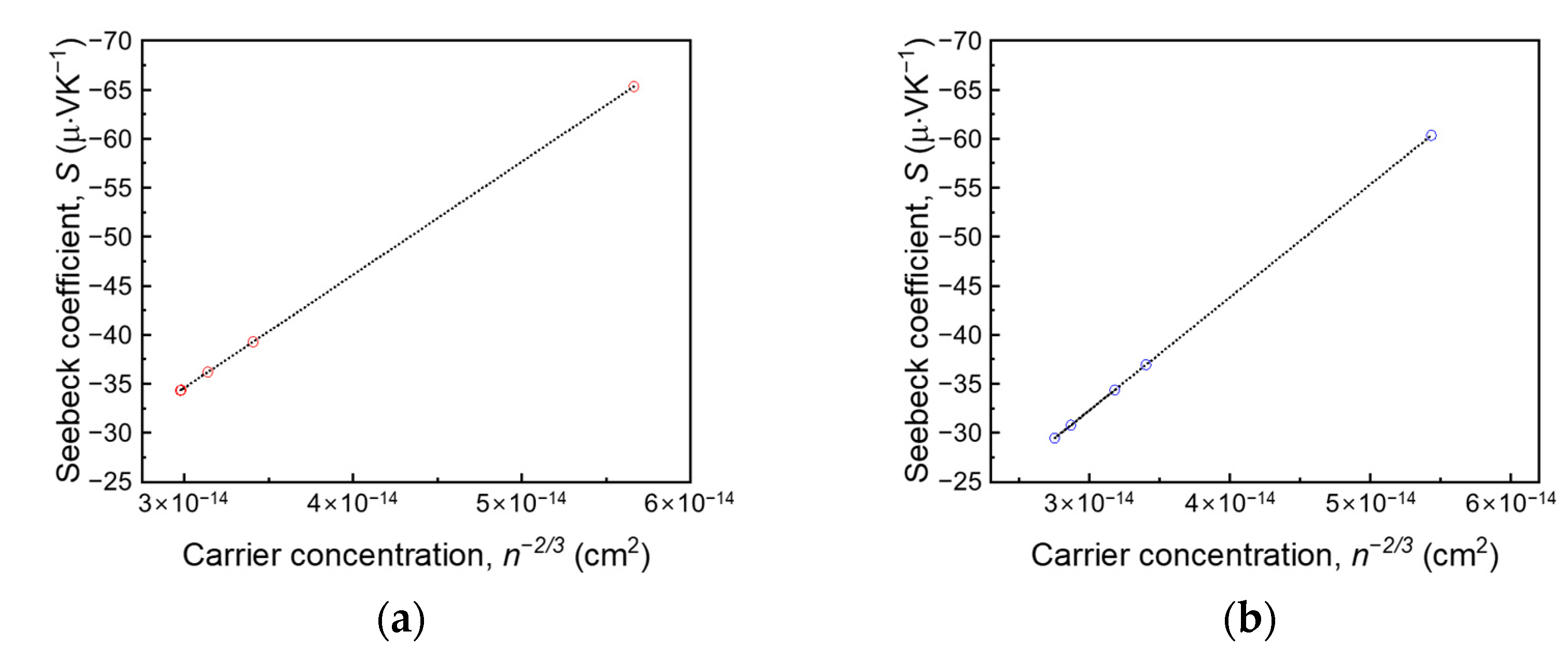
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mahmoudinezhad, S.; Atouei, S.A.; Cotfas, P.; Cotfas, D.; Rosendahl, L.A.; Rezania, A. Experimental and numerical study on the transient behavior of multi-junction solar cell-thermoelectric generator hybrid system. Energy Convers. Manag. 2019, 184, 448–455. [Google Scholar] [CrossRef]
- Park, K.-I.; Xu, S.; Liu, Y.; Hwang, G.-T.; Kang, S.-J.L.; Wang, Z.L.; Lee, K.J. Piezoelectric BaTiO3 thin film nanogenerator on plastic substrates. Nano Lett. 2010, 10, 4939–4943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Wang, A.C.; Ding, W.; Guo, H.; Wang, Z.L. Triboelectric nanogenerator: A foundation of the energy for the new era. Adv. Energy Mater. 2019, 9, 1802906. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, S.; Zhang, Z.; Yurchenko, D. High-performance piezoelectric wind energy harvester with Y-shaped attachments. Energy Convers. Manag. 2019, 181, 645–652. [Google Scholar] [CrossRef]
- Yarlagadda, V.; Carpenter, M.K.; Moylan, T.E.; Kukreja, R.S.; Koestner, R.; Gu, W.; Thompson, L.; Kongkanand, A. Boosting fuel cell performance with accessible carbon mesopores. ACS Energy Lett. 2018, 3, 618–621. [Google Scholar] [CrossRef] [Green Version]
- Juang, Z.-Y.; Tseng, C.-C.; Shi, Y.; Hsieh, W.-P.; Ryuzaki, S.; Saito, N.; Hsiung, C.-E.; Chang, W.-H.; Hernandez, Y.; Han, Y.; et al. Graphene-Au nanoparticle based vertical heterostructures: A novel route towards high-ZT Thermoelectric devices. Nano Energy 2017, 38, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Lan, M.; Li, G.; Piao, Y.; Ahmoum, H.; Wang, Q. Breaking the tradeoff among thermoelectric parameters by multi composite of porosity and CNT in AZO films. Energy 2021, 225, 120320. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.; Lan, M.; Zhu, M.; Mori, T.; Wang, Q. Improvement of Thermoelectric Properties of Evaporated ZnO:Al Films by CNT and Au Nanocomposites. J. Phys. Chem. C 2020, 124, 12713–12722. [Google Scholar] [CrossRef]
- Tambasov, I.A.; Voronin, A.S.; Evsevskaya, N.P.; Volochaev, M.N.; Fadeev, Y.V.; Simunin, M.M.; Aleksandrovsky, A.S.; Smolyarova, T.E.; Abelian, S.R.; Tambasova, E.V.; et al. Thermoelectric properties of low-cost transparent single wall carbon nanotube thin films obtained by vacuum filtration. Phys. E 2019, 114, 113619. [Google Scholar] [CrossRef]
- Tomeda, A.; Ishibe, T.; Taniguchi, T.; Okuhata, R.; Watanabe, K.; Nakamura, Y. Enhanced thermoelectric performance of Ga-doped ZnO film by controlling crystal quality for transparent thermoelectric films. Thin Solid Films 2018, 666, 185–190. [Google Scholar] [CrossRef]
- Yang, C.; Souchay, D.; Kneiß, M.; Bogner, M.; Wei, H.M.; Lorenz, M.; Oeckler, O.; Benstetter, G.; Fu, Y.Q.; Grundmann, M. Transparent flexible thermoelectric material based on non-toxic earth-abundant p-type copper iodide thin film. Nat. Commun. 2017, 8, 16076. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.E. Cooling, heating, generating power, and recovering waste heat with thermoelectric systems. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.-E.; Lee, S.-K.; Yoon, S.-G. Enhanced thermoelectric properties of thermal treated Sb2Te3 thin films. J. Alloys Compd. 2014, 583, 111–115. [Google Scholar] [CrossRef]
- Jeong, M.-W.; Na, S.; Shin, H.; Park, H.-B.; Lee, H.-J.; Joo, Y.-C. Thermomechanical in situ monitoring of Bi2Te3 thin film and its relationship with microstructure and thermoelectric performances. Electron. Mater. Lett. 2018, 14, 426–431. [Google Scholar] [CrossRef]
- Loureiro, J.; Neves, N.; Barros, R.; Mateus, T.; Santos, R.; Filonovich, S.; Reparaz, S.; Sotomayor-Torres, C.M.; Wyczisk, F.; Divay, L. Transparent aluminium zinc oxide thin films with enhanced thermoelectric properties. J. Mater. Chem. A 2014, 2, 6649–6655. [Google Scholar] [CrossRef]
- Ali, H. Characterization of a new transparent-conducting material of ZnO doped ITO thin films. Phys. Status Solidi A 2005, 202, 2742–2752. [Google Scholar] [CrossRef]
- Chung, S.M.; Shin, J.H.; Cheong, W.-S.; Hwang, C.-S.; Cho, K.I.; Kim, Y.J. Characteristics of Ti-doped ITO films grown by DC magnetron sputtering. Ceram. Int. 2012, 38, S617–S621. [Google Scholar] [CrossRef]
- Fallah, H.R.; Ghasemi, M.; Hassanzadeh, A. Influence of heat treatment on structural, electrical, impedance and optical properties of nanocrystalline ITO films grown on glass at room temperature prepared by electron beam evaporation. Phys. E 2007, 39, 69–74. [Google Scholar] [CrossRef]
- Hu, Y.; Diao, X.; Wang, C.; Hao, W.; Wang, T. Effects of heat treatment on properties of ITO films prepared by rf magnetron sputtering. Vacuum 2004, 75, 183–188. [Google Scholar] [CrossRef]
- Wu, C.; Wu, C.; Sturm, J.; Kahn, A. Surface modification of indium tin oxide by plasma treatment: An effective method to improve the efficiency, brightness, and reliability of organic light emitting devices. Appl. Phys. Lett. 1997, 70, 1348–1350. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-H.; Lee, S.-C.; Lin, T.-C.; Zhuang, W.-Y. Opto-electronic properties of titanium-doped indium–tin-oxide films deposited by RF magnetron sputtering at room temperature. Mat. Sci. Eng. B-Adv. 2006, 134, 68–75. [Google Scholar] [CrossRef]
- Kang, Y.; Kwon, S.; Choi, J.; Cho, Y.; Song, P. Properties of Ce-doped ITO films deposited on polymer substrate by DC magnetron sputtering. Thin Solid Films 2010, 518, 3081–3084. [Google Scholar] [CrossRef]
- Nomura, K.; Ohta, H.; Takagi, A.; Kamiya, T.; Hirano, M.; Hosono, H. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 2004, 432, 488–492. [Google Scholar] [CrossRef]
- Lee, H.Y.; Yang, I.J.; Yoon, J.-H.; Jin, S.-H.; Kim, S.; Song, P.K. Thermoelectric Properties of Zinc-Doped Indium Tin Oxide Thin Films Prepared Using the Magnetron Co-Sputtering Method. Coatings 2019, 9, 788. [Google Scholar] [CrossRef] [Green Version]
- Cocemasov, A.; Brinzari, V.; Jeong, D.-G.; Korotcenkov, G.; Vatavu, S.; Lee, J.-S.; Nika, D.L. Thermal transport evolution due to nanostructural transformations in Ga-doped indium-tin-oxide thin films. Nanomaterials 2021, 11, 1126. [Google Scholar] [CrossRef]
- Lan, J.-L.; Liu, Y.; Lin, Y.-H.; Nan, C.-W.; Cai, Q.; Yang, X. Enhanced thermoelectric performance of In2O3-based ceramics via Nanostructuring and Point Defect Engineering. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004; Volume 85. [Google Scholar]
- Yagi, T.; Tamano, K.; Sato, Y.; Taketoshi, N.; Baba, T.; Shigesato, Y. Analysis on thermal properties of tin doped indium oxide films by picosecond thermoreflectance measurement. J. Vac. Sci. Technol. A 2005, 23, 1180–1186. [Google Scholar] [CrossRef]
- Carreras, P.; Antony, A.; Roldán, R.; Nos, O.; Frigeri, P.A.; Asensi, J.M.; Bertomeu, J. Transparent conducting thin films by co-sputtering of ZnO-ITO targets. Phys. Status Solidi C 2010, 7, 953–956. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.-S.; Wu, C.-C.; Lee, C.-T. A transparent and conductive film prepared by RF magnetron cosputtering system at room temperature. Jpn. J. Appl. Phys. 2005, 44, 5119. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Ishibe, T.; Tomeda, A.; Komatsubara, Y.; Kitaura, R.; Uenuma, M.; Uraoka, Y.; Yamashita, Y.; Nakamura, Y. Carrier and phonon transport control by domain engineering for high-performance transparent thin film thermoelectric generator. Appl. Phys. Lett. 2021, 118, 151601. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.; Lan, M.; Zhu, M.; Miyazaki, K.; Wang, Q. Role of intrinsic defects on thermoelectric properties of ZnO:Al films. Ceram. Int. 2021, 47, 17760–17767. [Google Scholar] [CrossRef]
- Petit, T.; Puskar, L. FTIR spectroscopy of nanodiamonds: Methods and interpretation. Diam. Relat. Mater. 2018, 89, 52–66. [Google Scholar] [CrossRef]
- Winiarski, J.; Tylus, W.; Winiarska, K.; Szczygieł, I.; Szczygieł, B. XPS and FT-IR characterization of selected synthetic corrosion products of zinc expected in neutral environment containing chloride ions. J. Spectrosc. 2018, 2018, 2079278. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.C.; Shi, E.W.; Zheng, Y.Q.; Li, W.J. Effect of pH of medium on hydrothermal synthesis of nanocrystalline cerium (IV) oxide powders. J. Am. Ceram. Soc. 2002, 85, 2462–2468. [Google Scholar] [CrossRef]
- Zamiri, R.; Abbastabar Ahangar, H.; Kaushal, A.; Zakaria, A.; Zamiri, G.; Tobaldi, D.; Ferreira, J. Dielectrical properties of CeO2 nanoparticles at different temperatures. PLoS ONE 2015, 10, e0122989. [Google Scholar]
- Petrov, T.; Markova-Deneva, I.; Chauvet, O.; Nikolov, R.; Denev, I. Sem and Ft-Ir Spectroscopy Study of Cu, Sn AND Cu-Sn Nanoparticles. J. Chem. Technol. Metall. 2012, 47, 2. [Google Scholar]
- Zhu, G.; Guo, L.; Shen, X.; Ji, Z.; Chen, K.; Zhou, H. Monodispersed In2O3 mesoporous nanospheres: One-step facile synthesis and the improved gas-sensing performance. Sens. Actuators B Chem. 2015, 220, 977–985. [Google Scholar] [CrossRef]
- Chang, K.-C.; Tsai, T.-M.; Chang, T.-C.; Zhang, R.; Chen, K.-H.; Chen, J.-H.; Chen, M.-C.; Huang, H.-C.; Zhang, W.; Lin, C.-Y. Improvement of resistive switching characteristic in silicon oxide-based RRAM through hydride-oxidation on indium tin oxide electrode by supercritical CO2 fluid. IEEE Electron. Device Lett. 2015, 36, 558–560. [Google Scholar] [CrossRef]
- Raja, K.; Ramesh, P.; Geetha, D. Structural, FTIR and photoluminescence studies of Fe doped ZnO nanopowder by co-precipitation method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 131, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ansari, A.A.; Kaushik, A.; Solanki, P.R.; Barik, A.; Pandey, M.; Malhotra, B. Nanostructured zinc oxide film for urea sensor. Mater. Lett. 2009, 63, 2473–2475. [Google Scholar] [CrossRef]
- Liu, W.; Shi, X.; Hong, M.; Yang, L.; Moshwan, R.; Chen, Z.-G.; Zou, J. Ag doping induced abnormal lattice thermal conductivity in Cu2Se. J. Mater. Chem. C 2018, 6, 13225–13231. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.J.; Lee, H.Y.; Kim, S.; Song, P.K. Controlled Lattice Thermal Conductivity of Transparent Conductive Oxide Thin Film via Localized Vibration of Doping Atoms. Nanomaterials 2021, 11, 2363. https://doi.org/10.3390/nano11092363
Choi YJ, Lee HY, Kim S, Song PK. Controlled Lattice Thermal Conductivity of Transparent Conductive Oxide Thin Film via Localized Vibration of Doping Atoms. Nanomaterials. 2021; 11(9):2363. https://doi.org/10.3390/nano11092363
Chicago/Turabian StyleChoi, Young Joong, Ho Yun Lee, Seohan Kim, and Pung Keun Song. 2021. "Controlled Lattice Thermal Conductivity of Transparent Conductive Oxide Thin Film via Localized Vibration of Doping Atoms" Nanomaterials 11, no. 9: 2363. https://doi.org/10.3390/nano11092363
APA StyleChoi, Y. J., Lee, H. Y., Kim, S., & Song, P. K. (2021). Controlled Lattice Thermal Conductivity of Transparent Conductive Oxide Thin Film via Localized Vibration of Doping Atoms. Nanomaterials, 11(9), 2363. https://doi.org/10.3390/nano11092363