Preparation of a ZnO Nanostructure as the Anode Material Using RF Magnetron Sputtering System
Abstract
:1. Introduction
2. Experiment Details
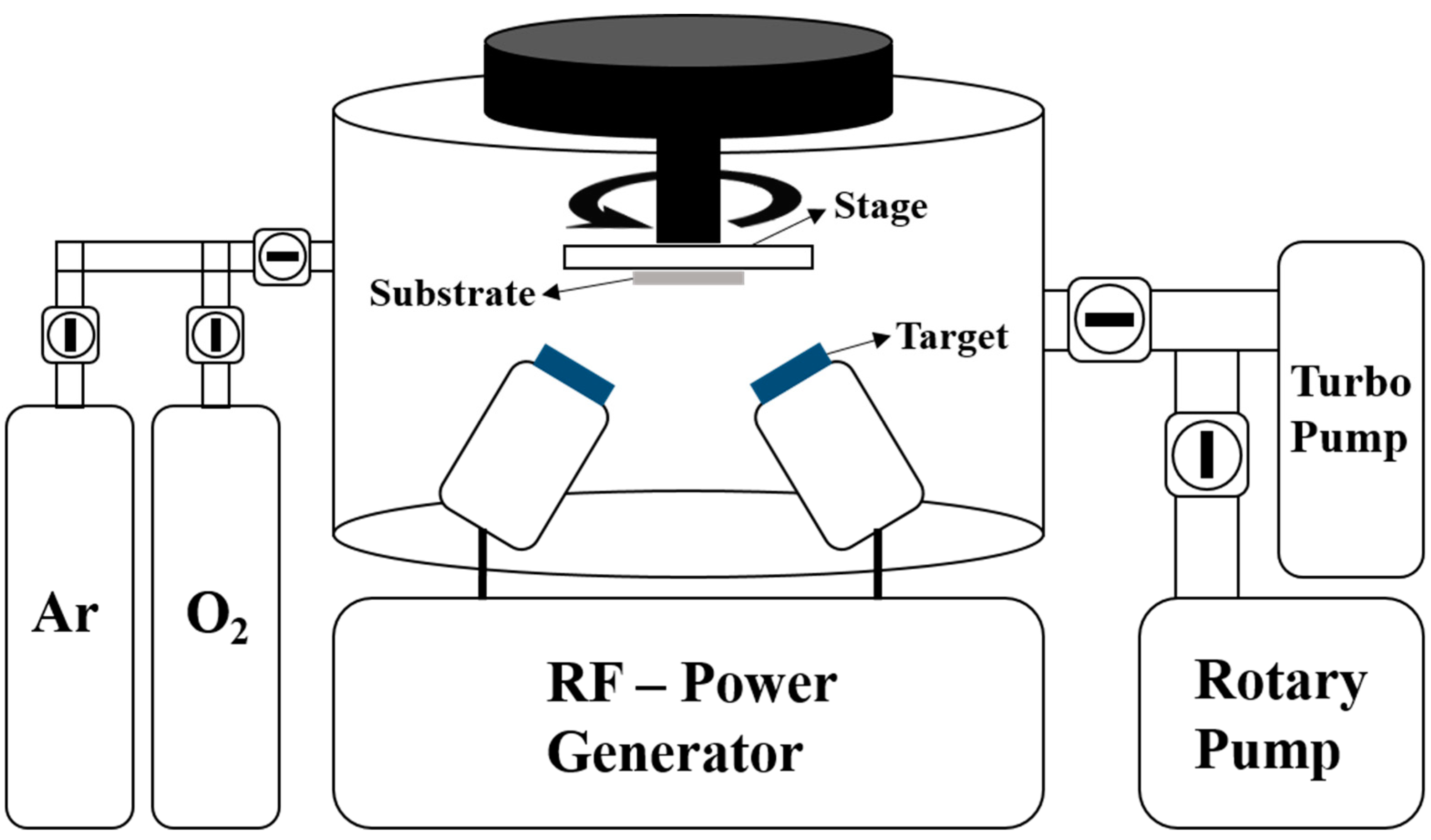
2.1. Preparation of Substrates and Copper (Cu) Layer Deposition
2.2. ZnO Film Deposition
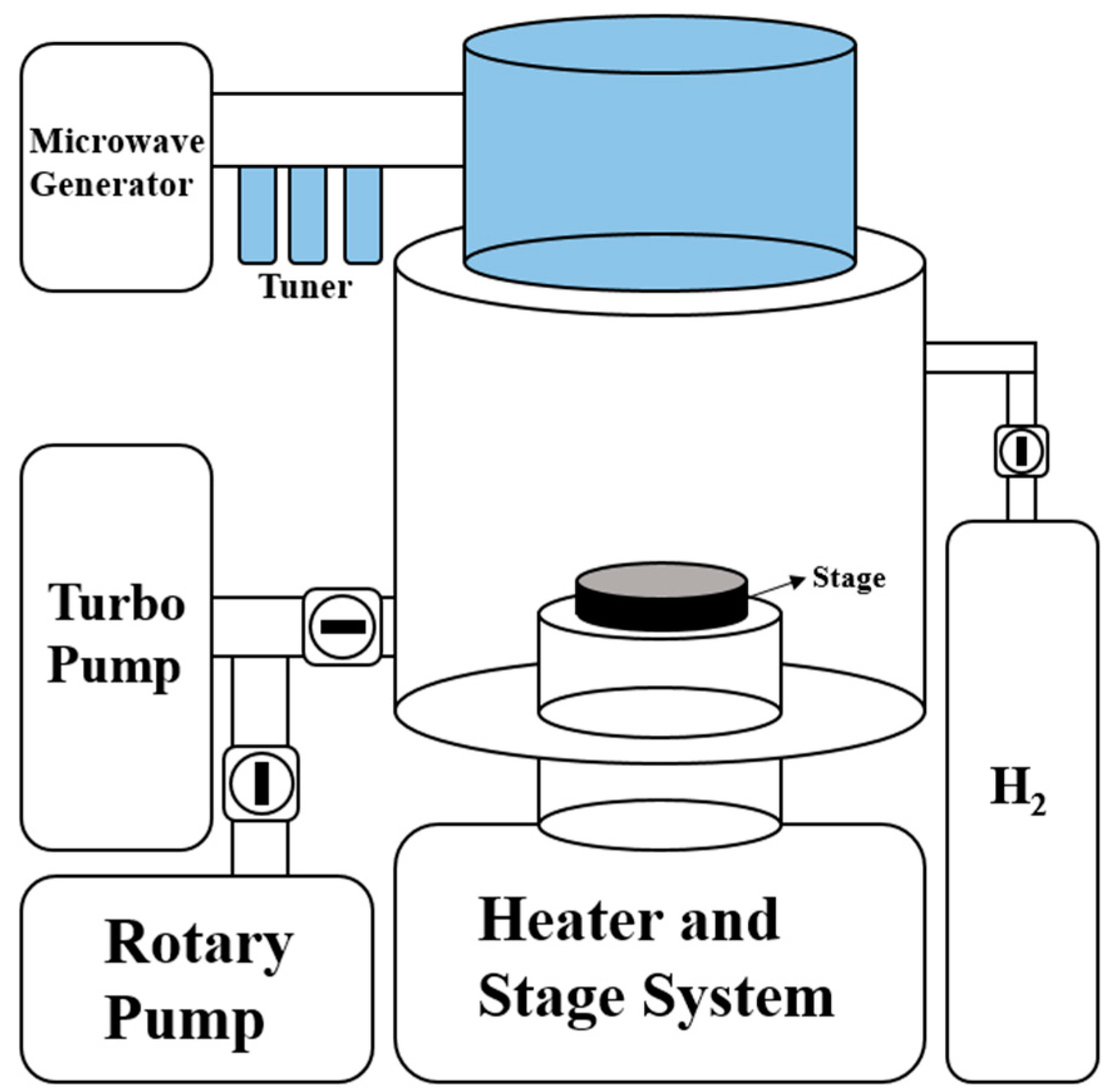
2.3. H2 Reduction Process
2.4. Analysis
3. Results and Discussion
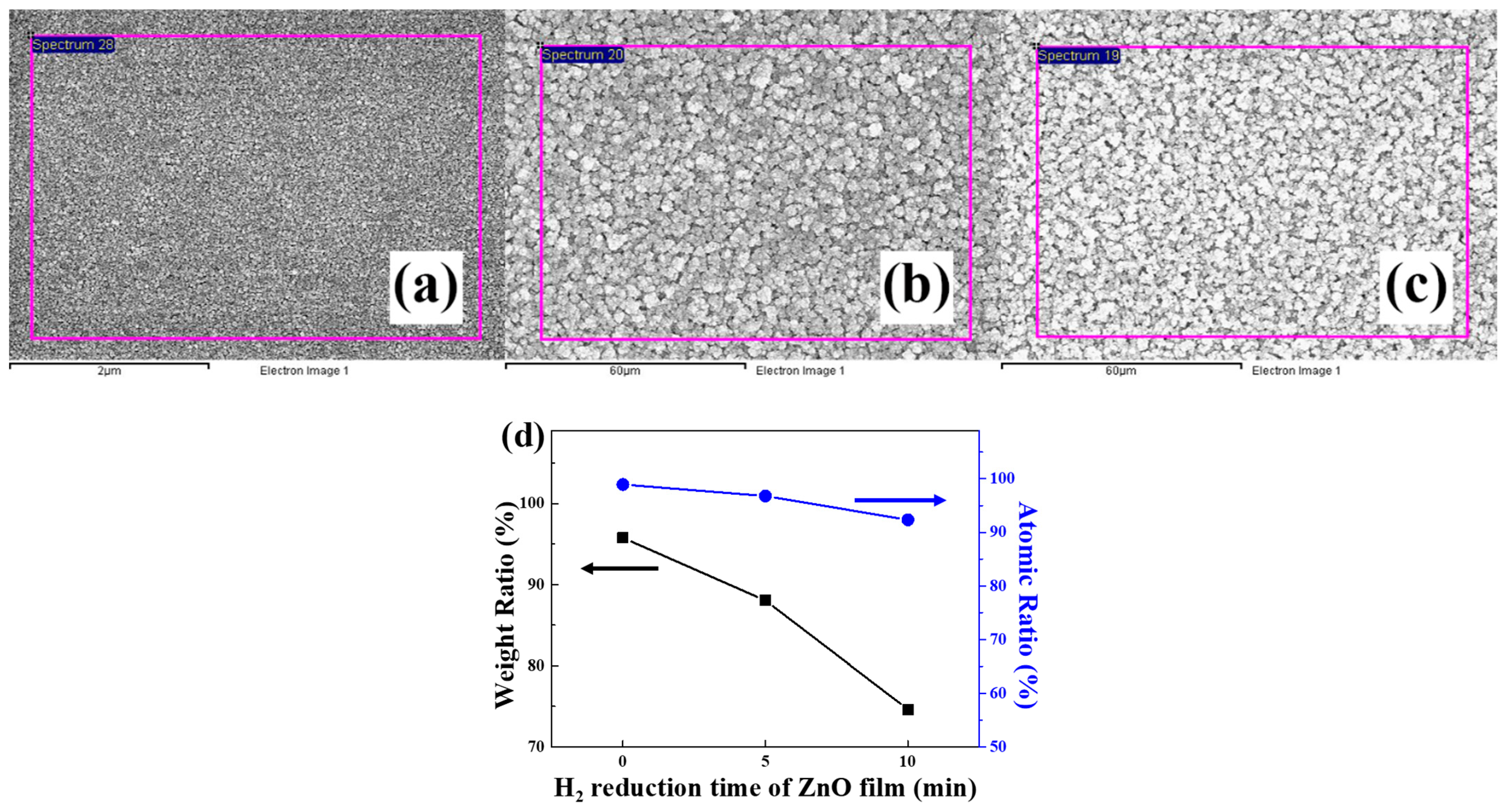
3.1. Field Emission Scanning Electron Microscopy (FESEM)
3.2. Energy Dispersive Spectrometry (EDS)
3.3. Hall Measurement
3.4. Atomic Force Microscopy (AFM)
3.5. Cyclic Voltammetry (CV)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, L.; Li, E.; Yi, J.; Meng, W.; Wang, S.; Deng, B.; Liu, J. Enhancing the performance of nanostructured ZnO as an anode material for lithium-ion batteries by polydopamine-derived carbon coating and confined crystallization. J. Alloys Compd. 2018, 764, 545. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Wang, Y.; Li, H.; Peng, Y. The application of nanostructured transition metal sulfides as anodes for lithium ion batteries. J. Energy Chem. 2018, 27, 1536. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Jaffer, S.; Yu, H. Transition metal oxides for sodium-ion batteries. Energy Storage Mater. 2016, 5, 116. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, B.; Yan, C.; Kang, D. Decorating ZnO nanoflakes on carbon cloth: Free-standing, highly stable lithium-ion battery anodes. Ceram. Int. 2019, 45, 15906. [Google Scholar] [CrossRef]
- Wei, J.S.; Zhu, Z.Y.; Zhao, X.; Song, T.B.; Huang, J.H.; Zhang, Y.X.; Chen, L.; Niu, X.Q.; Wang, Y.G.; Xiong, H.M. Self-assembled ZnO-carbon dots anode materials for high performance nickel-zinc alkaline batteries. Chem. Eng. J. 2021, 425, 130660. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Wang, Z.; Zhao, W.; Qin, C.; Yu, H.; Wang, X.; Bakenov, Z.; Zhang, Y. Defective ZnOx@porous carbon nanofiber network inducing dendrite-free zinc plating as zinc metal anode for high-performance aqueous rechargeable Zn/Na4Mn9O18 battery based on hybrid electrolyte. J. Power Sources 2022, 518, 230761. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Pham, T.N.; Hur, J.; Lee, Y.C. Review of ZnO Binary and Ternary Composite Anodes for Lithium-Ion Batteries. Nanomaterials 2021, 11, 2001. [Google Scholar] [CrossRef]
- Deckenbach, D.; Schmeider, J.J. A 3D hierarchically porous nanoscale ZnO anode for high-energy rechargeable zinc-air batteries. J. Power Sources 2021, 488, 229393. [Google Scholar] [CrossRef]
- Joshi, N.; Silva, L.; Shimizu, F.M.; Mastelaro, V.R.; M’Pekoo, J.C.; Lin, L.; Oliveira, O.N., Jr. UV-assisted chemiresistors made with gold modified ZnO nanorods to detect ozone gas at room temperature. Microchim. Acta 2019, 186, 418. [Google Scholar] [CrossRef]
- Yudha, C.S.; Hutama, A.P.; Rahmawati, M.; Widiyandari, H.; Nursukatmo, H.; Nilasary, H.; Oktaviano, H.S.; Purwanto, A. Synthesis and Characterization of ZnO from Thermal Decomposition of Precipitated Zinc Oxalate Dihydrate as an Anode Material of Li-Ion Batteries. Energies 2021, 14, 5980. [Google Scholar] [CrossRef]
- Shah, D.K.; KC, D.; Akhtar, M.S.; Kim, C.Y.; Yang, O.B. Vertically Arranged Zinc Oxid-e Nanorods as Antireflection Layer for Crystalline Silicon Solar Cell: A Simulation Study of Photovoltaic Properties. Appl. Sci. 2020, 10, 6062. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, B.; Shen, Y. Blue Luminescent ZnO Nanoclusters Stabilized by Esterifiable Polyamidoamine Dendrimers and their UV-Shielding Applications. Asian J. Chem. 2014, 26. [Google Scholar] [CrossRef]
- Han, Z.; Li, Y.; Chen, F.; Tang, S.; Wang, P. Preparation of ZnO/Ag2O Nano-fibers by Coaxial Electrospinning and Study of Their Photocatalytic Properties. Chem. J. Chin. Univ.-Chin. 2020, 41, 308–316. [Google Scholar]
- Han, Z.Y.; Li, Y.J.; Lin, X.; Wang, Z.Y.; Li, Z.Q.; Wang, H. Preparation and photoelectrocatalytic performance of Fe2O3/ZnO composite electrode loading on conductive glass. Chem. Res. Chin. Univ. 2018, 39, 771–778. [Google Scholar]
- Chen, S.; Zhou, S.; Fu, J.; Tang, S.; Wu, X.; Zhao, P.; Zhang, Z. A near infrared fluorescence imprinted sensor based on zinc oxide nanorods for rapid determination of ketoprofen. Anal. Methods 2021, 13, 2836–2846. [Google Scholar] [CrossRef] [PubMed]
- Bhati, V.; Ranwa, S.; Fanetti, M.; Valant, M.; Kumar, M. Efficient hydrogen sensor based on Ni-doped ZnO nanostructures by RF sputtering. Sens. Actuators B Chem. 2018, 255, 588. [Google Scholar] [CrossRef]
- Joshi, K.; Rawat, M.; Gautam, S.; Singh Ramola, R.; Singh, F. Band gap widening and narrowing in Cu-doped ZnO thin films. J. Alloys Compd. 2018, 680, 252–258. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, X.; Chen, C.; Hao, Y.; Agrawal, R.; Wang, C.; Li, W.; Yu, H.; Yu, Y.; Zhu, X.; et al. Facile fabrication of three-dimensional porous ZnO thin films on Ni foams for lithium ion battery anodes. Mater. Lett. 2017, 190, 37. [Google Scholar] [CrossRef] [Green Version]
- Quartarone, E.; Dall’Asta, V.; Resmini, A.; Tealdi, C.; Tredici, I.G.; Tamburini, U.A.; Mustarelli, P. Graphite-coated ZnO nanosheets as high-capacity, highly stable, and binder-free anodes for lithium-ion batteries. J. Power Sources 2016, 320, 314. [Google Scholar] [CrossRef]
- Wang, D.; Guo, J.; Cui, C.; Ma, J.; Cao, A. Controllable synthesis of CNT@ZnO composites with enhanced electrochemical properties for lithium-ion battery. Mater. Res. Bull. 2018, 101, 305. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Wang, X.; Liu, W.; Yu, K.; Liang, C. Simple synthesis of rice husk hollow carbon-coated flower ZnO for the anode in a high performance lithium-ion battery. J. Phys. Chem. Solids 2020, 145, 109540. [Google Scholar] [CrossRef]
- Huang, X.; Xia, X.; Yuan, Y.; Zhou, F. Porous ZnO nanosheets grown on copper substrates as anodes for lithium ion batteries. Port. Electrochim. Acta 2011, 56, 4960. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Y.; Li, H.; Zhao, Y.; Yin, F.; Wang, X. Simple fabrication of free-standing ZnO/graphene/carbon nanotube composite anode for lithium-ion batteries. Mater. Lett. 2016, 184, 235. [Google Scholar] [CrossRef]
- Yun, Y.H. Characterization of Ga-doped ZnO thin films prepared by RF magnetron sputtering method. J. Korean Cryst. Growth Cryst. Technol. 2021, 31, 73–77. [Google Scholar]
- Westwood, W.D. Magnetrons. In Sputter Deposition; AVS: New York, NY, USA, 2003; pp. 43–74. [Google Scholar]




| Substrate | Target | RF Power (W) | Base Pressure (Torr) | Working Pressure (Torr) | Deposition Time (min) | Temperature °C |
|---|---|---|---|---|---|---|
| Si (100) | Cu | 300 | 15 | 250 |
| Substrate | Target | RF Power (W) | Base Pressure | Working Pressure | Deposition Time (min) | Temperature °C |
|---|---|---|---|---|---|---|
| Si wafer/Cu layer | ZnO | 150 | 20 | 250 |
| Samples | Substrate | H2 (sccm) | Base Pressure | Temperature °C | Process Time (min) |
|---|---|---|---|---|---|
| Sample 1 | Si wafer/Cu layer/ZnO film | 100 | 600 | 5 | |
| Sample 2 | Si wafer/Cu layer/ZnO film | 100 | 600 | 10 |
| Samples | Zn Weight (%) | O2 Weight (%) | Zn Atomic (%) | O2 Atomic (%) |
|---|---|---|---|---|
| Cu-ZnO film | 4.24 | 95.76 | 1.07 | 98.93 |
| ZnO NS (5 min) | 11.97 | 88.03 | 3.22 | 96.78 |
| ZnO NS (10 min) | 25.37 | 74.63 | 7.68 | 92.32 |
| Samples | Ra | Rq | Skewness (Rsk) | Kurtosis (Rku) |
|---|---|---|---|---|
| ZnO NS (5 min) | 3.117 | 3.970 | −0.373 | 4 |
| ZnO NS (10 min) | 3.418 | 4.427 | −0.612 | 4.721 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Joung, Y.-H.; Yoon, Y.-K.; Choi, W. Preparation of a ZnO Nanostructure as the Anode Material Using RF Magnetron Sputtering System. Nanomaterials 2022, 12, 215. https://doi.org/10.3390/nano12020215
Lee S, Joung Y-H, Yoon Y-K, Choi W. Preparation of a ZnO Nanostructure as the Anode Material Using RF Magnetron Sputtering System. Nanomaterials. 2022; 12(2):215. https://doi.org/10.3390/nano12020215
Chicago/Turabian StyleLee, Seokwon, Yeon-Ho Joung, Yong-Kyu Yoon, and Wonseok Choi. 2022. "Preparation of a ZnO Nanostructure as the Anode Material Using RF Magnetron Sputtering System" Nanomaterials 12, no. 2: 215. https://doi.org/10.3390/nano12020215
APA StyleLee, S., Joung, Y.-H., Yoon, Y.-K., & Choi, W. (2022). Preparation of a ZnO Nanostructure as the Anode Material Using RF Magnetron Sputtering System. Nanomaterials, 12(2), 215. https://doi.org/10.3390/nano12020215







