Diamond-Based Electrodes for Detection of Metal Ions and Anions
Abstract
:1. Introduction
2. Diamond-Based Electrodes in Metal Ions Detection
3. Diamond-Based Electrodes in Anions Discrimination
4. Optimization Requirements
- 1)
- Generally speaking, synthesis of diamond-based nanomaterials is mostly carried out by CVD techniques [126], which require careful optimization in operating temperatures and chamber pressures to deliver the appropriate nanostructures and morphology.
- 2)
- 3)
- In the fabrication of specific atom- or nanoparticle-doped electrodes (for example, BDD, AuNPs–BDD, Ir-BDD, and Pt-implanted BDD), cautious optimization with doping concentrations is essential to attain reproducible results [130].
- 4)
- When the diamond-based electrodes, such as BDD electrodes, are used for detection, it is important to select the suitable reference (example: Ag/AgCl or SCE) and counter electrodes (example: Pt, graphite, GCE, etc.). Therefore, great attention is required in selection of the reference and counter electrodes [131].
- 5)
- 6)
- To achieve repeatable results to specific analytes, the supporting electrolytes play a crucial role in enhancing the redox process that leads to the electrochemical signals [134]. Thus, optimization is required in determining the suitable supporting electrolyte to enhance the redox reaction.
- 7)
- 8)
- Finally, optimization in maintaining highly reliable and reproducible electrochemical data signals (such as CV, ASV, DPV, LSV, DPASV, and SWASV), scan range, scan rate, etc., under the aforementioned conditions is mandatory.
5. Advantages
- 1)
- Due to their unique structural and electrochemical resistivity features, diamond-based electrodes can be operated effectively towards specific metal ions or anions detections in suitable aqueous media and under operable conditions.
- 2)
- By tuning the B-atom doping concentration in the BDD electrode, it can be used effectively in detection and quantification of diverse metal analytes with specificity.
- 3)
- Modifications of the BDD electrodes by foreign materials or metallic nanoparticles can lead to different structural and morphological features, which allow the accumulation of specific metal ions and anions and lead to electrochemical responses via redox reaction.
- 4)
- By inclusion of the graphitic or nanographitic shells in the diamond-based electrodes, the conductivity can be enhanced by tuning the B/C and sp2/sp3 ratios; therefore, highly responsive signals can be obtained.
- 5)
- 6)
- In terms of the lowest LODs for toxic heavy metal ions and anions, the diamond-based electrodes open up a new path to quantify those species with specificity.
- 7)
- Recent reports on diamond-based electrodes demonstrate the exclusive features for simultaneous detection of multiple metal ions and anions, which are highly beneficial.
6. Limitations
- 1)
- Analyte quantitation by the diamond-based electrodes is limited to the availability of sophisticated electrochemical instruments and laboratory environment.
- 2)
- Synthesis of diamond nanostructures requires CVD tactics operated at high temperatures. Moreover, the electrode fabrication with certain film thickness is limited by many complicated procedures.
- 3)
- Morphological investigations on the diamond-based electrodes require microscopic investigations, thereby limiting by the use of costly equipment, such as SEM, TEM, AFM, etc.
- 4)
- Reproducibility of electrochemical signals to specific analytes is limited by the prescribed reference and counter electrodes, supporting electrolyte, pH value, and temperature conditions. Changes in the measurement conditions may result in loss of data.
- 5)
- The electrochemical results obtained from the diamond-based electrodes could possibly be affected due to changes in electrode surface and loss of signal by some highly concentrated unknown interferences existing in diverse real samples.
- 6)
- Use of hazardous acidic electrolytes, such as HCl, H2SO4, buffer solutions, and toxic Hg/Hg2Cl2 electrode, in the detection process is harmful to the environment, thereby limiting the real-time electrochemical interrogations.
- 7)
- Though the diamond-based electrodes are able to detect metal ions and anions down to picomolar level, their biological applicability still needs to be validated in many cases.
7. Conclusions and Perspectives
- 1)
- The diamond/BDD electrodes are typically fabricated by high-temperature CVD techniques. Thus, alternative wet-chemical routes must be established for easy synthesis and cost-effective commercialization.
- 2)
- Though reports on the diamond/BDD-based electrodes for detecting the metal ions and anions are impressive, currently there is no “state-of the art” procedure for commercialization.
- 3)
- Some reports did not provide information on the B-doping concentration, particle size modified over the electrode surface, and the effectiveness of the doping and modification on electrochemical interrogations. This should be rectified in upcoming research reports.
- 4)
- Similar to the case of implantation and metallic nanoparticles modifications, the diamond/BDD electrodes can be modified by certain emerging materials, such as MOFs and COFs, to further tune the detection signals towards specific metal ions and anions. However, this requires careful optimization procedure.
- 5)
- A few reports on the metal ions detection did not provide proper explanations regarding the redox process, suitable electrolytes, sensitivity, role of pH, temperature, and information on limitations. The missing information still requires further clarification.
- 6)
- The use of toxic SCE electrodes, highly concentrated acidic electrolytes, and buffer solution must be reduced to become truly environmentally friendly.
- 7)
- Demonstrations of the diamond/BDD-based electrodes towards quantification of Co2+, Mn2+, Pd2+, Ru3+, Al3+, Ga3+, In3+, etc., are not yet available, which should be addressed by upcoming researchers.
- 8)
- Demonstrations of the diamond/BDD-based electrodes towards electrochemical detection of lanthanides and actinides must be launched to increase the social impact.
- 9)
- Some reports on the diamond/BDD-based electrodes towards anionic species detection did not have any clear information on the electron transport or generation information, which require more evidence to understand the underlying mechanisms.
- 10)
- Reports on the diamond/BDD-based electrodes for anions detection are still insufficient, thereby requiring more research work toward this direction.
- 11)
- Detection of anions, such as Br−, CN−, ClO4−, H2PO4−, S2O32−, CO32−, PO43−, P2O74−, etc., by the diamond/BDD-based electrodes has not yet been reported, which should be the focus in future.
Author Contributions
Funding
Conflicts of Interest
References
- Rathi, B.S.; Kumar, P.S.; Vo, D.-V.N. Critical Review on Hazardous Pollutants in Water Environment: Occurrence, Monitoring, Fate, Removal Technologies and Risk Assessment. Sci. Total Environ. 2021, 797, 149134. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Inorganic-Diverse Nanostructured Materials for Volatile Organic Compound Sensing. Sensors 2021, 21, 633. [Google Scholar] [CrossRef]
- Suhito, I.R.; Koo, K.-M.; Kim, T.-H. Recent Advances in Electrochemical Sensors for the Detection of Biomolecules and Whole Cells. Biomedicines 2021, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Koduru, J.R.; Desai, M.L.; Park, T.J.; Singhal, R.K.; Basu, H. Recent Progress on Surface Chemistry of Plasmonic Metal Nanoparticles for Colorimetric Assay of Drugs in Pharmaceutical and Biological Samples. TrAC Trends Anal. Chem. 2018, 105, 106–120. [Google Scholar] [CrossRef]
- Du, H.; Xie, Y.; Wang, J. Nanomaterial-Sensors for Herbicides Detection Using Electrochemical Techniques and Prospect Applications. TrAC Trends Anal. Chem. 2021, 135, 116178. [Google Scholar] [CrossRef]
- Ahmed, M.; Faisal, M.; Ihsan, A.; Naseer, M.M. Fluorescent Organic Nanoparticles (FONs) as Convenient Probes for Metal Ion Detection in Aqueous Medium. Analyst 2019, 144, 2480–2497. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Caltagirone, C. Fluorescent and colorimetric sensors for anionic species. Coord. Chem. Rev. 2018, 354, 2–27. [Google Scholar] [CrossRef]
- Liu, X.; Huang, D.; Lai, C.; Zeng, G.; Qin, L.; Wang, H.; Yi, H.; Li, B.; Liu, S.; Zhang, M.; et al. Recent Advances in Covalent Organic Frameworks (COFs) as A Smart Sensing Material. Chem. Soc. Rev. 2019, 48, 5266–5302. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.-W. Progress in Metal-Organic Frameworks Facilitated Mercury Detection and Removal. Chemosensors 2021, 9, 101. [Google Scholar] [CrossRef]
- Zeiri, O. Metallic-Nanoparticle-Based Sensing: Utilization of Mixed-Ligand Monolayers. ACS Sens. 2020, 5, 3806–3820. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Review on Sensing Applications of Perovskite Nanomaterials. Chemosensors 2020, 8, 55. [Google Scholar] [CrossRef]
- Shellaiah, M.; Chen, Y.-T.; Thirumalaivasan, N.; Aazaad, B.; Awasthi, K.; Sun, K.W.; Wu, S.-P.; Lin, M.-C.; Ohta, N. Pyrene-Based AIEE Active Nanoprobe for Zn2+ and Tyrosine Detection Demonstrated by DFT, Bioimaging, and Organic Thin-Film Transistor. ACS Appl. Mater. Interfaces 2021, 13, 28610–28626. [Google Scholar] [CrossRef] [PubMed]
- Cichosz, S.; Masek, A.; Zaborski, M. Polymer-Based Sensors: A Review. Polym. Test. 2018, 67, 342–348. [Google Scholar] [CrossRef]
- Torregrosa, D.; Grindlay, G.; Gras, L.; Mora, J. Immunoassays Based on Inductively Coupled Plasma Mass Spectrometry Detection: So Far So Good, So What? Microchem. J. 2021, 166, 106200. [Google Scholar] [CrossRef]
- Olsen, B.A.; Castle, B.C.; Myers, D.P. Advances in HPLC Technology for the Determination of Drug Impurities. TrAC Trends Anal. Chem. 2006, 25, 796–805. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent Progress in Food Flavor Analysis Using Gas Chromatography–Ion Mobility Spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.H.; Pisonero, J.; Smith, C.M.M.; Taylor, R.N. Atomic Spectrometry Update: Review of Advances in Atomic Spectrometry and Related Techniques. J. Anal. At. Spectrom. 2020, 35, 830–851. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Niu, Q.; Gu, X.; Yang, N.; Zhao, G. Recent Progress on Carbon Nanomaterials for the Electrochemical Detection and Removal of Environmental Pollutants. Nanoscale 2019, 11, 11992–12014. [Google Scholar] [CrossRef] [PubMed]
- Manciu, F.S.; Oh, Y.; Barath, A.; Rusheen, A.E.; Kouzani, A.Z.; Hodges, D.; Guerrero, J.; Tomshine, J.; Lee, K.H.; Bennet, K.E. Analysis of Carbon-Based Microelectrodes for Neurochemical Sensing. Materials 2019, 12, 3186. [Google Scholar] [CrossRef] [Green Version]
- Di Nardo, F.; Chiarello, M.; Cavalera, S.; Baggiani, C.; Anfossi, L. Ten Years of Lateral Flow Immunoassay Technique Applications: Trends, Challenges and Future Perspectives. Sensors 2021, 21, 5185. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yu, R.; Yan, Y.; Zeng, H.; Luo, S.; Liu, N.; Morrin, A.; Luo, X.; Li, W. A Review of Ratiometric Electrochemical Sensors: From Design Schemes to Future Prospects. Sens. Actuators B 2018, 274, 501–516. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karimi, F.; Alizadeh, M.; Sanati, A.L. Electrochemical Sensors, a Bright Future in the Fabrication of Portable Kits in Analytical Systems. Chem. Rec. 2020, 20, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Jadon, N.; Jain, R.; Sharma, S.; Singh, K. Recent Trends in Electrochemical Sensors for Multianalyte Detection—A Review. Talanta 2016, 161, 894–916. [Google Scholar] [CrossRef] [PubMed]
- Muzyka, K.; Sun, J.; Fereja, T.H.; Lan, Y.; Zhang, W.; Xu, G. Boron-Doped Diamond: Current Progress and Challenges in View of Electroanalytical Applications. Anal. Methods 2019, 11, 397–414. [Google Scholar] [CrossRef]
- Boumya, W.; Taoufik, N.; Achak, M.; Barka, N. Chemically Modified Carbon-Based Electrodes for the Determination of Paracetamol in Drugs and Biological Samples. J. Pharmaceut. Anal. 2021, 11, 138–154. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-Based Composite Materials for Supercapacitor Electrodes: A Review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Sarakhman, O.; Švorc, Ľ. A Review on Recent Advances in the Applications of Boron-Doped Diamond Electrochemical Sensors in Food Analysis. Crit. Rev. Anal. Chem. 2020, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Yence, M.; Cetinkaya, A.; Ozcelikay, G.; Kaya, S.I.; Ozkan, S.A. Boron-Doped Diamond Electrodes: Recent Developments and Advances in View of Electrochemical Drug Sensors. Crit. Rev. Anal. Chem. 2021, 1–17. [Google Scholar] [CrossRef]
- Németh, P.; McColl, K.; Garvie, L.A.J.; Salzmann, C.G.; Murri, M.; McMillan, P.F. Complex Nanostructures in Diamond. Nat. Mater. 2020, 19, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Foord, J.S.; Jiang, X. Diamond Electrochemistry at the Nanoscale: A Review. Carbon 2016, 99, 90–110. [Google Scholar] [CrossRef]
- Huang, H.-J.; Seenithurai, S.; Chai, J.-D. TAO-DFT Study on the Electronic Properties of Diamond-Shaped Graphene Nanoflakes. Nanomaterials 2020, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Tian, W.; Zhang, H.; Sun, H.; Ao, Z.; Shao, Z.; Wang, S. sp2/sp3 Framework from Diamond Nanocrystals: A Key Bridge of Carbonaceous Structure to Carbocatalysis. ACS Catal. 2019, 9, 7494–7519. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Diamond Nanowire Synthesis, Properties and Applications. In Nanowires-Synthesis, Properties and Applications; Rackauskas, S., Ed.; IntechOpen (Ch2): London, UK, 2019; pp. 19–37. [Google Scholar]
- Uthappa, U.T.; Arvind, O.R.; Sriram, G.; Losic, D.; Ho Young, J.; Kigga, M.; Kurkuri, M.D. Nanodiamonds and Their Surface Modification Strategies for Drug Delivery Applications. J. Drug Del. Sci. Technol. 2020, 60, 101993. [Google Scholar] [CrossRef]
- Chauhan, S.; Jain, N.; Nagaich, U. Nanodiamonds with Powerful Ability for Drug Delivery and Biomedical Applications: Recent Updates on In Vivo Study and Patents. J. Pharmaceut. Anal. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Torelli, M.D.; Nunn, N.A.; Shenderova, O.A. A Perspective on Fluorescent Nanodiamond Bioimaging. Small 2019, 15, 1902151. [Google Scholar] [CrossRef] [PubMed]
- Morales-Zavala, F.; Casanova-Morales, N.; Gonzalez, R.B.; Chandía-Cristi, A.; Estrada, L.D.; Alvizú, I.; Waselowski, V.; Guzman, F.; Guerrero, S.; Oyarzún-Olave, M.; et al. Functionalization of Stable Fluorescent Nanodiamonds Towards Reliable Detection of Biomarkers for Alzheimer’s Disease. J. Nanobiotechnol. 2018, 16, 60. [Google Scholar] [CrossRef] [Green Version]
- Shellaiah, M.; Simon, T.; Venkatesan, P.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Cysteamine-Modified Diamond Nanoparticles Applied in Cellular Imaging and Hg2+ Ions Detection. Appl. Surf. Sci. 2019, 465, 340–350. [Google Scholar] [CrossRef]
- Hurtado, C.R.; Hurtado, G.R.; Cena, G.L.D.; Queiroz, R.C.; Silva, A.V.; Diniz, M.F.; Santos, V.R.D.; Trava-Airoldi, V.; Baptista, M.D.S.; Tsolekile, N.; et al. Diamond Nanoparticles-Porphyrin mTHPP Conjugate as Photosensitizing Platform: Cytotoxicity and Antibacterial Activity. Nanomaterials 2021, 11, 1393. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Simon, T.; Venkatesan, P.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Nanodiamonds Conjugated to Gold Nanoparticles for Colorimetric Detection of Clenbuterol and Chromium(III) in Urine. Microchim. Acta 2017, 185, 74. [Google Scholar] [CrossRef] [PubMed]
- Afandi, A.; Howkins, A.; Boyd, I.W.; Jackman, R.B. Nanodiamonds for Device Applications: An Investigation of the Properties of Boron-Doped Detonation Nanodiamonds. Sci. Rep. 2018, 8, 3270. [Google Scholar] [CrossRef] [PubMed]
- Liao, M. Progress in Semiconductor Diamond Photodetectors and MEMS Sensors. Funct. Diam. 2021, 1, 29–46. [Google Scholar] [CrossRef]
- Zhao, F.; Vrajitoarea, A.; Jiang, Q.; Han, X.; Chaudhary, A.; Welch, J.O.; Jackman, R.B. Graphene-Nanodiamond Heterostructures and their application to High Current Devices. Sci. Rep. 2015, 5, 13771. [Google Scholar] [CrossRef] [Green Version]
- Radulaski, M.; Zhang, J.L.; Tzeng, Y.-K.; Lagoudakis, K.G.; Ishiwata, H.; Dory, C.; Fischer, K.A.; Kelaita, Y.A.; Sun, S.; Maurer, P.C.; et al. Nanodiamond Integration with Photonic Devices. Laser Photon. Rev. 2019, 13, 1800316. [Google Scholar] [CrossRef] [Green Version]
- Shellaiah, M.; Chen, T.H.; Simon, T.; Li, L.-C.; Sun, K.W.; Ko, F.-H. An Affordable Wet Chemical Route to Grow Conducting Hybrid Graphite-Diamond Nanowires: Demonstration by A Single Nanowire Device. Sci. Rep. 2017, 7, 11243. [Google Scholar] [CrossRef] [Green Version]
- Shellaiah, M.; Chen, Y.-C.; Simon, T.; Li, L.-C.; Sun, K.W.; Ko, F.-H. Effect of Metal Ions on Hybrid Graphite-Diamond Nanowire Growth: Conductivity Measurements from a Single Nanowire Device. Nanomaterials 2019, 9, 415. [Google Scholar] [CrossRef] [Green Version]
- Handschuh-Wang, S.; Wang, T.; Tang, Y. Ultrathin Diamond Nanofilms—Development, Challenges, and Applications. Small 2021, 17, 2007529. [Google Scholar] [CrossRef] [PubMed]
- Pleskov, Y.V.; Krotova, M.D.; Ekimov, E.A. The Compacts of Boron-Doped Synthetic Diamond: Methods for the Increasing of Their Electrochemical Activity. J. Electroanal. Chem. 2021, 888, 115203. [Google Scholar] [CrossRef]
- Wang, T.; Huang, L.; Liu, Y.; Li, X.; Liu, C.; Handschuh-Wang, S.; Xu, Y.; Zhao, Y.; Tang, Y. Robust Biomimetic Hierarchical Diamond Architecture with a Self-Cleaning, Antibacterial, and Antibiofouling Surface. ACS Appl. Mater. Interfaces 2020, 12, 24432–24441. [Google Scholar] [CrossRef]
- Guo, C.; Zheng, J.; Deng, H.; Shi, P.; Zhao, G. Photoelectrocatalytic Interface of Boron-Doped Diamond: Modification, Functionalization and Environmental Applications. Carbon 2021, 175, 454–466. [Google Scholar] [CrossRef]
- Simcox, L.J.; Pereira, R.P.A.; Wellington, E.M.H.; Macpherson, J.V. Boron Doped Diamond as a Low Biofouling Material in Aquatic Environments: Assessment of Pseudomonas aeruginosa Biofilm Formation. ACS Appl. Mater. Interfaces 2019, 11, 25024–25033. [Google Scholar] [CrossRef]
- Speranza, G. Carbon Nanomaterials: Synthesis, Functionalization and Sensing Applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef]
- Lourencao, B.C.; Brocenschi, R.F.; Medeiros, R.A.; Fatibello-Filho, O.; Rocha-Filho, R.C. Analytical Applications of Electrochemically Pretreated Boron-Doped Diamond Electrodes. ChemElectroChem 2020, 7, 1291–1311. [Google Scholar] [CrossRef]
- Wei, M.; Terashima, C.; Lv, M.; Fujishima, A.; Gu, Z.-Z. Boron-Doped Diamond Nanograss Array for Electrochemical Sensors. Chem. Commun. 2009, 3624–3626. [Google Scholar] [CrossRef]
- Baluchová, S.; Daňhel, A.; Dejmková, H.; Ostatná, V.; Fojta, M.; Schwarzová-Pecková, K. Recent Progress in the Applications of Boron Doped Diamond Electrodes in Electroanalysis of Organic Compounds and Biomolecules—A Review. Anal. Chim. Acta 2019, 1077, 30–66. [Google Scholar] [CrossRef] [PubMed]
- Trellu, C.; Chakraborty, S.; Nidheesh, P.V.; Oturan, M.A. Environmental Applications of Boron-Doped Diamond Electrodes: 2. Soil Remediation and Sensing Applications. ChemElectroChem 2019, 6, 2143–2156. [Google Scholar] [CrossRef]
- Silva, K.N.O.; Araújo, K.C.F.; da Silva, D.R.; Martínez-Huitle, C.A.; Santos, E.V.D. Persulfate-Soil Washing: The Green Use of Persulfate Electrochemically Generated with Diamond Electrodes for Depolluting Soils. J. Electroanal. Chem. 2021, 895, 115498. [Google Scholar] [CrossRef]
- Purcell, E.K.; Becker, M.F.; Guo, Y.; Hara, S.A.; Ludwig, K.A.; McKinney, C.J.; Monroe, E.M.; Rechenberg, R.; Rusinek, C.A.; Saxena, A.; et al. Next-Generation Diamond Electrodes for Neurochemical Sensing: Challenges and Opportunities. Nanomaterials 2021, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, H.; Zhao, Y.; Xi, Y.; Liu, B.; Xi, J.; Shao, G.; Fan, B.; Lu, H.; Xu, H.; et al. Preparation and Electrochemical Properties of Boron-Doped Polycrystalline Diamond Film with Five-Fold Twin Structure. Appl. Surf. Sci. 2021, 568, 150977. [Google Scholar] [CrossRef]
- Ochiai, T.; Tago, S.; Hayashi, M.; Fujishima, A. Highly Sensitive Measurement of Bio-Electric Potentials by Boron-Doped Diamond (BDD) Electrodes for Plant Monitoring. Sensors 2015, 15, 26921–26928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponnuswamy, T.; Chen, J.-J.; Xu, F.; Chyan, O. Monitoring Metal Ion Contamination Onset in Hydrofluoric Acid Using Silicon–Diamond and Dual Silicon Sensing Electrode Assembly. Analyst 2001, 126, 877–880. [Google Scholar] [CrossRef]
- Maldonado, V.Y.; Espinoza-Montero, P.J.; Rusinek, C.A.; Swain, G.M. Analysis of Ag(I) Biocide in Water Samples Using Anodic Stripping Voltammetry with a Boron-Doped Diamond Disk Electrode. Anal. Chem. 2018, 90, 6477–6485. [Google Scholar] [CrossRef] [PubMed]
- Culková, E.; Lukáčová-Chomisteková, Z.; Bellová, R.; Melicherčíková, D.; Durdiak, J.; Rievaj, M.; Vojs, M.; Tomčík, P. Voltammetric Detection of Silver in Commercial Products on Boron Doped Diamond Electrode: Stripping at Lowered Potential in the Presence of Thiosulfate Ions. Mon. Chem. 2020, 151, 1009–1017. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, N.; Yang, B.; Wang, C.; Zhuang, H.; Tian, Q.; Zhai, Z.; Liu, L.; Jiang, X. Hybrid Diamond/Graphite Films as Electrodes for Anodic Stripping Voltammetry of Trace Ag+ and Cu2+. Sens. Actuators B 2016, 231, 194–202. [Google Scholar] [CrossRef]
- Foord, J.S.; Eaton, K.; Hao, W.; Crossley, A. Interaction Between Co-Deposited Metals During Stripping Voltammetry at Boron-Doped Diamond Electrodes. Phys. Chem. Chem. Phys. 2005, 7, 2787–2792. [Google Scholar] [CrossRef] [PubMed]
- Ivandini, T.A.; Sato, R.; Makide, Y.; Fujishima, A.; Einaga, Y. Electrochemical Detection of Arsenic(III) Using Iridium-Implanted Boron-Doped Diamond Electrodes. Anal. Chem. 2006, 78, 6291–6298. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Swain, G.M. Total Inorganic Arsenic Detection in Real Water Samples Using Anodic Stripping Voltammetry and A Gold-Coated Diamond Thin-Film Electrode. Anal. Chim. Acta 2007, 593, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Pungjunun, K.; Chaiyo, S.; Jantrahong, I.; Nantaphol, S.; Siangproh, W.; Chailapakul, O. Anodic Stripping Voltammetric Determination of Total Arsenic Using A Gold Nanoparticle-Modified Boron-Doped Diamond Electrode on A Paper-Based Device. Microchim. Acta 2018, 185, 324. [Google Scholar] [CrossRef]
- Fauzillah, N.A.; Abdullah, I.; Ivandini, T.A. Modification of Boron-Doped Diamond Electrode with Gold Nanoparticles Synthesized by Allyl Mercaptan as the Capping Agent for Arsenic Sensors. AIP Conf. Proc. 2020, 2242, 040031. [Google Scholar]
- Agustiany, T.; Khalil, M.; Einaga, Y.; Jiwanti, P.K.; Ivandini, T.A. Stable Iridium-Modified Boron-Doped Diamond Electrode for the Application in Electrochemical Detection of Arsenic (III). Mater. Chem. Phys. 2020, 244, 122723. [Google Scholar] [CrossRef]
- Sugitani, A.; Watanabe, T.; Ivandini, T.A.; Iguchi, T.; Einaga, Y. Controlling the Diffusion Profile of Electroactive Species for Selective Anodic Stripping Voltammetry of Cadmium at Boron-Doped Diamond Electrodes. Phys. Chem. Chem. Phys. 2013, 15, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, C.; Mao, B.; An, Y. Determination of Cd2+ By Ultrasound-Assisted Square Wave Anodic Stripping Voltammetry with A Boron-Doped Diamond Electrode. Ionics 2015, 21, 1761–1769. [Google Scholar] [CrossRef]
- Innuphat, C.; Chooto, P. Determination of Trace Levels of Cd(II) in Tap Water Samples by Anodic Stripping Voltammetry with An Electrografted Boron-Doped Diamond Electrode. ScienceAsia 2017, 43, 33. [Google Scholar] [CrossRef] [Green Version]
- Nantaphol, S.; Channon, R.B.; Kondo, T.; Siangproh, W.; Chailapakul, O.; Henry, C.S. Boron Doped Diamond Paste Electrodes for Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2017, 89, 4100–4107. [Google Scholar] [CrossRef]
- Pei, J.X.; Yu, X.; Zhang, C.; Liu, X.J. Development of a Boron-Doped Diamond Electrode for the Simultaneous Detection of Cd2+ and Pb2+ in Water. Int. J. Electrochem. Sci. 2019, 14, 3393–3407. [Google Scholar] [CrossRef]
- Štenclová, P.; Vyskočil, V.; Szabó, O.; Ižák, T.; Potocký, Š.; Kromka, A. Structured and Graphitized Boron Doped Diamond Electrodes: Impact on Electrochemical Detection of Cd2+ and Pb2+ Ions. Vacuum 2019, 170, 108953. [Google Scholar] [CrossRef]
- Zhou, M.; Zhai, Z.; Liu, L.; Zhang, C.; Yuan, Z.; Lu, Z.; Chen, B.; Shi, D.; Yang, B.; Wei, Q.; et al. Controllable Synthesized Diamond/CNWs Film as A Novel Nanocarbon Electrode with Wide Potential Window and Enhanced S/B Ratio for Electrochemical Sensing. Appl. Surf. Sci. 2021, 551, 149418. [Google Scholar] [CrossRef]
- Fierro, S.; Watanabe, T.; Akai, K.; Einaga, Y. Highly Sensitive Detection of Cr6+ on Boron Doped Diamond Electrodes. Electrochim. Acta 2012, 82, 9–11. [Google Scholar] [CrossRef]
- Xu, Y.; Xiong, C.; Gao, C.; Li, Y.; Bian, C.; Xia, S. Cathodically Pretreated AuNPs–BDD Electrode for Detection of Hexavalent Chromium. Micromachines 2020, 11, 1095. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Neodo, S.; Wharton, J.A.; Cranny, A.; Harris, N.R.; Wood, R.J.K.; Stokes, K.R. Electrochemical Detection of Cupric Ions with Boron-Doped Diamond Electrode for Marine Corrosion Monitoring. Electrochim. Acta 2016, 202, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Manivannan, A.; Seehra, M.S.; Tryk, D.A.; Fujishima, A. Electrochemical Detection of Ionic Mercury at Boron-Doped Diamond Electrodes. Anal. Lett. 2002, 35, 355–368. [Google Scholar] [CrossRef]
- McLaughlin, M.H.S.; Pakpour-Tabrizi, A.C.; Jackman, R.B. Diamond Electrodes for High Sensitivity Mercury Detection in the Aquatic Environment: Influence of Surface Preparation and Gold Nanoparticle Activity. Electroanalysis 2019, 31, 1775–1782. [Google Scholar] [CrossRef]
- McLaughlin, M.H.S.; Pakpour-Tabrizi, A.C.; Jackman, R.B. A Detailed EIS Study of Boron Doped Diamond Electrodes Decorated with Gold Nanoparticles for High Sensitivity Mercury Detection. Sci. Rep. 2021, 11, 9505. [Google Scholar] [CrossRef]
- Neodo, S.; Nie, M.; Wharton, J.A.; Stokes, K.R. Nickel-Ion Detection on A Boron-Doped Diamond Electrode in Acidic Media. Electrochim. Acta 2013, 88, 718–724. [Google Scholar] [CrossRef]
- Musyarofah, N.R.R.; Gunlazuardi, J.; Einaga, Y.; Ivandini, T.A. Anodic Stripping Voltammetry of Nickel Ions and Nickel hydroxide Nanoparticles at Boron-Doped Diamond Electrodes. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 012020. [Google Scholar] [CrossRef]
- Yuliani, T.; Saepudin, E.; Ivandini, T.A. Anodic Stripping Voltammetry of Ni(OH)2 Nanoparticles in Acid Solution Using Boron-Doped Diamond Electrodes. AIP Conf. Proc. 2018, 2023, 020096. [Google Scholar]
- Chooto, P.; Wararatananurak, P.; Innuphat, C. Determination of Trace Levels of Pb(II) in Tap Water by Anodic Stripping Voltammetry with Boron-Doped Diamond Electrode. ScienceAsia 2010, 36, 150–156. [Google Scholar] [CrossRef]
- Le, T.S.; Da Costa, P.; Huguet, P.; Sistat, P.; Pichot, F.; Silva, F.; Renaud, L.; Cretin, M. Upstream Microelectrodialysis For Heavy Metals Detection on Boron Doped Diamond. J. Electroanal. Chem. 2012, 670, 50–55. [Google Scholar] [CrossRef]
- Arantes, T.M.; Sardinha, A.; Baldan, M.R.; Cristovan, F.H.; Ferreira, N.G. Lead Detection Using Micro/Nanocrystalline Boron-Doped Diamond by Square-Wave Anodic Stripping Voltammetry. Talanta 2014, 128, 132–140. [Google Scholar] [CrossRef]
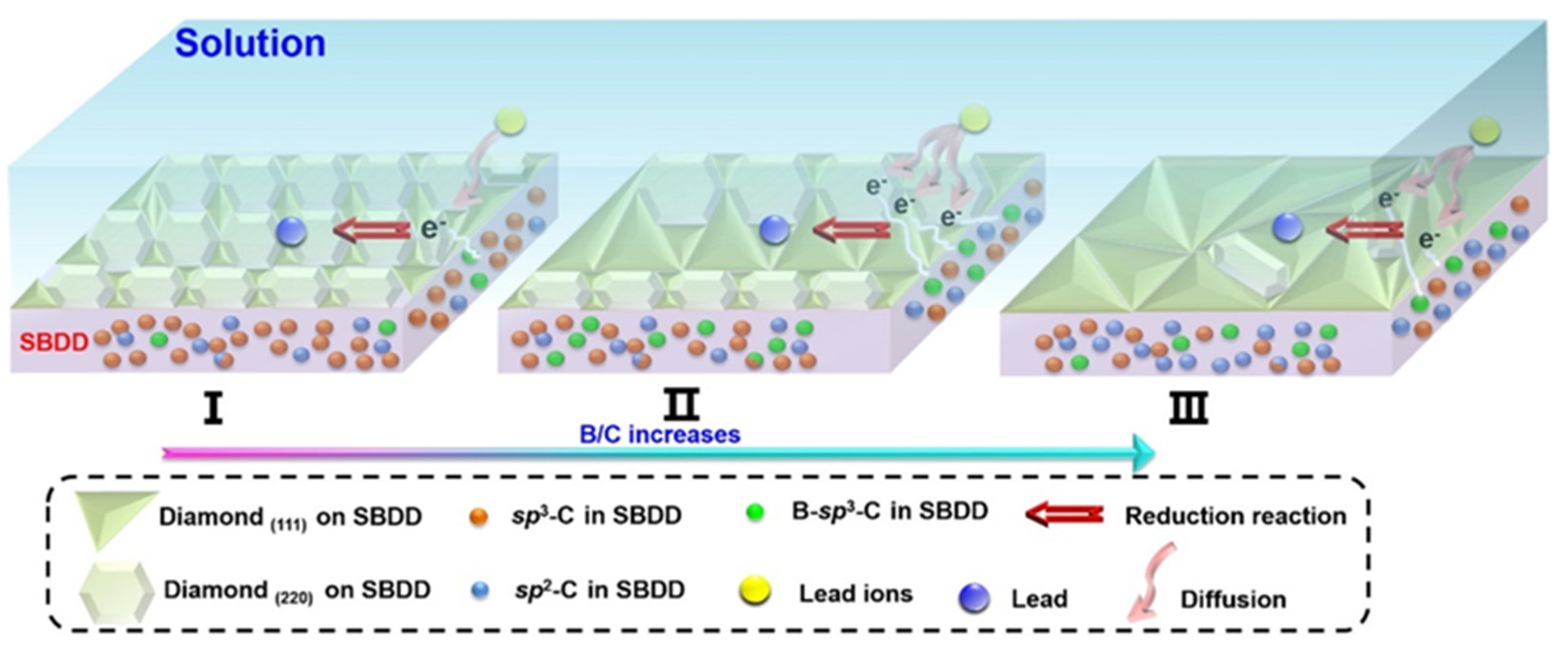
- Pei, J.; Yu, X.; Wei, S.; Boukherroub, R.; Zhang, Y. Double-Side Effect of B/C Ratio on BDD Electrode Detection for Heavy Metal Ion in Water. Sci. Total Environ. 2021, 771, 145430. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.; Sankaran, K.J.; Korneychuk, S.; Verbeeck, J.; McLaughlin, J.; Haenen, K.; Roy, S.S. Nanostructured Nitrogen Doped Diamond for the Detection of Toxic Metal Ions. Electrochim. Acta 2018, 283, 1871–1878. [Google Scholar] [CrossRef]
- Dos Santos, G.F.S.; Ferreira, R.D.Q. Determination of Pb(II) and Cu(II) in Microemulsified Biodiesel Using Boron-Doped Diamond Electrode. Microchem. J. 2020, 156, 104849. [Google Scholar] [CrossRef]
- Lukáčová-Chomisteková, Z.; Culková, E.; Bellová, R.; Melicherčíková, D.; Durdiak, J.; Beinrohr, E.; Rievaj, M.; Tomčík, P. Voltammetric Detection of Antimony in Natural Water on Cathodically Pretreated Microcrystalline Boron Doped Diamond Electrode: A Possibility How to Eliminate Interference of Arsenic Without Surface Modification. Talanta 2018, 178, 943–948. [Google Scholar] [CrossRef]
- Culková, E.; Švorc, L.; Tomčík, P.; Durdiak, J.; Rievaj, M.; Bustin, D.; Brescher, R.; Lokaj, J. Boron-Doped Diamond Electrode as Sensitive and Selective Green Electroanalytical Tool for Heavy Metals Environmental Monitoring: Zinc Detection in Rubber Industry Waste. Pol. J. Environ. Stud. 2013, 22, 1317–1323. [Google Scholar]
- Nurhayati, E.; Juang, Y.; Rajkumar, M.; Huang, C.; Hu, C.-C. Effects of Dynamic Polarization on Boron-Doped NCD Properties and on Its Performance for Electrochemical-Analysis of Pb(II), Cu(II) and Hg(II) in Aqueous Solution via Direct LSV. Sep. Purif. Technol. 2015, 156, 1047–1056. [Google Scholar] [CrossRef]
- Zazoua, A.; Khedimallah, N.; Jaffrezic-Renault, N. Electrochemical Determination of Cadmium, Lead, and Nickel Using a Polyphenol–Polyvinyl Chloride—Boron-Doped Diamond Electrode. Anal. Lett. 2018, 51, 336–347. [Google Scholar] [CrossRef]
- Marton, M.; Michniak, P.; Behul, M.; Rehacek, V.; Vojs Stanova, A.; Redhammer, R.; Vojs, M. Bismuth Modified Boron Doped Diamond Electrode for Simultaneous Determination of Zn, Cd and Pb Ions by Square Wave Anodic Stripping Voltammetry: Influence of Boron Concentration and Surface Morphology. Vacuum 2019, 167, 182–188. [Google Scholar] [CrossRef]
- Sonthalia, P.; McGaw, E.; Show, Y.; Swain, G.M. Metal Ion Analysis in Contaminated Water Samples Using Anodic Stripping Voltammetry and A Nanocrystalline Diamond Thin-Film Electrode. Anal. Chim. Acta 2004, 522, 35–44. [Google Scholar] [CrossRef]
- El Tall, O.; Jaffrezic-Renault, N.; Sigaud, M.; Vittori, O. Anodic Stripping Voltammetry of Heavy Metals at Nanocrystalline Boron-Doped Diamond Electrode. Electroanalysis 2007, 19, 1152–1159. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Yang, J.E.; Kim, J.P.; Bae, J.S.; Shim, Y.-B.; Won, M.-S. Simultaneous Detection of Cd (II), Pb (II), Cu (II), and Hg (II) Ions in Dye Waste Water Using a Boron Doped Diamond Electrode with DPASV. Bull. Kor. Chem. Soc. 2010, 31, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Li, J.; Yu, X.; Zhang, J.; Ren, Y. In-Situ Detection of Heavy Metal Pollution in Seawater with Diamond Coated Electrodes. Surf. Rev. Lett. 2019, 26, 1850179. [Google Scholar] [CrossRef]
- Zhai, Z.; Huang, N.; Zhuang, H.; Liu, L.; Yang, B.; Wang, C.; Gai, Z.; Guo, F.; Li, Z.; Jiang, X. A Diamond/Graphite Nanoplatelets Electrode for Anodic Stripping Voltammetric Trace Determination of Zn(II), Cd(II), Pb(II) and Cu(II). Appl. Surf. Sci. 2018, 457, 1192–1201. [Google Scholar] [CrossRef]
- Ferreira, R.; Chaar, J.; Baldan, M.; Braga, N. Simultaneous Voltammetric Detection of Fe3+, Cu2+, Zn2+, Pb2+ e Cd2+ in Fuel Ethanol Using Anodic Stripping Voltammetry and Boron-Doped Diamond Electrodes. Fuel 2021, 291, 120104. [Google Scholar] [CrossRef]
- Xu, J.; Swain, G.M. Oxidation of Azide Anion at Boron-Doped Diamond Thin-Film Electrodes. Anal. Chem. 1998, 70, 1502–1510. [Google Scholar] [CrossRef]
- Ivandini, T.A.; Sato, R.; Makide, Y.; Fujishima, A.; Einaga, Y. Pt-Implanted Boron-Doped Diamond Electrodes and the Application for Electrochemical Detection of Hydrogen Peroxide. Diam. Relat. Mater. 2005, 14, 2133–2138. [Google Scholar] [CrossRef]
- Komkova, M.A.; Pasquarelli, A.; Andreev, E.A.; Galushin, A.A.; Karyakin, A.A. Prussian Blue Modified Boron-Doped Diamond Interfaces for Advanced H2O2 Electrochemical Sensors. Electrochim. Acta 2020, 339, 135924. [Google Scholar] [CrossRef]
- Irkham; Fiorani, A.; Valenti, G.; Kamoshida, N.; Paolucci, F.; Einaga, Y. Electrogenerated Chemiluminescence by in Situ Production of Coreactant Hydrogen Peroxide in Carbonate Aqueous Solution at a Boron-Doped Diamond Electrode. J. Am. Chem. Soc. 2020, 142, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Stefan, R.-I.; Ghebru Bairu, S.; van Staden, J.F. Diamond Paste Based Electrodes for the Determination of Iodide in Vitamins and Table Salt. Anal. Lett. 2003, 36, 1493–1500. [Google Scholar] [CrossRef]
- Fierro, S.; Comninellis, C.; Einaga, Y. Simultaneous Detection of Iodine and Iodide on Boron Doped Diamond Electrodes. Talanta 2013, 103, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Culková, E.; Tomčík, P.; Švorc, Ľ.; Cinková, K.; Chomisteková, Z.; Durdiak, J.; Rievaj, M.; Bustin, D. Indirect Voltammetric Sensing Platforms for Fluoride Detection on Boron-Doped Diamond Electrode Mediated via [FeF6]3− and [CeF6]2− Complexes Formation. Electrochim. Acta 2014, 148, 317–324. [Google Scholar] [CrossRef]
- Lucio, A.J.; Meyler, R.E.P.; Edwards, M.A.; Macpherson, J.V. Investigation of sp2-Carbon Pattern Geometry in Boron-Doped Diamond Electrodes for the Electrochemical Quantification of Hypochlorite at High Concentrations. ACS Sens. 2020, 5, 789–797. [Google Scholar] [CrossRef]
- Lucio, A.J.; Macpherson, J.V. Combined Voltammetric Measurement of pH and Free Chlorine Speciation Using a Micro-Spot sp2 Bonded Carbon–Boron Doped Diamond Electrode. Anal. Chem. 2020, 92, 16072–16078. [Google Scholar] [CrossRef] [PubMed]
- Julião, M.S.D.S.; Ferreira, E.I.; Ferreira, N.G.; Serrano, S.H.P. Voltammetric Detection of the Interactions Between RNO2− and Electron Acceptors in Aqueous Medium at Highly Boron Doped Diamond Electrode (HBDDE). Electrochim. Acta 2006, 51, 5080–5086. [Google Scholar] [CrossRef]
- Sahraoui, Y.; Sbartai, A.; Chaliaa, S.; Maaref, A.; Haddad, A.; Jaffrezic-Renault, N. A Nitrite Electrochemical Sensor Based on Boron-Doped Diamond Planar Electrochemical Microcells Modified with a Monolacunary Silicotungstate Polyoxoanion. Electroanalysis 2015, 27, 1359–1367. [Google Scholar] [CrossRef]
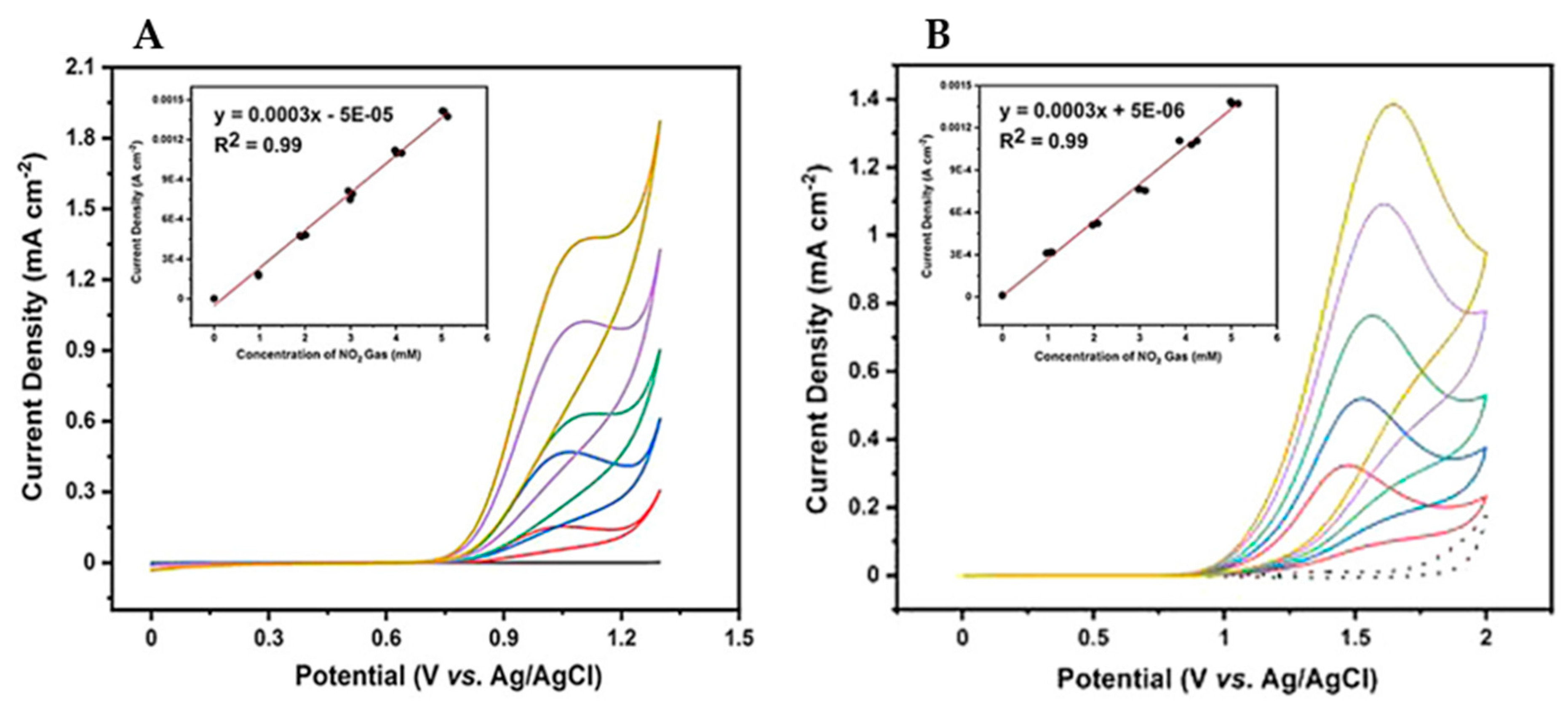
- Triana, Y.; Irkham; Einaga, Y. Electrochemical Oxidation Behavior of Nitrogen Dioxide for Gas Detection Using Boron Doped Diamond Electrodes. Electroanalysis 2021. [Google Scholar] [CrossRef]
- Welch, C.M.; Hyde, M.E.; Banks, C.E.; Compton, R.G. The Detection of Nitrate Using In-Situ Copper Nanoparticle Deposition at a Boron Doped Diamond Electrode. Anal. Sci. 2005, 21, 1421–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, P.; Natsui, K.; Feng, C.; Einaga, Y. Electrochemical Reduction of Nitrate on Boron-Doped Diamond Electrodes: Effects of Surface Termination and Boron-Doping Level. Chemosphere 2020, 251, 126364. [Google Scholar] [CrossRef] [PubMed]
- Khamis, D.; Mahé, E.; Dardoize, F.; Devilliers, D. Peroxodisulfate Generation on Boron-Doped Diamond Microelectrodes Array and Detection by Scanning Electrochemical Microscopy. J. Appl. Electrochem. 2010, 40, 1829–1838. [Google Scholar] [CrossRef]
- Irkham; Watanabe, T.; Fiorani, A.; Valenti, G.; Paolucci, F.; Einaga, Y. Co-reactant-on-Demand ECL: Electrogenerated Chemiluminescence by the in-Situ Production of S2O82− at Boron-Doped Diamond Electrodes. J. Am. Chem. Soc. 2016, 138, 15636–15641. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Niwano, Y.; Tamura, A.; Ivandini, T.A.; Einaga, Y.; Tryk, D.A.; Fujishima, A.; Kawai, T. Sensitive Electrochemical Detection of Oxalate at a Positively Charged Boron-Doped Diamond Surface. Electroanalysis 2008, 20, 1556–1564. [Google Scholar] [CrossRef]
- Lawrence, N.S.; Thompson, M.; Prado, C.; Jiang, L.; Jones, T.G.J.; Compton, R.G. Amperometric Detection of Sulfide at a Boron Doped Diamond Electrode: The Electrocatalytic Reaction of Sulfide with Ferricyanide in Aqueous Solution. Electroanalysis 2002, 14, 499–504. [Google Scholar] [CrossRef]
- Baciu, A.; Ardelean, M.; Pop, A.; Pode, R.; Manea, F. Simultaneous Voltammetric/Amperometric Determination of Sulfide and Nitrite in Water at BDD Electrode. Sensors 2015, 15, 14526–14538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.; Niu, T.; Shi, P.; Zhao, G. Boron-Doped Diamond for Hydroxyl Radical and Sulfate Radical Anion Electrogeneration, Transformation, and Voltage-Free Sustainable Oxidation. Small 2019, 15, 1900153. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, X.; Luo, G.; Niu, Y.; Zou, R.; Yin, C.; Huang, S.; Sun, W.; Li, G. Nano-Diamond Modified Electrode for the Investigation on Direct Electrochemistry and Electrocatalytic Behavior of Myoglobin. Diam. Relat. Mater. 2019, 97, 107453. [Google Scholar] [CrossRef]
- Cheng, H.; Sun, Y.; Shao, B.; Deng, Y.; Zhang, X.; Li, G.; Sun, W. Application of Gold Nanoparticles and Nano-Diamond Modified Electrode for Hemoglobin Electrochemistry. Int. J. Electrochem. Sci. 2020, 15, 11416–11426. [Google Scholar] [CrossRef]
- Bakharev, P.V.; Huang, M.; Saxena, M.; Lee, S.W.; Joo, S.H.; Park, S.O.; Dong, J.; Camacho-Mojica, D.C.; Jin, S.; Kwon, Y.; et al. Chemically Induced Transformation of Chemical Vapour Deposition Grown Bilayer Graphene into Fluorinated Single-Layer Diamond. Nat. Nanotechnol. 2020, 15, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Venkateshaiah, A.; Padil, V.V.T.; Nagalakshmaiah, M.; Waclawek, S.; Černík, M.; Varma, R.S. Microscopic Techniques for the Analysis of Micro and Nanostructures of Biopolymers and Their Derivatives. Polymers 2020, 12, 512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scipioni, R.; Jørgensen, P.S.; Ngo, D.-T.; Simonsen, S.B.; Liu, Z.; Yakal-Kremski, K.J.; Wang, H.; Hjelm, J.; Norby, P.; Barnett, S.A.; et al. Electron Microscopy Investigations of Changes in Morphology and Conductivity of LiFePO4/C Electrodes. J. Power Sources 2016, 307, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, J.V. A Practical Guide to Using Boron Doped Diamond in Electrochemical Research. Phys. Chem. Chem. Phys. 2015, 17, 2935–2949. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Rusinek, C.A.; Thompson, C.H.; Setien, M.; Guo, Y.; Rechenberg, R.; Gong, Y.; Weber, A.J.; Becker, M.F.; Purcell, E.; et al. Flexible, Diamond-Based Microelectrodes Fabricated Using the Diamond Growth Side for Neural Sensing. Microsys. Nanoeng. 2020, 6, 42. [Google Scholar] [CrossRef]
- Kee, Y.; Suzuki, Y.; Ishigaki, N.; Motoyama, M.; Kimura, Y.; Amezawa, K.; Iriyama, Y. An Appropriate Reference and Counter Electrode in an All-Solid-State Battery Using NASICON-Structured Solid Electrolyte. Electrochem. Commun. 2021, 130, 107108. [Google Scholar] [CrossRef]
- Song, C.W.; Cho, D.S.; Lee, J.M.; Song, P.K. Effect of Boron Doping on Diamond Film and Electrochemical Properties of BDD According to Thickness and Morphology. Coatings 2020, 10, 331. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, K.; Kondo, T.; Sugai, S.; Tei, T.; Nishikawa, M.; Tojo, T.; Yuasa, M. Boron-doped Nanodiamond as an Electrode Material for Aqueous Electric Double-layer Capacitors. Sci. Rep. 2019, 9, 17846. [Google Scholar] [CrossRef] [Green Version]
- Aquino, J.M.; Rodrigo, M.A.; Rocha-Filho, R.C.; Sáez, C.; Cañizares, P. Influence of the Supporting Electrolyte on the Electrolyses of Dyes with Conductive-Diamond Anodes. Chem. Eng. J. 2012, 184, 221–227. [Google Scholar] [CrossRef]
- Liu, T.; Xue, Q.; Jia, J.; Liu, F.; Zou, S.; Tang, R.; Chen, T.; Li, J.; Qian, Y. New Insights into the Effect of pH on the Mechanism of Ofloxacin Electrochemical Detection in Aqueous Solution. Phys. Chem. Chem. Phys. 2019, 21, 16282–16287. [Google Scholar] [CrossRef] [PubMed]
- Oje, A.I.; Ogwu, A.A.; Oje, A.M. Effect of Temperature on the Electrochemical Performance of Silver Oxide Thin Films Supercapacitor. J. Electroanal. Chem. 2021, 882, 115015. [Google Scholar] [CrossRef]
- Park, S.-H.; Kwon, N.; Lee, J.-H.; Yoon, J.; Shin, I. Synthetic Ratiometric Fluorescent Probes for Detection of Ions. Chem. Soc. Rev. 2020, 49, 143–179. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Thirumalaivasan, N.; Aazaad, B.; Awasthi, K.; Sun, K.W.; Wu, S.-P.; Lin, M.-C.; Ohta, N. Novel Rhodamine Probe for Colorimetric and Fluorescent Detection of Fe3+ Ions in Aqueous Media with Cellular Imaging. Spectrochim. Acta A 2020, 242, 118757. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, I.; Miller, K.; Diepenheim, G.; Cantrell, K.; Hall, W.P. Nanoparticle Design Rules for Colorimetric Plasmonic Sensors. ACS Appl. Nano Mater. 2020, 3, 4342–4350. [Google Scholar] [CrossRef]
- Shellaiah, M.; Thirumalaivasan, N.; Sun, K.W.; Wu, S.-P. A pH Cooperative Strategy for Enhanced Colorimetric Sensing of Cr(III) Ions Using Biocompatible L-Glutamic Acid Stabilized Gold Nanoparticles. Microchem. J. 2021, 160, 105754. [Google Scholar] [CrossRef]
- Lou, Y.; Zhao, Y.; Zhu, J.-J. Ultrasensitive Optical Detection of Anions by Quantum Dots. Nanoscale Horiz. 2016, 1, 125–134. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Luminescent Metal Nanoclusters for Potential Chemosensor Applications. Chemosensors 2017, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Shellaiah, M.; Simon, T.; Thirumalaivasan, N.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Cysteamine-Capped Gold-Copper Nanoclusters for Fluorometric Determination and Imaging of Chromium(VI) and Dopamine. Microchim. Acta 2019, 186, 788. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Zhou, Y.; Leblanc, R.M.; Peng, Z. Recent Developments of Carbon Dots in Biosensing: A Review. ACS Sens. 2020, 5, 2724–2741. [Google Scholar] [CrossRef] [PubMed]

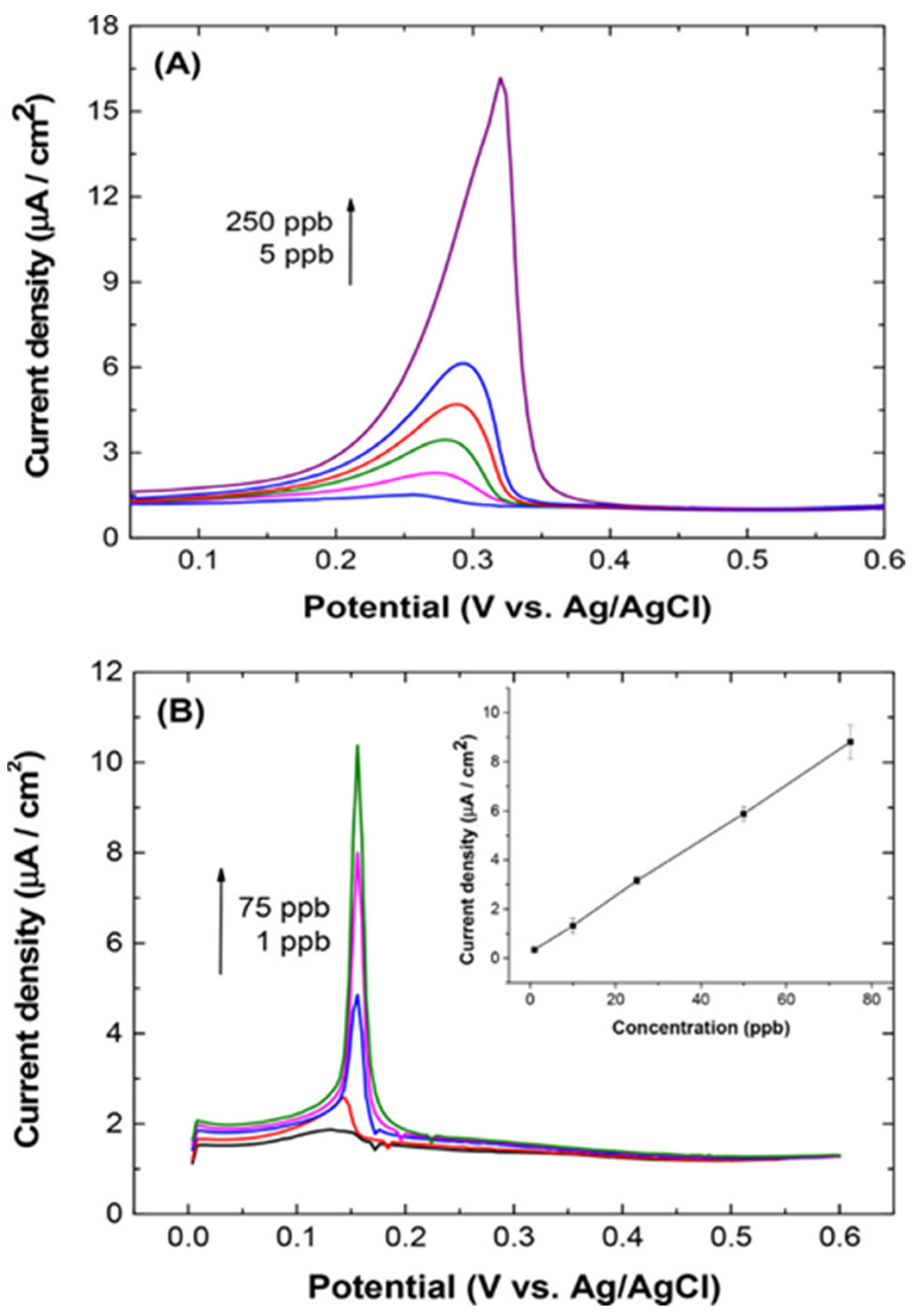
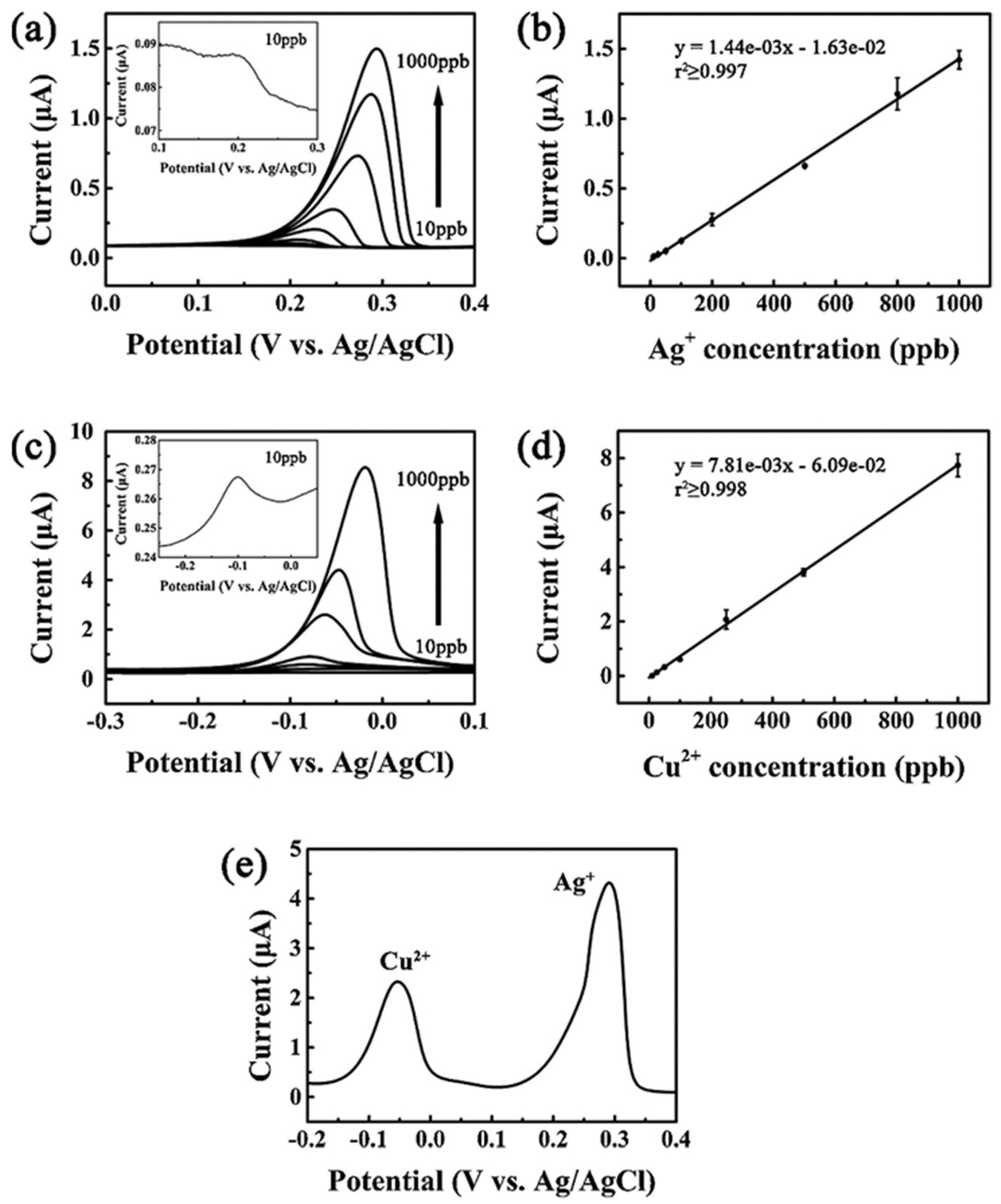
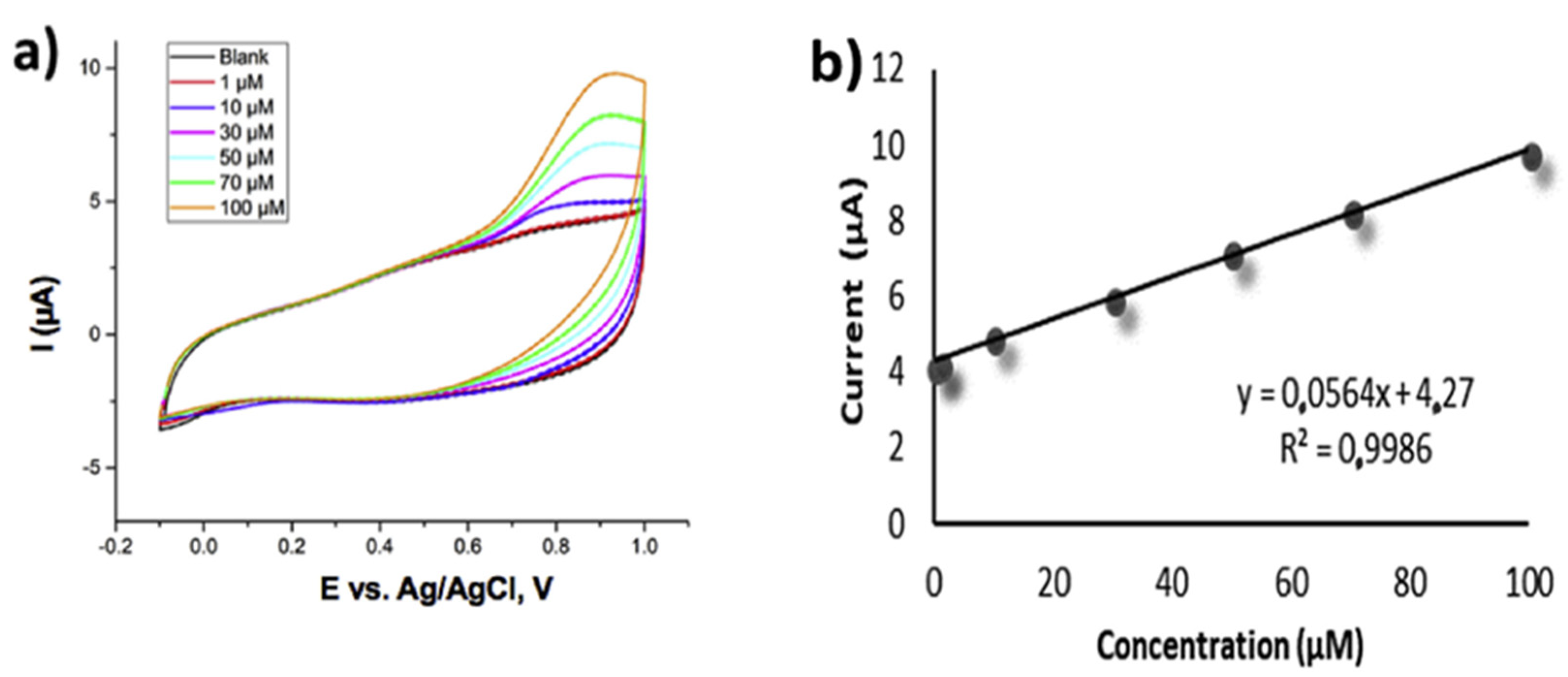
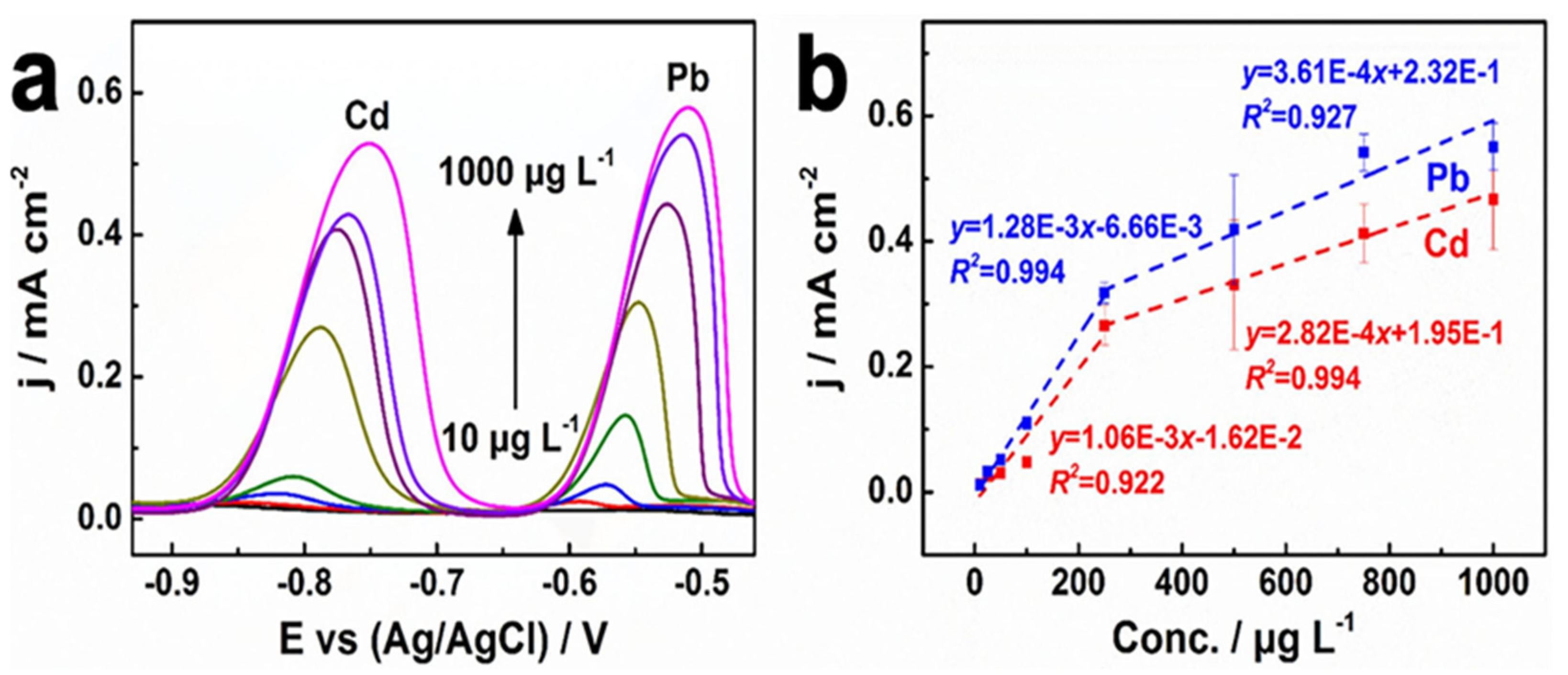
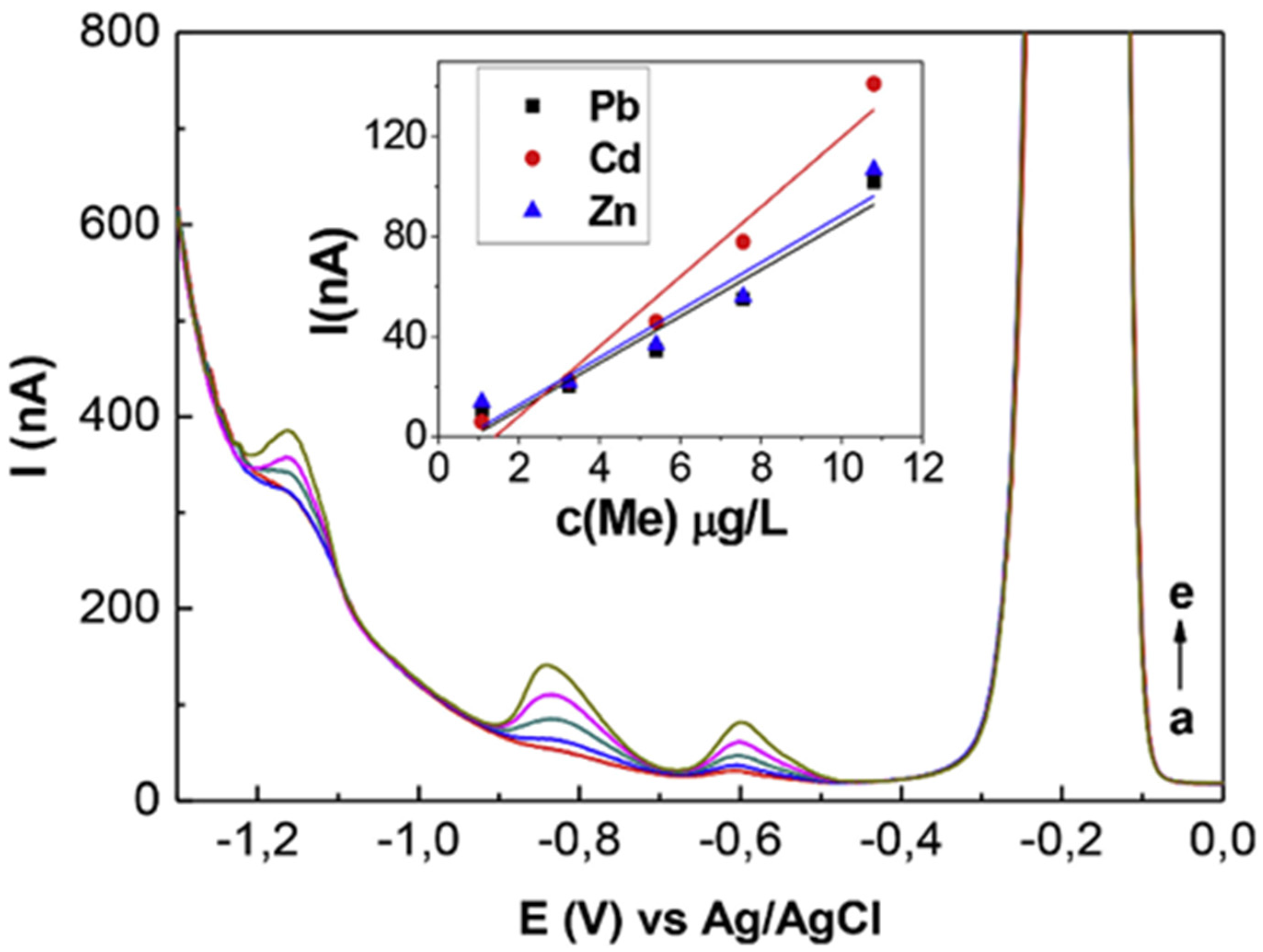
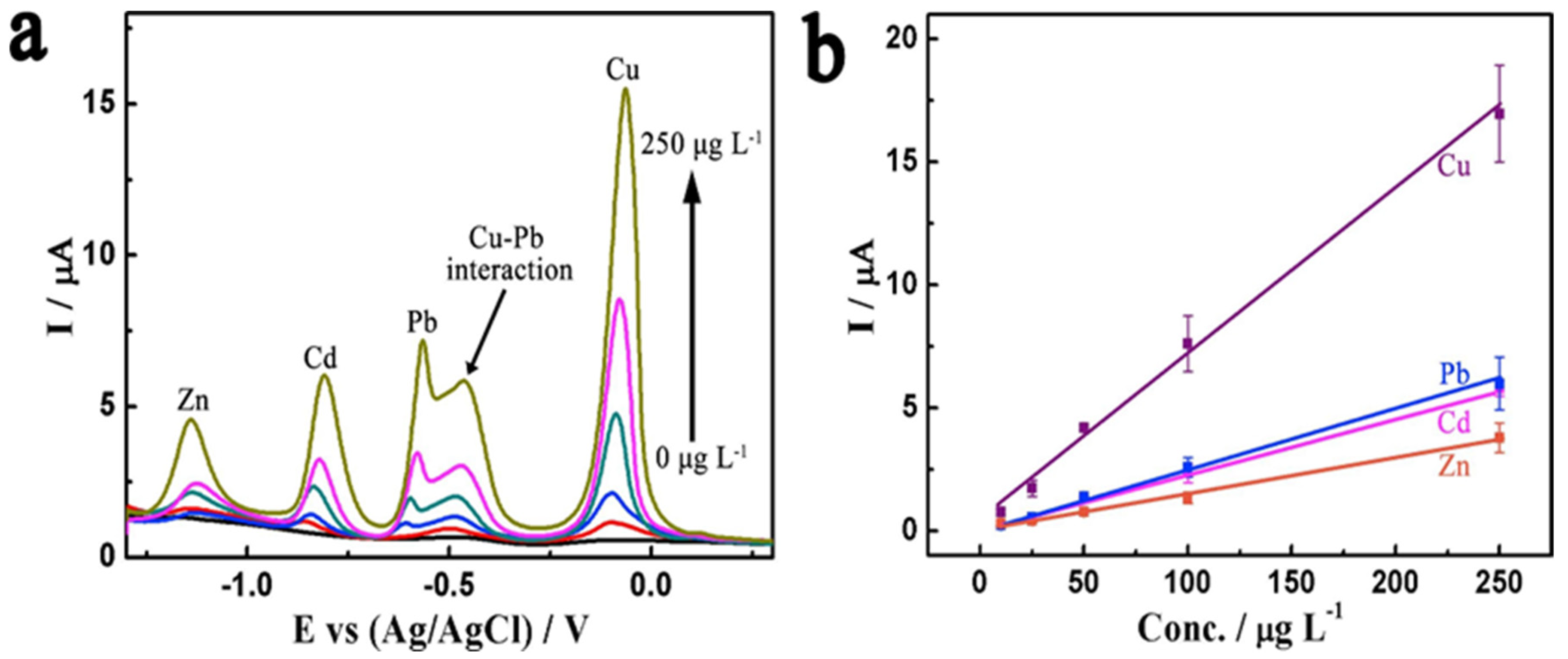
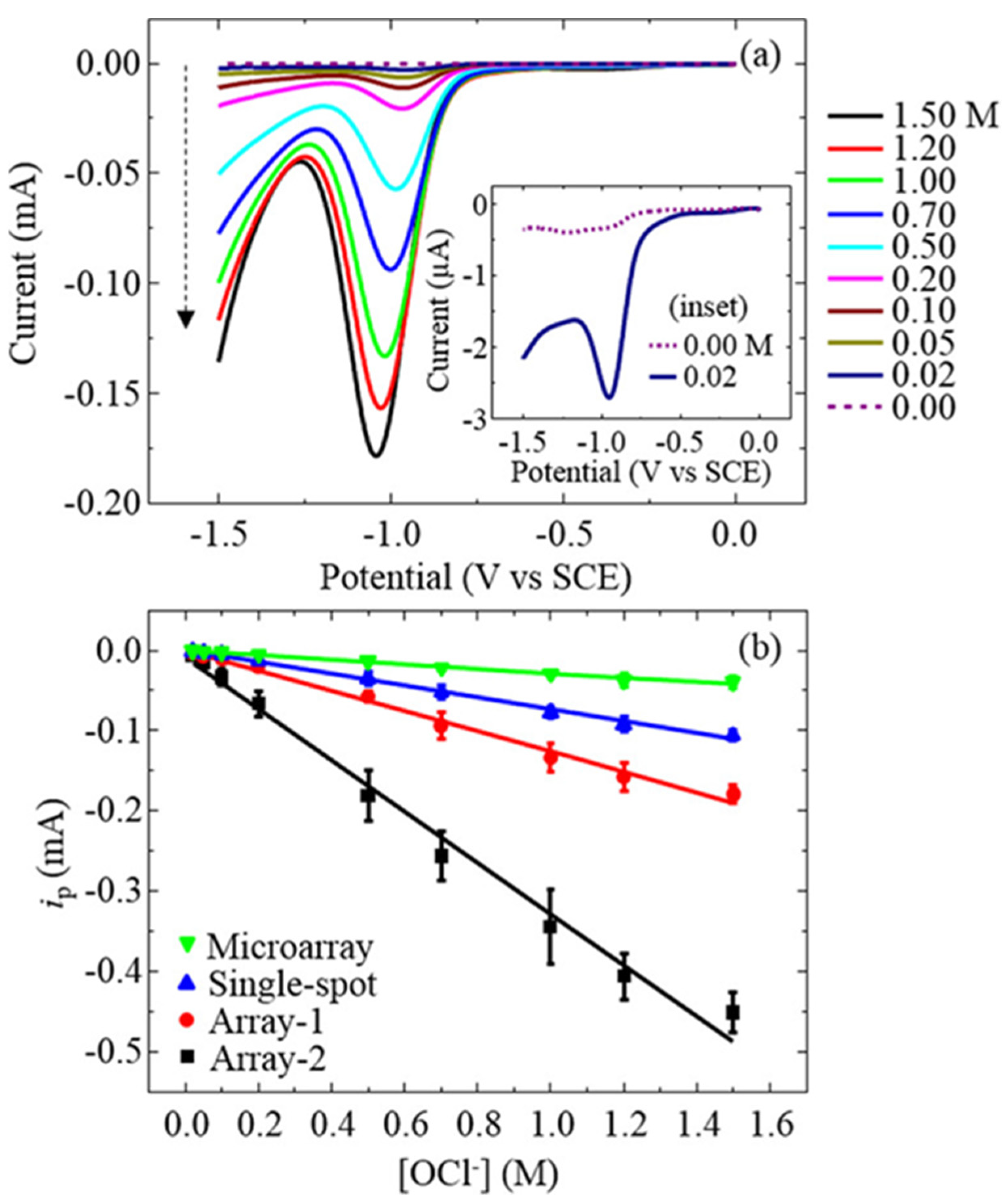
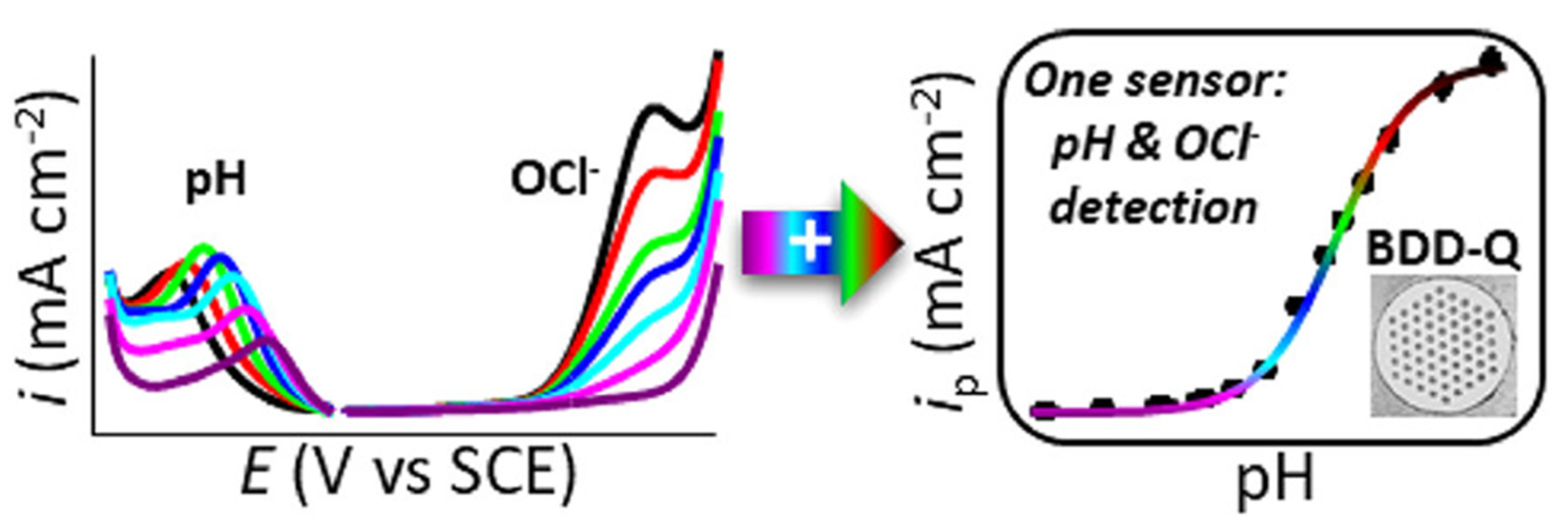
| Analyte | Electrode | Doping Concentration/Doping Atom | Method of Detection | Linear Range | Detection Limit (LOD) | Ref. |
|---|---|---|---|---|---|---|
| Ag+ | Planar BDD/Disk BDD | 10 ppm/Boron | DPASV | 45.5 nM–2.3 µM and 9.1–682 nM | 31 nM and 43 nM | [62] |
| Ag+ | BDD | 2000 ppm/Boron | DPASV | 1–7 nM | 0.2 nM | [63] |
| Ag+ and Cu2+ | Diamond/Graphite film | n/a | ASV | 0.91 pM–9 nM and 0.16 pM–15.7 nM, respectively | 0.52 pM and 0.88 pM, respectively | [64] |
| Ag+ and Pb2+ | BDD | n/a | ASV | 0.75–0.025 mM and 0.72–0.05 mM, respectively | n/a | [65] |
| As3+ | Ir-BDD | B/C = 1:100/Boron; implanted with 800 keV Ir+ | Amperometry | 0.1–100 µM | 20 nM | [66] |
| As3+ | Au-BDD | 1020 cm−3 | DPASV | 0.133–534 pM | 0.067 pM | [67] |
| As3+ | AuNPs/BDD | n/a | SWASV | 1.33–20 nM | 13.35 nM | [68] |
| As3+ | AuNPs- BDD | B/C = 104 ppm/Boron | ASV | 0–100 µM | 64 nM | [69] |
| As3+ | stable Ir-BDD | B/C = 0.1%/Boron | CV | 0–100 µM | 4.64 µM | [70] |
| Cd2+ | BDD | B/C = 1%/Boron | ASV | 0–0.1 mM | 3. 94 nM | [71] |
| Cd2+ | BDD | 8000 ppm/Boron | SWASV | 0.18–2.17 µM | 8.9 nM | [72] |
| Cd2+ | BDD | n/a | SWASV | 18–445 pM | 1.8 pM | [73] |
| Cd2 + and Pb2 + | BDD | B/C = 2.4%/Boron | SWASV | 0.05–4 µM and 0.05–8 µM, respectively | 30.16 nM and 17.47 nM, respectively | [75] |
| Cd2+ and Pb2+ | BDD | 10000 ppm/Boron | DPASV | n/a | n/a | [76] |
| Cd2+ and Pb2+ | D/CNWs | D/CNWs = 3%, 5%, 7% and 9% | DPASV | 0.089–8.9 µM, and 0.048–4.83 µM, respectively | 89 nM and 48 nM, respectively | [77] |
| Cr6+ | BDD | B/C = 0.1%/Boron | LSV | 0.2–96 nM | 0.57 pM | [78] |
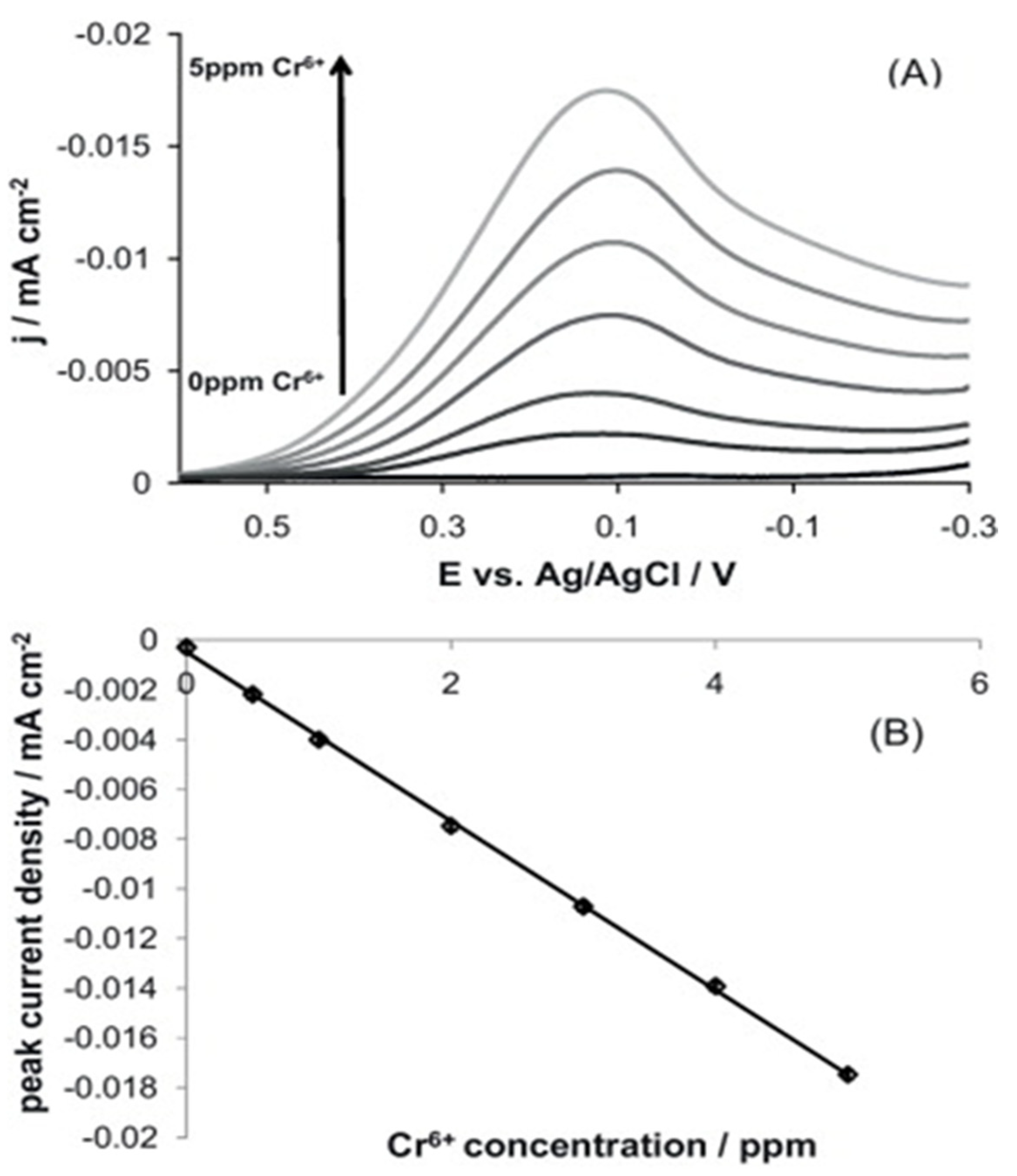
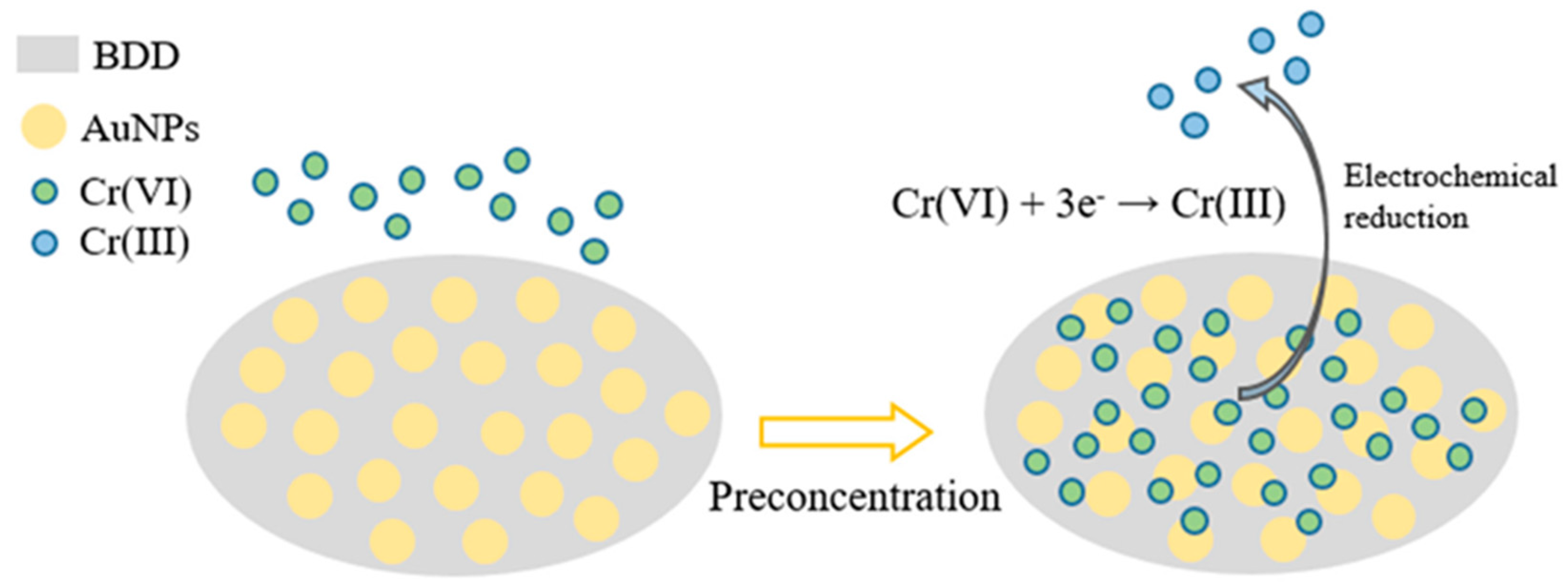
| Cr6+ | AuNPs–BDD | n/a | SWCSV | 0.193–19.3 µM | 22.91 nM | [79] |
| Cu2+ | BDD | 2000 ppm/Boron | DPV | 10 µM–100 mM | 10 µM | [80] |
| Hg2+ | BDD | n/a | DPV | 10 nM–10 µM | 68 nM | [81] |
| Hg2+ | AuNPs–BDD | >1020 cm−3/Boron | SWASV | 0.5–100 µM | 5 µM | [82] |
| Hg2+ | AuNPs–BDD | >1020 cm−3/Boron | EIS | 1 pM–1 mM | n/a | [83] |
| Ni2+ | BDD | ~1020 cm−3/Boron | DPV | 10–500 µM | 26.1 µM | [84] |
| Ni2+ and Ni(OH)2 NPs | BDD | n/a | ASV | 5–25 mM | 5.73 µM | [85] |
| Ni(OH)2 NPs | BDD | n/a | ASV | 10–25 mM | 0.42 µM | [86] |
| Pb2 + | BDD | n/a | SWASV | 9.65–145 nM | 1.45 nM | [87] |
| Pb2+ | BDD | B/C = 1000 ppm/Boron | SWASV | 96–482 pM | 19 pM | [88] |
| Pb2+ | BDD | B/C = 2000 ppm/Boron | SWASV | 4.83–48.3 nM | < 4.83 nM | [89] |
| Pb2+ | SBDD | B/C = 0.05%, 0.1%, 0.2% and 0.4%/Boron | SWASV | 15–362 nM | 3.38 nM | [90] |
| Pb2+ and Cd2+ | N-DNRs | n/a | SWASV | 50 nM–1 µM and 10 nM–1.1 µM, respectively | 50 and 10 nM, respectively | [91] |
| Pb2+ and Cu2+ | BDD | 8000 ppm/Boron | SWASV | 30–180 nM (for both) | 27 and 4 nM, respectively | [92] |
| Sb3+ | BDD | 1000 ppm or 1020 cm−3/Boron | DPASV | 2.44–7.31 µM | 108 nM | [93] |
| Zn2+ | BDD | n/a | DPASV | 0.5 nM–5 µM | 0.47 nM | [94] |
| Pb2+, Cu2+, and Hg2+ | BD-NCD | B/C = 0.3%/Boron | LSV | 1–22.5 µM (for Pb2+ and Cu2+) and 1–10 µM (for Hg2+) | 1.399, 0.102, and 0.666 µM, respectively | [95] |
| Ni2+, Cd2+, and Pb2+ | PVC-BDD | 7000–8000 ppm/Boron | DPASV | 0–100 nM, 0–40 nM, and 0–150 nM, respectively | 0.00424, 0.0221, and 0.25 nM, respectively | [96] |
| Zn2+, Cd2+, and Pb2+ | Bismuth modified BDD | 10000 ppm/Boron | SWASV | 15–183 nM, 9–105 nM, and 5–58 nM, respectively | 1.97, 0.57, and 0.51 nM, respectively | [97] |
| Ag+, Cu2+, Pb2+, Cd2+, and Zn2+ | NCD | n/a | DPASV | n/a | n/a | [98] |
| Zn2+, Cd2+, Pb2+, and Cu2+ | BDD | 1300 ppm/Boron | DPASV | 76–306 pM, 11–219 pM, 18.3–217 pM, and 47.2–315 pM, respectively | 24, 3.16, 5.55, and 14.2 pM, respectively | [99] |
| Cd2+, Pb2+, Cu2+, and Hg2+ | BDD | n/a | DPASV | 0.088–0.88 nM, 0.048–0.48 nM, 0.157–1.57 nM, and 0.05–0.5 nM, respectively | 30, 9.65, 1.57, and 3.49 pM, respectively | [100] |
| Pb2+, Cd2+, Zn2+, and Cu2+ | BDD | 7000–8000 ppm/Boron | ASV | n/a | n/a | [101] |
| Zn2+, Cd2+, Pb2+, and Cu2+ | D/G nanoplatelets | n/a | DPASV | 0.153–3.8 µM, 0.088–2.2 µM, 0.121–1.21 µM, and 0.157–3.93 µM, respectively | 26.3, 4.13, 23.5, and 7.1 nM, respectively | [102] |
| Fe3+, Cu2+, Zn2+, Pb2+, and Cd2+ | BDD | 8000 ppm/Boron | SWASV | 36–716 nM, 31–629 nM, 31–612 nM, 9.6–193 nM, and 17.56–351 nM, respectively | 35.45, 22.34, 27, 8.4, and 14 nM, respectively | [103] |
| Analyte | Electrode | Doping Concentration/Doping Atom | Method of Detection | Linear Range | Detection Limit (LOD) | Ref |
|---|---|---|---|---|---|---|
| N3− | BDD | 1 × 1019 cm−3/Boron | LSV, DPV, and flow injection analysis | 3.3 mM–0.30 µM | 8 nM | [104] |
| H2O2 | Pt-BDD | B/C = 1: 100/Boron and 5 × 1014 cm−2/Platinum | CV and flow injection analysis | 0.1 to 10 µM | 30 nM | [105] |
| H2O2 | BDD/PB | 1019–1020 cm−3/Boron | CV and flow injection analysis | n/a | n/a | [106] |
| I− | Diamond paste electrode | n/a | DPV | At pM–nM level | Subnanomolar level | [108] |
| I−/I2 | BDD | 0.1% wt/Boron | CV | 0–1.2 mM/0–0.6 mM | 20 µM/10 µM | [109] |
| F− at [FeF6]3−/[CeF6]2−/[FeF6] | BDD | n/a | LSV and SWV | n/a | 5 µM (LSV, [FeF6]3−), and 0.6 µM (SWV, [CeF6]2−) | [110] |
| OCl− | BDD | 1020 atoms cm−3/Boron | SWV and LSV | 0.02–1.5 M (by both) | n/a | [111] |
| OCl− | sp2-bonded carbon microspot-BDD | 1020 atoms cm−3/Boron | SWV and LSV | 58.31 µM–1.9 mM | 58.31 µM | [112] |
| RNO2− | BDD | n/a | DPV | 0.99–17 µM (absence of O2 at pH 4.0) and 0.99–11 µM (presence of O2 at pH 8.0) | 0.41 µM (absence of O2 at pH 4.0) and 0.34 µM (presence of O2 at pH 8.0) | [113] |
| NO2− | BDD | 7000–8000 ppm/Boron | SWV | 4 µM–4 mM | 20 µM | [114] |
| NO2− and HONO | BDD | B/C = 1% | CV | 1–5 mM (for both) | 0.24 and 1.27 nM, respectively | [115] |
| NO3− | BDD | n/a | LSV | 0–100 µM | 1.5 µM | [116] |
| NO3− | BDD | B/C = 0.1%, 1%, 2%, and 3% | LSV | n/a | n/a | [117] |
| S2O82− | BDD | 500–8000 ppm/Boron | SWV | n/a | n/a | [118] |
| C2O42− | ATAB-BDD | B/C = 10,000 ppm | Amperometry | 0.8–100 µM | 32 nM | [120] |
| S2− and NO2− | BDD | B/C = 0.1% | CV, SWV and DPV | 0.02–0.1 mM | n/a | [122] |
| NaNO2, CCl3, COOH, and H2O2 | Nafion/Mb/ND/CILE | n/a | CV | 0.02–6.60 mM, 1.1–30 mM, and 0.3–19 mM, respectively | 6.67, 370, and 100 µM, respectively | [124] |
| NaNO2, CCl3, COOH, and KBrO3 | Nafion/Hb/AuNPs/ND/CILE | n/a | CV | 0.07–2.6 mM, 1–500 mM, and 0.35–12 mM, respectively | 27, 330, and 3.3 µM, respectively | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shellaiah, M.; Sun, K.W. Diamond-Based Electrodes for Detection of Metal Ions and Anions. Nanomaterials 2022, 12, 64. https://doi.org/10.3390/nano12010064
Shellaiah M, Sun KW. Diamond-Based Electrodes for Detection of Metal Ions and Anions. Nanomaterials. 2022; 12(1):64. https://doi.org/10.3390/nano12010064
Chicago/Turabian StyleShellaiah, Muthaiah, and Kien Wen Sun. 2022. "Diamond-Based Electrodes for Detection of Metal Ions and Anions" Nanomaterials 12, no. 1: 64. https://doi.org/10.3390/nano12010064







