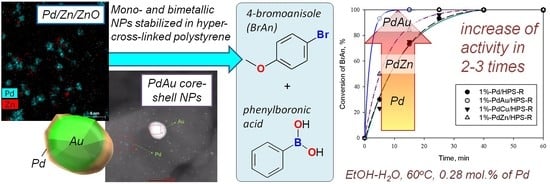
Mono- and Bimetallic Nanoparticles Stabilized by an Aromatic Polymeric Network for a Suzuki Cross-Coupling Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Synthesis and Characterization
2.3. Reaction Procedure and Analysis of the Reaction Mixture
- (i)
- Stirring rate from 500 rpm to 1300 rpm using 1 mmol of BrAn, 1.5 mmol of PBA, 1.5 mmol of NaOH, and 30 mg of catalyst (corresponds to 0.28 mol.% of Pd with respect to BrAn) at 60 °C;
- (ii)
- Catalyst loading in the range from 15 mg (0.14 mol.% of Pd) up to 50 mg (0.47 mol.% of Pd) using 1 mmol of BrAn, 1.5 mmol of PBA, and 1.5 mmol of NaOH at 60 °C and 900 rpm;
- (iii)
- Amounts of PBA (1.5–3.0 mmol) and NaOH (1.5–3.8 mmol) using 1 mmol of BrAn and 30 mg of catalyst at 60 °C and 900 rpm;
- (iv)
- Reaction temperature (40–70 °C) using 1 mmol of BrAn, 2.5 mmol of PBA, 3.0 mmol of NaOH, and 30 mg of catalyst at 900 rpm.
2.4. Catalysts Separation from the Reaction Mixture for Reuse
2.5. Hot-Filtration Test
3. Results
3.1. Catalyst Characterization
3.2. Suzuki Cross-Coupling
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, M.; Yang, R.; Wei, S.; Hu, X.; Xu, D.; Yang, J.; Dong, Z. Ultra-fine Pd nanoparticles confined in a porous organic polymer: A leaching-and-aggregation-resistant catalyst for the efficient reduction of nitroarenes by NaBH4. J. Colloid Interface Sci. 2019, 538, 720–730. [Google Scholar] [CrossRef]
- Karakhanov, E.; Maximov, A.; Terenina, M.; Vinokurov, V.; Kulikov, L.; Makeeva, D.; Glotov, A. Selective hydrogenation of terminal alkynes over palladium nanoparticles within the pores of amino-modified porous aromatic frameworks. Catal. Today 2020, 357, 176–184. [Google Scholar] [CrossRef]
- Masoumi, H.; Ghaemi, A.; Gilani, H.G. Evaluation of hyper-cross-linked polymers performances in the removal of hazardous heavy metal ions: A review. Sep. Purif. Technol. 2021, 260, 118221. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Zhu, G. Multifunctional porous aromatic frameworks: State of the art and opportunities. Energy Chem. 2020, 2, 100037. [Google Scholar] [CrossRef]
- Shao, L.; Sang, Y.; Liu, N.; Wei, Q.; Wang, F.; Zhan, P.; Luo, W.; Huang, J.; Chen, J. One-step synthesis of N-containing hyper-cross-linked polymers by two crosslinking strategies and their CO2 adsorption and iodine vapor capture. Sep. Purif. Technol. 2021, 262, 118352. [Google Scholar] [CrossRef]
- Wang, K.; Cui, W.; Bian, Z.; Liu, Y.; Jiang, S.; Zhou, Y.; Wang, J. Size and stability modulation of Pd nanoparticles on porous hypercrosslinked ionic polymer for heterogeneous aerobic oxidative coupling of diaryl ether. Appl. Catal. B Environ. 2021, 281, 119425. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.; Gao, S.; Wang, H.; He, Z.; Xu, Y.; Huang, K. In situ encapsulated ultrafine Pd nanoparticles in nitrogen-doped porous carbon derived from hyper-crosslinked polymers effectively catalyse hydrogenation. J. Catal. 2021, 396, 342–350. [Google Scholar] [CrossRef]
- Wang, K.; Jia, Z.; Yang, X.; Wang, L.; Gu, Y.; Tan, B. Acid and base coexisted heterogeneous catalysts supported on hypercrosslinked polymers for one-pot cascade reactions. J. Catal. 2017, 348, 168–176. [Google Scholar] [CrossRef]
- Wang, G.; Wu, Z.; Liang, Y.; Liu, W.; Zhan, H.; Song, M.; Sun, Y. Exploring the coordination confinement effect of divalent palladium/zero palladium doped polyaniline-networking: As an excellent-performance nanocomposite catalyst for C–C coupling reactions. J. Catal. 2020, 384, 177–188. [Google Scholar] [CrossRef]
- Kashin, A.S.; Ananikov, V.P. Catalytic C-C and C-heteroatom bond formation reactions: In situ generated or preformed catalysts? Complicated mechanistic picture behind well-known experimental procedures. J. Org. Chem. 2013, 78, 11117–11125. [Google Scholar] [CrossRef]
- Eremin, D.B.; Ananikov, V.P. Understanding active species in catalytic transformations: From molecular catalysis to nanoparticles, leaching, “cocktails” of catalysts and dynamic systems. Coord. Chem. Rev. 2017, 346, 2–19. [Google Scholar] [CrossRef]
- D’Alterio, M.C.; Casals-Cruañas, È.; Tzouras, N.V.; Talarico, G.; Nolan, S.P.; Poater, A. Mechanistic aspects of the palladium-catalyzed Suzuki-Miyaura cross-coupling reaction. Chem. Eur. J. 2021, 27, 13481–13493. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.-P.; Jutand, A.; Tian, Z.-Q.; Amatore, C. Au-Pd core-shell nanoparticles catalyze Suzuki–Miyaura reactions in water through Pd leaching. Angew. Chem. Int. Ed. 2011, 50, 12184–12188. [Google Scholar] [CrossRef] [Green Version]
- Nasrollahzadeh, M.; Azarian, A.; Maham, M.; Ehsani, A. Synthesis of Au/Pd bimetallic nanoparticles and their application in the Suzuki coupling reaction. J. Ind. Eng. Chem. 2015, 21, 746–748. [Google Scholar] [CrossRef]
- Wen, M.C.; Takakura, S.; Fuku, K.; Mori, K.; Yamashita, H. Enhancement of Pd-catalyzed Suzuki-Miyaura coupling reaction assisted by localized surface plasmon resonance of Au nanorods. Catal. Today 2015, 242, 381–385. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Chen, H.; Jiang, R.; Sun, L.-D.; Li, Q.; Wang, J.; Yu, J.C.; Yan, C.-H. Plasmonic harvesting of light energy for Suzuki coupling reactions. J. Am. Chem. Soc. 2013, 135, 5588–5601. [Google Scholar] [CrossRef]
- Nemygina, N.; Nikoshvili, L.; Tiamina, I.; Bykov, A.; Smirnov, I.; LaGrange, T.; Kaszkur, Z.; Matveeva, V.; Sulman, E.; Kiwi-Minsker, L. Au(core)/Pd(shell) bimetallic nanoparticles immobilized within hyper-crosslinked polystyrene for mechanistical study of Suzuki cross-coupling: Homogeneous or heterogeneous catalysis? Org. Proc. Res. Dev. 2018, 22, 1606–1613. [Google Scholar] [CrossRef]
- Mohan, M.K.; Sunajadevi, K.R.; Daniel, N.K.; Gopi, S.; Sugunan, S.; Perumparakunnel, N.C. Cu/Pd bimetallic supported on mesoporous TiO2 for Suzuki coupling reaction. Bull. Chem. React. Eng. Catal. 2018, 13, 287–294. [Google Scholar] [CrossRef]
- Deng, J.Z.; Paone, D.V.; Ginnetti, A.T.; Kurihara, H.; Dreher, S.D.; Weissman, S.A.; Stauffer, S.R.; Burgey, C.S. Copper-Facilitated Suzuki Reactions: Application to 2-Heterocyclic Boronates. Org. Lett. 2009, 11, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Crowley, B.M.; Potteiger, C.M.; Deng, J.Z.; Prier, C.K.; Paone, D.V.; Burgey, C.S. Expanding the scope of the Cu assisted Suzuki–Miyaura reaction. Tetrahedron Lett. 2011, 52, 5055–5059. [Google Scholar] [CrossRef]
- Procter, R.J.; Dunsford, J.J.; Rushworth, P.J.; Hulcoop, D.G.; Layfield, R.A.; Ingleson, M.J. A zinc catalyzed C(sp3)-C(sp2) Suzuki–Miyaura cross-coupling reaction mediated by aryl-zincates. Chem. Eur. J. 2017, 23, 15889–15893. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.-X.; Hu, M.; Liu, B.; Liu, J.; Wang, P.; Yao, J.; Zhang, X.; He, M.; Song, W. Pd-Zn alloy nanoparticles encapsulated into mesoporous silica with confinement effect for highly selective semi-hydrogenation of phenylacetylene. ChemCatChem 2021, 13, 868–873. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Xu, H. Interaction between Pd and ZnO during reduction of Pd/ZnO catalyst for steam reforming of methanol to hydrogen. Chin. J. Catal. 2006, 27, 217–222. [Google Scholar] [CrossRef]
- Barrios, C.E.; Baltanás, M.A.; Bosco, M.V.; Bonivardi, A.L. On the surface nature of bimetallic PdZn particles supported on a ZnO-CeO2 nanocomposite for the methanol steam reforming reaction. Catal. Lett. 2018, 148, 2233–2246. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowker, M.; Jones, W.; Hayward, J.; Ruiz Esquius, J.; Morgan, D.J.; Hutchings, G.J. PdZn catalysts for CO2 hydrogenation to methanol using chemical vapour impregnation (CVI). Faraday Discuss. 2017, 197, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Kast, P.; Friedrich, M.; Girgsdies, F.; Kröhnert, J.; Teschner, D.; Lunkenbein, T.; Behrens, M.; Schlögl, R. Strong metal-support interaction and alloying in Pd/ZnO catalysts for CO oxidation. Catal. Today 2016, 260, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Sulman, E.M.; Nikoshvili, L.Z.; Matveeva, V.G.; Tyamina, I.Y.; Sidorov, A.I.; Bykov, A.V.; Demidenko, G.N.; Stein, B.D.; Bronstein, L.M. Palladium containing catalysts based on hypercrosslinked polystyrene for selective hydrogenation of acetylene alcohols. Top. Catal. 2012, 55, 492–497. [Google Scholar] [CrossRef]
- Nikoshvili, L.; Nemygina, N.; Bykov, A.; Sidorov, A.; Matveeva, V.; Tyamina, I.; Sulman, M.; Sulman, E.; Stein, B. Synthesis of 4-methoxybiphenyl using Pd-containing catalysts based on polymeric matrix of functionalized hypercrosslinked polystyrene. Bull. Chem. React. Eng. Catal. 2015, 10, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Pentsak, E.O.; Eremin, D.B.; Gordeev, E.G.; Ananikov, V.P. Phantom reactivity in organic and catalytic reactions as a consequence of microscale destruction and contamination-trapping effects of magnetic stir bars. ACS Catal. 2019, 9, 3070–3081. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST). X-Ray Photoelectron Spectroscopy Database. Version 4.1; 2012. Available online: http://srdata.nist.gov/xps/ (accessed on 21 November 2020).
- Wu, T.; Kaden, W.E.; Kunkel, W.A.; Anderson, S.L. Size-dependent oxidation of Pdn (n ≤ 13) on alumina/NiAl(110): Correlation with Pd core level binding energies. Surf. Sci. 2009, 603, 2764–2770. [Google Scholar] [CrossRef]
- Rupp, H.; Weser, U. Copper(I) and copper(II) in complexes of biochemical significance studied by X-ray photoelectron spectroscopy. BBA—Protein Struct. 1976, 446, 151–156. [Google Scholar] [CrossRef]
- Mar, G.L.; Timbrell, P.Y.; Lamb, R.N. Formation of zinc oxide thin films by the thermal decomposition of zinc acetate. In Surface Science. Springer Proceedings in Physics; Howe, R.F., Lamb, R.N., Wandelt, K., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; Volume 73, pp. 177–192. [Google Scholar]
- Nikoshvili, L.; Bakhvalova, E.S.; Bykov, A.V.; Sidorov, A.I.; Vasiliev, A.L.; Matveeva, V.G.; Sulman, M.G.; Sapunov, V.N.; Kiwi-Minsker, L. Study of deactivation in Suzuki reaction of polymer-stabilized Pd nanocatalysts. Processes 2020, 8, 1653. [Google Scholar] [CrossRef]
- Xiao, Q.; Sarina, S.; Jaatinen, E.; Jia, J.; Arnold, D.P.; Liu, H.; Zhu, H. Efficient photocatalytic Suzuki cross-coupling reactions on Au-Pd alloy nanoparticles under visible light irradiation. Green Chem. 2014, 16, 4272–4285. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bhattacharya, S.K. Size-dependent catalytic activity and fate of palladium nanoparticles in Suzuki-Miyaura coupling reactions. ACS Omega 2018, 3, 12905–12913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chistyakov, A.V.; Tsodikov, M.V.; Murzin, V.Y.; Yandieva, F.A.; Zubavichus, Y.V.; Kozitsyna, N.Y.; Gekhman, A.E.; Kriventsov, V.V.; Moiseev, I.I. Cocatalytic effect of palladium and zinc in the condensation of alcohol carbon backbones into hydrocarbons. Kinet. Catal. 2011, 52, 258–272. [Google Scholar] [CrossRef]
- Nemygina, N.; Nikoshvili, L.; Bykov, A.; Sidorov, A.; Molchanov, V.; Sulman, M.; Tiamina, I.; Stein, B.; Matveeva, V.; Sulman, E.; et al. Catalysts of Suzuki cross-coupling based on functionalized hyper-cross-linked polystyrene: Influence of precursor nature. Org. Process Res. Dev. 2016, 20, 1453–1460. [Google Scholar] [CrossRef]
- Crawford, K.A.; Cowley, A.H.; Humphrey, S.M. Bis(imino)acenaphthene (BIAN)-supported palladium(II) carbene complexes as effective C–C coupling catalysts and solvent effects in organic and aqueous media. Catal. Sci. Technol. 2014, 4, 1456–1464. [Google Scholar] [CrossRef] [Green Version]
- Bykov, A.V.; Demidenko, G.N.; Nikoshvili, L.Z.; Sulman, M.G.; Kiwi-Minsker, L. Hydrogenation of benzene-toluene mixture using metal nanoparticles stabilized by hyper-cross-linked aromatic polymer. Chem. Eng. Technol. 2021, 44, 1955–1961. [Google Scholar] [CrossRef]
- Bykov, A.V.; Nikoshvili, L.Z.; Lyubimova, N.A.; Komar, K.P. Effect of the conditions of thermal reduction on the formation, stability, and catalytic properties of polymer-stabilized palladium nanoparticles in the selective hydrogenation of acetylene alcohols. Catal. Ind. 2014, 6, 182–189. [Google Scholar] [CrossRef]
- Speziali, M.G.; da Silva, A.G.M.; Vaz de Miranda, D.M.; Monteiro, A.L.; Robles-Dutenhefner, P.A. Air stable ligandless heterogeneous catalyst systems based on Pd and Au supported in SiO2 and MCM-41 for Suzuki-Miyaura cross-coupling in aqueous medium. Appl. Catal. A Gen. 2013, 39, 462–463. [Google Scholar] [CrossRef]
- Heugebaert, T.S.A.; De Corte, S.; Sabbe, T.; Hennebel, T.; Verstraete, W.; Boon, N.; Stevens, C.V. Biodeposited Pd/Au bimetallic nanoparticles as novel Suzuki catalysts. Tetrahedron Lett. 2012, 53, 1410–1412. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; Jiang, H.; Wu, L.; Ding, N.; Jiang, P.; Zhang, H.; Zhao, L.; Yin, F.; Yang, Q. Anchoring Pd(OAc)2 on amide-bonded covalent organic frameworks: An efficient heterogeneous Pd@OC-MA catalyst for Suzuki-Miyaura coupling reactions in water. Tetrahedron 2020, 76, 131664. [Google Scholar] [CrossRef]
- Yadav, C.; Maka, V.K.; Payra, S.; Moorthy, J.N. Multifunctional porous organic polymers (POPs): Inverse adsorption of hydrogen over nitrogen, stabilization of Pd(0) nanoparticles, and catalytic cross-coupling reactions and reductions. J. Catal. 2020, 384, 61–71. [Google Scholar] [CrossRef]













| Sample | SSABET, m2/g | SSAt-plot, m2/g 1 |
|---|---|---|
| HPS | 793 | 189 2; 603 3 |
| 1%-Pd/HPS | 668 | 104 2; 572 3 |
| 1%-Pd/HPS-R | 595 | 133 2; 459 3 |
| 0.5%-PdAu/HPS | 713 | 141 2; 593 3 |
| 0.5%-PdAu/HPS-R | 655 | 148 2; 507 3 |
| 1%-PdAu/HPS | 744 | 187 2; 556 3 |
| 1%-PdCu/HPS | 872 | 123 2; 747 3 |
| 1%-PdCu/HPS-R | 414 | 264 2; 148 3 |
| 1%-PdZn/HPS | 851 | 179 2; 672 3 |
| 1%-PdZn/HPS-R | 732 | 166 2; 588 3 |
| Sample | Maximum Conversion of BrAn, % | Yield of MBP, % | R0, molBrAn/(molPd·min) 2 |
|---|---|---|---|
| 1%-Pd/HPS | 99 | 89 | 26.2 |
| 1%-Pd/HPS-R | ~100 | 89 | 21.3 |
| 1%-Pd/HPS-R 1 | 92 | 87 | 11.0 |
| 0.5%-PdAu/HPS-R | 99 | 93 | 63.0 |
| 0.5%-PdAu/HPS-R 1 | 100 | 92 | 61.6 |
| 1%-PdAu/HPS | 100 | 91 | 66.6 |
| 1%-PdAu/HPS-R | 100 | 85 | 73.5 |
| 1%-PdAu/HPS-R 1 | ~100 | 94 | 65.9 |
| 1%-PdCu/HPS | 100 | 93 | 38.5 |
| 1%-PdCu/HPS-R | 100 | 92 | 13.7 |
| 1%-PdZn/HPS | 100 | 89 | 8.7 |
| 1%-PdZn/HPS-R | 100 | 90 | 23.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikoshvili, L.Z.; Shkerina, K.N.; Bykov, A.V.; Sidorov, A.I.; Vasiliev, A.L.; Sulman, M.G.; Kiwi-Minsker, L. Mono- and Bimetallic Nanoparticles Stabilized by an Aromatic Polymeric Network for a Suzuki Cross-Coupling Reaction. Nanomaterials 2022, 12, 94. https://doi.org/10.3390/nano12010094
Nikoshvili LZ, Shkerina KN, Bykov AV, Sidorov AI, Vasiliev AL, Sulman MG, Kiwi-Minsker L. Mono- and Bimetallic Nanoparticles Stabilized by an Aromatic Polymeric Network for a Suzuki Cross-Coupling Reaction. Nanomaterials. 2022; 12(1):94. https://doi.org/10.3390/nano12010094
Chicago/Turabian StyleNikoshvili, Linda Zh., Kristina N. Shkerina, Alexey V. Bykov, Alexander I. Sidorov, Alexander L. Vasiliev, Mikhail G. Sulman, and Lioubov Kiwi-Minsker. 2022. "Mono- and Bimetallic Nanoparticles Stabilized by an Aromatic Polymeric Network for a Suzuki Cross-Coupling Reaction" Nanomaterials 12, no. 1: 94. https://doi.org/10.3390/nano12010094
APA StyleNikoshvili, L. Z., Shkerina, K. N., Bykov, A. V., Sidorov, A. I., Vasiliev, A. L., Sulman, M. G., & Kiwi-Minsker, L. (2022). Mono- and Bimetallic Nanoparticles Stabilized by an Aromatic Polymeric Network for a Suzuki Cross-Coupling Reaction. Nanomaterials, 12(1), 94. https://doi.org/10.3390/nano12010094