Tackling Structural Complexity in Li2S-P2S5 Solid-State Electrolytes Using Machine Learning Potentials
Abstract
:1. Introduction
2. Methods
Computational Details
3. Results
3.1. Lithium Ion Mobility
3.2. LiPS Phase Transition
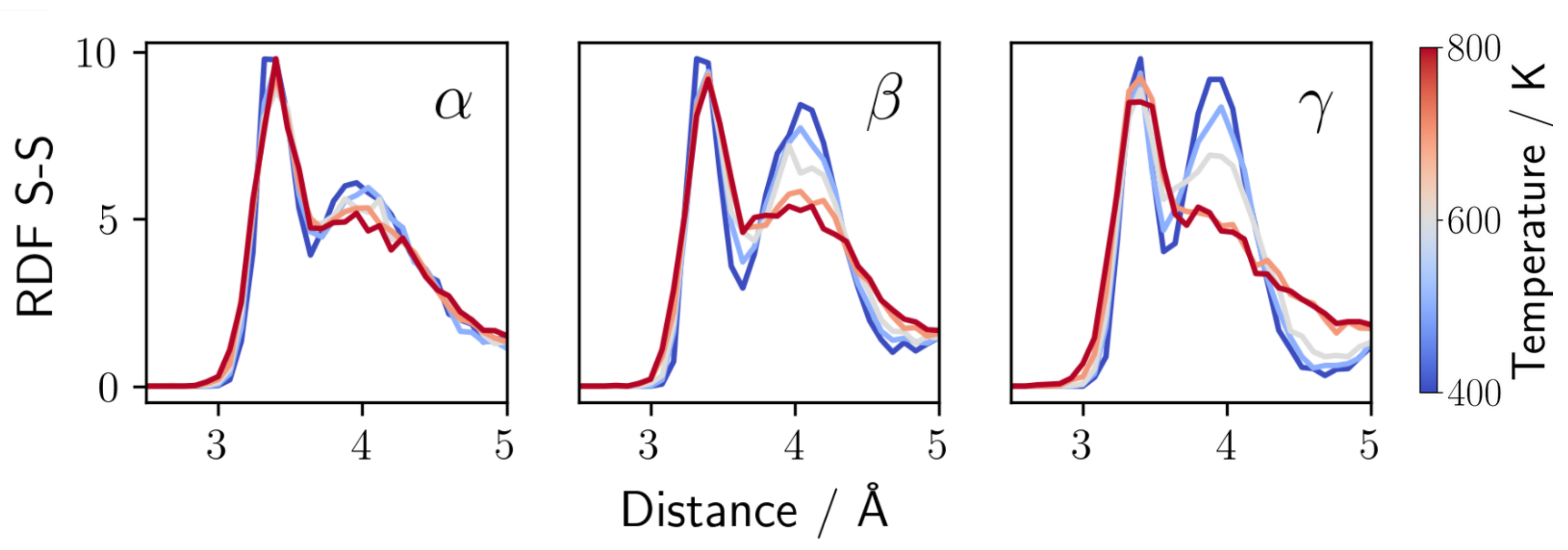
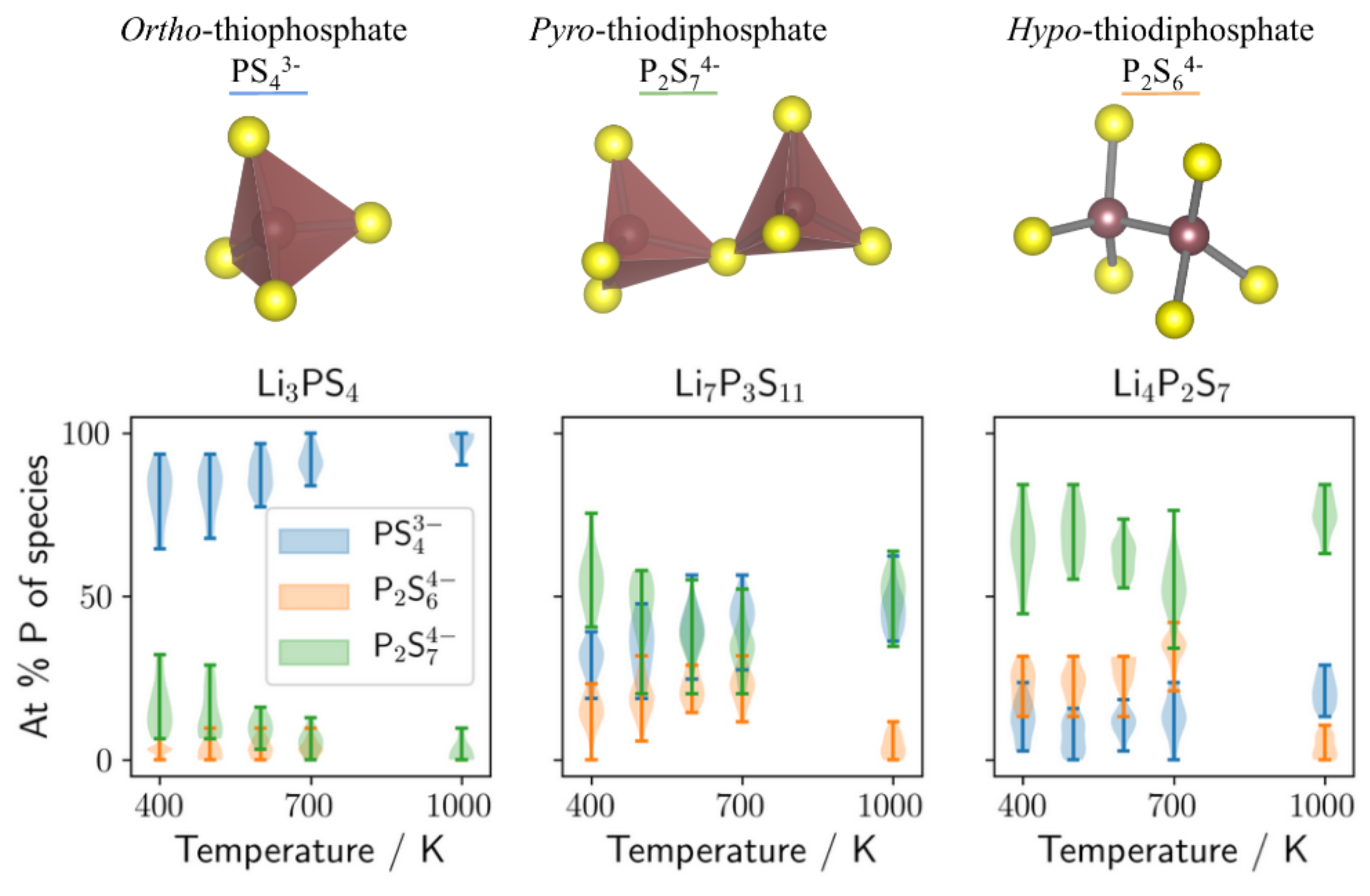
3.3. The Role of Anion Composition in LiS-PS Glasses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LPS | Lithium thiophosphate |
| SSE | Solid-state electrolytes |
| GAP | Gaussian Approximation Potential |
| ASS-LIB | All-solid-state lithium-ion batteries |
| NEB | Nudged-Elastic-Band |
| ML | Machine Learning |
| MD | Molecular Dynamics |
| DFT | Density-functional theory |
| MSD | Mean-square-displacement |
| RDF | Radial distribution function |
| HCP | Hexagonal close-packed |
References
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Luntz, A.C.; Voss, J.; Reuter, K. Interfacial challenges in solid-state Li ion batteries. J. Phys. Chem. Lett. 2015, 6, 4599–4604. [Google Scholar] [CrossRef] [PubMed]
- Oudenhoven, J.F.; Baggetto, L.; Notten, P.H. All-solid-state lithium-ion microbatteries: A review of various three-dimensional concepts. Adv. Energy Mater. 2011, 1, 10–33. [Google Scholar] [CrossRef]
- Shoji, M.; Cheng, E.J.; Kimura, T.; Kanamura, K. Recent progress for all solid state battery using sulfide and oxide solid electrolytes. J. Phys. D Appl. Phys. 2019, 52, 103001. [Google Scholar] [CrossRef]
- Kudu, Ö.U.; Famprikis, T.; Fleutot, B.; Braida, M.D.; Le Mercier, T.; Islam, M.S.; Masquelier, C. A review of structural properties and synthesis methods of solid electrolyte materials in the Li2S-P2S5 binary system. J. Power Sources 2018, 407, 31–43. [Google Scholar] [CrossRef]
- Robinson, A.L.; Janek, J. Solid-state batteries enter EV fray. MRS Bull. 2014, 39, 1046–1047. [Google Scholar] [CrossRef]
- Kim, K.J.; Balaish, M.; Wadaguchi, M.; Kong, L.; Rupp, J.L. Solid-state Li–metal batteries: Challenges and horizons of oxide and sulfide solid electrolytes and their interfaces. Adv. Energy Mater. 2021, 11, 2002689. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Q.; Urban, A.; Artrith, N. Artificial Intelligence-Aided Mapping of the Structure–Composition–Conductivity Relationships of Glass–Ceramic Lithium Thiophosphate Electrolytes. Chem. Mater. 2022, 34, 6702–6712. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef]
- Aono, H.; Sugimoto, E.; Sadaoka, Y.; Imanaka, N.; Adachi, G.-y. Ionic Conductivity of Solid Electrolytes Based on Lithium Titanium Phosphate. J. Electrochem. Soc. 1990, 137, 1023–1027. [Google Scholar] [CrossRef]
- Tachez, M.; Malugain, J.; Mercire, R.; Robert, G. Ionic conductivity of and phase transition in lithium thiophosphate Li3PS4. Solid State Ion. 1984, 14, 181–185. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Q.; Cui, Y.; Chen, Y.; Shi, S.; Wang, R.Z.; Yan, H. Elastic Properties, Defect Thermodynamics, Electrochemical Window, Phase Stability, and Li+ Mobility of Li3PS4: Insights from First-Principles Calculations. ACS Appl. Mater. Interfaces 2016, 8, 25229–25242. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, H.; Mori, S.; Morimoto, H.; Hayashi, A.; Tatsumisago, M. Direct observation of a non-crystalline state of Li2S–P2S5 solid electrolytes. Sci. Rep. 2017, 7, 4142. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, W.; Payzant, E.A.; Yu, X.; Wu, Z.; Dudney, N.J.; Kiggans, J.; Hong, K.; Rondinone, A.J.; Liang, C. Anomalous High Ionic Conductivity of Nanoporous β-Li3PS4. J. Am. Chem. Soc. 2013, 135, 975–978. [Google Scholar] [CrossRef]
- Wenzel, S.; Weber, D.A.; Leichtweiss, T.; Busche, M.R.; Sann, J.; Janek, J. Interphase formation and degradation of charge transfer kinetics between a lithium metal anode and highly crystalline Li7P3S11 solid electrolyte. Solid State Ion. 2016, 286, 24–33. [Google Scholar] [CrossRef]
- Dietrich, C.; Weber, D.A.; Sedlmaier, S.J.; Indris, S.; Culver, S.P.; Walter, D.; Janek, J.; Zeier, W.G. Lithium ion conductivity in Li2S-P2S5 glasses–building units and local structure evolution during the crystallization of superionic conductors Li3PS4, Li7P3S11 and Li4P2S7. J. Mater. Chem. A 2017, 5, 18111–18119. [Google Scholar] [CrossRef]
- Kim, J.S.; Jung, W.D.; Choi, S.; Son, J.W.; Kim, B.K.; Lee, J.H.; Kim, H. Thermally Induced S-Sublattice Transition of Li3PS4 for Fast Lithium-Ion Conduction. J. Phys. Chem. Lett. 2018, 9, 5592–5597. [Google Scholar] [CrossRef]
- Staacke, C.G.; Heenen, H.H.; Scheurer, C.; Csányi, G.; Reuter, K.; Margraf, J.T. On the Role of Long-Range Electrostatics in Machine-Learned Interatomic Potentials for Complex Battery Materials. ACS Appl. Energy Mater. 2021, 4, 12562–12569. [Google Scholar] [CrossRef]
- Timmermann, J.; Lee, Y.; Staacke, C.G.; Margraf, J.T.; Scheurer, C.; Reuter, K. Data-efficient iterative training of Gaussian approximation potentials: Application to surface structure determination of rutile IrO2 and RuO2. J. Chem. Phys. 2021, 155, 244107. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Blum, V.; Gehrke, R.; Hanke, F.; Havu, P.; Havu, V.; Ren, X.; Reuter, K.; Scheffler, M. Ab initio molecular simulations with numeric atom-centered orbitals. Comput. Phys. Commun. 2009, 180, 2175–2196. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Bartók, A.P.; Payne, M.C.; Kondor, R.; Csányi, G. Gaussian approximation potentials: The accuracy of quantum mechanics, without the electrons. Phys. Rev. Lett. 2010, 104, 136403. [Google Scholar] [CrossRef] [PubMed]
- Bartók, A.P.; Kondor, R.; Csányi, G. On representing chemical environments. Phys. Rev. B 2013, 87, 184115. [Google Scholar] [CrossRef]
- Larsen, A.H.; Mortensen, J.J.; Blomqvist, J.; Castelli, I.E.; Christensen, R.; Dułak, M.; Friis, J.; Groves, M.N.; Hammer, B.; Hargus, C.; et al. The atomic simulation environment—A Python library for working with atoms. J. Phys. Condens. Matter 2017, 29, 273002. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar] [CrossRef]
- Wang, Y.; Richards, W.D.; Ong, S.P.; Miara, L.J.; Kim, J.C.; Mo, Y.; Ceder, G. Design principles for solid-state lithium superionic conductors. Nat. Mater. 2015, 14, 1026–1031. [Google Scholar] [CrossRef]
- Chu, I.H.; Nguyen, H.; Hy, S.; Lin, Y.C.; Wang, Z.; Xu, Z.; Deng, Z.; Meng, Y.S.; Ong, S.P. Insights into the performance limits of the Li7P3S11 superionic conductor: A combined first-principles and experimental study. ACS Appl. Mater. Interfaces 2016, 8, 7843–7853. [Google Scholar] [CrossRef]
- Scheurer, C. Model Structures for Glass Phases of the Solid-State Electrolyte LPS. 2022. Available online: https://edmond.mpdl.mpg.de/dataset.xhtml?persistentId=doi:10.17617/3.VZHSXS (accessed on 17 August 2022). [CrossRef]
- Homma, K.; Yonemura, M.; Kobayashi, T.; Nagao, M.; Hirayama, M.; Kanno, R. Crystal structure and phase transitions of the lithium ionic conductor Li3PS4. Solid State Ion. 2011, 182, 53–58. [Google Scholar] [CrossRef]
- Phuc, N.H.H.; Totani, M.; Morikawa, K.; Muto, H.; Matsuda, A. Preparation of Li3PS4 solid electrolyte using ethyl acetate as synthetic medium. Solid State Ion. 2016, 288, 240–243. [Google Scholar] [CrossRef]
- Busche, M.R.; Weber, D.A.; Schneider, Y.; Dietrich, C.; Wenzel, S.; Leichtweiss, T.; Schröder, D.; Zhang, W.; Weigand, H.; Walter, D.; et al. In Situ Monitoring of Fast Li-Ion Conductor Li7P3S11 Crystallization Inside a Hot-Press Setup. Chem. Mater. 2016, 28, 6152–6165. [Google Scholar] [CrossRef]
- Hayashi, A.; Minami, K.; Ujiie, S.; Tatsumisago, M. Preparation and ionic conductivity of Li7P3S11-z glass-ceramic electrolytes. J. Non-Cryst. Solids 2010, 356, 2670–2673. [Google Scholar] [CrossRef]
- Ohara, K.; Mitsui, A.; Mori, M.; Onodera, Y.; Shiotani, S.; Koyama, Y.; Orikasa, Y.; Murakami, M.; Shimoda, K.; Mori, K.; et al. Structural and electronic features of binary Li2S-P2S5 glasses. Sci. Rep. 2016, 6, 21302. [Google Scholar] [CrossRef]
- Dietrich, C.; Weber, D.A.; Culver, S.; Senyshyn, A.; Sedlmaier, S.J.; Indris, S.; Janek, J.; Zeier, W.G. Synthesis, Structural Characterization, and Lithium Ion Conductivity of the Lithium Thiophosphate Li2P2S6. Inorg. Chem. 2017, 56, 6681–6687. [Google Scholar] [CrossRef]
- Smith, J.G.; Siegel, D.J. Low-temperature paddlewheel effect in glassy solid electrolytes. Nat. Comm. 2020, 11, 1483. [Google Scholar] [CrossRef]
- Timmermann, J.; Kraushofer, F.; Resch, N.; Li, P.; Wang, Y.; Mao, Z.; Riva, M.; Lee, Y.; Staacke, C.; Schmid, M.; et al. IrO2 Surface Complexions Identified through Machine Learning and Surface Investigations. Phys. Rev. Lett. 2020, 125, 206101. [Google Scholar] [CrossRef]
- Staacke, C.G.; Wengert, S.; Kunkel, C.; Csányi, G.; Reuter, K.; Margraf, J.T. Kernel charge equilibration: Efficient and accurate prediction of molecular dipole moments with a machine-learning enhanced electron density model. Mach. Learn. Sci. Technol. 2022, 3, 015032. [Google Scholar] [CrossRef]
- Stegmaier, S.; Schierholz, R.; Povstugar, I.; Barthel, J.; Rittmeyer, S.P.; Yu, S.; Wengert, S.; Rostami, S.; Hans, K.; Reuter, K.; et al. Nano-Scale Complexions Facilitate Li Dendrite-Free Operation in LATP Solid-State Electrolyte. Adv. Energy Mater 2021, 11, 2100707. [Google Scholar] [CrossRef]
- Türk, H.; Schmidt, F.P.; Götsch, T.; Girgsdies, F.; Hammud, A.; Ivanov, D.; Vinke, I.C.; de Haart, L.; Eichel, R.A.; Reuter, K.; et al. Complexions at the Electrolyte/Electrode Interface in Solid Oxide Cells. Adv. Mater. Interfaces 2021, 8, 2100967. [Google Scholar] [CrossRef]
- Mehrer, H. Diffusion in Solids: Fundamentals, Methods, Materials, Diffusion-Controlled Processes; Springer Science & Business Media: Cham, Switzerland, 2007; Volume 155. [Google Scholar]
- Frenkel, D.; Smit, B. Understanding Molecular Simulation: From Algorithms to Applications; Elsevier: Alpharetta, GA, USA, 2001; Volume 1. [Google Scholar]
- Seino, Y.; Ota, T.; Takada, K.; Hayashi, A.; Tatsumisago, M. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 2014, 2, 627–631. [Google Scholar] [CrossRef]
- De Klerk, N.; van der Maas, E.; Wagemaker, M. Analysis of Diffusion in Solid-State Electrolytes through MD Simulations, Improvement of the Li-Ion Conductivity in β-Li3PS4 as an Example. ACS Appl. Energy Mater. 2018, 2, 3230–3242. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Kawamura, Y. Structure and ionic conductivity of Li2S–P2S5 glass electrolytes simulated with first-principles molecular dynamics. Front. Energy Res. 2016, 4, 22. [Google Scholar] [CrossRef]
- Shiotani, S.; Ohara, K.; Tsukasaki, H.; Mori, S.; Kanno, R. Pair distribution function analysis of sulfide glassy electrolytes for all-solid-state batteries: Understanding the improvement of ionic conductivity under annealing condition. Sci. Rep. 2017, 7, 6972. [Google Scholar] [CrossRef]
- Seino, Y.; Nakagawa, M.; Senga, M.; Higuchi, H.; Takada, K.; Sasaki, T. Analysis of the structure and degree of crystallisation of 70Li2S–30P2S5 glass ceramic. J. Mater. Chem. A 2015, 3, 2756–2761. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staacke, C.G.; Huss, T.; Margraf, J.T.; Reuter, K.; Scheurer, C. Tackling Structural Complexity in Li2S-P2S5 Solid-State Electrolytes Using Machine Learning Potentials. Nanomaterials 2022, 12, 2950. https://doi.org/10.3390/nano12172950
Staacke CG, Huss T, Margraf JT, Reuter K, Scheurer C. Tackling Structural Complexity in Li2S-P2S5 Solid-State Electrolytes Using Machine Learning Potentials. Nanomaterials. 2022; 12(17):2950. https://doi.org/10.3390/nano12172950
Chicago/Turabian StyleStaacke, Carsten G., Tabea Huss, Johannes T. Margraf, Karsten Reuter, and Christoph Scheurer. 2022. "Tackling Structural Complexity in Li2S-P2S5 Solid-State Electrolytes Using Machine Learning Potentials" Nanomaterials 12, no. 17: 2950. https://doi.org/10.3390/nano12172950
APA StyleStaacke, C. G., Huss, T., Margraf, J. T., Reuter, K., & Scheurer, C. (2022). Tackling Structural Complexity in Li2S-P2S5 Solid-State Electrolytes Using Machine Learning Potentials. Nanomaterials, 12(17), 2950. https://doi.org/10.3390/nano12172950




