Biologically Synthesized Silver Nanoparticles and Their Diverse Applications
Abstract
:1. Introduction
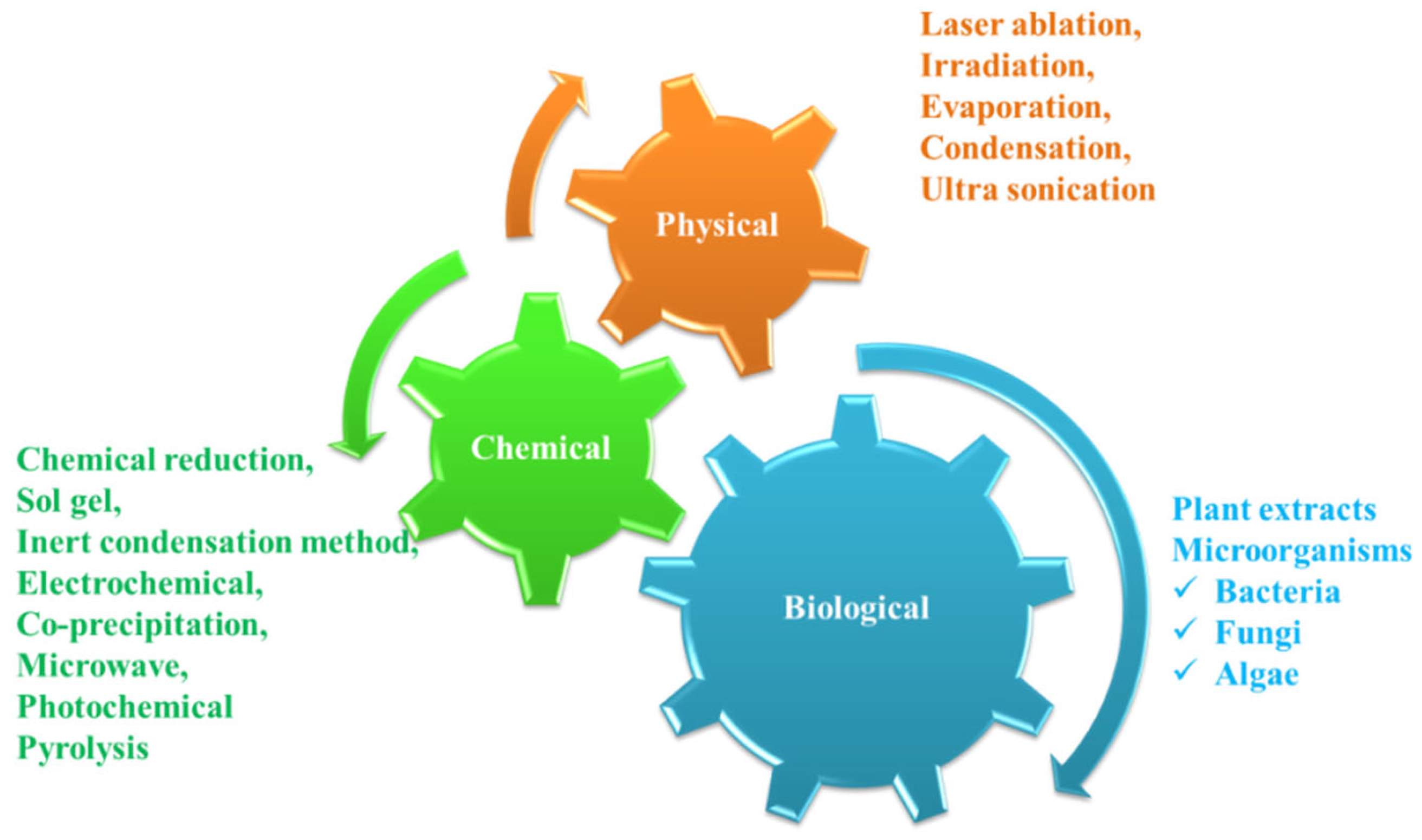
2. Types of AgNPs Synthesis
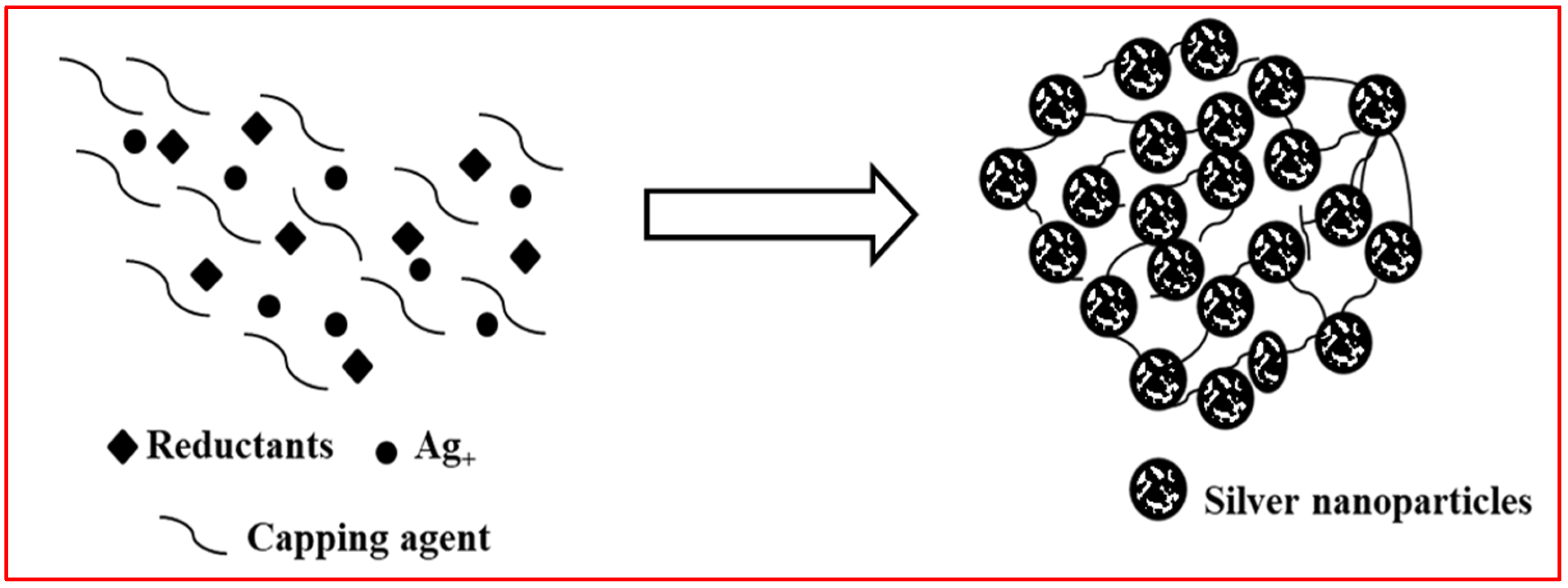
2.1. AgNPs Synthesis by Physical and Chemical Approaches
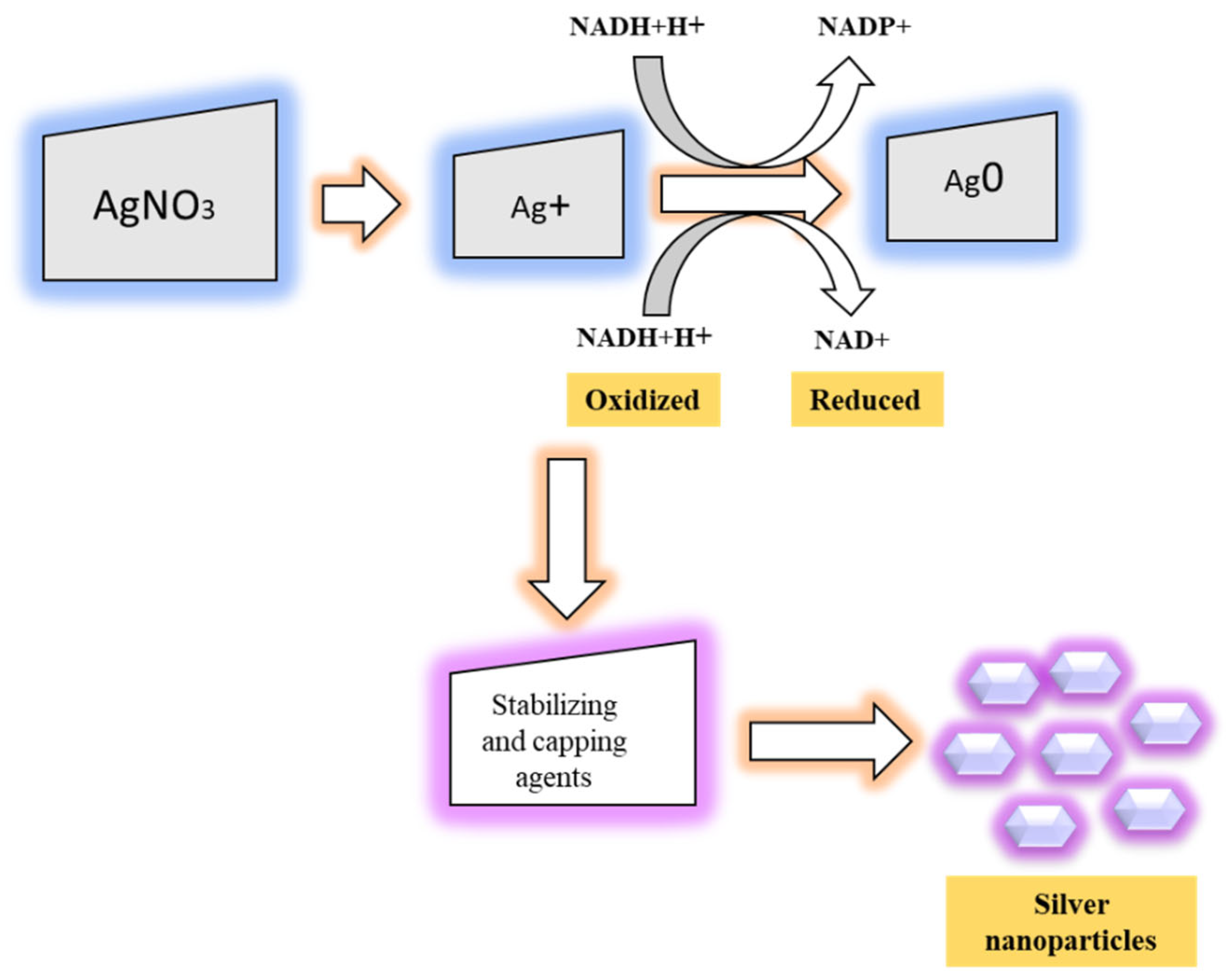
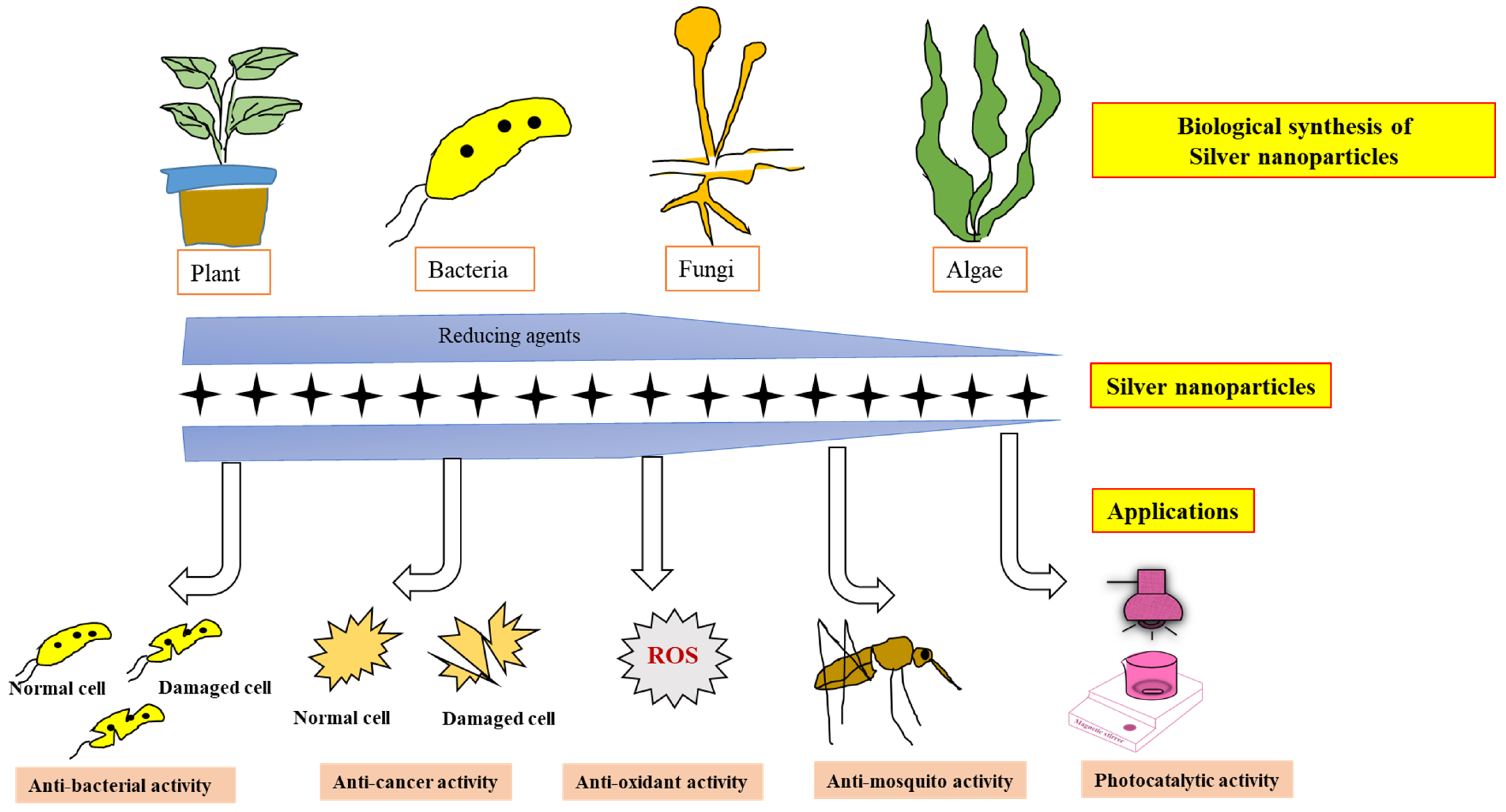
2.2. Synthesis of NPs from Biological Sources
2.3. Comprehensive Analyses of Commercial Products Involving Silver Nanoparticle Synthesis
3. Applications of AgNPs
3.1. Antioxidant Properties
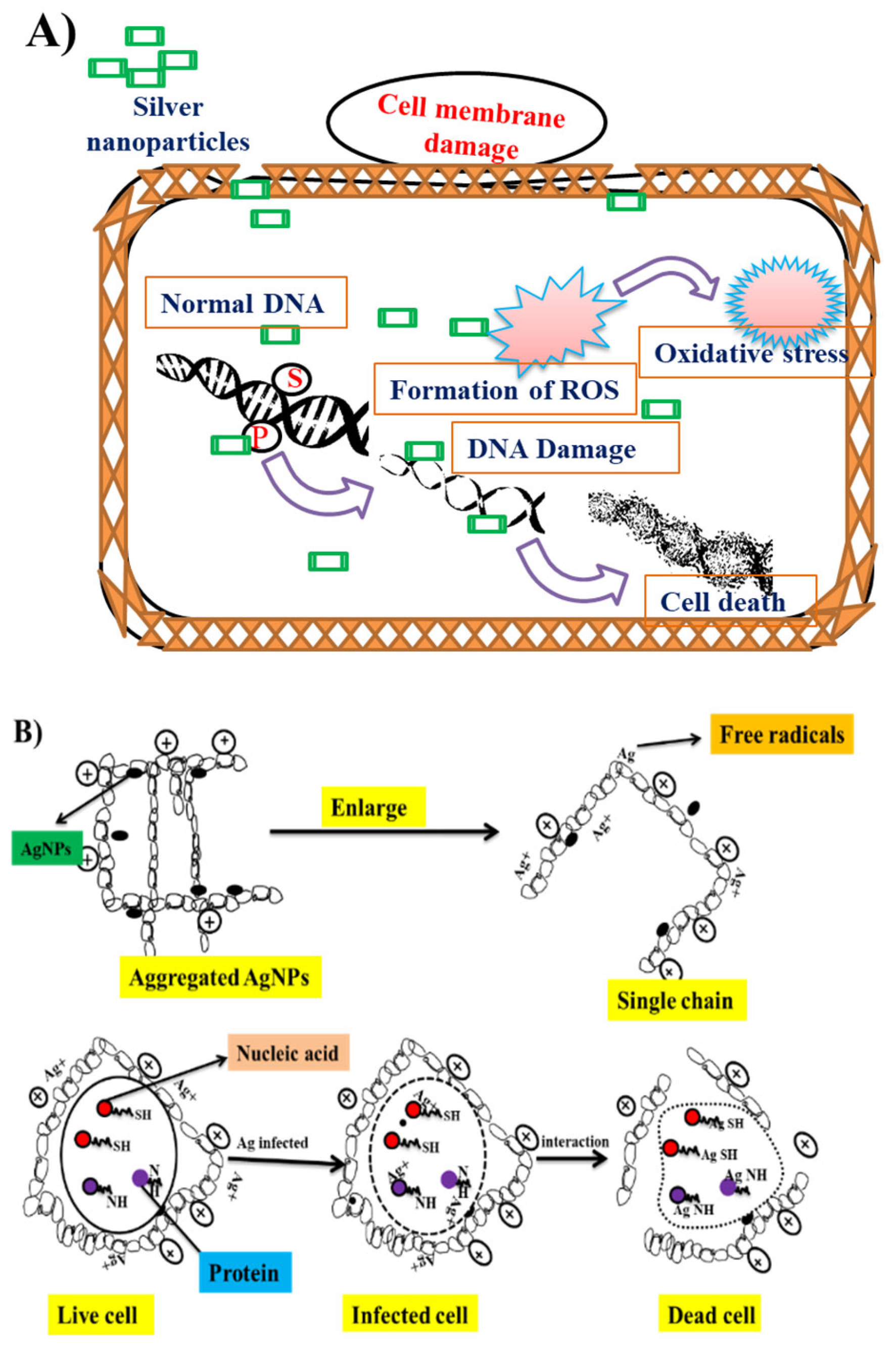
3.2. Antibacterial Properties of AgNPs
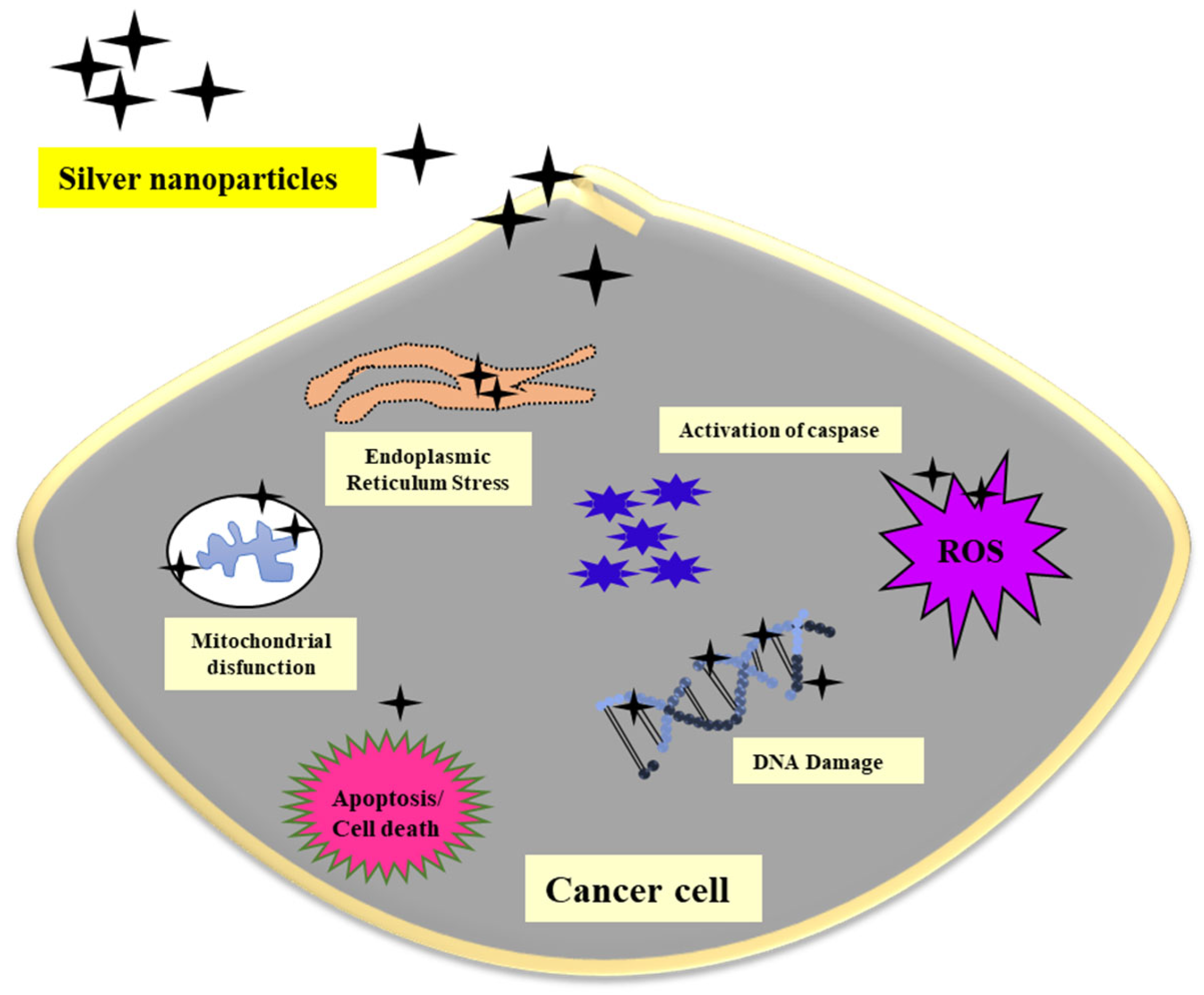
3.3. Use of AgNPs against Cancer
3.4. AgNPs Used for Controlling Mosquito Larvae
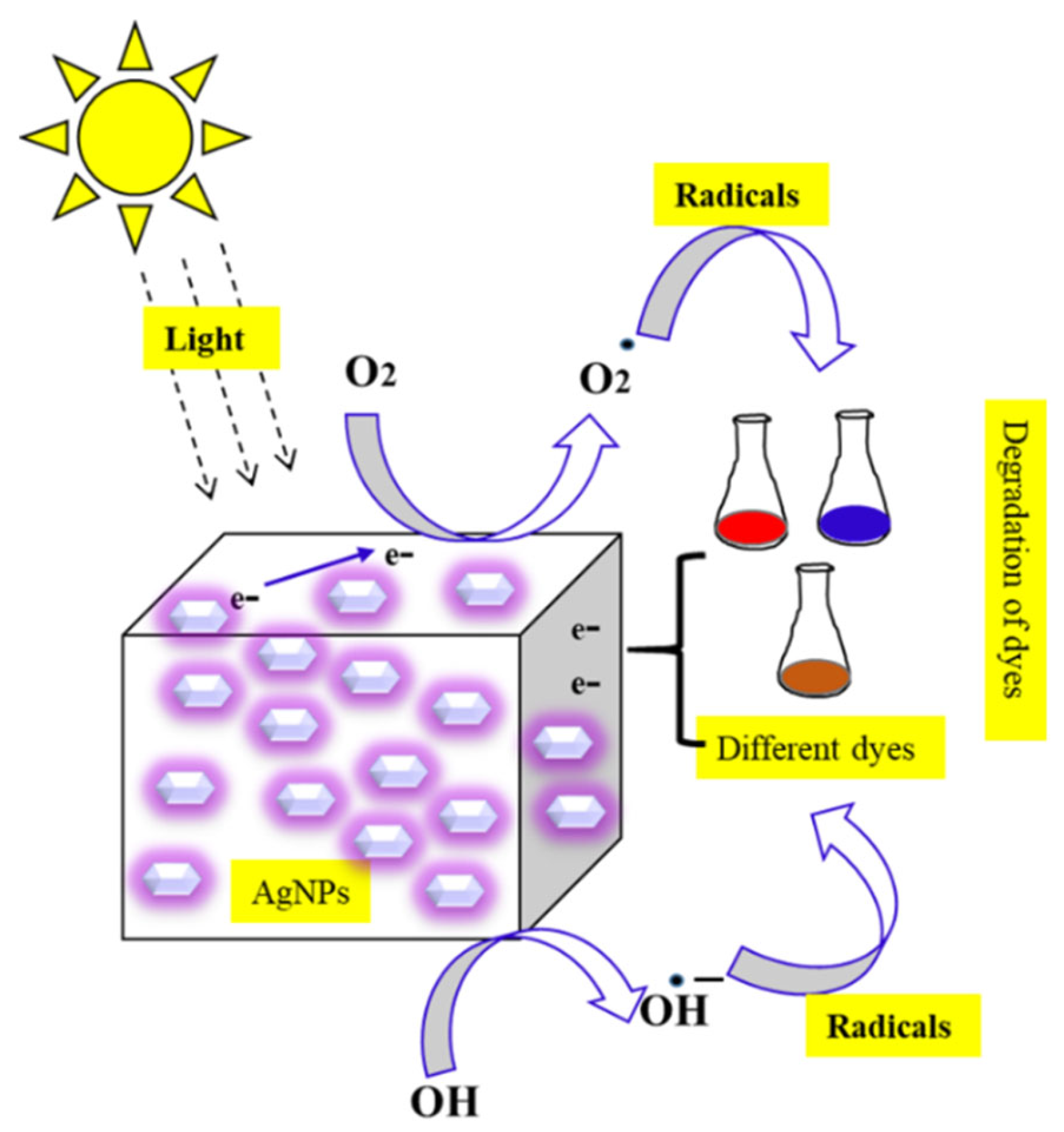
3.5. AgNPs Used for Environmental Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Patra, J.K.; Baek, K.-H. Green nanobiotechnology: Factors affecting synthesis and characterization techniques. J. Nanomater. 2014, 2014, 417305. [Google Scholar] [CrossRef]
- Chen, L.; Xu, W.-H.; Zhao, Y.-G.; Kang, Y.; Liu, S.-H.; Zhang, Z.-Y. Synthesis of vaterite and aragonite crystals using biomolecules of tomato and capsicum. Russ. J. Phys. Chem. A 2012, 86, 2071–2075. [Google Scholar] [CrossRef]
- Rao, A.; Bankar, A.; Shinde, A.; Kumar, A.R.; Gosavi, S.; Zinjarde, S. Phyto-inspired silica nanowires: Characterization and application in lipase immobilization. ACS Appl. Mater. Interfaces 2012, 4, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Swati; Verma, R.; Chauhan, A.; Shandilya, M.; Li, X.; Kumar, R.; Kulshrestha, S. Antimicrobial potential of Ag-doped ZnO nanostructure synthesized by the green method using moringa oleifera extract. J. Environ. Chem. Eng. 2020, 8, 103730. [Google Scholar] [CrossRef]
- Zuorro, A.; Iannone, A.; Natali, S.; Lavecchia, R. Green synthesis of silver nanoparticles using bilberry and red currant waste extracts. Processes 2019, 7, 193. [Google Scholar] [CrossRef]
- Barabadi, H.; Mahjoub, M.A.; Tajani, B.; Ahmadi, A.; Junejo, Y.; Saravanan, M. Emerging theranostic biogenic silver nanomaterials for breast cancer: A systematic review. J. Clust. Sci. 2019, 30, 259–279. [Google Scholar] [CrossRef]
- Sampath, G.; Govarthanan, M.; Rameshkumar, N.; Vo, D.-V.N.; Krishnan, M.; Sivasankar, P.; Kayalvizhi, N. Eco-friendly biosynthesis metallic silver nanoparticles using Aegle marmelos (Indian bael) and its clinical and environmental applications. Appl. Nanosci. 2021, 1–12. [Google Scholar] [CrossRef]
- Srivastava, S.; Bhargava, A. Biological synthesis of nanoparticles: Bacteria. In Green Nanoparticles: The Future of Nanobiotechnology; Springer: Singapore, 2022; pp. 77–79. [Google Scholar] [CrossRef]
- Chauhan, A.; Anand, J.; Parkash, V.; Rai, N. Biogenic synthesis: A sustainable approach for nanoparticles synthesis mediated by fungi. Inorg. Nano-Metal Chem. 2022, 1–14. [Google Scholar] [CrossRef]
- Dudhagara, P.; Alagiya, J.; Bhagat, C.; Dudhagara, D.; Ghelani, A.; Desai, J.; Patel, R.; Vansia, A.; Nhiem, D.N.; Chen, Y.-Y.; et al. Biogenic synthesis of antibacterial, hemocompatible, and antiplatelets lysozyme functionalized silver nanoparticles through the one-step process for therapeutic applications. Processes 2022, 10, 623. [Google Scholar] [CrossRef]
- Singh, P.; Ahn, S.; Kang, J.-P.; Veronika, S.; Huo, Y.; Singh, H.; Chokkaligam, M.; Farh, M.E.-A.; Aceituno, V.C.; Kim, Y.J.; et al. In vitro anti-inflammatory activity of spherical silver nanoparticles and monodisperse hexagonal gold nanoparticles by fruit extract of Prunus serrulata: A green synthetic approach. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2022–2032. [Google Scholar] [CrossRef]
- Jaiswal, S.; Mishra, P. Antimicrobial and antibiofilm activity of curcumin-silver nanoparticles with improved stability and selective toxicity to bacteria over mammalian cells. Med. Microbiol. Immunol. 2018, 207, 39–53. [Google Scholar] [CrossRef]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Leporatti, S. Silver nanoparticles: Synthetic routes, in vitro toxicity and theranostic applications for cancer disease. Nanomaterials 2018, 8, 319. [Google Scholar] [CrossRef]
- Kalaimurugan, D.; Sivasankar, P.; Lavanya, K.; Shivakumar, M.S.; Venkatesan, S. Antibacterial and larvicidal activity of Fusarium proliferatum (YNS2) whole cell biomass mediated copper nanoparticles. J. Clust. Sci. 2019, 30, 1071–1080. [Google Scholar] [CrossRef]
- Fahimirad, S.; Ajalloueian, F.; Ghorbanpour, M. Synthesis and therapeutic potential of silver nanomaterials derived from plant extracts. Ecotoxicol. Environ. Saf. 2019, 168, 260–278. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.A.; Sarma, S.J.; Brar, S.K. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, M. Silver Nanoparticles: Synthesis and Applications. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–14. [Google Scholar] [CrossRef]
- Mohamed, I.; Shuid, A.; Borhanuddin, B.; Fozi, N. The application of phytomedicine in modern drug development. Internet J. Herb. Plant Med. 2012, 1, 1–9. [Google Scholar]
- Iyer, M.; Gujjari, A.K.; Rao, R.N.; Gowda, D.V.; Srivastava, A. Biomedical applications of phytomedicines: Dental perspective. Dent. Hypotheses 2016, 7, 34. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, V.; Rishi, P.; Sehrawat, N.; Dilawari, R.; Kumar, P.; Aggarwal, N.K. Phytomedicine: History, Scope and Future Prospects. In Industrial Biotechnology: Plant Systems, Resources and Products; Yadav, M., Kumar, V., Sehrawat, N., Eds.; De Gruyter: Boston, MA, USA, 2019; pp. 105–120. [Google Scholar] [CrossRef]
- Souza, A.O.; Oliveira, J.W.D.F.; Moreno, C.J.G.; de Medeiros, M.J.C.; Fernandes-Negreiros, M.M.; Souza, F.R.M.; Pontes, D.L.; Silva, M.S.; Rocha, H.A.O. Silver nanoparticles containing fucoidan synthesized by green method have anti-Trypanosoma cruzi activity. Nanomaterials 2022, 12, 2059. [Google Scholar] [CrossRef] [PubMed]
- Sampath, G.; Shyu, D.J.H.; Rameshkumar, N.; Krishnan, M.; Sivasankar, P.; Kayalvizhi, N. Synthesis and characterization of pyrogallol capped silver nanoparticles and evaluation of their in vitro anti-bacterial, anti-cancer profile against AGS cells. J. Clust. Sci. 2021, 32, 549–557. [Google Scholar] [CrossRef]
- Vanaraj, S.; Keerthana, B.B.; Preethi, K. Biosynthesis, characterization of silver nanoparticles using quercetin from Clitoria ternatea L. to enhance toxicity against bacterial biofilm. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1412–1422. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, W.; Xue, J.; Liu, Y.; Liu, Y.; Yan, P.; Liu, J.; Tang, J. Recent advances in synthetic methods and applications of silver nanostructures. Nanoscale Res. Lett. 2018, 13, 54. [Google Scholar] [CrossRef]
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Lamichhane, G.; Bhattarai, D.P.; Parajuli, N. Current research on silver nanoparticles: Synthesis, characterization, and applications. J. Nanomater. 2021, 2021, 6687290. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Gaffet, E.; Tachikart, M.; El Kedim, O.; Rahouadj, R. Nanostructural materials formation by mechanical alloying: Morphologic analysis based on transmission and scanning electron microscopic observations. Mater. Charact. 1996, 36, 185–190. [Google Scholar] [CrossRef]
- Amulyavichus, A.; Daugvila, A.; Davidonis, R.; Sipavichus, C. Study of chemical composition of nanostructural materials prepared by laser cutting of metals. Fiz. Met. Metalloved. 1998, 85, 111–117. [Google Scholar]
- Evanoff, J.A.D.D.; Chumanov, G. Size-controlled synthesis of nanoparticles. 2. Measurement of extinction, scattering, and absorption cross sections. J. Phys. Chem. B 2004, 108, 13957–13962. [Google Scholar] [CrossRef]
- Merga, G.; Wilson, R.; Lynn, G.; Milosavljevic, B.H.; Meisel, D. Redox catalysis on “naked” silver nanoparticles. J. Phys. Chem. C 2007, 111, 12220–12226. [Google Scholar] [CrossRef]
- Wiley, B.; Sun, Y.; Mayers, B.; Xia, Y. Shape-controlled synthesis of metal nanostructures: The case of silver. Chem. A Eur. J. 2005, 11, 454–463. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Lee, K.D. Synthesis of plant-mediated silver nanoparticles using Dioscorea batatas rhizome extract and evaluation of their antimicrobial activities. J. Nanomater. 2011, 2011, 573429. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, S.; Singh, V.N.; Shamsi, S.F.; Fatma, A.; Mehta, B.R. Biosynthesis of silver nanoparticles from Desmodium triflorum: A novel approach towards weed utilization. Biotechnol. Res. Int. 2011, 2011, 454090. [Google Scholar] [CrossRef]
- Nauman, B.; Abbasi, A.S. Review: Green synthesis of silver and gold nanoparticles. Middle East J. Sci. Res. 2014, 19, 834–842. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K. Plant mediated green synthesis: Modified approaches. Nanoscale 2013, 5, 10155–10162. [Google Scholar] [CrossRef]
- Dumur, F.; Guerlin, A.; Dumas, E.; Bertin, D.; Gigmes, D.; Mayer, C.R. Controlled spontaneous generation of gold nanoparticles assisted by dual reducing and capping agents. Gold Bull. 2011, 44, 119–137. [Google Scholar] [CrossRef]
- Rao, A.; Mahajan, K.; Bankar, A.; Srikanth, R.; Kumar, A.R.; Gosavi, S.; Zinjarde, S. Facile synthesis of size-tunable gold nanoparticles by pomegranate (Punica granatum) leaf extract: Applications in arsenate sensing. Mater. Res. Bull. 2013, 48, 1166–1173. [Google Scholar] [CrossRef]
- Saklani, V.; Suman, J.V.; Jain, K. Microbial synthesis of silver nanoparticles: A review. J. Biotechnol. Biomater. 2012, s13, 007. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanoparticle Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: A novel biological approach to nanoparticle synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: A green method. Carbohydr. Res. 2004, 339, 2627–2631. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Shavandi, A.; Raie, D.S.; Sangeetha, J.; Soleimani, M.; Hajibehzad, S.S.; Thangadurai, D.; Hospet, R.; Popoola, J.O.; Arzani, A.; et al. Plant molecular farming: Production of metallic nanoparticles and therapeutic proteins using green factories. Green Chem. 2019, 21, 1845–1865. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Xie, A.; Yu, X.; Qiu, L.; Zhang, L.; Zhang, Q. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007, 9, 852–858. [Google Scholar] [CrossRef]
- Moores, A.; Goettmann, F. The plasmon band in noble metal nanoparticles: An introduction to theory and applications. New J. Chem. 2006, 30, 1121–1132. [Google Scholar] [CrossRef]
- Pitarke, J.M.; Silkin, V.M.; Chulkov, E.V.; Echenique, P.M. Surface plasmons in metallic structures. J. Opt. A Pure Appl. Opt. 2005, 7, S73–S84. [Google Scholar] [CrossRef] [Green Version]
- Tasca, F.; Antiochia, R. Biocide activity of green quercetin-mediated synthesized silver nanoparticles. Nanomaterials 2020, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Pascu, B.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Nemeş, N.S.; Seiman, C.; Marian, E.; Micle, O. A green, simple and facile way tosynthesize silver nanoparticles using soluble starch. pH studies and antimicrobial applications. Materials 2021, 14, 4765. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, G.; Chen, G.; Mi, T.; Tai, J. Green synthesis of silver nanoparticles with glucose for conductivity enhancement of conductive ink. BioResources 2017, 12, 608–621. [Google Scholar] [CrossRef]
- Filippo, E.; Serra, A.; Buccolieri, A.; Manno, D. Green synthesis of silver nanoparticles with sucrose and maltose: Morphological and structural characterization. J. Non-Cryst. Solids 2010, 356, 344–350. [Google Scholar] [CrossRef]
- Qin, Y.; Ji, X.; Jing, J.; Liu, H.; Wu, H.; Yang, W. Size control over spherical silver nanoparticles by ascorbic acid reduction. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 172–176. [Google Scholar] [CrossRef]
- Karan, T.; Erenler, R.; Bozer, B.M. Synthesis and characterization of silver nanoparticles using curcumin: Cytotoxic, apoptotic, and necrotic effects on various cell lines. Z. Naturforsch. C J. Biosci. 2022, 77, 343–350. [Google Scholar] [CrossRef]
- Yerragopu, P.S.; Hiregoudar, S.; Nidoni, U.; Ramappa, K.T.; Sreenivas, A.G.; Doddagoudar, S.R. Chemical Synthesis of Silver Nanoparticles Using Tri-sodium Citrate, Stability Study and Their Characterization. Int. Res. J. Pure Appl. Chem. 2020, 21, 37–50. [Google Scholar] [CrossRef]
- Venkatesham, M.; Ayodhya, D.; Madhusudhan, A.; Babu, N.V.; Veerabhadram, G. A novel green one-step synthesis of silver nanoparticles using chitosan: Catalytic activity and antimicrobial studies. Appl. Nanosci. 2012, 4, 113–119. [Google Scholar] [CrossRef]
- Rao, S.S.; Saptami, K.; Venkatesan, J.; Rekha, P. Microwave-assisted rapid synthesis of silver nanoparticles using fucoidan: Characterization with assessment of biocompatibility and antimicrobial activity. Int. J. Biol. Macromol. 2020, 163, 745–755. [Google Scholar] [CrossRef]
- Kim, T.Y.; Cha, S.-H.; Cho, S.; Park, Y. Tannic acid-mediated green synthesis of antibacterial silver nanoparticles. Arch. Pharm. Res. 2016, 39, 465–473. [Google Scholar] [CrossRef]
- Barnaby, S.N.; Yu, S.M.; Fath, K.R.; Tsiola, A.; Khalpari, O.; Banerjee, I.A. Ellagic acid promoted biomimetic synthesis of shape-controlled silver nanochains. Nanotechnology 2011, 22, 225605. [Google Scholar] [CrossRef]
- Bhatt, S.; Vyas, G.; Paul, P. Rosmarinic acid-capped silver nanoparticles for colorimetric detection of CN– and Redox-modulated surface reaction-aided detection of Cr(VI) in water. ACS Omega 2022, 7, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.K.; Valand, N.N.; Solanki, K.B.; Menon, S.K. Picric acid capped silver nanoparticles as a probe for colorimetric sensing of creatinine in human blood and cerebrospinal fluid samples. Analyst 2016, 141, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Cha, S.-H.; Cho, I.; Park, S.; Park, Y.; Cho, S.; Park, Y. Antibacterial nanocarriers of resveratrol with gold and silver nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Karimi, E.; Oskoueian, E.; Es-Haghi, A.; Yazdi, M.E.T. Comparative study on the biological effects of sodium citrate-based and apigenin-based synthesized silver nanoparticles. Nutr. Cancer 2021, 73, 1511–1519. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; Zavala-Alonso, N.V.; Lara, R.H.; Reyes-López, S.Y.; Martínez-Castañón, G.A.; Loyola-Rodríguez, J.P.; Niño-Martínez, N.; Ruiz, F. Bovine serum albumin and chitosan coated silver nanoparticles and its antimicrobial activity against oral and nonoral bacteria. J. Nanomater. 2015, 2015, 420853. [Google Scholar] [CrossRef]
- Tian, S.; Hu, Y.; Chen, X.; Liu, C.; Xue, Y.; Han, B. Green synthesis of silver nanoparticles using sodium alginate and tannic acid: Characterization and anti-S. aureus activity. Int. J. Biol. Macromol. 2022, 195, 515–522. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Bhubhanil, S.; Talodthaisong, C.; Khongkow, M.; Namdee, K.; Wongchitrat, P.; Yingmema, W.; Hutchison, J.A.; Lapmanee, S.; Kulchat, S. Enhanced wound healing properties of guar gum/curcumin-stabilized silver nanoparticle hydrogels. Sci. Rep. 2021, 11, 21836. [Google Scholar] [CrossRef]
- Attallah, N.G.M.; Elekhnawy, E.; Negm, W.A.; Hussein, I.A.; Mokhtar, F.A.; Al-Fakhrany, O.M. In vivo and in vitro antimicrobial activity of biogenic silver nanoparticles against Staphylococcus aureus clinical isolates. Pharmaceuticals 2022, 15, 194. [Google Scholar] [CrossRef]
- El-Deeb, N.M.; Abo-Eleneen, M.A.; Awad, O.A.; Abo-Shady, A.M. Arthrospira platensis-mediated green biosynthesis of silver nano-particles as breast cancer controlling agent: In vitro and in vivo safety approaches. Appl. Biochem. Biotechnol. 2022, 194, 2183–2203. [Google Scholar] [CrossRef] [PubMed]
- Anbazhagan, P.; Murugan, K.; Jaganathan, A.; Sujitha, V.; Samidoss, C.M.; Jayashanthani, S.; Amuthavalli, P.; Higuchi, A.; Kumar, S.; Wei, H.; et al. Mosquitocidal, antimalarial and antidiabetic potential of Musa paradisiaca-synthesized silver nanoparticles: In vivo and in vitro approaches. J. Clust. Sci. 2016, 28, 91–107. [Google Scholar] [CrossRef]
- Demling, R.H.; Desanti, L. Effects of silver on wound management. Wounds 2001, 13, 4–15. [Google Scholar]
- Dunn, K.; Edwards-Jones, V. The role of Acticoat™ with nanocrystalline silver in the management of burns. Burns 2004, 30, S1–S9. [Google Scholar] [CrossRef]
- Fong, J. The use of silver products in the management of burn wounds: Change in practice for the burn unit at Royal Perth Hospital. Prim. Intent. Aust. J. Wound Manag. 2005, 13, S16–S22. [Google Scholar]
- Klasen, H.J. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 2000, 26, 131–138. [Google Scholar] [CrossRef]
- Lin, J.; Chen, R.; Feng, S.; Pan, J.; Li, Y.; Chen, G.; Cheng, M.; Huang, Z.; Yu, Y.; Zeng, H. A novel blood plasma analysis technique combining membrane electrophoresis with silver nanoparticle-based SERS spectroscopy for potential applications in noninvasive cancer detection. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 655–663. [Google Scholar] [CrossRef]
- Kwan, K.H.; Liu, X.; To, M.K.; Yeung, K.; Ho, C.-M.; Wong, K.K. Modulation of collagen alignment by silver nanoparticles results in better mechanical properties in wound healing. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 497–504. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Sampath, G.; Shyu, D.J.H.; Rameshkumar, N.; Krishnan, M.; Durairaj, K.; Kayalvizhi, N. Fabrication and Characterization of pH-mediated Labeo rohita fish scale extract capped silver nanoparticles and its antibacterial activity. J. Clust. Sci. 2021, 33, 1553–1560. [Google Scholar] [CrossRef]
- Sivasankar, P.; Poongodi, S.; Seedevi, P.; Kalaimurugan, D.; Sivakumar, M.; Loganathan, S. Nanoparticles from actinobacteria: A potential target to antimicrobial therapy. Curr. Pharm. Des. 2019, 25, 2626–2636. [Google Scholar] [CrossRef]
- Rolim, W.R.; Pieretti, J.C.; Renó, D.L.S.; Lima, B.A.; Nascimento, M.H.M.; Ambrosio, F.N.; Lombello, C.B.; Brocchi, M.; De Souza, A.C.S.; Seabra, A.B. Antimicrobial activity and cytotoxicity to tumor cells of nitric oxide donor and silver nanoparticles containing PVA/PEG films for topical applications. ACS Appl. Mater. Interfaces 2019, 11, 6589–6604. [Google Scholar] [CrossRef] [PubMed]
- Nasar, S.; Murtaza, G.; Mehmood, A.; Bhatti, T.M.; Raffi, M. Environmentally benign and economical phytofabrication of silver nanoparticles using Juglans regia leaf extract for antibacterial study. J. Electron. Mater. 2019, 48, 3562–3569. [Google Scholar] [CrossRef]
- Yuan, Y.-G.; Zhang, S.; Hwang, J.-Y.; Kong, I.-K. Silver nanoparticles potentiates cytotoxicity and apoptotic potential of camptothecin in human cervical cancer cells. Oxid. Med. Cell. Longev. 2018, 2018, 6121328. [Google Scholar] [CrossRef] [PubMed]
- Feichtmeier, N.S.; Ruchter, N.; Zimmermann, S.; Sures, B.; Leopold, K. A direct solid sampling analysis method for the detection of silver nanoparticles in biological matrices. Anal. Bioanal. Chem. 2016, 408, 295–305. [Google Scholar] [CrossRef]
- Pang, C.; Hristozov, D.; Zabeo, A.; Pizzol, L.; Tsang, M.P.; Sayre, P.; Marcomini, A. Probabilistic approach for assessing infants’ health risks due to ingestion of nanoscale silver released from consumer products. Environ. Int. 2017, 99, 199–207. [Google Scholar] [CrossRef]
- Chirag, P.J.; Tyagi, S.; Halligudi, N.; Yadav, J.; Pathak, S.; Singh, S.P.; Shankar, P. Antioxidant activity of herbal plants: A recent review. J. Drug Deliv. Ther. 2013, 1, 1–8. [Google Scholar]
- Men’shikova, E.B.; Zenkov, N.K.; Lankin, V.Z.; Bondar, I.A.; Trufanin, V.A. Okislitel’nyi Stress: Patologicheskie Sostoyaniya i Zabolevaniya [Oxidation Stress: Pathological States and Diseases]; ARTA: Novosibirsk, Russia, 2008; p. 284. [Google Scholar]
- Knight, J.A. Diseases related to oxygen-derived free radicals. Ann. Clin. Lab. Sci. 1995, 25, 111–121. [Google Scholar]
- Adeshina, G.O.; Onaolapo, J.A.; Ehinmidu, J.O.; Odama, L.E. Phytochemical and antimicrobial studies of the ethyl acetate extract of Alchornea cordifolia leaf found in Abuja, Nigeria. J. Med. Plant Res. 2010, 4, 649–658. [Google Scholar]
- Thanh, N.C.; Pugazhendhi, A.; Chinnathambi, A.; Alharbi, S.A.; Subramani, B.; Brindhadevi, K.; Whangchai, N.; Pikulkaew, S. Silver nanoparticles (AgNPs) fabricating potential of aqueous shoot extract of Aristolochia bracteolata and assessed their antioxidant efficiency. Environ. Res. 2022, 208, 112683. [Google Scholar] [CrossRef]
- Thomas, B.; Vithiya, B.S.M.; Prasad, T.A.A.; Mohamed, S.B.; Magdalane, C.M.; Kaviyarasu, K.; Maaza, M. Antioxidant and photocatalytic activity of aqueous leaf extract mediated green synthesis of silver nanoparticles using Passiflora edulis f. flavicarpa. J. Nanosci. Nanotechnol. 2019, 19, 2640–2648. [Google Scholar] [CrossRef]
- Chinnasamy, G.; Chandrasekharan, S.; Bhatnagar, S. Biosynthesis of silver nanoparticles from Melia azedarach: Enhancement of antibacterial, wound healing, antidiabetic and antioxidant activities. Int. J. Nanomed. 2019, 14, 9823–9836. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Shin, H.-S. Facile green biosynthesis of silver nanoparticles using Pisum sativum L. outer peel aqueous extract and its antidiabetic, cytotoxicity, antioxidant, and antibacterial activity. Int. J. Nanomed. 2019, 14, 6679–6690. [Google Scholar] [CrossRef]
- Barabadi, H.; Ovais, M.; Shinwari, Z.K.; Saravanan, M. Anti-cancer green bionanomaterials: Present status and future prospects. Green Chem. Lett. Rev. 2017, 10, 285–314. [Google Scholar] [CrossRef]
- Mortezaee, K.; Najafi, M.; Samadian, H.; Barabadi, H.; Azarnezhad, A.; Ahmadi, A. Redox interactions and genotoxicity of metal-based nanoparticles: A comprehensive review. Chem. Biol. Interact. 2019, 312, 108814. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial activity of silver nanoparticles: Structural effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Wyatt, D.; McGowan, D.N.; Najarian, M.P. Comparison of a hydrocolloid dressing and silver sulfadiazine cream in the outpatient management of second-degree burns. J. Trauma Inj. Infect. Crit. Care 1990, 30, 857–865. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Silver nanoparticles: Partial oxidation and antibacterial activities. JBIC J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef]
- Thomas, V.; Yallapu, M.M.; Sreedhar, B.; Bajpai, S. A versatile strategy to fabricate hydrogel–silver nanocomposites and investigation of their antimicrobial activity. J. Colloid Interface Sci. 2007, 315, 389–395. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Liau, S.Y.; Read, D.C.; Pugh, W.J.; Furr, J.R.; Russell, A.D. Interaction of silver nitrate with readily identifiable groups: Relationship to the antibacterialaction of silver ions. Lett. Appl. Microbiol. 1997, 25, 279–283. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Quinteros, M.A.; Aristizábal, V.C.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. In Vitro 2016, 36, 216–223. [Google Scholar] [CrossRef]
- Singhal, G.; Bhavesh, R.; Kasariya, K.; Sharma, A.R.; Singh, R.P. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J. Nanoparticle Res. 2011, 13, 2981–2988. [Google Scholar] [CrossRef]
- Masurkar, S.A.; Chaudhari, P.R.; Shidore, V.B.; Kamble, S.P. Rapid biosynthesis of silver nanoparticles using Cymbopogan Citratus (Lemongrass) and its antimicrobial activity. Nano-Micro Lett. 2011, 3, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Gopinath, V.; MubarakAli, D.; Priyadarshini, S.; Priyadharsshini, N.M.; Thajuddin, N.; Velusamy, P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: A novel biological approach. Colloids Surf. B Biointerfaces 2012, 96, 69–74. [Google Scholar] [CrossRef]
- Swamy, V.S.; Prasad, R. Green synthesis of silver nanoparticles from the leaf extract of Santalum album and its antimicrobial activity. J. Optoelectron. Biomed. Mater. 2012, 4, 53–59. [Google Scholar]
- Amin, M.; Anwar, F.; Janjua, M.R.S.A.; Iqbal, M.A.; Rashid, U. Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berry extract: Characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori. Int. J. Mol. Sci. 2012, 13, 9923–9941. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, G.M.; Mohammed, W.H.; Marzoog, T.R.; Al-Amiery, A.A.A.; Kadhum, A.A.H.; Mohamad, A.B. Green synthesis, antimicrobial and cytotoxic effects of silver nanoparticles using Eucalyptus chapmaniana leaves extract. Asian Pac. J. Trop. Biomed. 2013, 3, 58–63. [Google Scholar] [CrossRef]
- Gnanajobitha, G.; Rajeshkumar, S.; Annadurai, G.; Kannan, C. Preparation and characterization of fruit-mediated silver nanoparticles using pomegranate extract and assessment of its antimicrobial activities. J. Environ. Nanotechnol. 2013, 2, 04–10. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S. Biosynthesis of silver nanoparticles using Plectranthus amboinicus leaf extract and its antimicrobial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 128, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.A.; Palanichamy, V.; Roopan, S.M. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 127, 168–171. [Google Scholar] [CrossRef]
- Amin, M.; Hameed, S.; Ali, A.; Anwar, F.; Shahid, S.A.; Shakir, I.; Yaqoob, A.; Hasan, S.; Khan, S.A.; Rahman, S.U. Green synthesis of silver nanoparticles: Structural features and in vivo and in vitro therapeutic effects against Helicobacter pylori induced gastritis. Bioinorg. Chem. Appl. 2014, 2014, 135824. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Saravanan, M.; Badathala, V. Green synthesis of silver nanoparticles using aqueous extract of Taraxacum officinale and its antimicrobial activity. South Indian J. Biol. Sci. 2015, 21, 115–118. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeong, J.-K.; Han, J.W.; Zhang, X.-F.; Park, J.H.; Kim, J.-H. Multidimensional effects of biologically synthesized silver nanoparticles in Helicobacter pylori, Helicobacter felis, and human lung (L132) and lung carcinoma A549 cells. Nanoscale Res. Lett. 2015, 10, 35. [Google Scholar] [CrossRef]
- Ojo, O.A.; Oyinloye, B.E.; Ojo, A.B.; Afolabi, O.B.; Peters, O.A.; Olaiya, O.; Fadaka, A.; Jonathan, J.; Osunlana, O. Green synthesis of silver nanoparticles (AgNPs) using Talinum triangulare (Jacq.) Willd. leaf extract and monitoring their antimicrobial activity. J. Bionanosci. 2017, 11, 292–296. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Elumalai, S.; Kumar, V.; Kumar, S.; Sangwan, R.S. Nano silver particle synthesis using Swertia paniculata herbal extract and its antimicrobial activity. Microb. Pathog. 2018, 114, 402–408. [Google Scholar] [CrossRef]
- Escárcega-González, C.E.; Garza-Cervantes, J.A.; Vazquez-Rodríguez, A.; Montelongo-Peralta, L.Z.; Treviño-Gonzalez, M.T.; Castro, E.D.B.; Saucedo-Salazar, E.M.; Morales, R.M.C.; Regalado-Soto, D.I.; Treviño-González, F.M.; et al. In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. Int. J. Nanomed. 2018, 13, 2349–2363. [Google Scholar] [CrossRef]
- Ontong, J.C.; Paosen, S.; Shankar, S.; Voravuthikunchai, S.P. Eco-friendly synthesis of silver nanoparticles using Senna alata bark extract and its antimicrobial mechanism through enhancement of bacterial membrane degradation. J. Microbiol. Methods 2019, 165, 105692. [Google Scholar] [CrossRef]
- Ahmad, S.; Tauseef, I.; Haleem, K.S.; Khan, K.; Shahzad, M.; Ali, M.; Sultan, F. Synthesis of silver nanoparticles using leaves of Catharanthus roseus and their antimicrobial activity. Appl. Nanosci. 2020, 10, 4459–4464. [Google Scholar] [CrossRef]
- Singh, A.; Gaud, B.; Jaybhaye, S. Optimization of synthesis parameters of silver nanoparticles and its antimicrobial activity. Mater. Sci. Energy Technol. 2020, 3, 232–236. [Google Scholar] [CrossRef]
- Ogunsile, B.O.; Seyinde, D.O.; Salako, B.A. Green synthesis of silver nanoparticles from leaf extract of Tetrapleura tetraptera and its antimicrobial activity. IOP Conf. Ser. Mater. Sci. Eng. 2020, 805, 012032. [Google Scholar] [CrossRef]
- Mirsadeghi, S.; Koudehi, M.F.; Rajabi, H.R.; Pourmortazavi, S.M. Green and simple synthesis of silver nanoparticles by aqueous extract of Perovskia abrotanoides: Characterization, optimization and antimicrobial activity. Curr. Pharm. Biotechnol. 2020, 21, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Sanpui, P.; Chattopadhyay, A.; Ghosh, S.S. Induction of apoptosis in cancer cells at low silver nanoparticle concentrations using chitosan nanocarrier. ACS Appl. Mater. Interfaces 2011, 3, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Lee, K.-J.; Kalishwaralal, K.; Sheikpranbabu, S.; Vaidyanathan, R.; Eom, S.H. Antiangiogenic properties of silver nanoparticles. Biomaterials 2009, 30, 6341–6350. [Google Scholar] [CrossRef]
- Dwivedi, S.; Siddiqui, M.A.; Farshori, N.N.; Ahamed, M.; Musarrat, J.; Al-Khedhairy, A.A. Synthesis, characterization and toxicological evaluation of iron oxide nanoparticles in human lung alveolar epithelial cells. Colloids Surf. B Biointerfaces 2014, 122, 209–215. [Google Scholar] [CrossRef]
- Bin-Jumah, M.; Monera, A.A.; Albasher, G.; Alarifi, S. Effects of green gilver nanoparticles on apoptosis and oxidative stress in normal and cancerous human hepatic cells in vitro. Int. J. Nanomed. 2020, 15, 1537–1548. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Das, J.; Das, S.; Samadder, A.; Das, D.; De, A.; Khuda-Bukhsh, A.R. Rapid green synthesis of silver nanoparticles from silver nitrate by a homeopathic mother tincture Phytolacca Decandra. Chin. J. Integr. Med. 2012, 10, 546–554. [Google Scholar] [CrossRef]
- Devi, J.S.; Bhimba, B.V. Anticancer Activity of Silver Nanoparticles synthesized by the seaweed Ulva lactuca Invitro. Open Access Sci. Rep. 2012, 1, 242. [Google Scholar] [CrossRef]
- Shawkey, A.M.; Rabeh, M.A.; Abdulall, A.K.; Abdellatif, A.O. Green nanotechnology: Anticancer activity of silver nanopar-ticles using Citrullus colocynthis aqueous extracts. Adv. Life Sci. Technol. 2013, 13, 60–70. [Google Scholar]
- Kathiravan, V.; Ravi, S.; Ashokkumar, S. Synthesis of silver nanoparticles from Melia dubia leaf extract and their in vitro anticancer activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 116–121. [Google Scholar] [CrossRef]
- Nayak, D.; Pradhan, S.; Ashe, S.; Rauta, P.R.; Nayak, B. Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J. Colloid Interface Sci. 2015, 457, 329–338. [Google Scholar] [CrossRef]
- Sreekanth, T.V.M.; Pandurangan, M.; Kim, D.H.; Lee, Y.R. Green Synthesis: In-vitro anticancer activity of silver nanoparticles on human cervical cancer cells. J. Clust. Sci. 2016, 27, 671–681. [Google Scholar] [CrossRef]
- Kummara, S.; Patil, M.B.; Uriah, T. Synthesis, characterization, biocompatible and anticancer activity of green and chemically synthesized silver nanoparticles—A comparative study. Biomed. Pharmacother. 2016, 84, 10–21. [Google Scholar] [CrossRef]
- Ramar, M.; Manikandan, B.; Marimuthu, P.N.; Raman, T.; Mahalingam, A.; Subramanian, P.; Karthick, S.; Munusamy, A. Synthesis of silver nanoparticles using Solanum trilobatum fruits extract and its antibacterial, cytotoxic activity against human breast cancer cell line MCF 7. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 140, 223–228. [Google Scholar] [CrossRef]
- Supraja, S.; Arumugam, P. Antibacterial and anticancer activity of silver nanoparticles synthesized from Cynodon dactylon leaf extract. J. Acad. Ind. Res. 2015, 3, 629–631. [Google Scholar]
- Venugopal, K.; Rather, H.A.; Rajagopal, K.; Shanthi, M.P.; Sheriff, K.; Illiyas, M.; Rather, R.A.; Manikandan, E.; Uvarajan, S.; Bhaskar, M.; et al. Synthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. J. Photochem. Photobiol. B Biol. 2017, 167, 282–289. [Google Scholar] [CrossRef]
- Vijayan, R.; Joseph, S.; Mathew, B. Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties. Artif. Cells Nanomed. Biotechnol. 2018, 46, 861–871. [Google Scholar] [CrossRef] [Green Version]
- Bethu, M.S.; Netala, V.R.; Domdi, L.; Tartte, V.; Janapala, V.R. Potential anticancer activity of biogenic silver nanoparticles using leaf extract of Rhynchosia suaveolens: An insight into the mechanism. Artif. Cells Nanomed. Biotechnol. 2018, 46, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Anandan, M.; Poorani, G.; Boomi, P.; Varunkumar, K.; Anand, K.; Chuturgoon, A.A.; Saravanan, M.; Prabu, H.G. Green synthesis of anisotropic silver nanoparticles from the aqueous leaf extract of Dodonaea viscosa with their antibacterial and anticancer activities. Process Biochem. 2019, 80, 80–88. [Google Scholar] [CrossRef]
- Erdogan, O.; Abbak, M.; Demirbolat, G.M.; Birtekocak, F.; Aksel, M.; Pasa, S.; Cevik, O. Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: The characterization, anticancer potential with photodynamic therapy in MCF7 cells. PLoS ONE 2019, 14, e0216496. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Kumar, D.; Agrawal, V. Green synthesis of silver nanoparticles using Indian Belladonna extract and their potential antioxidant, anti-inflammatory, anticancer and larvicidal activities. Plant Cell Rep. 2020, 39, 921–939. [Google Scholar] [CrossRef]
- Nayaka, S.; Bhat, M.P.; Chakraborty, B.; Pallavi, S.S.; Airodagi, D.; Muthuraj, R.; Halaswamy, H.M.; Dhanyakumara, S.B.; Shashiraj, K.N.; Kupaneshi, K.N.S.A.C. Seed extract-mediated synthesis of silver nanoparticles from Putranjiva roxburghii Wall., Phytochemical characterization, antibacterial activity and anticancer activity against MCF-7 Cell Line. Indian J. Pharm. Sci. 2020, 82, 260–269. [Google Scholar] [CrossRef]
- World Health Organization. Dengue Transmission Research in WHO Bulletin. Available online: http://whqlibdoc.who.int/hq/2005/WHO-CDS-WHOPES-GCDPP-2005.13.pdf (accessed on 25 June 2010).
- Vinoth, S.; Shankar, S.G.; Gurusaravanan, P.; Janani, B.; Devi, J.K. Anti-larvicidal activity of silver nanoparticles synthesized from Sargassum polycystum against mosquito vectors. J. Clust. Sci. 2019, 30, 171–180. [Google Scholar] [CrossRef]
- Benelli, G.; Maggi, F.; Pavela, R.; Murugan, K.; Govindarajan, M.; Vaseeharan, B.; Petrelli, R.; Cappellacci, L.; Kumar, S.; Hofer, A.; et al. Mosquito control with green nanopesticides: Towards the one health approach? A review of non-target effects. Environ. Sci. Pollut. Res. 2017, 25, 10184–10206. [Google Scholar] [CrossRef]
- Gnanadesigan, M.; Anand, M.; Ravikumar, S.; Maruthupandy, M.; Vijayakumar, V.; Selvam, S.; Dhineshkumar, M.; Kumaraguru, A. Biosynthesis of silver nanoparticles by using mangrove plant extract and their potential mosquito larvicidal property. Asian Pac. J. Trop. Med. 2011, 4, 799–803. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Rajakumar, G.; Kirthi, A.V.; Santhoshkumar, T.; Marimuthu, S.; Bagavan, A.; Kamaraj, C.; Zahir, A.A.; Elango, G. Synthesis of pediculocidal and larvicidal silver nanoparticles by leaf extract from heartleaf moonseed plant, Tinospora cordifolia Miers. Parasitol. Res. 2011, 109, 185–194. [Google Scholar] [CrossRef]
- Marimuthu, S.; Rahuman, A.A.; Rajakumar, G.; Santhoshkumar, T.; Kirthi, A.V.; Jayaseelan, C.; Bagavan, A.; Zahir, A.A.; Elango, G.; Kamaraj, C. Evaluation of green synthesized silver nanoparticles against parasites. Parasitol. Res. 2010, 108, 1541–1549. [Google Scholar] [CrossRef]
- Santhoshkumar, T.; Rahuman, A.A.; Rajakumar, G.; Marimuthu, S.; Bagavan, A.; Jayaseelan, C.; Zahir, A.A.; Elango, G.; Kamaraj, C. Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol. Res. 2010, 108, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, K.A.; Murugan, K.; Panneerselvam, C.; Ponarulselvam, S.; Sekar, P.; Hwang, J.-S.; Nicoletti, M. Biolarvicidal and pupicidal potential of silver nanoparticles synthesized using Euphorbia hirta against Anopheles stephensi Liston (Diptera: Culicidae). Parasitol. Res. 2012, 111, 997–1006. [Google Scholar] [CrossRef]
- Patil, C.; Borase, H.P.; Patil, S.; Salunkhe, R.B.; Salunke, B.K. Larvicidal activity of silver nanoparticles synthesized using Pergularia daemia plant latex against Aedes aegypti and Anopheles stephensi and nontarget fish Poecillia reticulata. Parasitol. Res. 2012, 111, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Haldar, K.M.; Haldar, B.; Chandra, G. Fabrication, characterization and mosquito larvicidal bioassay of silver nanoparticles synthesized from aqueous fruit extract of putranjiva, Drypetes roxburghii (Wall.). Parasitol. Res. 2013, 112, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Poopathi, S.; De Britto, L.J.; Praba, V.L.; Mani, C.; Praveen, M. Synthesis of silver nanoparticles from Azadirachta indica—A most effective method for mosquito control. Environ. Sci. Pollut. Res. Int. 2015, 22, 2956–2963. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaran, U.; Govindarajan, M.; Rajeswary, M. Green synthesis of silver nanoparticles from Cassia roxburghii—A most potent power for mosquito control. Parasitol. Res. 2015, 114, 4385–4395. [Google Scholar] [CrossRef] [PubMed]
- Deepak, P.; Sowmiya, R.; Ramkumar, R.; Balasubramani, G.; Aiswarya, D.; Perumal, P. Structural characterization and evaluation of mosquito-larvicidal property of silver nanoparticles synthesized from the seaweed, Turbinaria ornata (Turner) J. Agardh Artif. Cells Nanomed. Biotechnol. 2017, 45, 990–998. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, G.; Agrawal, V. Green synthesis of silver nanoparticles using Holarrhena antidysenterica (L.) Wall.bark extract and their larvicidal activity against dengue and filariasis vectors. Parasitol. Res. 2018, 117, 377–389. [Google Scholar] [CrossRef]
- Parthiban, E.; Manivannan, N.; Ramanibai, R.; Mathivanan, N. Green synthesis of silver-nanoparticles from Annona reticulata leaves aqueous extract and its mosquito larvicidal and anti-microbial activity on human pathogens. Biotechnol. Rep. 2019, 21, e00297. [Google Scholar] [CrossRef]
- Chowdhury, N.; Ghosh, A.; Chandra, G. Mosquito larvicidal activities of Solanum villosum berry extract against the dengue vector Stegomyia aegypti. BMC Complement. Altern. Med. 2008, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Amala, V.E.; Krishnaveni, R. Biogenic synthesis of silver nanoparticles: Characterizations, antibacterial and larvicidal bioassay. Mater. Today Proc. 2022, 49, A7–A11. [Google Scholar] [CrossRef]
- Kumar, V.A.; Ammani, K.; Jobina, R.; Parasuraman, P.; Siddhardha, B. Larvicidal activity of green synthesized silver nanoparticles using Excoecaria agallocha L. (Euphorbiaceae) leaf extract against Aedes aegypti. IET Nanobiotechnol. 2016, 10, 382–388. [Google Scholar] [CrossRef]
- Sareen, S.J.; Pillai, R.K.; Chandramohanakumar, N.; Balagopalan, M. Larvicidal potential of biologically synthesised silver nanoparticles against Aedes albopictus. Res. J. Recent Sci. 2012, 1, 52–56. [Google Scholar]
- Cai, X.; Cai, Y.; Liu, Y.; Deng, S.; Wang, Y.; Wang, Y.; Djerdj, I. Photocatalytic degradation properties of Ni(OH)2 nanosheets/ZnO nanorods composites for azo dyes under visible-light irradiation. Ceram. Int. 2014, 40, 57–65. [Google Scholar] [CrossRef]
- Kim, S.; Umar, A.; Kumar, R.; Ibrahim, A.A.; Kumar, G. Facile synthesis and photocatalytic activity of cocoon-shaped CuO nanostructures. Mater. Lett. 2015, 156, 138–141. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hensley, R. Effective control of chlorination and dechlorination at wastewater treatment plants using redox potential. Water Environ. Res. 1997, 69, 1008–1014. [Google Scholar] [CrossRef]
- Chaudhary, D.; Vigneswaran, S.; Jegatheesan, V.; Ngo, H.H.; Moon, H.; Shim, W.; Kim, S. Granular activated carbon (GAC) adsorption in tertiary wastewater treatment: Experiments and models. Water Sci. Technol. 2003, 47, 113–120. [Google Scholar] [CrossRef]
- Butler, E.; Hung, Y.-T.; Yeh, R.Y.-L.; Al Ahmad, M.S. Electrocoagulation in Wastewater Treatment. Water 2011, 3, 495–525. [Google Scholar] [CrossRef]
- Rana; Padhi, B.S. Pollution due to synthetic dyes toxicity & carcinogenicity studies and remediation. Int. J. Environ. Sci. 2012, 3, 940–955. [Google Scholar] [CrossRef]
- Tamuly, C.; Hazarika, M.; Bordoloi, M. Biosynthesis of Au nanoparticles by Gymnocladus assamicus and its catalytic activity. Mater. Lett. 2013, 108, 276–279. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of nanomaterials in the treatment of wastewater: A review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Garg, D.; Sarkar, A.; Chand, P.; Bansal, P.; Gola, D.; Sharma, S.; Khantwal, S.; Surabhi; Mehrotra, R.; Chauhan, N.; et al. Synthesis of silver nanoparticles utilizing various biological systems: Mechanisms and applications—A review. Prog. Biomater. 2020, 9, 81–95. [Google Scholar] [CrossRef]
- Sumi, M.B.; Devadiga, A.; Shetty, K.V.; Saidutta, M.B. Solar photocatalytically active, engineered silver nanoparticle synthesis using aqueous extract of mesocarp of Cocos nucifera (Red Spicata Dwarf). J. Exp. Nanosci. 2016, 12, 14–32. [Google Scholar] [CrossRef]
- Malaikozhundan, B.; Vijayakumar, S.; Vaseeharan, B.; Jenifer, A.A.; Chitra, P.; Prabhu, N.M.; Kannapiran, E. Two potential uses for silver nanoparticles coated with Solanum nigrum unripe fruit extract: Biofilm inhibition and photodegradation of dye effluent. Microb. Pathog. 2017, 111, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Elemike, E.E.; Onwudiwe, D.C.; Ekennia, A.C.; Ehiri, R.C.; Nnaji, N.J. Phytosynthesis of silver nanoparticles using aqueous leaf extracts of Lippia citriodora: Antimicrobial, larvicidal and photocatalytic evaluations. Mater. Sci. Eng. C 2017, 75, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Vishnudevan, M. Rapid Green aynthesis of amalgamated silver nanoparticles and its photocatalytic activity of dye degradation. J. Environ. Nanotechnol. 2017, 6, 28–33. [Google Scholar] [CrossRef]
- Saraswathi, V.S.; Santhakumar, K. Green synthesis of silver nanoparticles mediated using Lagerstroemia speciosa and photo-catalytic activity against azo dye. Mech. Mater. Sci. Eng. 2017, 9, hal-01500536. [Google Scholar]
- Karthik, R.; Govindasamy, M.; Chen, S.-M.; Cheng, Y.-H.; Muthukrishnan, P.; Padmavathy, S.; Elangovan, A. Biosynthesis of silver nanoparticles by using Camellia japonica leaf extract for the electrocatalytic reduction of nitrobenzene and photocatalytic degradation of Eosin-Y. J. Photochem. Photobiol. B Biol. 2017, 170, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Anupama, N.; Madhumitha, G. Green synthesis and catalytic application of silver nanoparticles using Carissa carandas fruits. Inorg. Nano-Metal Chem. 2017, 47, 116–120. [Google Scholar] [CrossRef]
- Arya, G.; Kumari, R.M.; Gupta, N.; Kumar, A.; Chandra, R.; Nimesh, S. Green synthesis of silver nanoparticles using Prosopis juliflora bark extract: Reaction optimization, antimicrobial and catalytic activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 985–993. [Google Scholar] [CrossRef]
- Kathiravan, V. Green synthesis of silver nanoparticles using different volumes of Trichodesma indicum leaf extract and their antibacterial and photocatalytic activities. Res. Chem. Intermed. 2018, 44, 4999–5012. [Google Scholar] [CrossRef]
- Chokkalingam, M.; Rupa, E.J.; Huo, Y.; Mathiyalagan, R.; Anandapadmanaban, G.; Ahn, J.C.; Park, J.K.; Lu, J.; Yang, D.C. Photocatalytic degradation of industrial dyes using Ag and Au nanoparticles synthesized from Angelica gigas ribbed stem extracts. Optik 2019, 185, 1213–1219. [Google Scholar] [CrossRef]
- Thatikayala, D.; Jayarambabu, N.; Banothu, V.; Ballipalli, C.B.; Park, J.; Rao, K.V. Biogenic synthesis of silver nanoparticles mediated by Theobroma cacao extract: Enhanced antibacterial and photocatalytic activities. J. Mater. Sci. Mater. Electron. 2019, 30, 17303–17313. [Google Scholar] [CrossRef]
- Kannan, D.S.; Mahboob, S.; Al-Ghanim, K.A.; Venkatachalam, P. Antibacterial, antibiofilm and photocatalytic activities of biogenic silver nanoparticles from Ludwigia octovalvis. J. Clust. Sci. 2020, 32, 255–264. [Google Scholar] [CrossRef]
- Nyabola, A.O.; Kareru, P.G.; Madivoli, E.S.; Wanakai, S.I.; Maina, E.G. Formation of silver nanoparticles via Aspilia pluriseta extracts their antimicrobial and catalytic activity. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3493–3501. [Google Scholar] [CrossRef]
- Panja, S.; Choudhuri, I.; Khanra, K.; Pati, B.; Bhattacharyya, N. Biological and photocatalytic activity of silver nanoparticle synthesized from Ehretia laevis Roxb. leaves extract. Nano Biomed. Eng. 2020, 12, 104–113. [Google Scholar] [CrossRef]

| Agents | Particle Size (nm) | Synthetic Time | Optimization Parameters Concentration/Temperature Used | Reference |
|---|---|---|---|---|
| Quercetin | 8.4 ± 0.3 | 20 min | 350 µM | [53] |
| Starch | 20–60 | 100 min | pH = 12 | [54] |
| Glucose | 80–100 | 180 min | 80 °C | [55] |
| Sucrose Maltose | 6 62.4 ± 9.5 | 40 min 36 min | - | [56] |
| Ascorbic acid | 31.5 | 15 min | 6 × 10−4 M pH = 10.5 | [57] |
| Curcumin | 51.13 | 2.5 h | 50 °C | [58] |
| Trisodium citrate | 22.14 | 20 min | 90 °C | [59] |
| Chitosan | 5 to 30 | 50 min | Autoclave at a pressure of 15 psi at 120 °C | [60] |
| Fucoidan | 39.99 ± 12.39 | 4 min | Microwave irradiation RT | [61] |
| Tannic acid | 27.7–46.7 | 30 min | 85 °C oven | [62] |
| Ellagic acid | - | 20 min | 5 to 15 µM | [63] |
| Rosmarinic acid | 2–5 | 10 min | RT | [64] |
| Picric acid | 30 | 4 min | - | [65] |
| Resveratrol | 11.5 ± 3.18 | 2 h | 80 °C oven; 0.7 µM | [66] |
| Sodium citrate Epigenin | 95.5 93.94 | 12 h | RT | [67] |
| Bovine serum albumin | 113.3 | 12 h | RT | [68] |
| Tannic acid and Sodium alginate | 18.52 ± 0.07 | 200 W ultrasound for 10 min | 1 mM | [69] |
| Plant Name | Part Used | Antibacterial Activity * | Reference |
|---|---|---|---|
| Ocimum santum | Leaf | E. coli, S. aureus | [107] |
| Cymbopogan citratus | Leaf | E. coli, S. aureus, S. typhi, C. albicans | [108] |
| Tribulus terrestris | Fruit bodies | S. pyogenes, P. aeruginosa, E. coli, B. subtilis, S. aureus | [109] |
| Santalum album | Leaf | E. coli, S. aureus, P. aeruginosa, A. chroococcum, B. licheniformis 9555 | [110] |
| Solanum xanthocarpum | Berry | H. pylori | [111] |
| Eucalyptus chapmaniana | Leaf | E. coli, P. aeruginosa, K. pneumoniae, Proteus volgaris, S. aureus, C. albicans | [112] |
| Pomegranate | Fruit | B. subtilis, K. planticola | [113] |
| Plectranthus amboinicus | Leaf | E. coli, Penicillium spp. | [114] |
| Alternathera dentate | Leaf | E. coli, P. aeruginosa, K. pneumoniae, Enterococcus faecalis | [115] |
| Peganum harmala | Seed | H. pylori | [116] |
| Taraxacum officinale | Floral | E. faecalis, P. aeruginosa | [117] |
| Artemisia princeps | Leaves | H. pylori | [118] |
| Talinum triangulare | Leaf | S. aureus, E. coli, C. albicans | [119] |
| Swertia paniculata | Aerial parts | P. aeruginosa, K. pneumoniae, S. aureus | [120] |
| Acacia rigidula | Stem Root | E. coli ATCC11229 P. auruginosa, B. subtilis | [121] |
| Senna alata | Bark | S. aureus, A. baumannii, E. coli, K. pneumoniae, P. auruginosa, C. albicans | [122] |
| Catharanthus roseus | Leaf | S. dysenteriae, K. pneumoniae, B. anthraces, S. aureus, P. aeruginosa | [123] |
| Hibiscus rosasinesis | Leaf | E. coli, S. aureus | [124] |
| Tetrapleura tetraptera | Leaf | S. aureus, E. coli, Salmonalla spp. | [125] |
| Perovskia abrotanoides | Plant | S. aureus, B. cereus, E. coli | [126] |
| Plant Name | Extract Used | Type of Cancer Cells * | IC50 Value (µg/mL) | Reference |
|---|---|---|---|---|
| Phytolacca decandra | Root ethanol | A549 | 80 | [131] |
| Ulva lactuca (Marine Macroalgae) | Aqueous | MCF-7, HT-29, Hep-2, Vero cells | 37 49 12.5 95 | [132] |
| Citrullus colocynthis | Fruit-Aqueous | MCF-7 Hep-G2 | 22.4 17.2 | [133] |
| Melia dubia | Leaf-Aqueous | MCF-7 | 31.2 | [134] |
| Cucurbita maxima Moringa oleifera Acorus calamus | Petal Leaf Rhizome | A431 | 82.39 ± 31.1 83.57 ± 3.9 78.58 ± 2.7 | [135] |
| Saccharina japonica | Plant-Aqueous | HeLa | - | [136] |
| Azadirachta indica | Leaf-Aqueous | A549 | 30 | [137] |
| Solanum trilobatum | Unripe-fruit-Aqueous | MCF-7 | - | [138] |
| Cynodon dectylon | Leaf-Aqueous | HepG-2 | 45. 6 | [139] |
| Syzygium aromaticum | Cloves-Aqueous | MCF-7 HEp-2 | 60 50 | [140] |
| Indigofera tinctoria | Leaf-Aqueous | A549 | 56.62 ± 0.86 | [141] |
| Rhynchosia suaveolens | Leaf-Aqueous | DU-145, PC-3 SKOV3 A549 | 4.35 7.72 4.2 24.7 | [142] |
| Dodonaea viscosa | Leaf -Methanol Acetone Acetonitrile Water | A549 | 14 3 80 4 | [143] |
| Cynara scolymus | Leaf | MCF-7 | - | [144] |
| Atropa acuminate | Leaf-Aqueous | HeLa | 5.418 | [145] |
| Putranjiva roxburghii wall | Seed-Aqueous | MCF-7 | 72.32 | [146] |
| Plant Name | Type of Larvae * | LC50 Value | Reference |
|---|---|---|---|
| Rhizophora mucronaota | Aa, Cq | 0.585, 0.891 (mg/L) | [150] |
| Tinospora cordifolia | As, Cq | 6.43, 6.96 (mg/L) | [151] |
| Mimosa pudica | As, Cq | 13.90, 11.73 (mg/L) | [152] |
| Nelumbo nucifera | As, Cq | 0.69 ± 0.54, 1.10 ± 0.68 (mg/L) | [153] |
| Euphorbia hirta | As | 16.82 ppm | [154] |
| Pergularia daemia | Aa, As | 5.12 ± 0.31, 5.35 ± 0.34 (mg/L) | [155] |
| Drypetes roxbarghii | Cq, As | 0.8632, 0.13 ppm | [156] |
| Azadirachta Indica | Aa, Cq | 0.006, 0.047 (mg/L) | [157] |
| Cassia roxburghii | As, Aa, Cq | 26.35, 28.67, 31.27 (µg/mL) | [158] |
| Turbunaria ornata | Aa, As, Cq | 0.738, 1.134, 1.494 (µg/mL) | [159] |
| Holarrhena antidysenterica | Aa, Cq | 5.53, 9.3 ppm | [160] |
| Annona reticulata | Aa | 4.43 (µg/mL) | [161] |
| Plant Extract | Extract | Type of Dye Degradation * | Time | % Dye Degradation | Reference |
|---|---|---|---|---|---|
| Solanum nigrum | Unripe fruit | MO | 6 h | - | [176] |
| Lippia citriodora | Leaf | MB | 660 min | 68.7 | [177] |
| Moringa oleifera | Flower | MO | 52 h | 97 | [178] |
| Lagersteoemia speciosa | Leaves | MO | 310 min | 10 | [179] |
| Camellia japanica | Leaf | EY dye | 60 min | ˃97 | [180] |
| Carissa carandas | Fruit | CV | 150 min | 100 | [181] |
| Prosopis juliflora | Bark | 4-Nitrophenol | 80 min | 90 | [182] |
| Trichodwsma indicum | Leaf | MB | 210 min | 82 | [183] |
| Angelica gigas | Ribbed stem | EY MG | 180 min | 67 64 | [184] |
| Theobroma cacao | Pulp | MB | 180 min | 98.3 | [185] |
| Ludwigia octovalvis | Leaf | Alizarin red Congo red Rhodamine B MB | 6 h | 92.3 76 91.1 94.5 | [186] |
| Aspilia pluriseta | Leaf | Congo red | 30 h | 50 | [187] |
| Ehretia laevis Roxb | Leaves | Congo red | 8 h | 85 | [188] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampath, G.; Chen, Y.-Y.; Rameshkumar, N.; Krishnan, M.; Nagarajan, K.; Shyu, D.J.H. Biologically Synthesized Silver Nanoparticles and Their Diverse Applications. Nanomaterials 2022, 12, 3126. https://doi.org/10.3390/nano12183126
Sampath G, Chen Y-Y, Rameshkumar N, Krishnan M, Nagarajan K, Shyu DJH. Biologically Synthesized Silver Nanoparticles and Their Diverse Applications. Nanomaterials. 2022; 12(18):3126. https://doi.org/10.3390/nano12183126
Chicago/Turabian StyleSampath, Gattu, Yih-Yuan Chen, Neelamegam Rameshkumar, Muthukalingan Krishnan, Kayalvizhi Nagarajan, and Douglas J. H. Shyu. 2022. "Biologically Synthesized Silver Nanoparticles and Their Diverse Applications" Nanomaterials 12, no. 18: 3126. https://doi.org/10.3390/nano12183126
APA StyleSampath, G., Chen, Y.-Y., Rameshkumar, N., Krishnan, M., Nagarajan, K., & Shyu, D. J. H. (2022). Biologically Synthesized Silver Nanoparticles and Their Diverse Applications. Nanomaterials, 12(18), 3126. https://doi.org/10.3390/nano12183126








