Hysteresis in Heat Capacity of MWCNTs Caused by Interface Behavior
Abstract
:1. Introduction
2. Materials and Methods
3. Experimental Results and Discussion
3.1. Transmission Electron Microscopy
3.2. X-ray Diffraction
3.3. Raman
3.4. Thermogravimetric Analysis and Differential Scanning Calorimetry
3.5. Specific Heat
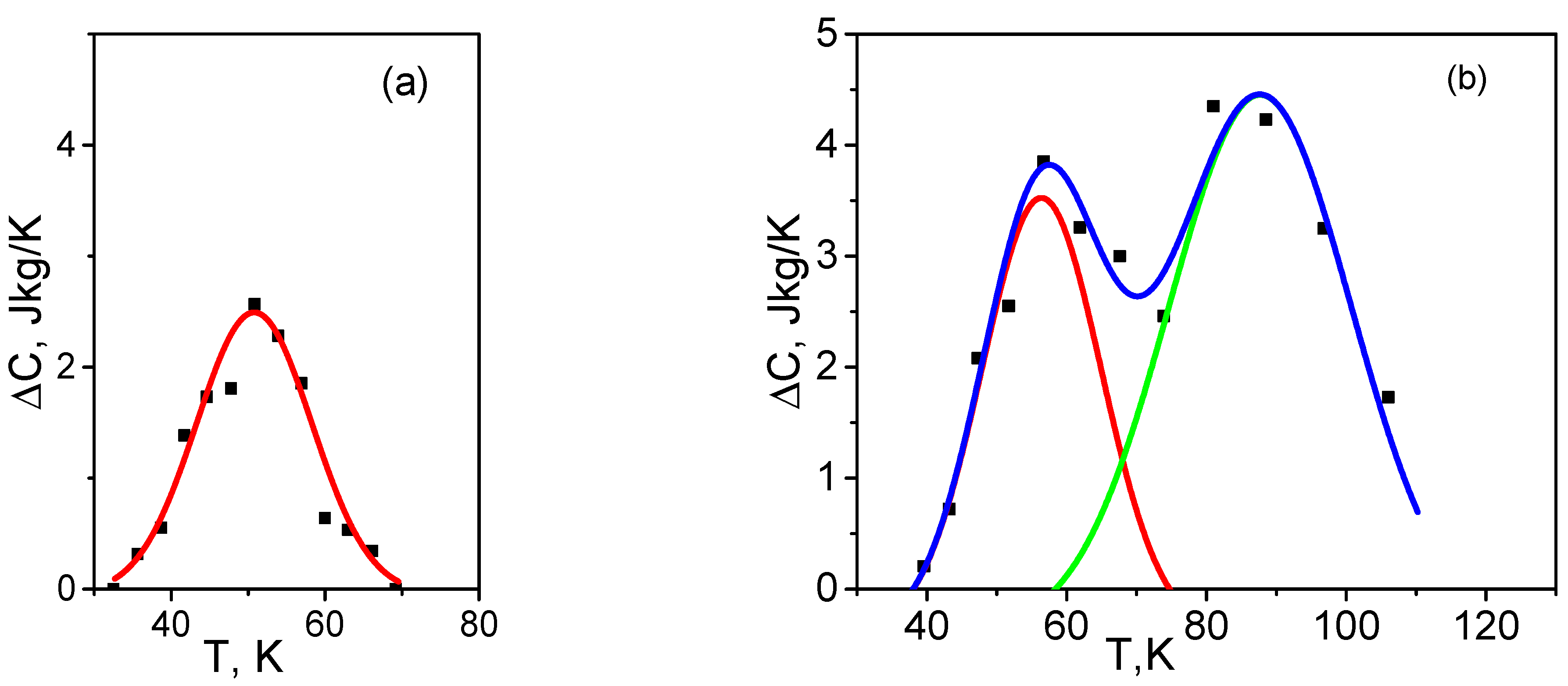
4. Energy Dissipation and Temperature Hysteresis of Heat Capacity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Slepičková Kasálková, N.; Slepička, P.; Švorčík, V. Carbon Nanostructures, Nanolayers, and Their Composites. Nanomaterials 2021, 11, 2368. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhang, X.; Liu, J.; Xu, H.; Wen, F.; Li, T.; Cui, J.; Liu, P.; Shen, L.; Cui, Y.; et al. Multiwalled Carbon Nanotube/Graphite Powder Film for Wearable Pressure Sensors with High Sensing Performance. Nanomaterials 2022, 12, 2637. [Google Scholar] [CrossRef]
- Kumar Jagadeesan, A.; Thangavelu, K.; Dhananjeyan, V. Carbon nanotubes: Synthesis, properties and applications. In 21st Century Surface Science—A Handbook; IntechOpen: London, UK, 2020. [Google Scholar]
- Iqbal, S.; Khatoon, H.; Pandit, A.H.; Ahmad, S. Recent development of carbon based materials for energy storage devices. Mater. Sci. Energy Technol. 2019, 2, 417–428. [Google Scholar] [CrossRef]
- Kuznetsov, V.L.; Bokova-Sirosh, S.N.; Moseenkov, S.I.; Ishchenko, A.; Krasnikov, D.; Kazakova, M.; Romanenko, A.; Tkachev, E.N.; Obraztsova, E. Raman spectra for characterization of defective CVD multi-walled carbon nanotubes. Phys. Status Solidi 2014, 251, 2444–2450. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Jang, J.-W. Direct curvature measurement of the compartments in bamboo-shaped multi-walled carbon nanotubes via scanning probe microscopy. Sci. Rep. 2021, 11, 701. [Google Scholar] [CrossRef]
- Flygare, M.; Svensson, K. Quantifying crystallinity in carbon nanotubes and its influence on mechanical behaviour. Mater. Today Commun. 2019, 18, 39–45. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Belin, T.; Epron, F. Characterization methods of carbon nanotubes: A review. Mater. Sci. Eng. B 2005, 119, 105–118. [Google Scholar] [CrossRef]
- Amelinckx, S.; Lucas, A.; Lambin, P. Electron diffraction and microscopy of nanotubes. Rep. Prog. Phys. 1999, 62, 1471–1524. [Google Scholar] [CrossRef]
- Watanabe, T.; Yamazaki, S.; Yamashita, S.; Inaba, T.; Muroga, S.; Morimoto, T.; Kobashi, K.; Okazaki, T. Comprehensive Characterization of Structural, Electrical, and Mechanical Properties of Carbon Nanotube Yarns Produced by Various Spinning Methods. Nanomaterials 2022, 12, 593. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.G. Defects and Disorder in Carbon Nanotubes; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Meng, T.; Sun, Y.; Tong, C.; Zhang, P.; Xu, D.; Yang, J.; Gu, P.; Yang, J.; Zhao, Y. Solid-state thermal memory of temperature-responsive polymer induced by hydrogen bonds. Nano Lett. 2021, 21, 3843–3848. [Google Scholar] [CrossRef] [PubMed]
- Hess, T.; Maier, L.M.; Bachmann, N.; Corhan, P.; Schäfer-Welsen, O.; Wöllenstein, J.; Bartholomé, K. Thermal hysteresis and its impact on the efficiency of first-order caloric materials. J. Appl. Phys. 2020, 127, 075103. [Google Scholar] [CrossRef]
- Gomez-Heredia, C.L.; Ramirez-Rincon, J.A.; Bhardwaj, D.; Rajasekar, P.; Tadeo, I.J.; Cervantes-Lopez, J.L.; Ordonez-Miranda, J.; Ares, O.; Umarji, A.M.; Drevillon, J.; et al. Measurement of the hysteretic thermal properties of W-doped and undoped nanocrystalline powders of VO2. Sci. Rep. 2019, 9, 14687. [Google Scholar] [CrossRef] [PubMed]
- Grivei, E.; Cassart, M.; Issi, J.; Langer, L.; Nysten, B.; Michenaud, J.-P.; Fabre, C.; Rassat, A. Anomalous specific heat of C60. Phys. Rev. B 1993, 48, 8514–8516. [Google Scholar] [CrossRef]
- Sumarokov, V.V.; Jeżowski, A.; Szewczyk, D. The low-temperature specific heat of MWCNTs. Low. Temp. Phys. 2019, 45, 347. [Google Scholar] [CrossRef]
- Shirai, K. Interpretation of the Apparent Activation Energy of Glass Transition. J. Phys. Commun. 2021, 5, 095013. [Google Scholar] [CrossRef]
- Zeng, Z.; Gui, X.; Gan, Q.; Lin, Z.; Zhu, Y.; Zhang, W.; Xiang, R.; Cao, A.; Tang, Z. Integrated random-aligned carbon nanotube layers: Deformation mechanism under compression. Nanoscale 2014, 6, 1748–1755. [Google Scholar] [CrossRef]
- Li, S.; Ding, X.; Ren, J.; Moya, X.; Li, J.; Sun, J.; Salje, E.K.H. Strain-controlled thermal conductivity in ferroic twinned films. Sci. Rep. 2014, 4, 6375. [Google Scholar] [CrossRef]
- Salje, E.; Zhang, H. Domain boundary engineering. Phase Transit. 2009, 82, 452–469. [Google Scholar] [CrossRef]
- Maschmann, M.R.; Ehlert, G.J.; Park, S.J.; Mollenhauer, D.; Maruyama, B.; Hart, A.J.; Baur, J.W. Visualizing strain evolution and coordinated buckling within CNT arrays by in situ digital image correlation. Adv. Funct. Mater. 2012, 22, 4686–4695. [Google Scholar] [CrossRef]
- Ponomarev, A.; Egorushkin, V.; Bobenko, N.; Barabashko, M.; Rezvanova, A.; Belosludtseva, A. On the possible nature of armchair-zigzag structure formation and heat capacity decrease in MWCNTs. Materials 2022, 15, 518. [Google Scholar] [CrossRef]
- Belosludtseva, A.A.; Bobenko, N.G.; Egorushkin, V.E.; Korusenko, P.; Melnikova, N.; Nesov, S. Oxygen functionalization and electronic band gap control in the disordered multi-walled carbon nanotubes. Synth. Met. 2021, 280, 116866. [Google Scholar] [CrossRef]
- Bobenko, N.G.; Bolotov, V.V.; Egorushkin, V.E.; Korusenko, P.; Melnikova, N.; Nesov, S.; Ponomarev, A.; Povoroznyuk, S. Experimental and theoretical study of electronic structure of disordered MWCNTs. Carbon 2019, 153, 40–51. [Google Scholar] [CrossRef]
- Kuznetsov, V.L.; Krasnikov, D.V.; Schmakov, A.N.; Elumeeva, K.V. In situ and ex situ time resolved study of multi-component Fe–Co oxide catalyst activation during MWNT synthesis. Phys. Status Solidi 2012, 249, 2390–2394. [Google Scholar] [CrossRef]
- Bokova-Sirosh, S.N.; Kuznetsov, V.L.; Romanenko, A.I.; Kazakova, M.A.; Krasnikov, D.V.; Tkachev, E.N.; Yuzyuk, Y.I.; Obraztsova, E.D. Investigation of Defectiveness of Multiwalled Carbon Nanotubes Produced with Fe–Co Catalysts of Different Composition. J. Nanophotonics 2016, 10, 012526. [Google Scholar] [CrossRef]
- Han, P.; Akagi, K.; Federici Canova, F.; Mutoh, H.; Shiraki, S.; Iwaya, K.; Weiss, P.S.; Asao, N.; Hitosugi, T. Bottom-up Graphene-Nanoribbon Fabrication Reveals Chiral Edges and Enantioselectivity. ACS Nano 2014, 8, 9181–9187. [Google Scholar] [CrossRef]
- Enoki, T.; Fujii, S.; Takai, K. Zigzag and Armchair Edges in Graphene. Carbon 2012, 50, 3141–3145. [Google Scholar] [CrossRef]
- Reznik, D.; Olk, C.H.; Neumann, D.A.; Copley, J.R.D. X-ray powder diffraction from carbon nanotubes and nanoparticles. Phys. Rev. B 1995, 52, 116–124. [Google Scholar] [CrossRef]
- Hsu, W.K.; Firth, S.; Redlich, P.; Terrones, M.; Terrones, H.; Zhu, Y.Q.; Grobert, N.; Schilder, A.; Clark, R.J.H.; Kroto, H.W.; et al. Boron-doping effects in carbon nanotubes. J. Mater. Chem. 2000, 10, 1425–1429. [Google Scholar] [CrossRef]
- Danilov, S.V. Modeling of the atomic structure and X-ray diffraction analysis of carbon nanotubes. Ph.D. Thesis, University of Petrozavodsk, Petrozavodsk, Russia, 2013. [Google Scholar]
- Singh, D.K.; Iyer, P.K.; Giri, P.K. Diameter dependence of interwall separation and strain in multiwalled carbon nanotubes probed by X-ray diffraction and Raman scattering studies. Diam. Relat. Mater. 2010, 19, 1281–1288. [Google Scholar] [CrossRef]
- Popov, V.N.; Henrard, L. Breathinglike phonon modes of multiwalled carbon nanotubes. Phys. Rev. B 2002, 65, 235415. [Google Scholar] [CrossRef]
- Parent, P.; Laffon, C.; Marhaba, I.; Ferry, D.; Regier, T.; Ortega, I.; Chazallon, B.; Carpentier, Y.; Focsa, C. Nanoscale characterization of aircraft soot: A high-resolution transmission electron microscopy, Raman spectroscopy, X-ray photoelectron and near-edge X-ray absorption spectroscopy study. Carbon 2016, 101, 86–100. [Google Scholar] [CrossRef]
- Gorelik, S.S.; Rastorguev, L.N.; Skakov, Y.A. X-ray Diffraction and Electron-Optical Analysis of Metals; Metallurgiya: Moscow, Russia, 1970. [Google Scholar]
- Landau, L.D.; Pitaevskii, L.P.; Lifshitz, E.M.; Kosevich, A.M. Theory of Elasticity, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 1984. [Google Scholar]
- Kim, J.; Lee, N.; Min, Y.H.; Noh, S.; Kim, N.-K.; Jung, S.; Joo, M.; Yamada, Y. Distinguishing zigzag and armchair edges on graphene nanoribbons by X-ray photoelectron and Raman spectroscopies. ACS Omega 2018, 3, 17789–17796. [Google Scholar] [CrossRef]
- Mohiuddin, T.M.G.; Lombardo, A.; Nair, R.R.; Bonetti, A.; Savini, G.; Jalil, R.; Bonini, N.; Basko, D.M.; Galiotis, C.; Marzari, N.; et al. Uniaxial strain in graphene by Raman spectroscopy:Gpeak splitting, Grüneisen parameters, and sample orientation. Phys. Rev. B 2009, 79, 205433. [Google Scholar] [CrossRef]
- Mowbray, D.; Fuchs, H.; Niles, D.; Cardona, M.; Thomsen, C.; Friedl, B. Raman Study of the Ge Phonon Side Band; Anastassakis, J.J.E., Ed.; World Scientific: Singapore, 1990; p. 2017. [Google Scholar]
- Mohr, M.; Machón, M.; Maultzsch, J.; Thomsen, C. Double-resonant Raman processes in germanium: Group theory andab initiocalculations. Phys. Rev. B 2006, 73, 035217. [Google Scholar] [CrossRef]
- Muratov, V.B.; Vasil’ev, O.O.; Kulikov, L.M.; Garbuz, V.V.; Nesterenko, Y.V.; Duda, T. Thermodynamic properties of multiwalled carbon nanotubes. J. Superhard Mater. 2012, 34, 173–178. [Google Scholar] [CrossRef]
- Jorge, G.A.; Bekeris, V.; Escobar, M.M.; Goyanes, S.; Zilli, D.; Cukierman, A.; Candal, R. A specific heat anomaly in multiwall carbon nanotubes as a possible sign of orientational order–disorder transition. Carbon 2010, 48, 525–530. [Google Scholar] [CrossRef]
- Yi, W.; Lu, L.; Zhang, D.; Pan, Z.W.; Xie, S.S. Linear specific heat of carbon nanotubes. Phys. Rev. B 1999, 59, R9015–R9018. [Google Scholar] [CrossRef]
- Masarapu, C.; Henry, L.L.; Wei, B. Specific heat of aligned multiwalled carbon nanotubes. Nanotechnology 2005, 16, 1490–1494. [Google Scholar] [CrossRef]
- Landau, L.D.; Lifshitz, E.M. Course of Theoretical Physics; Pergamon: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Pugachev, V.S. Probability Theory and Mathematical Statistics for Engineers; Pergamon: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Li, S.; Ding, X.; Ren, J.; Li, J.; Sun, J.; Salje, E.K.H. Mechanical controlled thermal switch and hysteresis with domain boundary engineered phonon transport. arXiv 2013, arXiv:1310.2879. [Google Scholar]
- Xie, Y.; Wang, T.; Zhu, B.; Yan, C.; Zhang, P.; Wang, X.; Eres, G. 19-Fold thermal conductivity increase of carbon nanotube bundles toward high-end thermal design applications. Carbon 2018, 139, 445–458. [Google Scholar] [CrossRef]
- Huang, X.; Wang, J.; Eres, G.; Wang, X. Thermophysical properties of multi-wall carbon nanotube bundles at elevated temperatures up to 830K. Carbon 2011, 49, 1680–1691. [Google Scholar] [CrossRef]
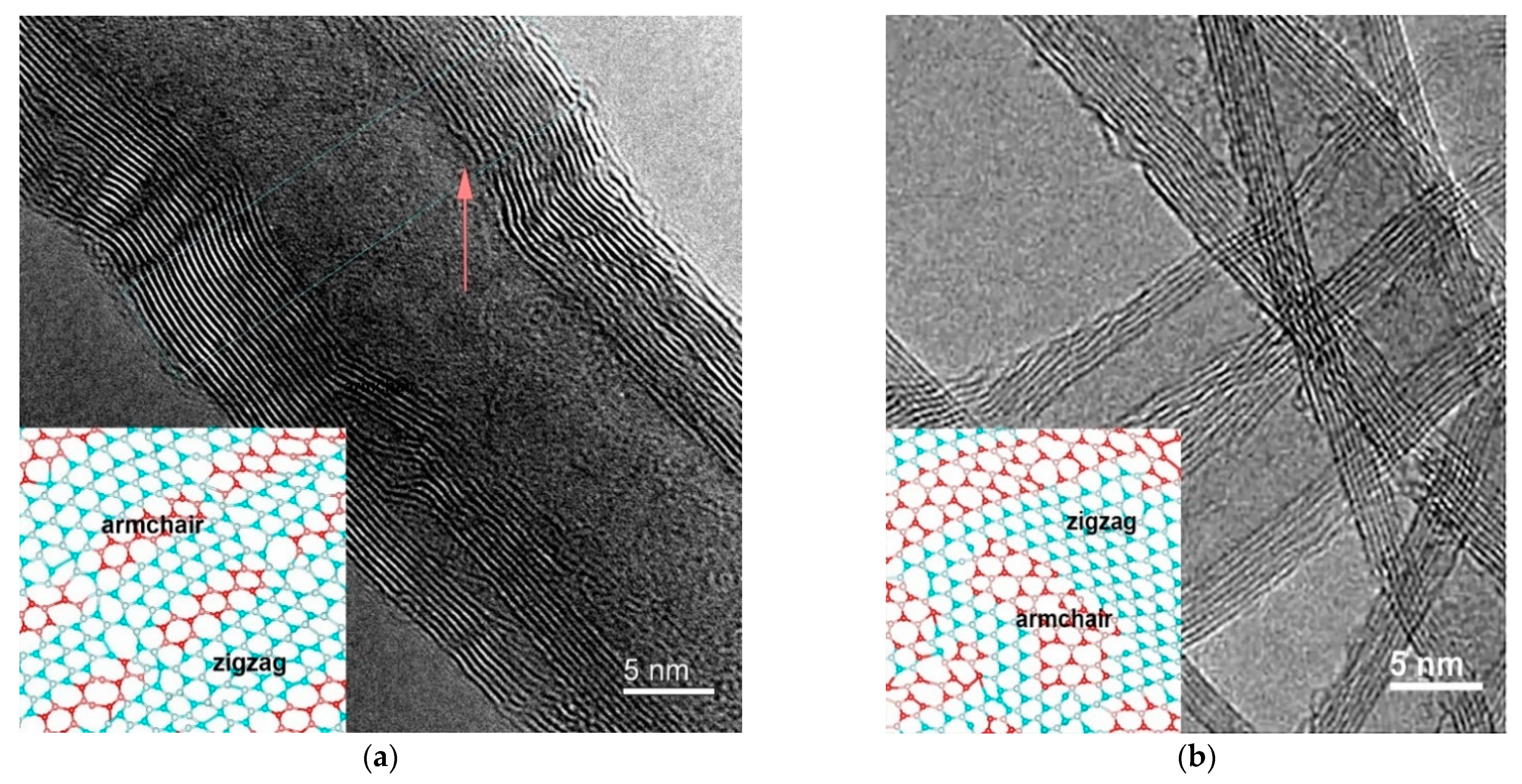
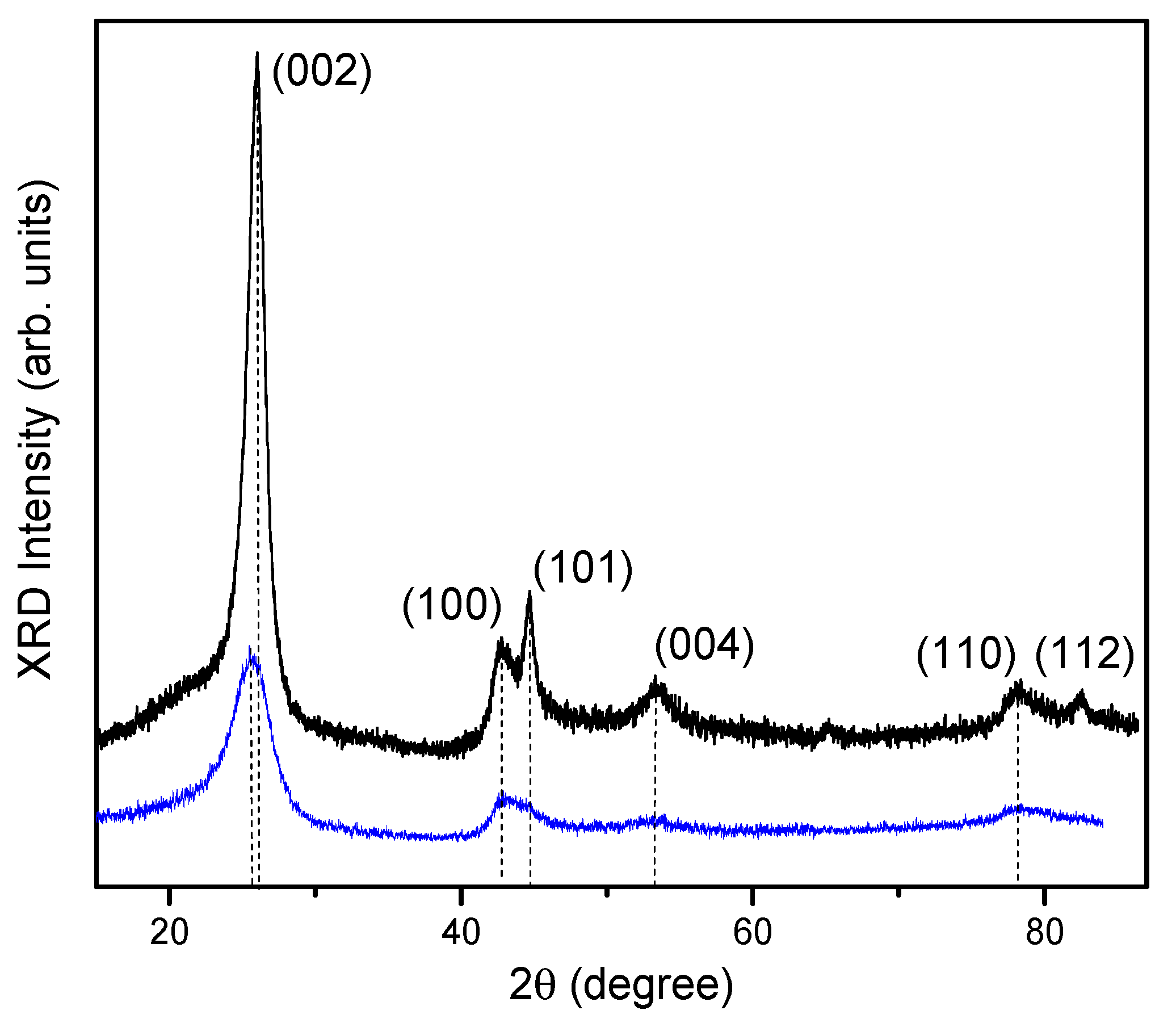
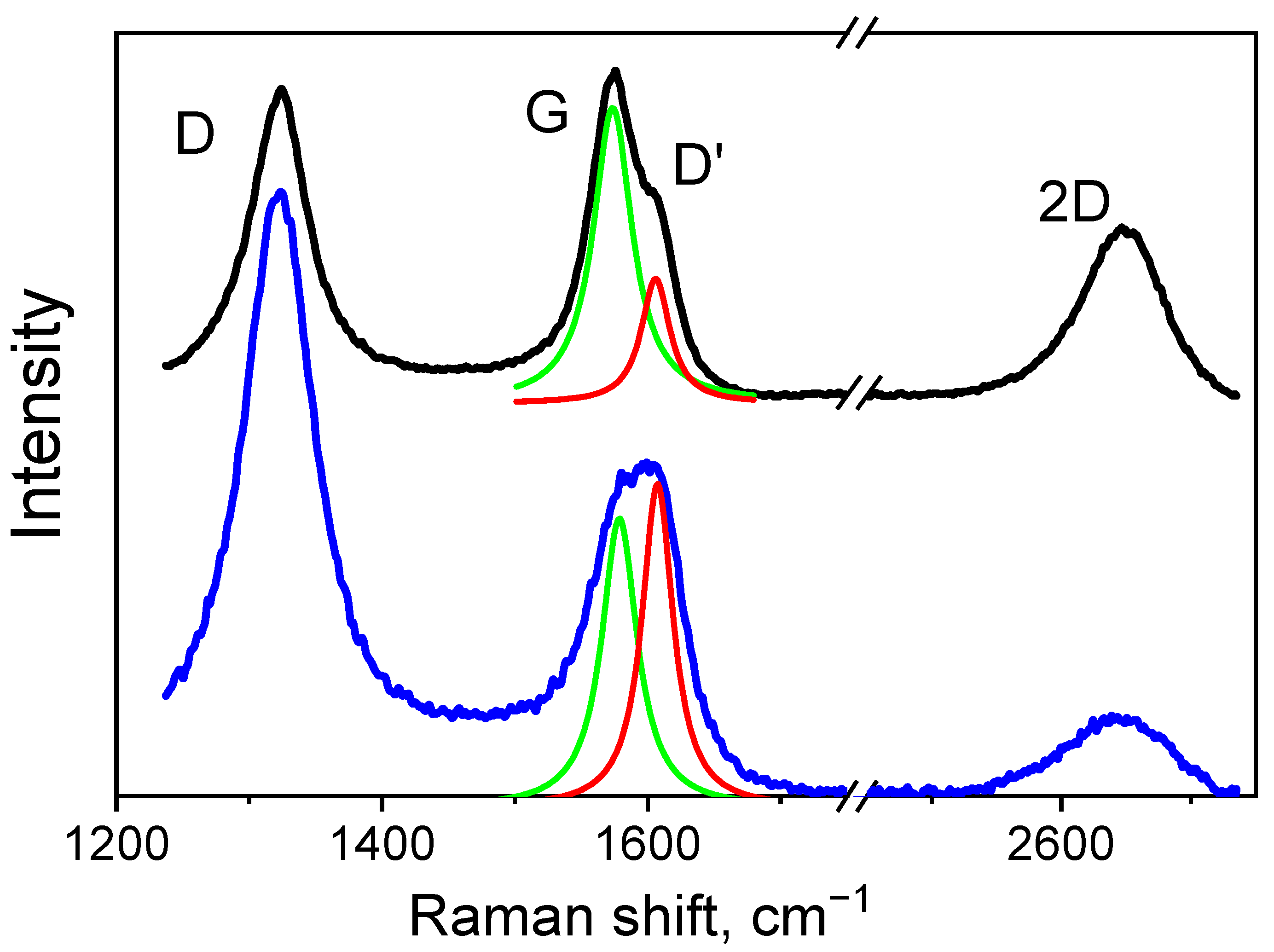

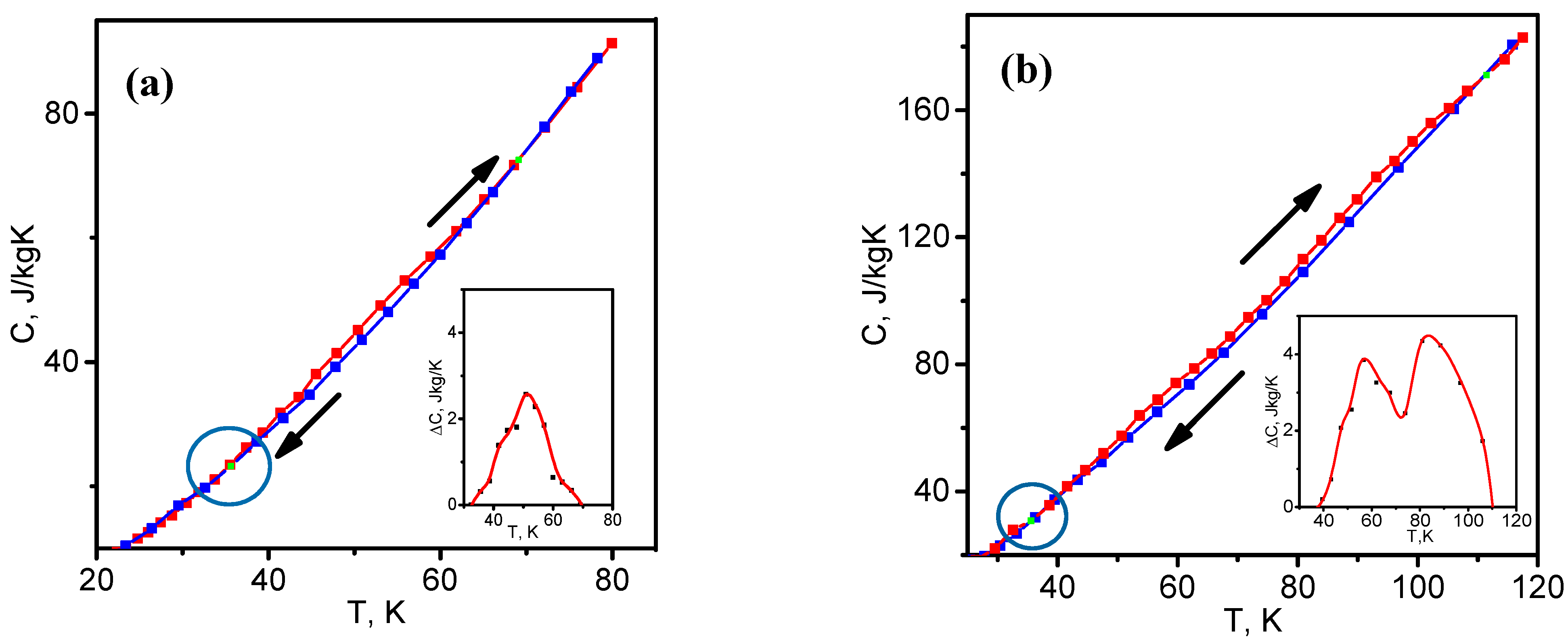
| Sample | 2θ | hkl | Intensity | FWHM | Interplanar Spacing d, Å | CSR Size (D), nm | Microstrain, <ε> |
|---|---|---|---|---|---|---|---|
| S1 | 25.953 | 002 | 100 | 1.4679 | 3.43 | 7 | 0.0649 |
| 42.84 | 100 | 28.83 | 1.0861 | 2.10 | 0.0126 | ||
| 44.71 | 101 | 34.96 | 1.2711 | - | |||
| 78.38 | 110 | 24.3 | 1.7938 | 0.0175 | |||
| 81.19 | 112 | 21.03 | - | 0.0065 | |||
| S2 | 25.531 | 002 | 100 | 2.9733 | 3.49 | 3 | 0.0534 |
| 42.84 | 100 | 36.72 | 1.2917 | 2.11 | 0.0179 | ||
| 44.71 | 101 | 33.66 | 1.9842 | - | |||
| 78.39 | 110 | 32.9 | 5.7241 | 0.0157 |
| Sample | D (cm−1) | G (cm−1) | D’ (cm−1) | 2D (cm−1) | ID/IG | La (nm) |
|---|---|---|---|---|---|---|
| S1 | 1323 | 1575 | 1606 | 2647 | 0.94 | 41 |
| S2 | 1322 | 1580 | 1607 | 2647 | 1.78 | 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobenko, N.; Egorushkin, V.; Ponomarev, A. Hysteresis in Heat Capacity of MWCNTs Caused by Interface Behavior. Nanomaterials 2022, 12, 3139. https://doi.org/10.3390/nano12183139
Bobenko N, Egorushkin V, Ponomarev A. Hysteresis in Heat Capacity of MWCNTs Caused by Interface Behavior. Nanomaterials. 2022; 12(18):3139. https://doi.org/10.3390/nano12183139
Chicago/Turabian StyleBobenko, Nadezhda, Valeriy Egorushkin, and Alexander Ponomarev. 2022. "Hysteresis in Heat Capacity of MWCNTs Caused by Interface Behavior" Nanomaterials 12, no. 18: 3139. https://doi.org/10.3390/nano12183139
APA StyleBobenko, N., Egorushkin, V., & Ponomarev, A. (2022). Hysteresis in Heat Capacity of MWCNTs Caused by Interface Behavior. Nanomaterials, 12(18), 3139. https://doi.org/10.3390/nano12183139









