Carbon Nanostructure/Zeolite Y Composites as Supports for Monometallic and Bimetallic Hydrocracking Catalysts
Abstract
:1. Introduction
2. Experimental
2.1. Catalyst Synthesis
2.2. Catalyst Characterization
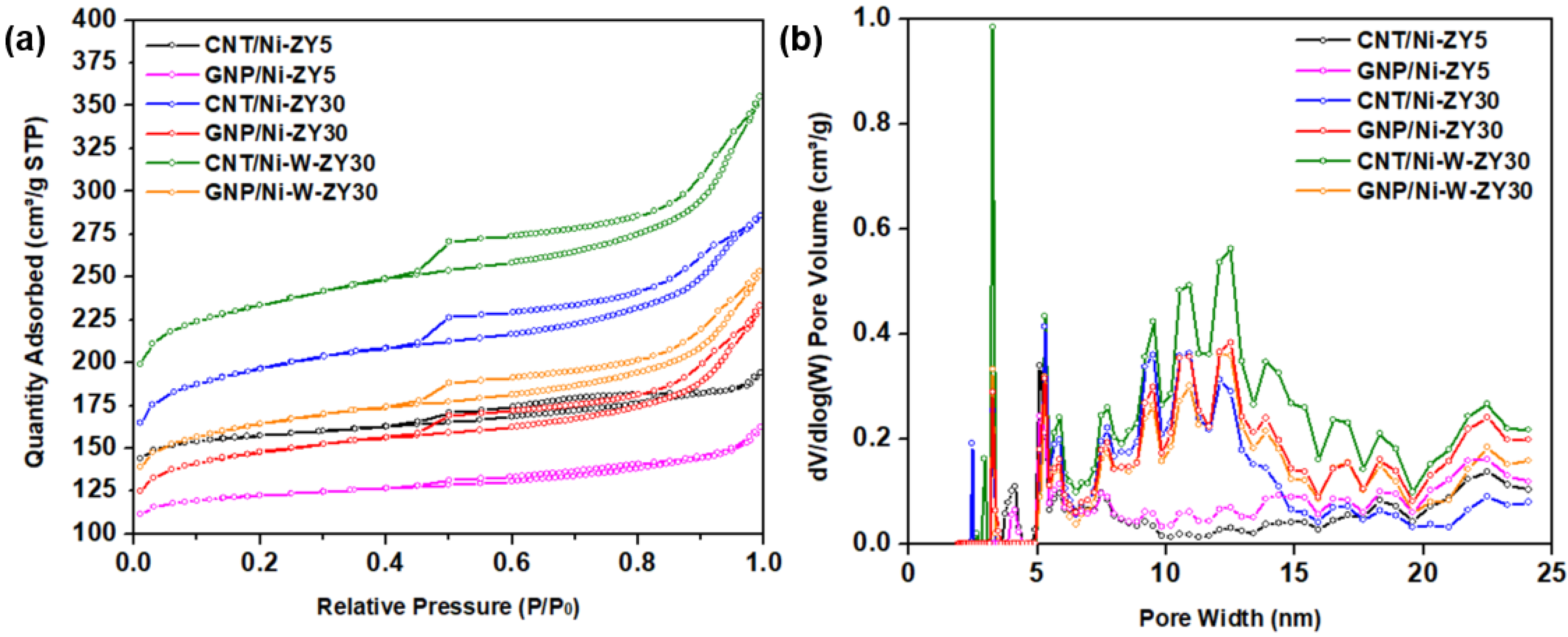
2.2.1. Textural Properties
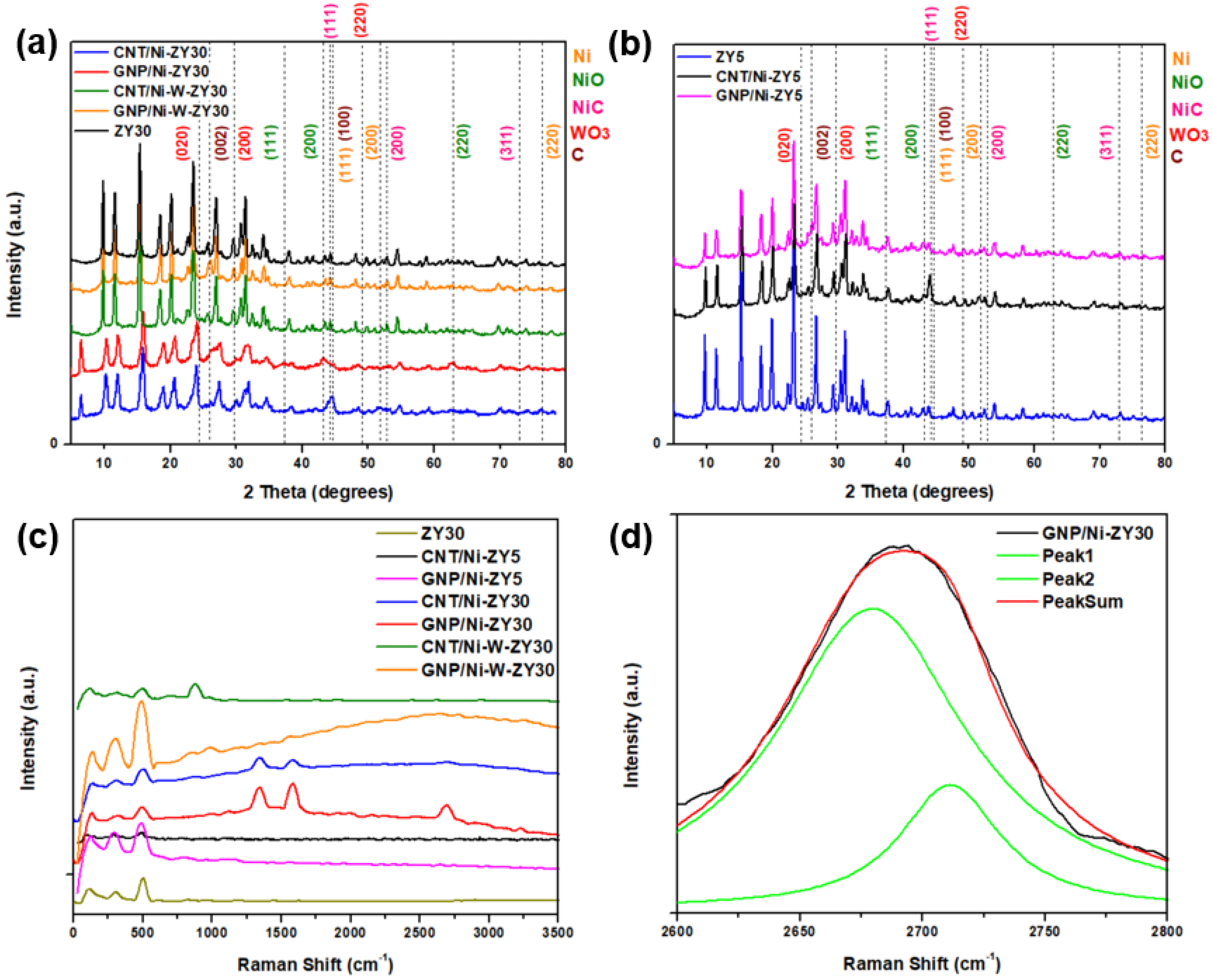
2.2.2. X-ray Diffraction (XRD)
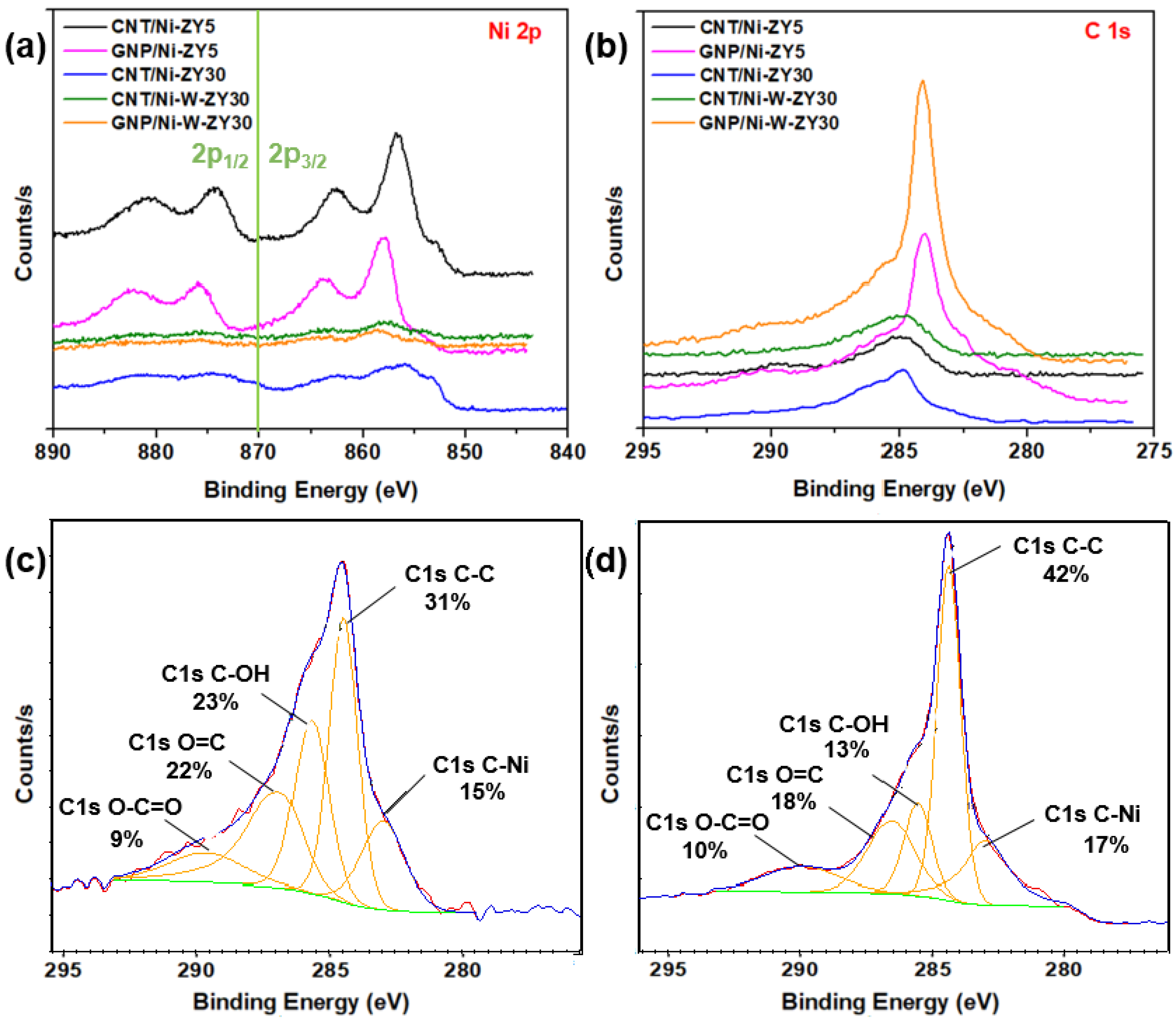
2.2.3. X-ray Photoelectron Spectroscopy (XPS)
2.2.4. Raman Spectroscopy
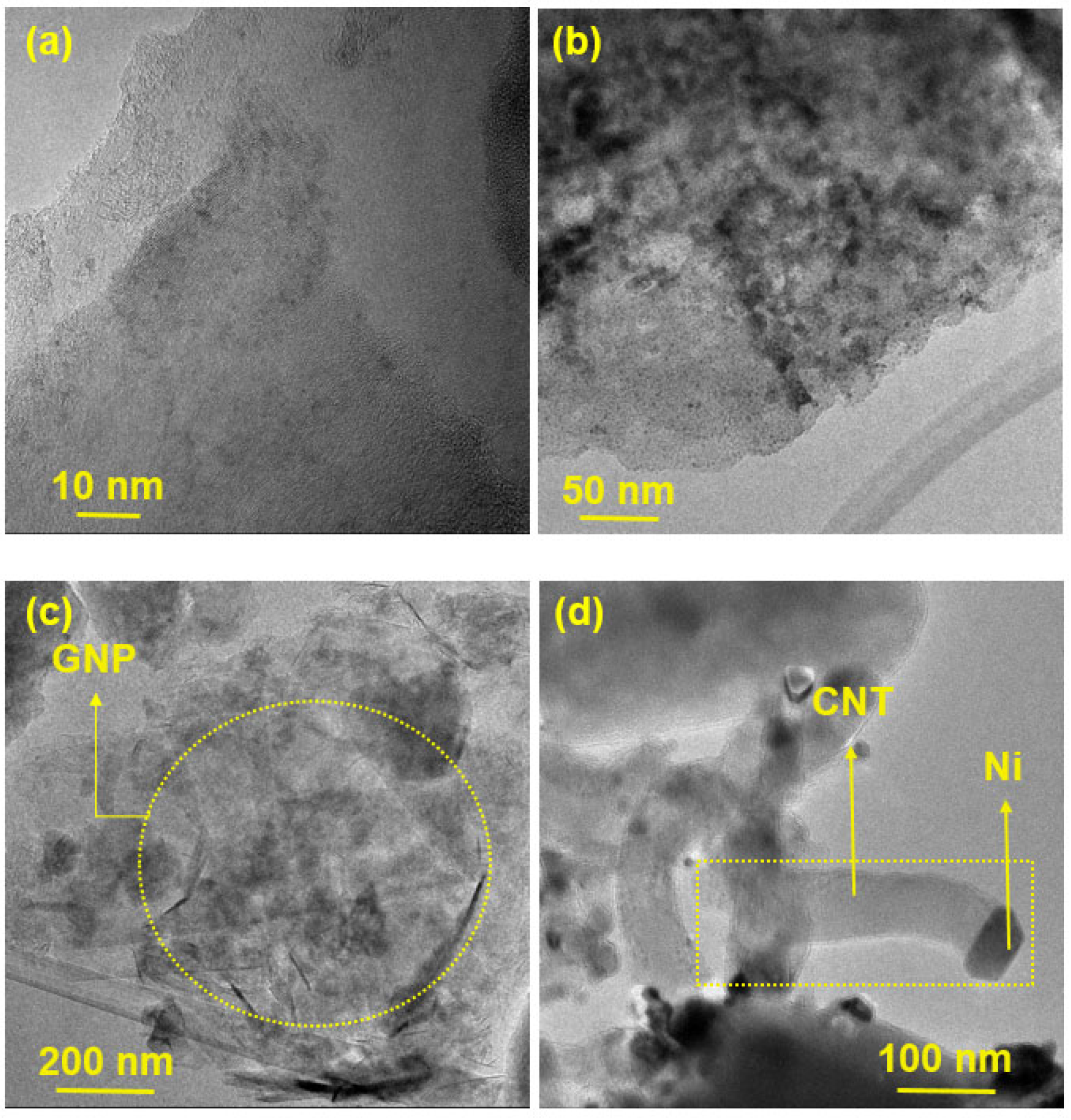
2.2.5. Scanning/Transmission Electron Microscopy (SEM/TEM)
2.2.6. Thermogravimetric Analysis (TGA)
2.3. Catalytic Testing
3. Results and Discussion
3.1. Textural Characterization
| Catalyst Composition | Ni (wt.%) | W (wt.%) | BET Surface Area (m2/g) | Pore Volume (cm3/g) | ||
|---|---|---|---|---|---|---|
| Micropore | Mesopore | Total | ||||
| Reference supports/catalysts | ||||||
| ZY5 | - | - | 399 | 0.21 | 0.01 | 0.22 |
| ZY30 | - | - | 560 | 0.23 | 0.14 | 0.37 |
| CNT | - | - | 476 * | ̶ | ̶ | ̶ |
| GNP | - | - | 300 ** | ̶ | ̶ | ̶ |
| Ni-ZY5 | 5 | - | 409 | 0.19 | 0.06 | 0.25 |
| Ni-ZY30 | 5 | - | 536 | 0.20 | 0.18 | 0.38 |
| Ni-W-ZY30 | 1.67 | 3.33 | 608 | 0.23 | 0.21 | 0.44 |
| CNT- and GNP-based catalysts | ||||||
| CNT/Ni-ZY5 | 5 | - | 479 | 0.21 | 0.09 | 0.30 |
| GNP/Ni-ZY5 | 5 | - | 372 | 0.16 | 0.09 | 0.25 |
| CNT/Ni-ZY30 | 5 | - | 612 | 0.22 | 0.22 | 0.44 |
| GNP/Ni-ZY30 | 5 | - | 458 | 0.17 | 0.19 | 0.36 |
| CNT/Ni-W-ZY30 | 1.67 | 3.33 | 725 | 0.27 | 0.28 | 0.55 |
| GNP/Ni-W-ZY30 | 1.67 | 3.33 | 511 | 0.18 | 0.21 | 0.39 |
3.2. Structure and Morphology Characterization
3.3. XPS Analysis
3.4. Thermal Analysis (TGA)
3.5. Catalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nguyen, M.T.; Nguyen, N.T.; Cho, J.; Park, C.; Park, S.; Jung, J.; Lee, C.W. A review on the oil-soluble dispersed catalyst for slurry-phase hydrocracking of heavy oil. J. Ind. Eng. Chem. 2016, 43, 1–12. [Google Scholar] [CrossRef]
- Kostoglou, N.; Lukovic, J.; Babic, B.; Matovic, B.; Photiou, D.; Constantinides, G.; Polychronopoulou, K.; Ryzhkov, V.; Grossmann, B.; Mitterer, C.; et al. Few-step synthesis, thermal purification and structural characterization of porous boron nitride nanoplatelets. Mater. Des. 2016, 110, 540–548. [Google Scholar] [CrossRef]
- Busca, G. Acidity and basicity of zeolites: A fundamental approach. Microporous Mesoporous Mater. 2017, 254, 3–16. [Google Scholar] [CrossRef]
- Khaleel, M.; Wagner, A.J.; Mkhoyan, K.A.; Tsapatsis, M. On the rotational intergrowth of hierarchical FAU/EMT zeolites. Angew. Chem.—Int. Ed. 2014, 53, 9456–9461. [Google Scholar] [CrossRef] [PubMed]
- Feliczak-Guzik, A. Hierarchical zeolites: Synthesis and catalytic properties. Microporous Mesoporous Mater. 2018, 259, 33–45. [Google Scholar] [CrossRef]
- Khaleel, M.; Xu, W.; Lesch, D.A.; Tsapatsis, M. Combining Pre- and Post-Nucleation Trajectories for the Synthesis of High FAU-Content Faujasite Nanocrystals from Organic-Free Sols. Chem. Mater. 2016, 28, 4204–4213. [Google Scholar] [CrossRef]
- Hasan, M.; Mohamed, A.M.; Al-Kandari, H. Semi-industrial studies of Tungsten-based catalyst for hydroisomerization/hydrocracking of n-hexane and n-heptane. Mol. Catal. 2018, 452, 1–10. [Google Scholar] [CrossRef]
- Blomsma, E.; Martens, J.A.; Jacobs, P.A. Isomerization and hydrocracking of heptane over bimetallic bifunctional PtPd/H-beta and PtPd/USY zeolite catalysts. J. Catal. 1997, 165, 241–248. [Google Scholar] [CrossRef]
- Fatima, S.; Singaravel, G.; Hashaikeh, R. Ni-W/nano zeolite Y catalysts for n-heptane hydrocracking. Mater. Chem. Phys. 2018, 212, 87–94. [Google Scholar]
- Saab, R.; Polychronopoulou, K.; Charisiou, N.; Goula, M.A.; Schiffer, A. Graphene Nanoplatelets-Based Ni-Zeolite Composite Catalysts for Heptane Hydrocracking. C 2020, 6, 31. [Google Scholar] [CrossRef]
- Saepurahman; Hashaikeh, R. Insight into ball milling for size reduction and nanoparticles production of H-Y zeolite. Mater. Chem. Phys. 2018, 220, 322–330. [Google Scholar]
- Zhuman, B.; Saepurahman; Anis, S.F.; Hashaikeh, R. Obtaining high crystalline ball milled H-Y zeolite particles with carbon nanostructures as a damping material. Microporous Mesoporous Mater. 2019, 273, 19–25. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Vaithilingam, B.V.; Al Wahedi, Y.; Joseph, T.; Singaravel, G.; Morin, S.; Berthod, M.; Alhassan, S.M. Synthesis and catalytic performance of zeolite-Y supported on silicon carbide in n-heptane cracking. Appl. Catal. A Gen. 2020, 608, 117866. [Google Scholar] [CrossRef]
- Kazakov, M.O.; Nadeina, K.A.; Danilova, I.G.; Dik, P.P.; Klimov, O.V.; Pereyma, V.Y.; Paukshtis, E.A.; Golubev, I.S.; Prosvirin, I.P.; Gerasimov, E.Y.; et al. Influence of USY Zeolite Recrystallization on Physicochemical Properties and Catalytic Performance of NiMo/USY-Al2O3 Hydrocracking Catalysts. Catal. Today 2019, 329, 108–115. [Google Scholar] [CrossRef]
- Saab, R.; Polychronopoulou, K.; Zheng, L.; Kumar, S.; Schiffer, A. Synthesis and performance evaluation of hydrocracking catalysts: A review. J. Ind. Eng. Chem. 2020, 89, 83–103. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Zhang, Q.; Shu, R.; Wang, H.; Xin, H.; Ma, L. Lignin-first depolymerization of native corn stover with an unsupported MoS2 catalyst. RSC Adv. 2018, 8, 1361–1370. [Google Scholar] [CrossRef]
- Ferraz, S.G.A.; Zotin, F.M.Z.; Araujo, L.R.R.; Zotin, J.L. Influence of support acidity of NiMoS catalysts in the activity for hydrogenation and hydrocracking of tetralin. Appl. Catal. A Gen. 2010, 384, 51–57. [Google Scholar] [CrossRef]
- Shin, J.; Oh, Y.; Choi, Y.; Lee, J.; Lee, J.K. Design of selective hydrocracking catalysts for BTX production from diesel-boiling-range polycyclic aromatic hydrocarbons. Appl. Catal. A Gen. 2017, 547, 12–21. [Google Scholar] [CrossRef]
- Kazakov, M.O.; Nadeina, K.A.; Danilova, I.G.; Dik, P.P.; Klimov, O.V.; Pereyma, V.Y.; Gerasimov, E.Y.; Dobryakova, I.V.; Knyazeva, E.E.; Ivanova, I.I.; et al. Hydrocracking of vacuum gas oil over NiMo/Y-Al2O3: Effect of mesoporosity introduced by zeolite Y recrystallization. Catal. Today 2018, 305, 117–125. [Google Scholar] [CrossRef]
- Francis, J.; Guillon, E.; Bats, N.; Pichon, C.; Corma, A.; Simon, L.J. Design of improved hydrocracking catalysts by increasing the proximity between acid and metallic sites. Appl. Catal. A Gen. 2011, 409–410, 140–147. [Google Scholar] [CrossRef]
- Mahamulkar, S.; Yin, K.; Agrawal, P.K.; Davis, R.J.; Jones, C.W.; Malek, A.; Shibata, H. Formation and Oxidation/Gasification of Carbonaceous Deposits: A Review. Ind. Eng. Chem. Res. 2016, 55, 9760–9818. [Google Scholar] [CrossRef]
- Babaei, M.; Anbia, M.; Kazemipour, M. Synthesis of zeolite/carbon nanotube composite for gas separation. Can. J. Chem. 2016, 95, 162–168. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal Conductivity of Carbon Nanotubes and their Polymer Nanocomposites: A Review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Han, Z.J.; Seo, D.H.; Yick, S.; Chen, J.H.; Ostrikov, K.K. MnOx/carbon nanotube/reduced graphene oxide nanohybrids as high-performance supercapacitor electrodes. NPG Asia Mater. 2014, 6, e140–e148. [Google Scholar] [CrossRef]
- Pal, G.; Kumar, S. Modeling of carbon nanotubes and carbon nanotube—Polymer composites. Prog. Aerosp. Sci. 2016, 80, 33–58. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, L.; Zhang, X.; Liu, G. Different roles of CNTs in hierarchical HZSM-5 synthesis with hydrothermal and steam-assisted crystallization. RSC Adv. 2015, 5, 78238–78246. [Google Scholar] [CrossRef]
- Tang, K.; Wang, Y.G.; Song, L.J.; Duan, L.H.; Zhang, X.T.; Sun, Z.L. Carbon nanotube templated growth of nano-crystalline ZSM-5 and NaY zeolites. Mater. Lett. 2006, 60, 2158–2160. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Controlling the diameter distribution of carbon nanotubes grown from camphor on a zeolite support. Carbon N. Y. 2005, 43, 533–540. [Google Scholar] [CrossRef]
- Kadlečíková, M.; Breza, J.; Jesenák, K.; Pastorková, K.; Luptáková, V.; Kolmačka, M.; Vojačková, A.; Michalka, M.; Vávra, I.; Križanová, Z. The growth of carbon nanotubes on montmorillonite and zeolite (clinoptilolite). Appl. Surf. Sci. 2008, 254, 5073–5079. [Google Scholar] [CrossRef]
- Zhao, W.; Lee, M.J.; Kim, H.T.; Kim, I.J. The synthesis of carbon nanotubes (CNTs) by catalytic CVD using a Fe/Co-supported zeolite template. Electron. Mater. Lett. 2011, 7, 139–144. [Google Scholar] [CrossRef]
- Shariaty, P.; Hashisho, Z. Carbon nanotube growth on zeolite Y to tailor its electric resistivity for resistive heating regeneration. Microporous Mesoporous Mater. 2019, 277, 171–178. [Google Scholar] [CrossRef]
- Gómez, M.J.; Loiácono, A.; Pérez, L.A.; Franceschini, E.A.; Lacconi, G.I. Highly Efficient Hybrid Ni/Nitrogenated Graphene Electrocatalysts for Hydrogen Evolution Reaction. ACS Omega 2019, 4, 2206–2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, O.S.G.P. Development of carbon materials as metal catalyst supports and metal-free catalysts for catalytic reduction of ions and advanced oxidation processes. Bol. Grupo Esp. Carbón 2016, 40, 36–41. [Google Scholar]
- Bandosz, T.J. Surface Chemistry of Carbon Materials. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 45–92. ISBN 9780470178850. [Google Scholar]
- Wong, W.Y.; Daud, W.R.W.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S.; Majlan, E.H. Recent progress in nitrogen-doped carbon and its composites as electrocatalysts for fuel cell applications. Int. J. Hydrogen Energy 2013, 38, 9370–9386. [Google Scholar] [CrossRef]
- Boehm, H.-P. Catalytic Properties of Nitrogen-Containing Carbons. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 219–265. [Google Scholar]
- Manrique, C.; Guzmán, A.; Pérez-Pariente, J.; Márquez-Álvarez, C.; Echavarría, A. Vacuum gas-oil hydrocracking performance of Beta zeolite obtained by hydrothermal synthesis using carbon nanotubes as mesoporous template. Fuel 2016, 182, 236–247. [Google Scholar] [CrossRef]
- Zhang, M.H.; Liu, Z.M.; Lin, G.D.; Zhang, H. Bin Pd/CNT-promoted CuZrO2/HZSM-5 hybrid catalysts for direct synthesis of DME from CO2/H2. Appl. Catal. A Gen. 2013, 451, 28–35. [Google Scholar] [CrossRef]
- Munthali, M.W.; Elsheikh, M.A.; Johan, E.; Matsue, N. Proton adsorption selectivity of zeolites in aqueous media: Effect of Si/Al ratio of zeolites. Molecules 2014, 19, 20468–20481. [Google Scholar] [CrossRef]
- Fritz, P.O.; Lunsford, J.H. The effect of sodium poisoning on dealuminated Y-type zeolites. J. Catal. 1989, 118, 85–98. [Google Scholar] [CrossRef]
- Xu, B.; Bordiga, S.; Prins, R.; van Bokhoven, J.A. Effect of framework Si/Al ratio and extra-framework aluminum on the catalytic activity of Y zeolite. Appl. Catal. A Gen. 2007, 333, 245–253. [Google Scholar] [CrossRef]
- Saab, R.; Polychronopoulou, K.; Anjum, D.H.; Charisiou, N.D.; Goula, M.A.; Hinder, S.J.; Baker, M.A.; Schiffer, A. Effect of SiO2/Al2O3 ratio in Ni/Zeolite-Y and Ni-W/Zeolite-Y catalysts on hydrocracking of heptane. Mol. Catal. 2022, 528, 112484. [Google Scholar] [CrossRef]
- Ben Tayeb, K.; Lamonier, C.; Lancelot, C.; Fournier, M.; Payen, E.; Bonduelle, A.; Bertoncini, F. Study of the active phase of NiW hydrocracking sulfided catalysts obtained from an innovative heteropolyanion based preparation. Catal. Today 2010, 150, 207–212. [Google Scholar] [CrossRef]
- Cui, G.; Wang, J.; Fan, H.; Sun, X.; Jiang, Y.; Wang, S.; Liu, D.; Gui, J. Towards understanding the microstructures and hydrocracking performance of sulfided Ni-W catalysts: Effect of metal loading. Fuel Process. Technol. 2011, 92, 2320–2327. [Google Scholar] [CrossRef]
- Gutberlet, L.C.; Bertolacini, R.J.; Kukes, S.G. Design of a Nickel–Tungsten Hydrocracking Catalyst. Energy Fuels 1994, 8, 227–233. [Google Scholar] [CrossRef]
- Saab, R.; Damaskinos, C.M.; Polychronopoulou, K.; Efstathiou, A.M.; Charisiou, N.; Goula, M.; Hinder, S.J.; Baker, M.A.; Schiffer, A. Ni/CNT/Zeolite-Y composite catalyst for efficient heptane hydrocracking: Steady-state and transient kinetic studies. Appl. Catal. A Gen. 2022, 630, 118437. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, Y.; Gao, Y.; Wu, J.; Hu, J.; Stein, A.; Tang, B. Nanocomposites of zeolitic imidazolate frameworks on graphene oxide for pseudocapacitor applications. J. Appl. Electrochem. 2016, 46, 441–450. [Google Scholar] [CrossRef]
- De Boer, J.H.; Linsen, B.G.; Van Der Plas, T.; Zondervan, G.J. Description Of Pore Dimensions Of Carbon Blacks By the t Method. J. Catal. 1965, 4, 649–653. [Google Scholar] [CrossRef]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.S.; Bandoch, G.F.G.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Minimum Surface Area Measurements with Micromeritics Physisorption Analyzers. 2020. Available online: https://www.micromeritics.com/Repository/Files/micro_tech_tip_14-surface-area-analyses.pdf (accessed on 2 February 2021).
- Alothman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- White, C.M.; Banks, R.; Hamerton, I.; Watts, J.F. Characterisation of commercially CVD grown multi-walled carbon nanotubes for paint applications. Prog. Org. Coat. 2016, 90, 44–53. [Google Scholar] [CrossRef]
- Osatiashtiani, A.; Puértolas, B.; Oliveira, C.C.S.; Manayil, J.C.; Barbero, B.; Isaacs, M.; Michailof, C.; Heracleous, E.; Pérez-Ramírez, J.; Lee, A.F.; et al. On the influence of Si:Al ratio and hierarchical porosity of FAU zeolites in solid acid catalysed esterification pretreatment of bio-oil. Biomass Convers. Biorefin. 2017, 7, 331–342. [Google Scholar] [CrossRef]
- Alshammari, A.; Kalevaru, V.N.; Martin, A. Bimetallic catalysts containing gold and palladium for environmentally important reactions. Catalysts 2016, 6, 97. [Google Scholar] [CrossRef]
- Sheng, J.; Yi, X.; Li, F.; Fang, W. Effects of tungsten on the catalytic activity of Ni-W catalysts for the hydrogenation of aromatic hydrocarbons. React. Kinet. Mech. Catal. 2010, 99, 371–379. [Google Scholar] [CrossRef]
- Choo, M.Y.; Juan, J.C.; Oi, L.E.; Ling, T.C.; Ng, E.P.; Rahman Noorsaadah, A.; Centi, G.; Lee, K.T. The role of nanosized zeolite y in the H2-free catalytic deoxygenation of triolein. Catal. Sci. Technol. 2019, 9, 772–782. [Google Scholar] [CrossRef]
- Furlan, A.; Lu, J.; Hultman, L.; Jansson, U.; Magnuson, M. Crystallization characteristics and chemical bonding properties of nickel carbide thin film nanocomposites. J. Phys. Condens. Matter 2014, 26, 415501. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M. Carbon Nanotube Synthesis and Growth Mechanism. In Carbon Nanotubes—Synthesis, Characterization, Applications; Yellampalli, S., Ed.; IntechOpen: London, UK, 2011; pp. 147–170. ISBN 978-953-307-497-9. [Google Scholar]
- de la Torre, A.I.R.; Banda, J.A.M.; García, U.P.; Alvarado, D.I.S.; García, M.A.C.; Martínez, B.P. Crystallographic Properties of the Unsupported Ni-Mo Carbides Phases. Adv. Mater. Phys. Chem. 2013, 03, 206–208. [Google Scholar] [CrossRef]
- Vakili, M.; Khosrojerdi, S.; Aghajannezhad, P.; Yahyaei, M. A hybrid artificial neural network-genetic algorithm modeling approach for viscosity estimation of graphene nanoplatelets nanofluid using experimental data. Int. Commun. Heat Mass Transf. 2017, 82, 40–48. [Google Scholar] [CrossRef]
- Ramoraswi, N.O.; Ndungu, P.G. Photo-Catalytic Properties of TiO2 Supported on MWCNTs, SBA-15 and Silica-Coated MWCNTs Nanocomposites. Nanoscale Res. Lett. 2015, 10, 1–16. [Google Scholar] [CrossRef]
- Ali, S.; Liu, T.F.; Lian, Z.; Li, B.; Su, D.S. The effect of defects on the catalytic activity of single Au atom supported carbon nanotubes and reaction mechanism for CO oxidation. Phys. Chem. Chem. Phys. 2017, 19, 22344–22354. [Google Scholar] [CrossRef]
- Ma, Y.; Lan, G.; Fu, W.; Lai, Y.; Han, W.; Tang, H.; Liu, H.; Li, Y. Role of surface defects of carbon nanotubes on catalytic performance of barium promoted ruthenium catalyst for ammonia synthesis. J. Energy Chem. 2020, 41, 79–86. [Google Scholar] [CrossRef]
- Triantafyllidis, K.S.; Karakoulia, S.A.; Gournis, D.; Delimitis, A.; Nalbandian, L.; Maccallini, E.; Rudolf, P. Formation of carbon nanotubes on iron/cobalt oxides supported on zeolite-Y: Effect of zeolite textural properties and particle morphology. Microporous Mesoporous Mater. 2008, 110, 128–140. [Google Scholar] [CrossRef]
- Casimir, D.; Alghamdi, H.; Ahmed, I.Y.; Garcia-Sanchez, R.; Misra, P. Raman Spectroscopy of Graphene, Graphite and Graphene Nanoplatelets. In 2D Materials; IntechOpen: London, UK, 2019; p. 13. [Google Scholar]
- Gokus, T.; Nair, R.; Bonetti, A.; Bohmler, M.; Lombardo, A.; Novoselov, K.; Geim, A..; Ferrari, A.; Hartschuh, A. Making Graphene Luminescent by Oxygen Plasma Treatment. ACS Nano 2009, 3, 3963–3968. [Google Scholar] [CrossRef] [PubMed]
- Tapasztó, O.; Balko, J.; Puchy, V.; Kun, P.; Dobrik, G.; Fogarassy, Z.; Horváth, Z.E.; Dusza, J.; Balázsi, K.; Balázsi, C.; et al. Highly wear-resistant and low-friction Si3N4 composites by addition of graphene nanoplatelets approaching the 2D limit. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Afify, H.H.; Hassan, S.A.; Obaida, M.; Moussa, I.; Abouelsayed, A. Preparation, characterization, and optical spectroscopic studies of nanocrystalline tungsten oxide WO3. Opt. Laser Technol. 2019, 111, 604–611. [Google Scholar] [CrossRef]
- Ayillath Kutteri, D.; Wang, I.W.; Samanta, A.; Li, L.; Hu, J. Methane decomposition to tip and base grown carbon nanotubes and COx-free H2 over mono- and bimetallic 3d transition metal catalysts. Catal. Sci. Technol. 2018, 8, 858–869. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Kim, K.S.; Winograd, N. X-ray photoelectron spectroscopic studies of nickel-oxygen surfaces using oxygen and argon ion-bombardment. Surf. Sci. 1974, 43, 625–643. [Google Scholar] [CrossRef]
- Ng, K.T.; Hercules, D.M. Studies of nickel-tungsten-alumina catalysts by X-ray photoelectron spectroscopy. J. Phys. Chem. 1976, 80, 2094–2102. [Google Scholar] [CrossRef]
- Gengenbach, T.R.; Major, G.H.; Linford, M.R.; Easton, C.D. Practical guides for X-ray photoelectron spectroscopy (XPS): Interpreting the carbon 1s spectrum. J. Vac. Sci. Technol. A 2021, 39, 013204. [Google Scholar] [CrossRef]
- Goto, Y.; Taniguchi, K.; Omata, T.; Otsuka-Yao-Matsuo, S.; Ohashi, N.; Ueda, S.; Yoshikawa, H.; Yamashita, Y.; Oohashi, H.; Kobayashi, K. Formation of Ni3C nanocrystals by thermolysis of nickel acetylacetonate in oleylamine: Characterization using hard X-ray photoelectron spectroscopy. Chem. Mater. 2008, 20, 4156–4160. [Google Scholar] [CrossRef]
- Hasha, D.; de Saldarriaga, L.S.; Saldarriaga, C.; Hathaway, P.E.; Cox, D.F.; Davis, M.E. Studies of Silicoaluminophosphates with the Sodalite Structure. J. Am. Chem. Soc. 1988, 110, 2127–2135. [Google Scholar] [CrossRef]
- Zhou, F.; Gao, Y.; Wu, G.; Ma, F.; Liu, C. Improved catalytic performance and decreased coke formation in post-treated ZSM-5 zeolites for methanol aromatization. Microporous Mesoporous Mater. 2017, 240, 96–107. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Kinage, A.; Banerjee, S.; Choudhary, V.R. Influence of Si/Ga and Si/Al ratios on propane aromatization over highly active H-GaAlMFI. Catal. Commun. 2006, 7, 166–169. [Google Scholar] [CrossRef]
- Auepattana-aumrung, C.; Márquez, V.; Wannakao, S.; Jongsomjit, B.; Panpranot, J.; Praserthdam, P. Role of Al in Na-ZSM-5 zeolite structure on catalyst stability in butene cracking reaction. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Venkatesha, N.J.; Bhat, Y.S.; Jai Prakash, B.S. Dealuminated BEA zeolite for selective synthesis of five-membered cyclic acetal from glycerol under ambient conditions. RSC Adv. 2016, 6, 18824–18833. [Google Scholar] [CrossRef]
- Verma, M.; Yadav, R.; Sinha, L.; Mali, S.S.; Hong, C.K.; Shirage, P.M. Pseudocapacitive-battery-like behavior of cobalt manganese nickel sulfide (CoMnNiS) nanosheets grown on Ni-foam by electrodeposition for realizing high capacity. RSC Adv. 2018, 8, 40198–40209. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Soultati, A.; Georgiadou, D.G.; Stergiopoulos, T.; Palilis, L.C.; Kennou, S.; Stathopoulos, N.A.; Davazoglou, D.; Argitis, P. Hydrogenated under-stoichiometric tungsten oxide anode interlayers for efficient and stable organic photovoltaics. J. Mater. Chem. A 2014, 2, 1738–1749. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, Y.; Zheng, S.; Ning, L.; Ye, Q.; Tao, M.; He, Y. Amine-functionalized low-cost industrial grade multi-walled carbon nanotubes for the capture of carbon dioxide. J. Energy Chem. 2014, 23, 111–118. [Google Scholar] [CrossRef]
- Shere, L.; Trivedi, S.; Roberts, S.; Sciacovelli, A.; Ding, Y. Synthesis and Characterization of Thermochemical Storage Material Combining Porous Zeolite and Inorganic Salts. Heat Transf. Eng. 2019, 40, 1176–1181. [Google Scholar] [CrossRef]
- Huang, D.; Xin, Q.; Ni, Y.; Shuai, Y.; Wang, S.; Li, Y.; Ye, H.; Lin, L.; Ding, X.; Zhang, Y. Synergistic effects of zeolite imidazole framework@graphene oxide composites in humidified mixed matrix membranes on CO2 separation. RSC Adv. 2018, 8, 6099–6109. [Google Scholar] [CrossRef]
- Thybaut, J.W.; Marin, G.B. Multiscale Aspects in Hydrocracking: From Reaction Mechanism Over Catalysts to Kinetics and Industrial Application. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 2016; Volume 59, pp. 109–238. [Google Scholar]
- Pérez-Ramírez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef]


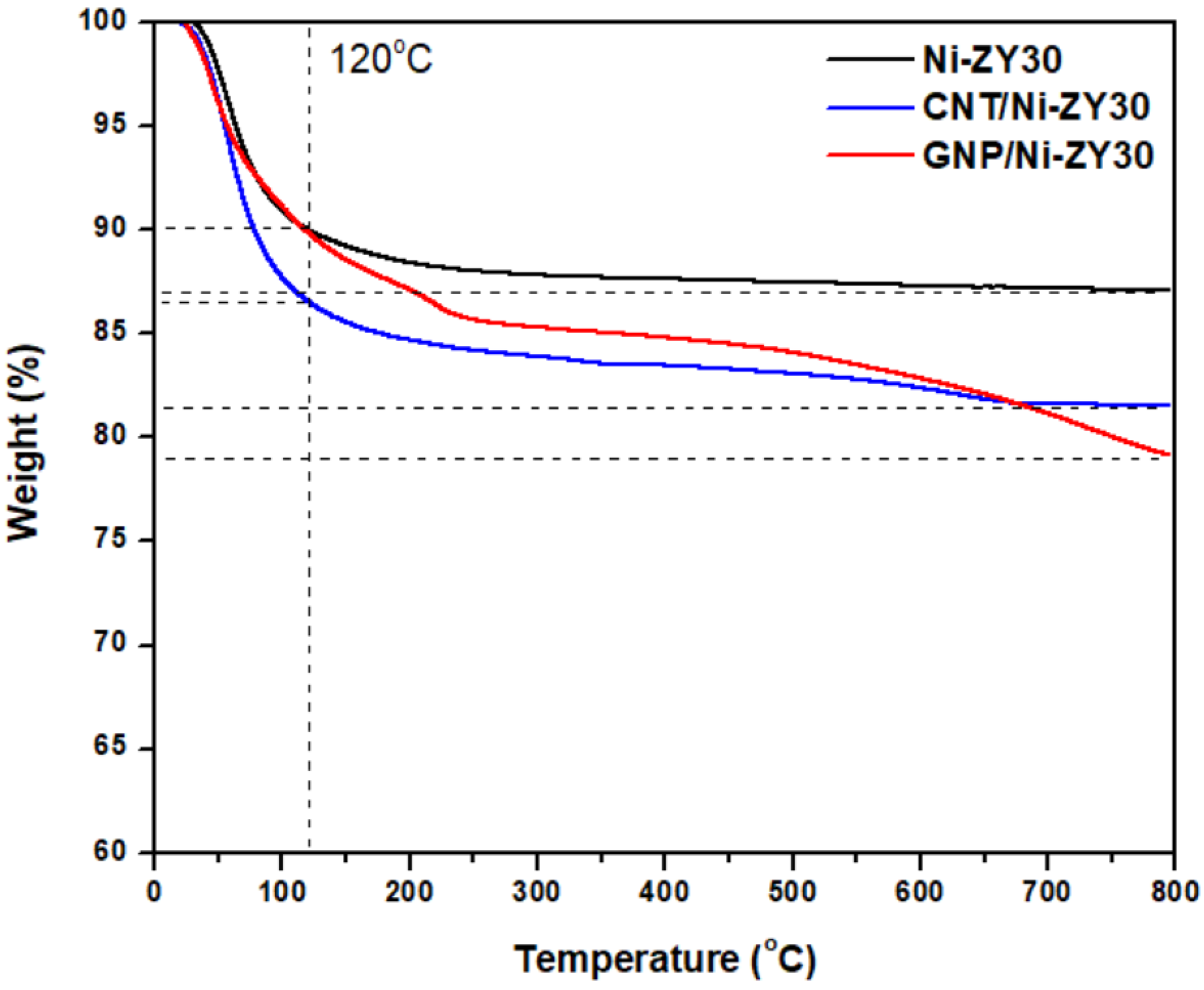

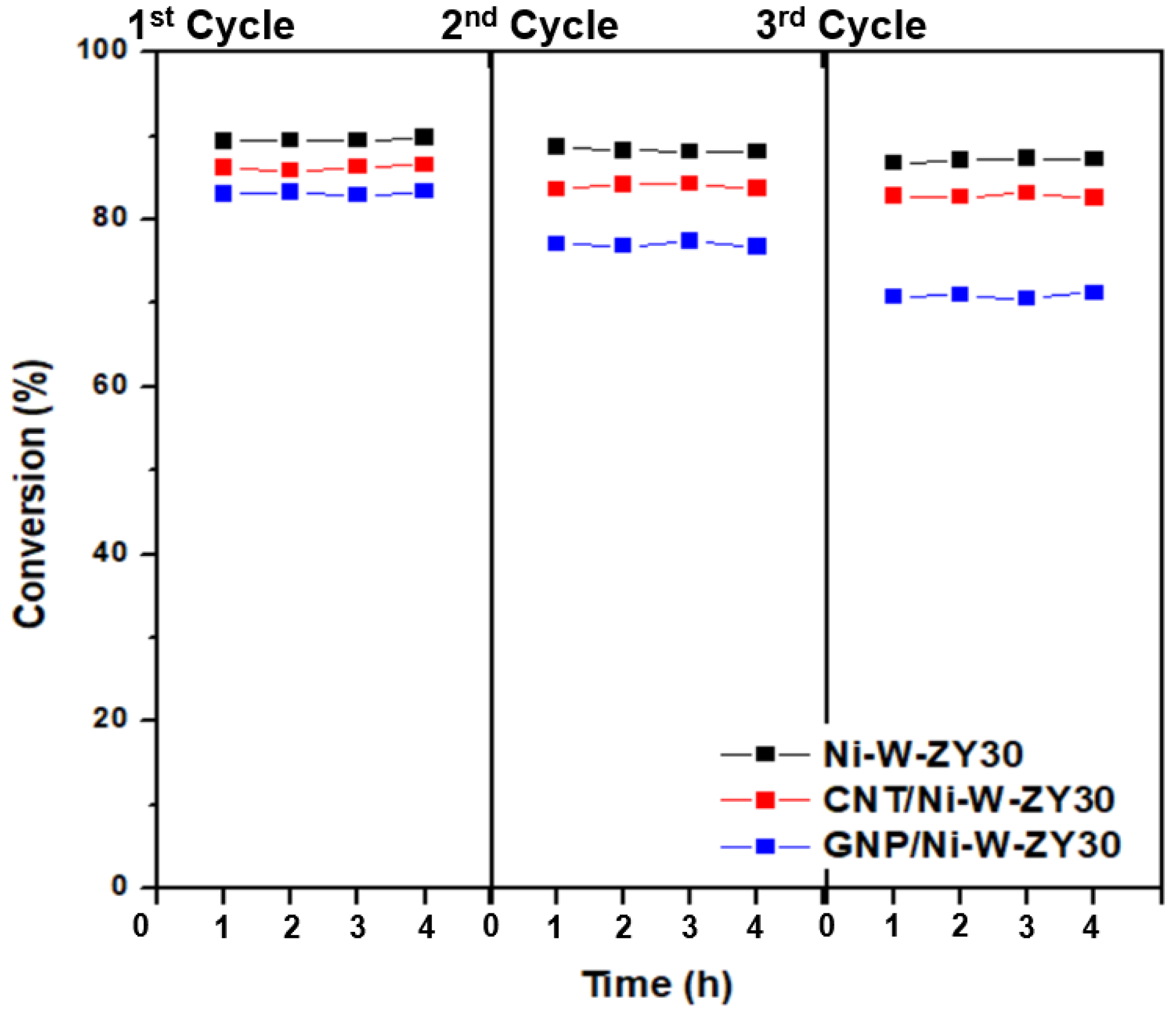
| Catalyst | Element | C 1s | O 1s | Si 2p | Al 2p | Ni 2p | W 4f | Si/Al (Atomic Ratio) | Si/Al (Nominal Ratio) |
|---|---|---|---|---|---|---|---|---|---|
| Reference catalysts | |||||||||
| Ni-ZY5 | Atomic % | 2.3 | 62.7 | 18.2 | 14.7 | 1.1 | - | 1.2 | 2.6 |
| BE (eV) | 285.0 | 532.0 | 102.7 | 74.7 | 857.1 | ||||
| Ni-ZY30 | Atomic % | 2.8 | 65.3 | 28.8 | 1.4 | 1.3 | - | 20.6 | 15.0 |
| BE (eV) | 285.0 | 532.9 | 103.6 | 74.9 | 855.9 | ||||
| Ni-W-ZY30 | Atomic % | 2.3 | 65.6 | 29.9 | 1.5 | 0.2 | 0.2 | 19.9 | 15.0 |
| BE (eV) | 285.0 | 532.8 | 103.4 | 75.0 | 858.0 | 36.3 | |||
| CNT- and GNP-based catalysts | |||||||||
| CNT/Ni-ZY5 | Atomic % | 2.5 | 62.5 | 16.2 | 15.4 | 2.3 | - | 1.1 | 2.6 |
| BE (eV) | 285.0 | 532.0 | 102.7 | 74.6 | 856.6 | ||||
| GNP/Ni-ZY5 | Atomic % | 10.2 | 56.8 | 15.3 | 14.4 | 2.4 | - | 1.1 | 2.6 |
| BE (eV) | 284.0 | 532.7 | 103.5 | 75.4 | 858.1 | ||||
| CNT/Ni-ZY30 | Atomic % | 5.8 | 63.5 | 27.9 | 1.1 | 1.3 | - | 25.4 | 15.0 |
| BE (eV) | 284.7 | 533.6 | 104.3 | 75.6 | 856.8 | ||||
| CNT/Ni-W-ZY30 | Atomic % | 2.5 | 64.8 | 30.2 | 1.8 | 0.3 | 0.2 | 16.8 | 15.0 |
| BE (eV) | 285.0 | 532.9 | 103.4 | 75.0 | 857.8 | 36.9 | |||
| GNP/Ni-W-ZY30 | Atomic % | 11.3 | 59.3 | 27.0 | 1.4 | 0.3 | 0.3 | 19.3 | 15.0 |
| BE (eV) | 284.1 | 533.6 | 104.1 | 75.7 | 858.6 | 37.5 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saab, R.; Polychronopoulou, K.; Anjum, D.H.; Charisiou, N.; Goula, M.A.; Hinder, S.J.; Baker, M.A.; Schiffer, A. Carbon Nanostructure/Zeolite Y Composites as Supports for Monometallic and Bimetallic Hydrocracking Catalysts. Nanomaterials 2022, 12, 3246. https://doi.org/10.3390/nano12183246
Saab R, Polychronopoulou K, Anjum DH, Charisiou N, Goula MA, Hinder SJ, Baker MA, Schiffer A. Carbon Nanostructure/Zeolite Y Composites as Supports for Monometallic and Bimetallic Hydrocracking Catalysts. Nanomaterials. 2022; 12(18):3246. https://doi.org/10.3390/nano12183246
Chicago/Turabian StyleSaab, Roba, Kyriaki Polychronopoulou, Dalaver H. Anjum, Nikolaos Charisiou, Maria A. Goula, Steven J. Hinder, Mark A. Baker, and Andreas Schiffer. 2022. "Carbon Nanostructure/Zeolite Y Composites as Supports for Monometallic and Bimetallic Hydrocracking Catalysts" Nanomaterials 12, no. 18: 3246. https://doi.org/10.3390/nano12183246
APA StyleSaab, R., Polychronopoulou, K., Anjum, D. H., Charisiou, N., Goula, M. A., Hinder, S. J., Baker, M. A., & Schiffer, A. (2022). Carbon Nanostructure/Zeolite Y Composites as Supports for Monometallic and Bimetallic Hydrocracking Catalysts. Nanomaterials, 12(18), 3246. https://doi.org/10.3390/nano12183246










