Biodistribution of Multimodal Gold Nanoclusters Designed for Photoluminescence-SPECT/CT Imaging and Diagnostic
Abstract
1. Introduction
2. Materials and Methods
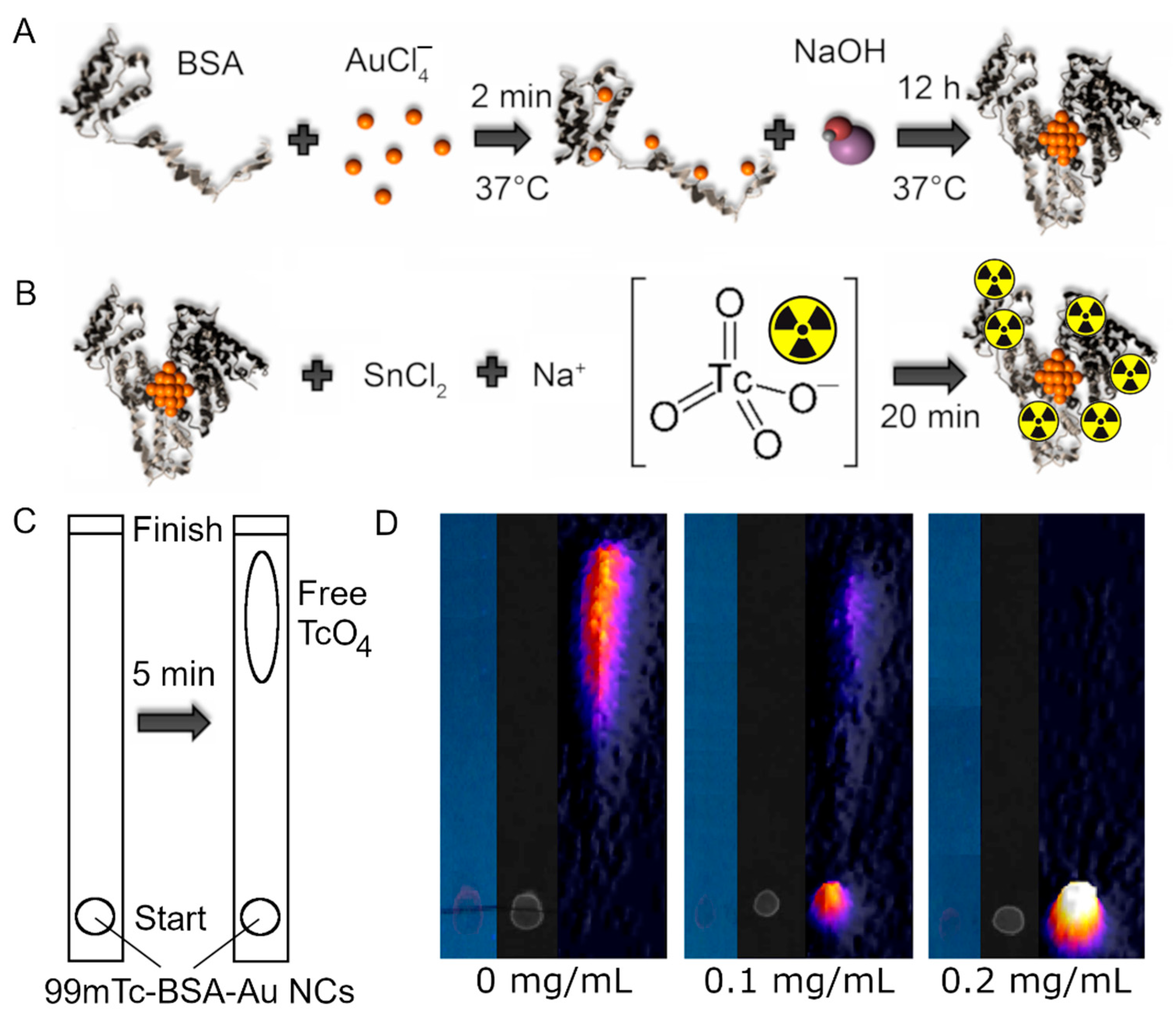
2.1. 99mTc-BSA-Au NCs Synthesis
2.2. Radiochemical Yield
2.3. Optical Spectroscopy Measurements
2.4. Animal Model
2.5. In Vivo Imaging
2.6. Nuclear Medicine Imaging Quantification
2.7. Histology
2.8. Laser Scanning Confocal Microscopy
2.9. Cell Culturing
2.10. Cytotoxicity
2.11. Accumulation in Cells
3. Results and Discussion
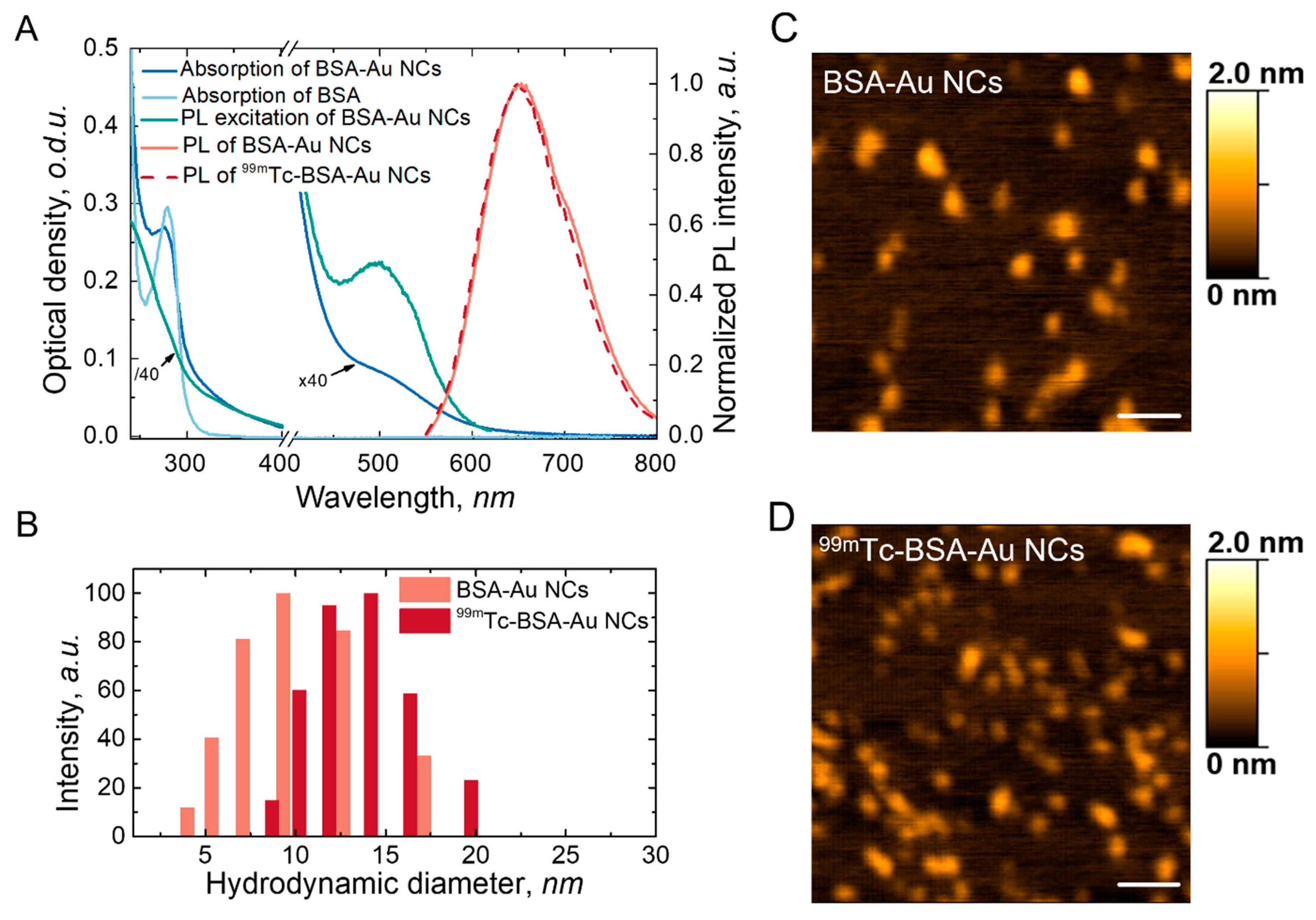
3.1. Characterisation
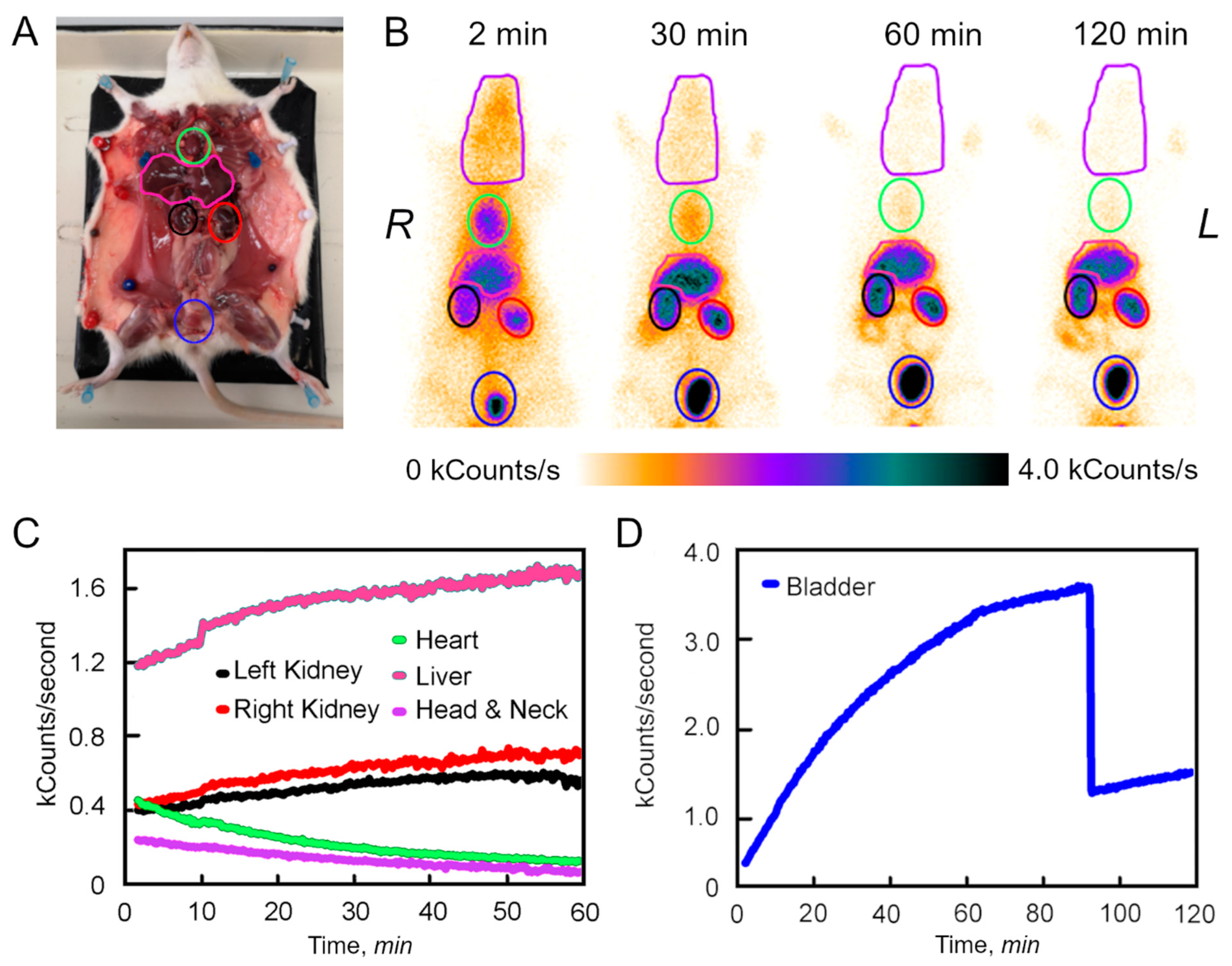
3.2. Dynamic Analysis of the 99mTc-BSA-Au NCs Biodistribution In Vivo
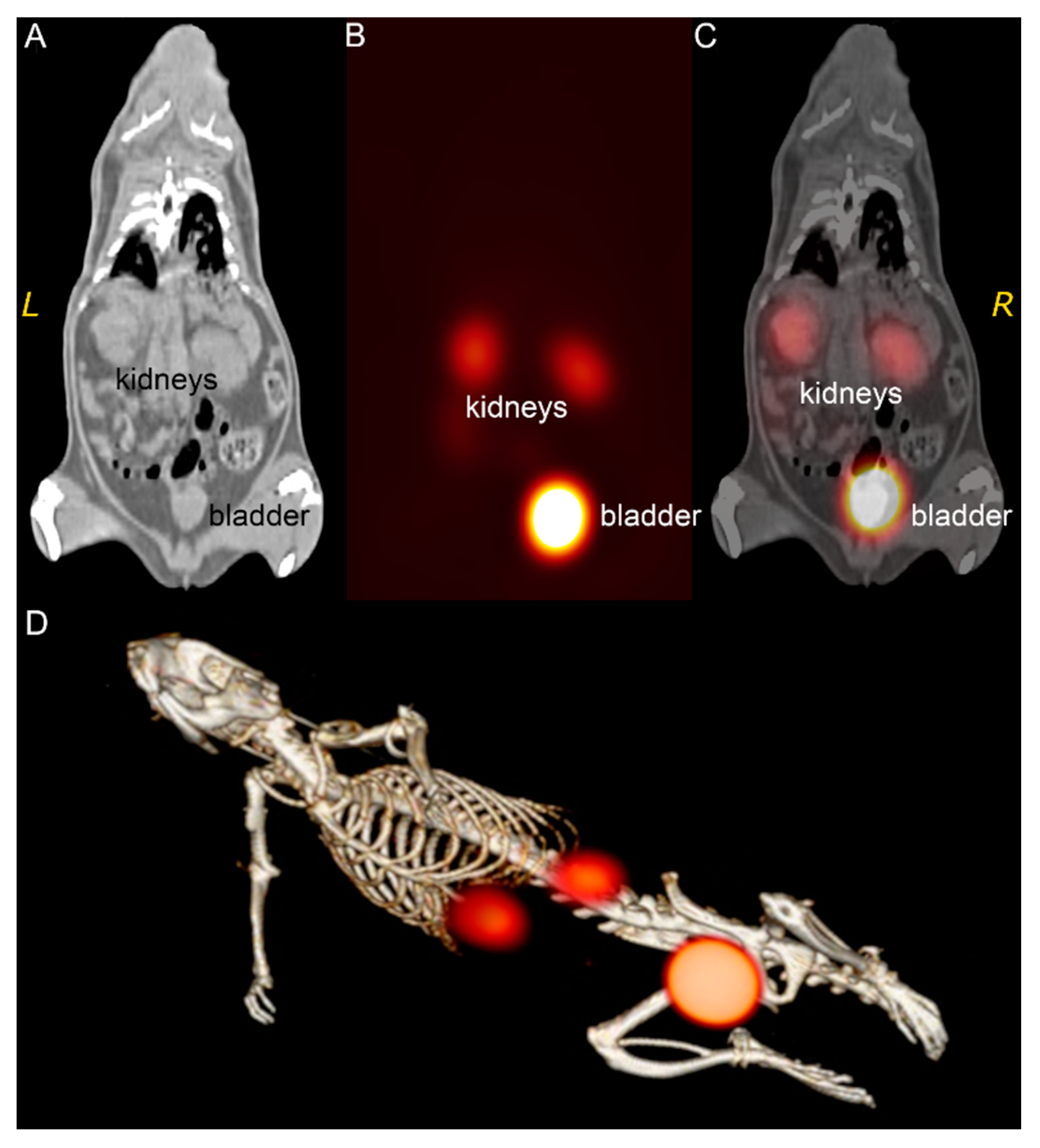
3.3. 99mTc-Au-BSA NCs Localization
3.4. Histology
3.5. Cellular Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Weissleder, R.; Mahmood, U. Molecular Imaging. Radiology 2001, 219, 316–333. [Google Scholar] [CrossRef]
- Chen, K.; Chen, X. Design and Development of Molecular Imaging Probes. Curr. Top. Med. Chem. 2010, 10, 1227–1236. [Google Scholar] [CrossRef]
- Massoud, T.F.; Gambhir, S.S. Molecular Imaging in Living Subjects: Seeing Fundamental Biological Processes in a New Light. Genes Dev. 2003, 17, 545–580. [Google Scholar] [CrossRef]
- James, M.L.; Gambhir, S.S. A Molecular Imaging Primer: Modalities, Imaging Agents, and Applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef]
- Xing, Y.; Zhao, J.; Conti, P.S.; Chen, K. Radiolabeled Nanoparticles for Multimodality Tumor Imaging. Theranostics 2014, 4, 290–306. [Google Scholar] [CrossRef]
- Mezzanotte, L.; van ’t Root, M.; Karatas, H.; Goun, E.A.; Löwik, C.W.G.M. In Vivo Molecular Bioluminescence Imaging: New Tools and Applications. Trends Biotechnol. 2017, 35, 640–652. [Google Scholar] [CrossRef]
- Wu, M.; Shu, J. Multimodal Molecular Imaging: Current Status and Future Directions. Contrast Media Mol. Imaging 2018, 2018, 1382183. [Google Scholar] [CrossRef]
- Walter, A.; Paul-Gilloteaux, P.; Plochberger, B.; Sefc, L.; Verkade, P.; Mannheim, J.G.; Slezak, P.; Unterhuber, A.; Marchetti-Deschmann, M.; Ogris, M.; et al. Correlated Multimodal Imaging in Life Sciences: Expanding the Biomedical Horizon. Front. Phys. 2020, 8, 47. [Google Scholar] [CrossRef]
- Azhdarinia, A.; Ghosh, P.; Ghosh, S.; Wilganowski, N.; Sevick-Muraca, E.M. Dual-Labeling Strategies for Nuclear and Fluorescence Molecular Imaging: A Review and Analysis. Mol. Imaging Biol. 2012, 14, 261–276. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-Directed Synthesis of Highly Fluorescent Gold Nanoclusters. J. Am. Chem Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef]
- Kubiliute, R.; Slektaite, A.; Burkanas, M.; Grigiene, R.; Rotomskis, R. Gold Nanoparticles as a Contrast Agent for X-ray Imaging. In Proceedings of the Medical Physics in the Baltic States; Adliene, D., Ed.; Kaunas University Technology Press: Kaunas, Lithuania, 2011; p. 27. [Google Scholar]
- Poderys, V.; Matulionytė-Safinė, M.; Rupšys, D.; Rotomskis, R. Protein Stabilized Au Nanoclusters: Spectral Properties and Photostability. Lith. J. Phys. 2016, 56, 55–65. [Google Scholar] [CrossRef]
- Su, D.; Gao, L.; Gao, F.; Zhang, X.; Gao, X. Peptide and Protein Modified Metal Clusters for Cancer Diagnostics. Chem. Sci. 2020, 11, 5614–5629. [Google Scholar] [CrossRef]
- Cifuentes-Rius, A.; Deepagan, V.G.; Xie, J.; Voelcker, N.H. Bright Future of Gold Nanoclusters in Theranostics. ACS Appl. Mater. Interfaces 2021, 13, 49581–49588. [Google Scholar] [CrossRef]
- Combes, G.F.; Vučković, A.-M.; Perić Bakulić, M.; Antoine, R.; Bonačić-Koutecky, V.; Trajković, K. Nanotechnology in Tumor Biomarker Detection: The Potential of Liganded Nanoclusters as Nonlinear Optical Contrast Agents for Molecular Diagnostics of Cancer. Cancers 2021, 13, 4206. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, J.; Gao, J.; Zhang, Z.; Zhu, H.; Wang, D. Gold Nanoparticles in Cancer Theranostics. Front. Bioeng. Biotechnol. 2021, 9, 647905. [Google Scholar] [CrossRef]
- Cole, L.E.; Ross, R.D.; Tilley, J.M.; Vargo-Gogola, T.; Roeder, R.K. Gold Nanoparticles as Contrast Agents in X-Ray Imaging and Computed Tomography. Nanomedicine 2015, 10, 321–341. [Google Scholar] [CrossRef]
- Zhang, A.; Tu, Y.; Qin, S.; Li, Y.; Zhou, J.; Chen, N.; Lu, Q.; Zhang, B. Gold Nanoclusters as Contrast Agents for Fluorescent and X-Ray Dual-Modality Imaging. J. Colloid Interface Sci. 2012, 372, 239–244. [Google Scholar] [CrossRef]
- Fang, X.; Wang, Y.; Ma, X.; Li, Y.; Zhang, Z.; Xiao, Z.; Liu, L.; Gao, X.; Liu, J. Mitochondria-Targeting Au Nanoclusters Enhance Radiosensitivity of Cancer Cells. J. Mater. Chem. B 2017, 5, 4190–4197. [Google Scholar] [CrossRef]
- Jarockyte, G.; Poderys, V.; Barzda, V.; Karabanovas, V.; Rotomskis, R. Blood Plasma Stabilized Gold Nanoclusters for Personalized Tumor Theranostics. Cancers 2022, 14, 1887. [Google Scholar] [CrossRef]
- Poderys, V.; Jarockyte, G.; Bagdonas, S.; Karabanovas, V.; Rotomskis, R. Protein-Stabilized Gold Nanoclusters for PDT: ROS and Singlet Oxygen Generation. J. Photochem. Photobiol. B Biol. 2020, 204, 111802. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Fu, C.; Tan, L.; Liu, H. Biosynthesis of Fluorescent Gold Nanoclusters for in Vitro and in Vivo Tumor Imaging. Opt. Commun. 2015, 355, 567–574. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Ikram, S.; Yudha, S.S. Biosynthesis of Gold Nanoparticles: A Green Approach. J. Photochem. Photobiol. B Biol. 2016, 161, 141–153. [Google Scholar] [CrossRef]
- El-Sayed, N.; Schneider, M. Advances in Biomedical and Pharmaceutical Applications of Protein-Stabilized Gold Nanoclusters. J. Mater. Chem. B 2020, 8, 8952–8971. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Q.; Liang, C.; Yang, Z.; Zhang, L.; Yi, X.; Dong, Z.; Chao, Y.; Chen, Y.; Liu, Z. Albumin-Templated Biomineralizing Growth of Composite Nanoparticles as Smart Nano-Theranostics for Enhanced Radiotherapy of Tumors. Nanoscale 2017, 9, 14826–14835. [Google Scholar] [CrossRef]
- Yoo, D.; Lee, D. Oligochitosan-Stabilized Photoluminescent Gold Nanoconstructs for Optical Bioimaging. Biomater. Res. 2017, 21, 20. [Google Scholar] [CrossRef]
- Matulionyte, M.; Dapkute, D.; Budenaite, L.; Jarockyte, G.; Rotomskis, R. Photoluminescent Gold Nanoclusters in Cancer Cells: Cellular Uptake, Toxicity, and Generation of Reactive Oxygen Species. Int. J. Mol. Sci 2017, 18, 378. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Zhang, C.; Jia, Y.; Luo, Y.; Gao, X. AuGd Integrated Nanoprobes for Optical/MRI/CT Triple-Modal in Vivo Tumor Imaging. Nanoscale 2017, 9, 4620–4628. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Zhai, J.; Gao, F.; Liu, R.; Gao, L.; Zhao, Y.; Chai, Z.; Gao, X. Label-Free Au Cluster Used for in Vivo 2D and 3D Computed Tomography of Murine Kidneys. Anal. Chem. 2015, 87, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Henry, M.; Trouillet, V.; Comby-Zerbino, C.; Bertorelle, F.; Sancey, L.; Antoine, R.; Coll, J.-L.; Josserand, V.; Le Guével, X. Zwitterion Functionalized Gold Nanoclusters for Multimodal near Infrared Fluorescence and Photoacoustic Imaging. APL Mater. 2017, 5, 053404. [Google Scholar] [CrossRef]
- Liang, G.; Xiao, L. Gd3+-Functionalized Gold Nanoclusters for Fluorescence–Magnetic Resonance Bimodal Imaging. Biomater. Sci. 2017, 5, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Xia, J.-M.; Hai, X.; Shu, Y.; Chen, X.-W.; Wang, J.-H. Protein-Stabilized Gadolinium Oxide-Gold Nanoclusters Hybrid for Multimodal Imaging and Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 6941–6949. [Google Scholar] [CrossRef]
- Han, W.; Yang, W.; Gao, F.; Cai, P.; Wang, J.; Wang, S.; Xue, J.; Gao, X.; Liu, Y. Iodine-124 Labeled Gold Nanoclusters for Positron Emission Tomography Imaging in Lung Cancer Model. J. Nanosci. Nanotechnol. 2020, 20, 1375–1382. [Google Scholar] [CrossRef]
- Ljungberg, M.; Pretorius, P.H. SPECT/CT: An Update on Technological Developments and Clinical Applications. Br. J. Radiol 2018, 91, 20160402. [Google Scholar] [CrossRef]
- Mariani, G.; Bruselli, L.; Kuwert, T.; Kim, E.E.; Flotats, A.; Israel, O.; Dondi, M.; Watanabe, N. A Review on the Clinical Uses of SPECT/CT. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1959–1985. [Google Scholar] [CrossRef]
- Hoogendam, J.P.; Veldhuis, W.B.; Hobbelink, M.G.G.; Verheijen, R.H.M.; van den Bosch, M.A.A.J.; Zweemer, R.P. 99mTc SPECT/CT Versus Planar Lymphoscintigraphy for Preoperative Sentinel Lymph Node Detection in Cervical Cancer: A Systematic Review and Metaanalysis. J. Nucl. Med. 2015, 56, 675–680. [Google Scholar] [CrossRef]
- Togami, S.; Kawamura, T.; Yanazume, S.; Kamio, M.; Kobayashi, H. Comparison of Lymphoscintigraphy and Single Photon Emission Computed Tomography with Computed Tomography (SPECT/CT) for Sentinel Lymph Node Detection in Endometrial Cancer. Int. J. Gynecol. Cancer 2020, 30, 626–630. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Chuang, M.-H.; Chiu, J.-S.; Cham, T.-M.; Chung, M.-I. On-Site Preparation of Technetium-99m Labeled Human Serum Albumin for Clinical Application. Tohoku J. Exp. Med. 2007, 211, 379–385. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Chen, Y.-C.; Li, D.-K.; Chuang, M.-H. Technetium-99m-Labeled Autologous Serum Albumin: A Personal-Exclusive Source of Serum Component. J. Biomed. Biotechnol. 2011, 2011, 413802. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ali, M.; Kumar, A.; Kumar, M.; Pandey, B.N. The Interaction of Human Serum Albumin with Selected Lanthanide and Actinide Ions: Binding Affinities, Protein Unfolding and Conformational Changes. Biochimie 2016, 123, 117–129. [Google Scholar] [CrossRef]
- Marenco, M.; Canziani, L.; De Matteis, G.; Cavenaghi, G.; Aprile, C.; Lodola, L. Chemical and Physical Characterisation of Human Serum Albumin Nanocolloids: Kinetics, Strength and Specificity of Bonds with 99mTc and 68Ga. Nanomaterials 2021, 11, 1776. [Google Scholar] [CrossRef]
- Rodewald, R.; Karnovsky, M.J. Porous Substructure of the Glomerular Slit Diaphragm in the Rat and Mouse. J. Cell Biol. 1974, 60, 423–433. [Google Scholar] [CrossRef]
- Wartiovaara, J.; Ofverstedt, L.-G.; Khoshnoodi, J.; Zhang, J.; Mäkelä, E.; Sandin, S.; Ruotsalainen, V.; Cheng, R.H.; Jalanko, H.; Skoglund, U.; et al. Nephrin Strands Contribute to a Porous Slit Diaphragm Scaffold as Revealed by Electron Tomography. J. Clin. Investig. 2004, 114, 1475–1483. [Google Scholar] [CrossRef]
- Tojo, A.; Onozato, M.L.; Kitiyakara, C.; Kinugasa, S.; Fukuda, S.; Sakai, T.; Fujita, T. Glomerular Albumin Filtration through Podocyte Cell Body in Puromycin Aminonucleoside Nephrotic Rat. Med. Mol. Morphol. 2008, 41, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, L.M.; Osicka, T.M.; Clavant, S.P.; Robinson, P.J.; Nikolic-Paterson, D.J.; Comper, W.D. Characterization of the Urinary Albumin Degradation Pathway in the Isolated Perfused Rat Kidney. J. Lab. Clin. Med. 2006, 147, 36–44. [Google Scholar] [CrossRef]
- Park, C.H. Time Course and Vectorial Nature of Albumin Metabolism in Isolated Perfused Rabbit PCT. Am. J. Physiol. 1988, 255, F520–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-D.; Wu, D.; Shen, X.; Liu, P.-X.; Fan, F.-Y.; Fan, S.-J. In Vivo Renal Clearance, Biodistribution, Toxicity of Gold Nanoclusters. Biomaterials 2012, 33, 4628–4638. [Google Scholar] [CrossRef]
- Chiu, N.T.; Lee, B.F.; Hwang, S.J.; Chang, J.M.; Liu, G.C.; Yu, H.S. Protein-Losing Enteropathy: Diagnosis with (99m)Tc-Labeled Human Serum Albumin Scintigraphy. Radiology 2001, 219, 86–90. [Google Scholar] [CrossRef]
- Furtado, A.K.; Cabral, V.L.R.; Santos, T.N.; Mansour, E.; Nagasako, C.K.; Lorena, S.L.; Pereira-Filho, R.A. Giardia Infection: Protein-Losing Enteropathy in an Adult with Immunodeficiency. World J. Gastroenterol. 2012, 18, 2430–2433. [Google Scholar] [CrossRef]
- Brouwer, O.R.; Buckle, T.; Vermeeren, L.; Klop, W.M.C.; Balm, A.J.M.; van der Poel, H.G.; van Rhijn, B.W.; Horenblas, S.; Nieweg, O.E.; van Leeuwen, F.W.B.; et al. Comparing the Hybrid Fluorescent-Radioactive Tracer Indocyanine Green-99mTc-Nanocolloid with 99mTc-Nanocolloid for Sentinel Node Identification: A Validation Study Using Lymphoscintigraphy and SPECT/CT. J. Nucl. Med. 2012, 53, 1034–1040. [Google Scholar] [CrossRef]
- KleinJan, G.H.; van Werkhoven, E.; van den Berg, N.S.; Karakullukcu, M.B.; Zijlmans, H.J.M.a.A.; van der Hage, J.A.; van de Wiel, B.A.; Buckle, T.; Klop, W.M.C.; Horenblas, S.; et al. The Best of Both Worlds: A Hybrid Approach for Optimal Pre- and Intraoperative Identification of Sentinel Lymph Nodes. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Zuckier, L.S.; Dohan, O.; Li, Y.; Chang, C.J.; Carrasco, N.; Dadachova, E.; Dohan, O. Kinetics of Perrhenate Uptake and Comparative Biodistribution of Perrhenate, Pertechnetate, and Iodide by NaI Symporter-Expressing Tissues in Vivo. J. Nucl. Med. 2004, 45, 500–507. [Google Scholar] [PubMed]
- Escudero-Francos, M.A.; Cepas, V.; González-Menéndez, P.; Badía-Laíño, R.; Díaz-García, M.E.; Sainz, R.M.; Mayo, J.C.; Hevia, D. Cellular Uptake and Tissue Biodistribution of Functionalized Gold Nanoparticles and Nanoclusters. J. Biomed. Nanotechnol. 2017, 13, 167–179. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, Y.; Yuan, Q.; Lu, C.; Zhang, X.; Yuan, J.; Hou, K.; Zhang, C.; Du, Z.; Gao, X.; et al. Gold Clusters Prevent Breast Cancer Bone Metastasis by Suppressing Tumor-Induced Osteoclastogenesis. Theranostics 2020, 10, 4042–4055. [Google Scholar] [CrossRef] [PubMed]
- Ungor, D.; Barbasz, A.; Czyżowska, A.; Csapó, E.; Oćwieja, M. Cytotoxicity Studies of Protein-Stabilized Fluorescent Gold Nanoclusters on Human Lymphocytes. Colloids Surf. B Biointerfaces 2021, 200, 111593. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarockyte, G.; Stasys, M.; Poderys, V.; Buivydaite, K.; Pleckaitis, M.; Bulotiene, D.; Matulionyte, M.; Karabanovas, V.; Rotomskis, R. Biodistribution of Multimodal Gold Nanoclusters Designed for Photoluminescence-SPECT/CT Imaging and Diagnostic. Nanomaterials 2022, 12, 3259. https://doi.org/10.3390/nano12193259
Jarockyte G, Stasys M, Poderys V, Buivydaite K, Pleckaitis M, Bulotiene D, Matulionyte M, Karabanovas V, Rotomskis R. Biodistribution of Multimodal Gold Nanoclusters Designed for Photoluminescence-SPECT/CT Imaging and Diagnostic. Nanomaterials. 2022; 12(19):3259. https://doi.org/10.3390/nano12193259
Chicago/Turabian StyleJarockyte, Greta, Marius Stasys, Vilius Poderys, Kornelija Buivydaite, Marijus Pleckaitis, Danute Bulotiene, Marija Matulionyte, Vitalijus Karabanovas, and Ricardas Rotomskis. 2022. "Biodistribution of Multimodal Gold Nanoclusters Designed for Photoluminescence-SPECT/CT Imaging and Diagnostic" Nanomaterials 12, no. 19: 3259. https://doi.org/10.3390/nano12193259
APA StyleJarockyte, G., Stasys, M., Poderys, V., Buivydaite, K., Pleckaitis, M., Bulotiene, D., Matulionyte, M., Karabanovas, V., & Rotomskis, R. (2022). Biodistribution of Multimodal Gold Nanoclusters Designed for Photoluminescence-SPECT/CT Imaging and Diagnostic. Nanomaterials, 12(19), 3259. https://doi.org/10.3390/nano12193259






