Polyvinylpyrrolidone-Capped Silver Nanoparticles for Highly Sensitive and Selective Optical Fiber-Based Ammonium Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Polyvinylpyrrolidone (PVP) Capped Silver Nanoparticles
2.2. Materials Characterization
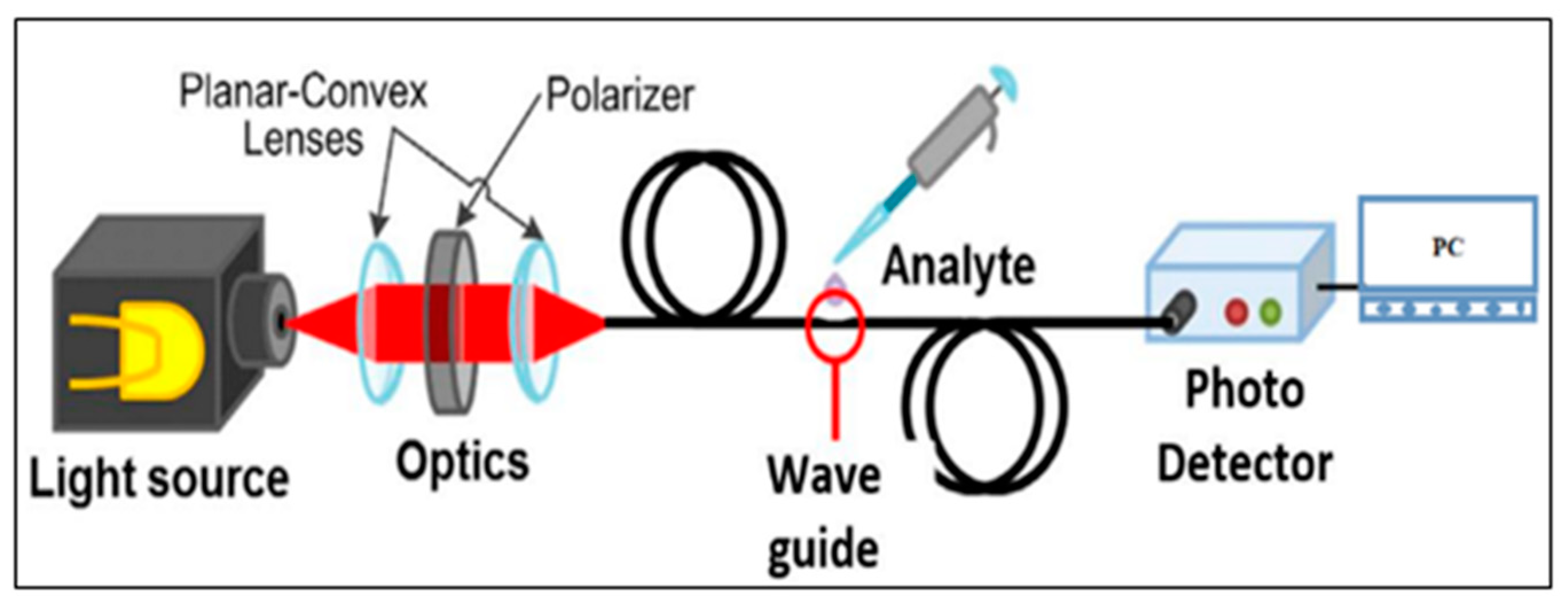
2.3. Sensor Characterization
3. Results and Discussion
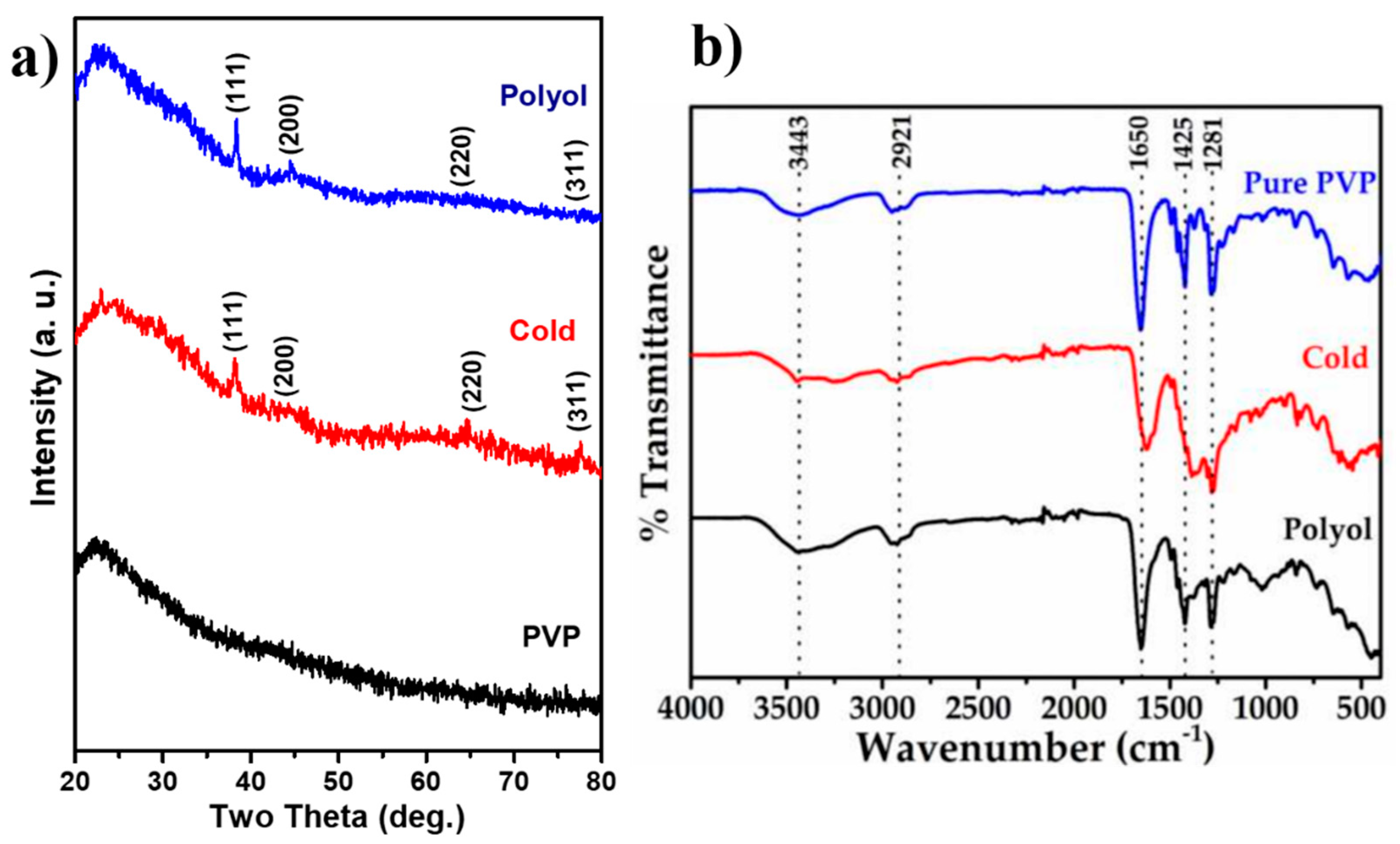
3.1. XRD and FTIR Analysis
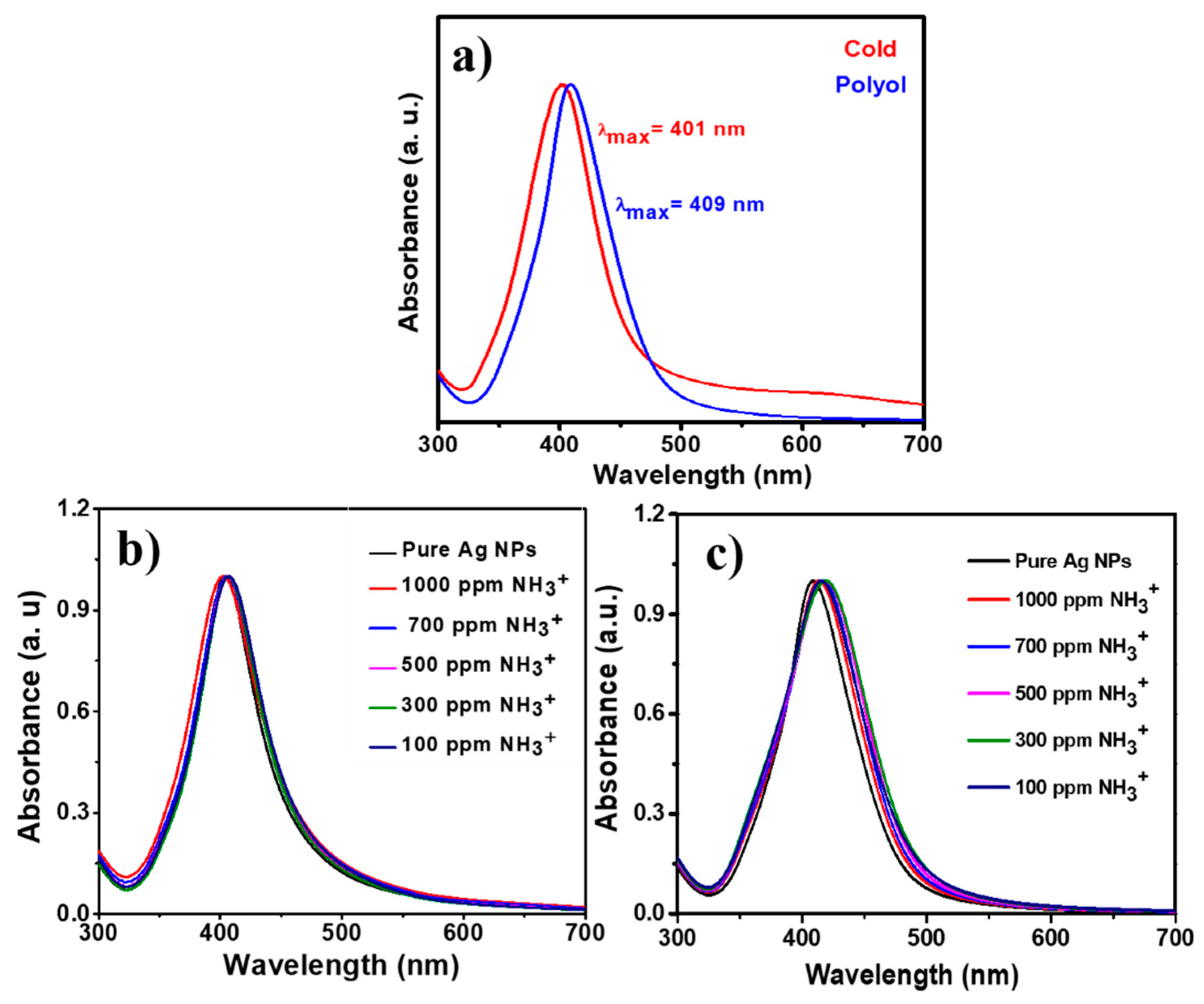
3.2. UV-Visible Analysis
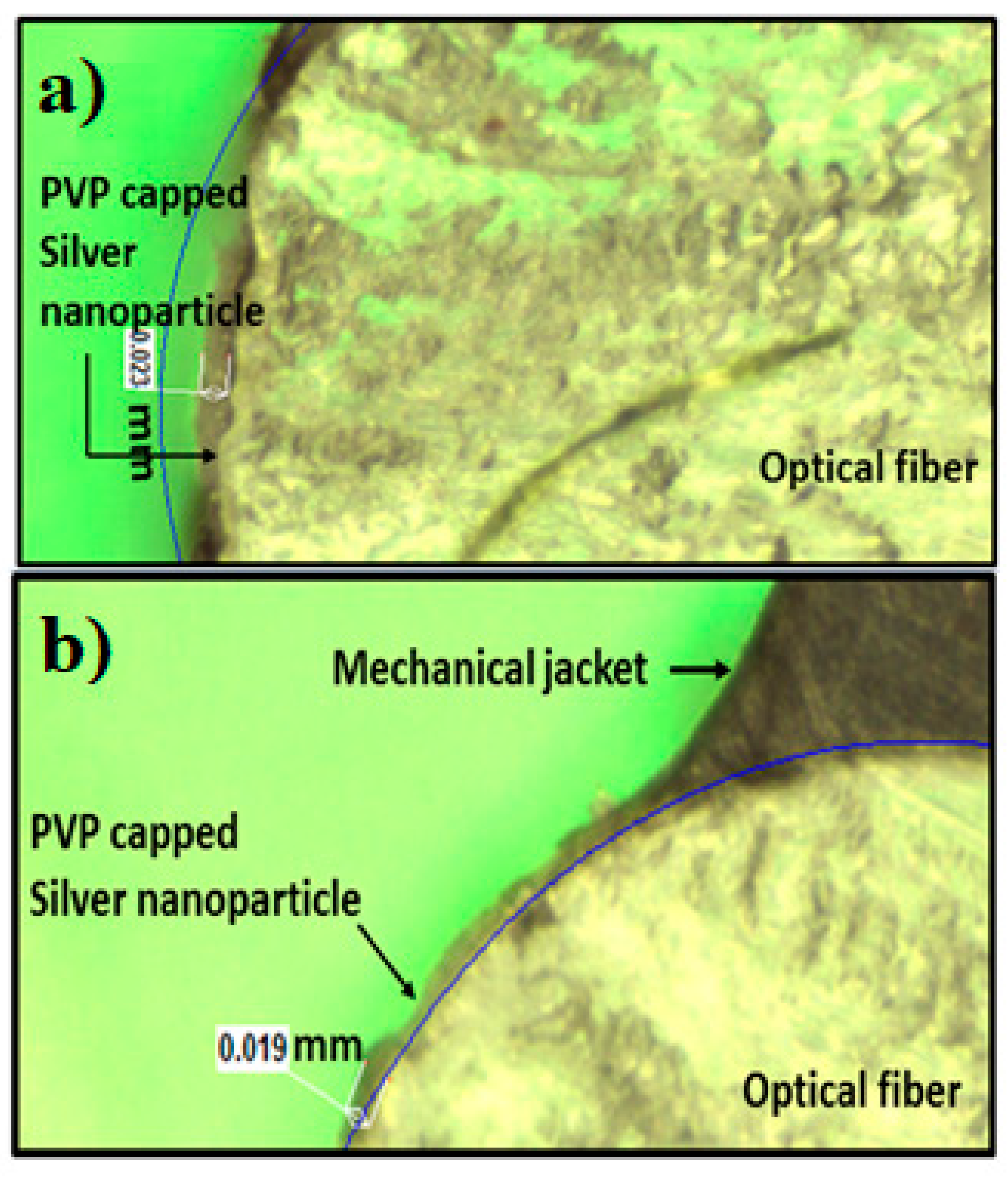
3.3. Optical Image Analysis
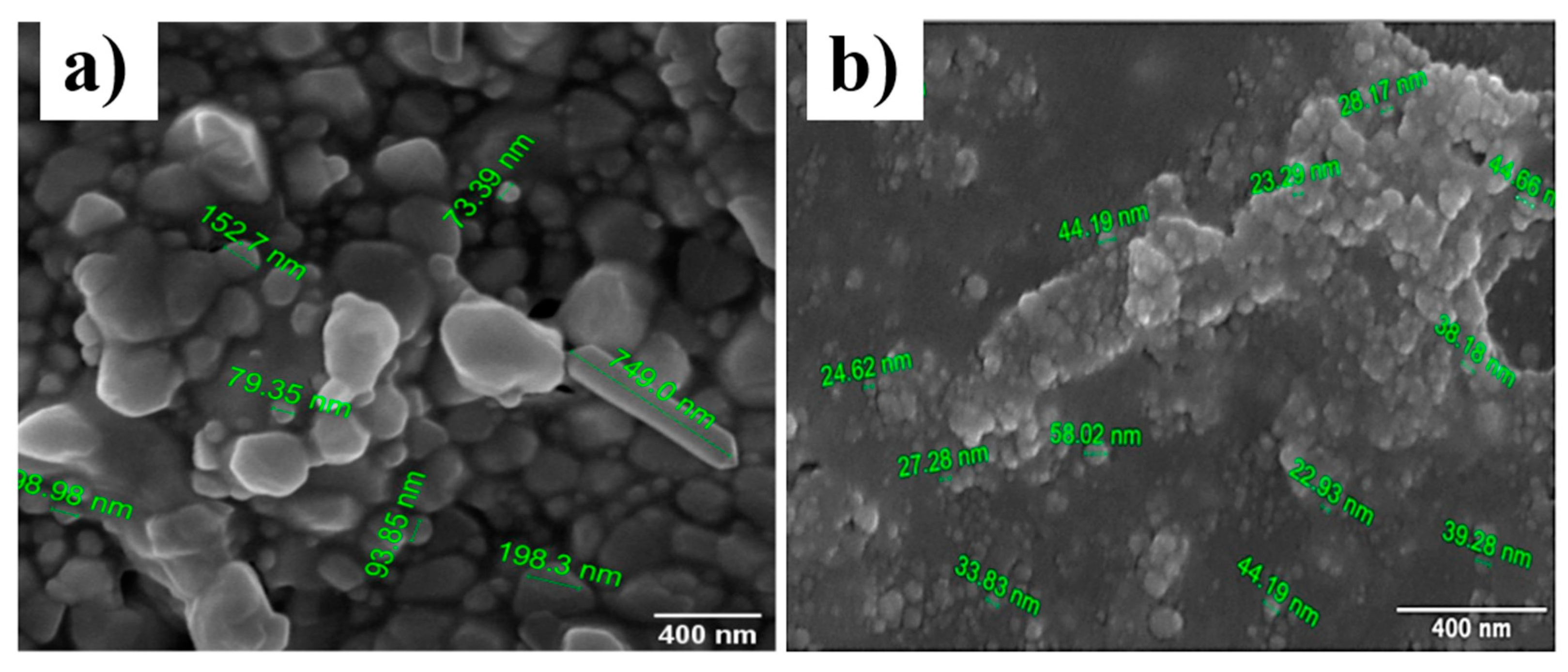
3.4. FESEM Image Analysis
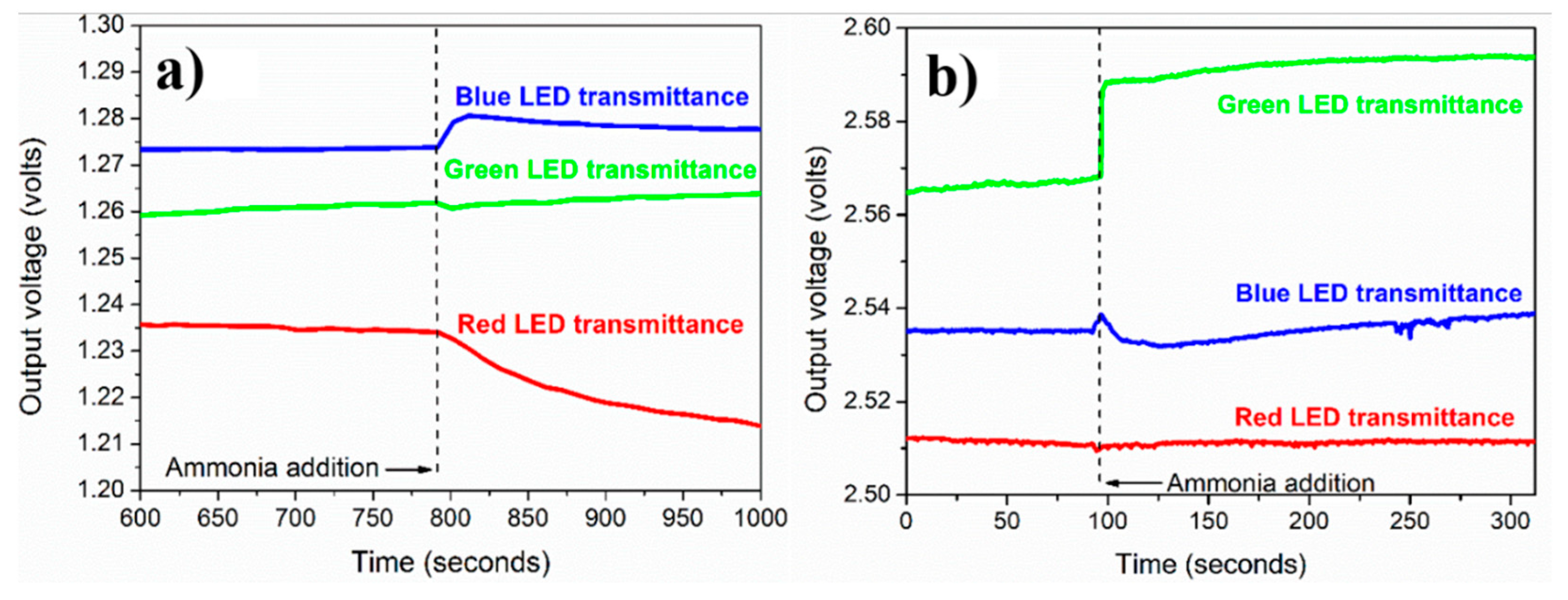
3.5. Ammonium Sensing Study
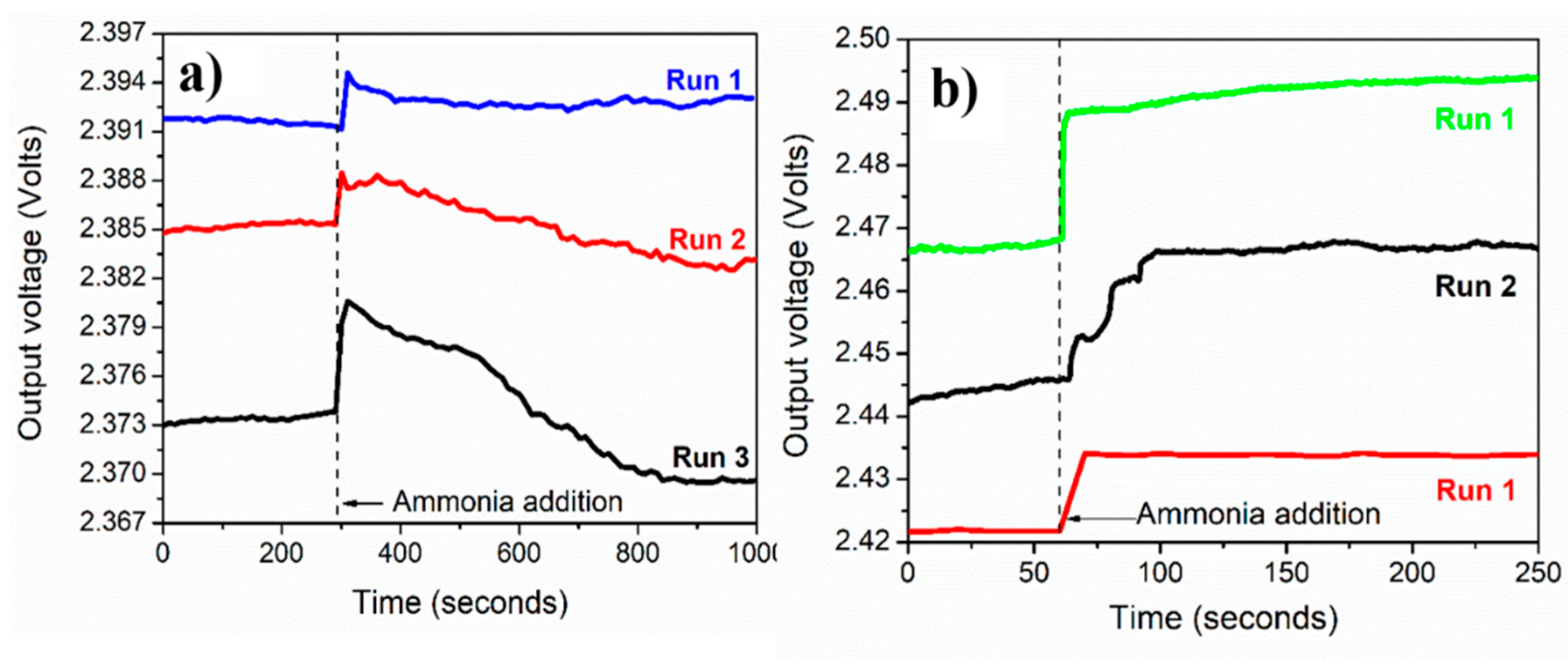
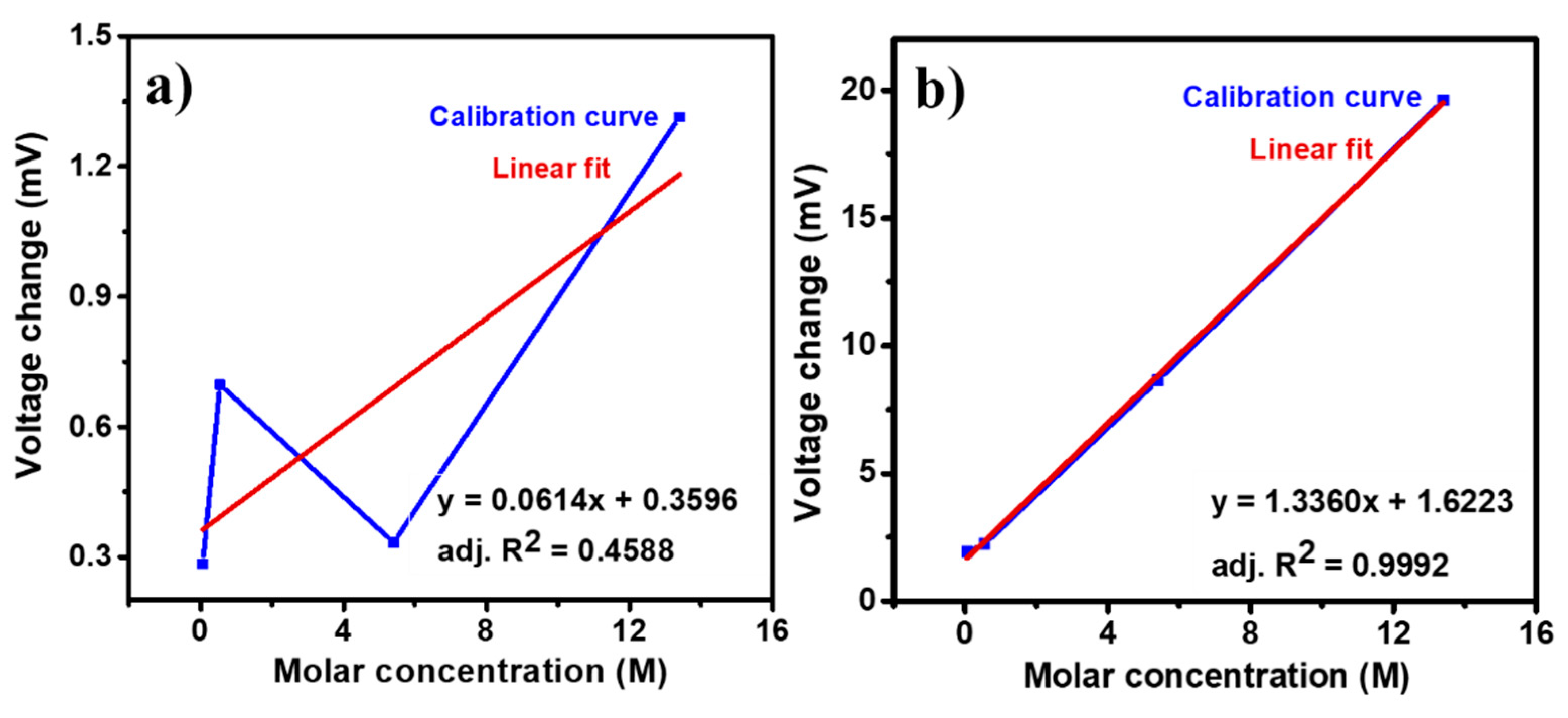
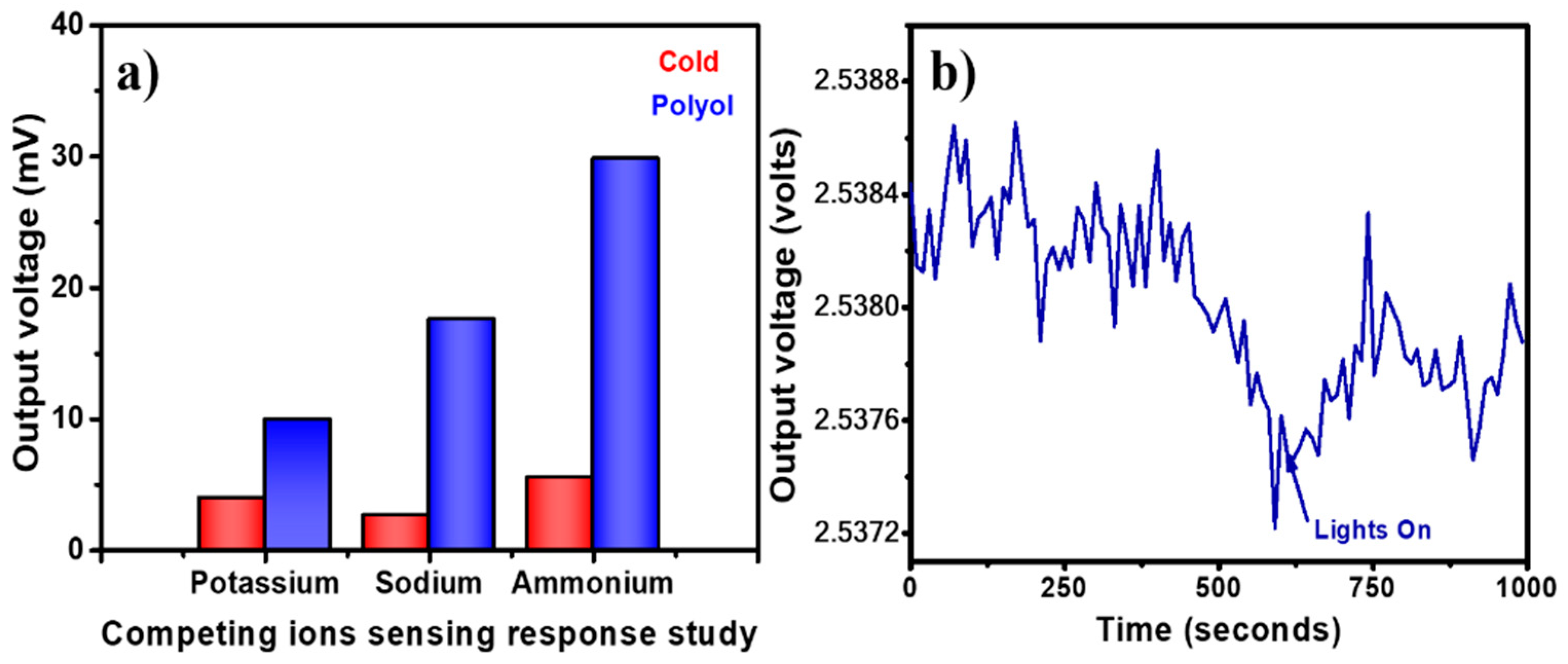
3.6. Sensing Repeatability, Calibration Curves, and Selectivity
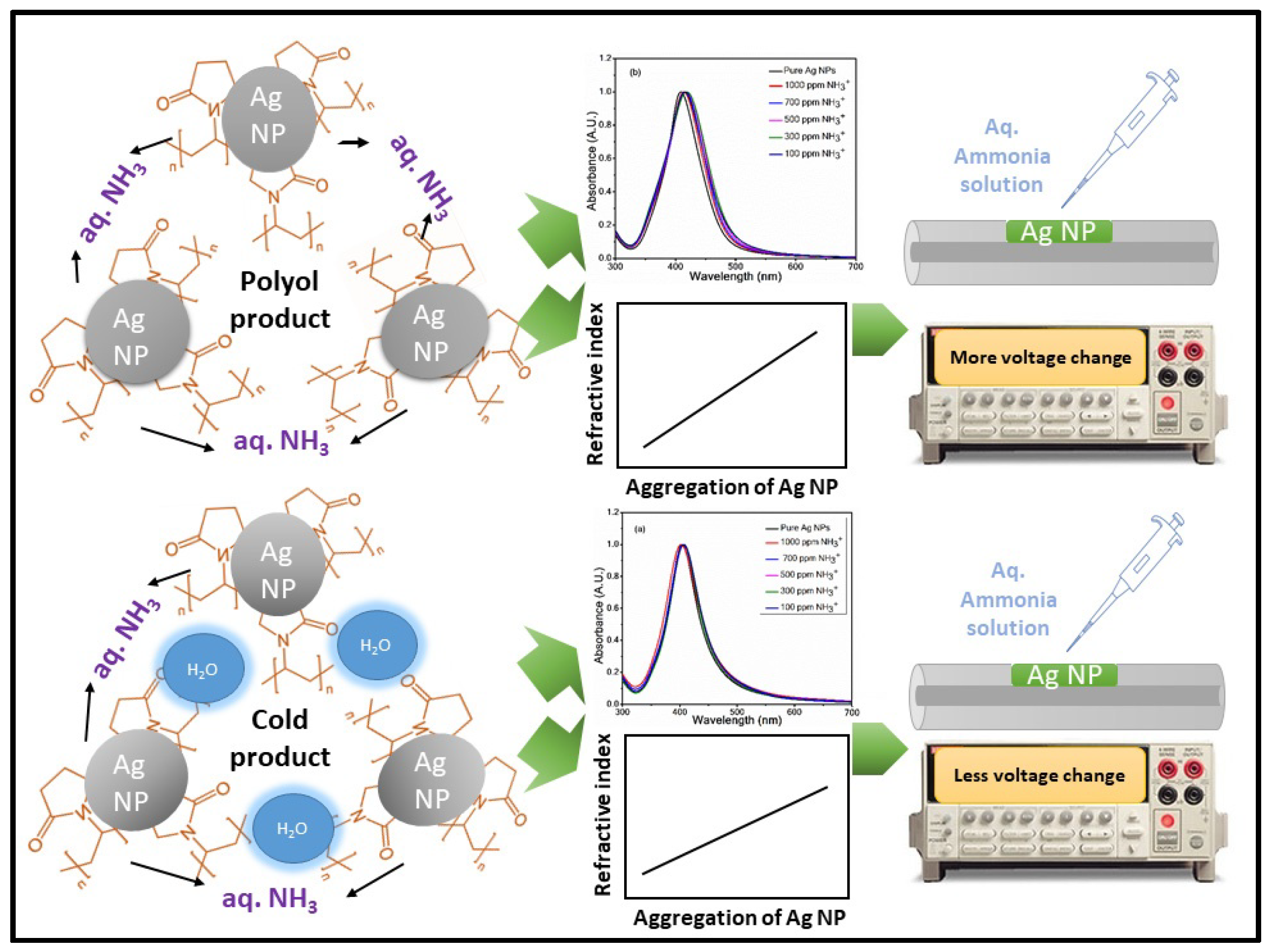
3.7. Sensing Mechanism
3.8. Comparison of Our Work with the Literature Review
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, Q.; Zheng, B.; Zhao, X.; Wang, L.; Liu, C. Ammonia pollution characteristics of centralized drinking water sources in China. J. Environ. Sci. 2012, 24, 1739–1743. [Google Scholar] [CrossRef]
- Li, D.; Xu, X.; Li, Z.; Wang, T.; Wang, C. Detection methods of ammonium nitrogen in water: A review. Trends Anal. Chem. 2020, 127, 115890. [Google Scholar] [CrossRef]
- Thomas, R.F.; Booth, R.L. Selective electrode measurement of ammonium in water and wastes. Environ. Sci. Technol. 1973, 7, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yan, C.; Guo, Q.; Xu, J.; Hu, H. Spectrophotometric determination of ammonium nitrogen in water by flow injection analysis based on NH3-o-phthalaldehyde-Na2SO3 reaction. Anal. Chem. React. 2016, 10, 1–8. [Google Scholar] [CrossRef]
- Duong, H.D.; Rhee, J.I. A ratiometric fluorescence sensor for the detection of ammonium in water. Sens. Actuators B Chem. 2014, 190, 768–774. [Google Scholar] [CrossRef]
- Jana, J.; Ganguly, M.; Pal, T. Enlightening surface Plasmon resonance effect of metal nanoparticles for practical spectroscopic application. RSC Adv. 2016, 6, 86174–86211. [Google Scholar]
- Urrutia, A.; Goicoechea, J.; Arregui, F. Optical fiber sensors based on nanoparticle-embedded coatings. J. Sens. 2015, 2015, 805053. [Google Scholar] [CrossRef]
- Ahn, H.; Song, H.; Choi, J.; Kim, K. A localized surface Plasmon resonance sensor using double-metal-complex nanostructures and a review of recent approaches. Sensors 2018, 18, 98. [Google Scholar] [CrossRef]
- Soelberg, S.D.; Chinowsky, T.; Geiss, G.; Spinelli, C.B.; Stevens, R.; Near, S.; Kauffman, P.; Yea, S.; Furlong, C.E. A portable surface Plasmon resonance sensor system for real-time monitoring of small to large analytes. J. Ind. Microbiol. Biotechnol. 2005, 32, 669–674. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Abdulhalim, I. SPR based blood glucose detection with improved sensitivity and stability. In Frontiers in Optics; Optica Publishing Group: Tucson, AZ, USA, 2014. [Google Scholar]
- Liu, L.; Xia, N.; Wang, J. Potential applications of SPR in early diagnosis and progression of Alzheimer’s disease. RSC Adv. 2012, 2, 2200–2204. [Google Scholar] [CrossRef]
- Monfared, Y. Overview of recent advances in the design of plasmonic fiber–optic biosensors. Biosensors 2020, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Bremer, K.; Roth, B. Fiber-optic surface Plasmon resonance sensor system designed for smartphones. Optics Express 2015, 23, 17179–17184. [Google Scholar] [CrossRef] [PubMed]
- Laskar, S.; Mukherjee, S. Optical sensing methods for assessment of soil macro-nutrients and other properties for application in precision agriculture: A review. ADBU J. Eng. Tech. 2016, 4, 206–210. [Google Scholar]
- Jharwal, M.; Devi, P.; Kumar, A. Structural analysis of PVP capped silver nanoparticles synthesized at room temperature for optical, electrical, and gas sensing properties. J. Mater. Sci. Mater. Electron. 2017, 28, 5014–5020. [Google Scholar]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. Characterization and optical studies of PVP- capped silver nanoparticles. J. Nanostruct. Chem. 2017, 7, 37–46. [Google Scholar] [CrossRef]
- Amirjani, A.; Fatmehsari, D. Colorimetric detection of ammonium using smartphones based on localized surface plasmon resonance of silver nanoparticles. Talanta 2018, 176, 242–246. [Google Scholar] [CrossRef]
- Khan, M.Q.; Kharaghani, D.; Nishat, N.; Ishikawa, T.; Ullah, S.; Lee, H.; Khatri, Z.; Kim, I.S. The development of nanofiber tubes based on nanocomposites of polyvinylpyrrolidone incorporated gold nanoparticles as scaffolds for neuroscience application in axons. Text. Res. J 2019, 89, 2713–2720. [Google Scholar] [CrossRef]
- Rahaman Mollick, M.M.; Bhowmick, B.; Maity, D.; Mondal, D.; Kanti Bain, M.; Bankura, K.; Sarkar, J.; Rana, D.; Acharya, K.; Chattopadhyay, D. Green synthesis of silver nanoparticles using paederia foetida L. leaf extract and assessment of their antimicrobial activities. Int. J. Green Nanotechnol. 2012, 4, 230–239. [Google Scholar] [CrossRef]
- Koohpeima, F.; Mokhtari, M.J.; Khalafi, S. The effect of silver nanoparticles on composite shear bond strength to dentin with different adhesion protocols. J. Appl. Oral Sci. 2017, 25, 367–373. [Google Scholar]
- Gharibshahi, L.; Saion, E.; Gharibshahi, E.; Shaari, A.H.; Matori, K.A. Influence of Poly(vinylpyrrolidone) concentration on properties of silver nanoparticles manufactured by modified thermal treatment method. PLoS ONE 2017, 12, e0186094. [Google Scholar] [CrossRef]
- Singh, S.; Bharti, A.; Meena, V.K. Structural, thermal, zeta potential, and electrical properties of disaccharide reduced silver nanoparticles. J. Mater. Sci. Mater. Electron. 2014, 25, 3747–3752. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, R.; Yu, S.; Zhang, Z.; Zhou, L.; Huang, H.; Du, R. Size-controlled preparation of silver nanoparticles by a modified polyol method. Colloids Surf. A Physicochem. Eng. Asp. 2010, 366, 197–202. [Google Scholar] [CrossRef]
- Kaur, A.; Kumar, R. Enhanced bactericidal efficacy of polymer stabilized silver nanoparticles in conjugation with different classes of antibiotics. RSC Adv. 2019, 9, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Tejamaya, M.; Romer, I.; Merrifield, R.C.; Lead, J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Muldarisnur, M.; Fridayanti, N.; Oktorina, E.; Zeni, E.; Elvaswer, E.; Syukri, S. Effect of nanoparticle geometry on sensitivity of metal nanoparticle based sensor. IOP Conf. Ser. Mater. Sci. Eng. 2019, 578, 012036. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Xiong, S.; Chin, L.K.; Zhang, J.B.; Ser, W.; Wu, J.H.; Chen, T.N.; Yang, Z.C.; Hao, Y.L.; Liu, A.Q. Determination of size and refractive index of single gold nanoparticles using an optofluidic chip. AIP Adv. 2017, 7, 095024. [Google Scholar] [CrossRef]
- Vijayan, A.; Fuke, M.; Hawaldar, R.; Kulkarni, M.; Amalnerkar, D.; Aiyer, R.C. Optical fiber based humidity sensor using Co-polyaniline clad. Sens. Actuators B 2008, 129, 106–112. [Google Scholar] [CrossRef]
- Ritthichai, T.; Pimpan, V. Ammonia sensing of silver nanoparticles synthesized using tannic acid combined with UV radiation: Effect of UV exposure time. J. King Suad Univ.-Sci. 2019, 31, 277–284. [Google Scholar] [CrossRef]
- Alzahrani, E. Colorimetric detection of ammonia using synthesized silver nanoparticles from durian fruit shell. J. Chem. 2020, 2020, 4712130. [Google Scholar] [CrossRef]
- Detsri, E.; Popanyasak, J. Fabrication of silver nanoparticles/Polyaniline composite thin films using Layer-by-Layer self-assembly technique for ammonia sensing. Colloids Surf. A Physiochem. Eng. Asp. 2015, 467, 57–65. [Google Scholar]
| Sr. No. | Materials Used | Detection Range | Sensitivity | Detection Principle | Reference |
|---|---|---|---|---|---|
| 1. | Silver nanoparticles synthesized using tannic acid | 0–500 ppm | 3.5/200 ppm | Colorimetric | [29] |
| 2. | Silver nanoparticles synthesized using durian fruit shell | 500–3000 ppm | 15/3000 ppm | Colorimetric | [30] |
| 3. | Silver nanoparticles/Polyaniline composite thin films | 0–800 mM | 0.2038 mM | Colorimetric | [31] |
| 4. | Silver nanoparticles synthesized using the polyol synthesis method and cold synthesis method | 0.054–13.4 M | 1.33 mV/M | Optical fiber evanescent absorption spectroscopy | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potdar, R.P.; Khollam, Y.B.; Shaikh, S.F.; More, P.S.; Rana, A.u.H.S. Polyvinylpyrrolidone-Capped Silver Nanoparticles for Highly Sensitive and Selective Optical Fiber-Based Ammonium Sensor. Nanomaterials 2022, 12, 3373. https://doi.org/10.3390/nano12193373
Potdar RP, Khollam YB, Shaikh SF, More PS, Rana AuHS. Polyvinylpyrrolidone-Capped Silver Nanoparticles for Highly Sensitive and Selective Optical Fiber-Based Ammonium Sensor. Nanomaterials. 2022; 12(19):3373. https://doi.org/10.3390/nano12193373
Chicago/Turabian StylePotdar, Revati P., Yogesh B. Khollam, Shoyebmohamad. F. Shaikh, Pravin S. More, and Abu ul Hassan S. Rana. 2022. "Polyvinylpyrrolidone-Capped Silver Nanoparticles for Highly Sensitive and Selective Optical Fiber-Based Ammonium Sensor" Nanomaterials 12, no. 19: 3373. https://doi.org/10.3390/nano12193373






