Disposal Immunosensor for Sensitive Electrochemical Detection of Prostate-Specific Antigen Based on Amino-Rich Nanochannels Array-Modified Patterned Indium Tin Oxide Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Measurements and Instrumentations
2.3. Preparation of NH2-VMSF/ITO Electrode
2.4. Fabrication of Label-Free Immunosensor
2.5. Electrochemical Determination of PSA
3. Results and Discussion
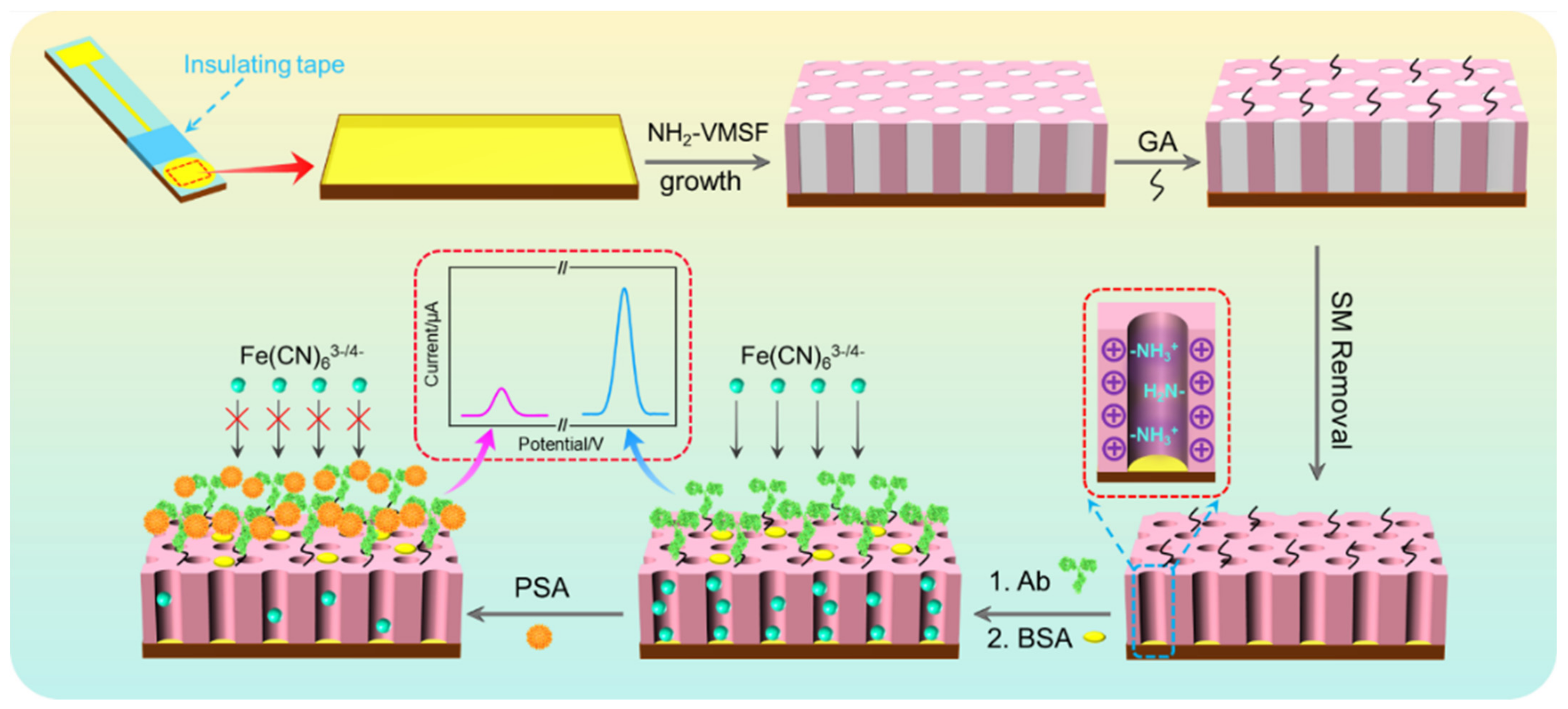
3.1. Fabrication of Immunosensor on Amino-Rich Nanochannel Array-Modified Electrode
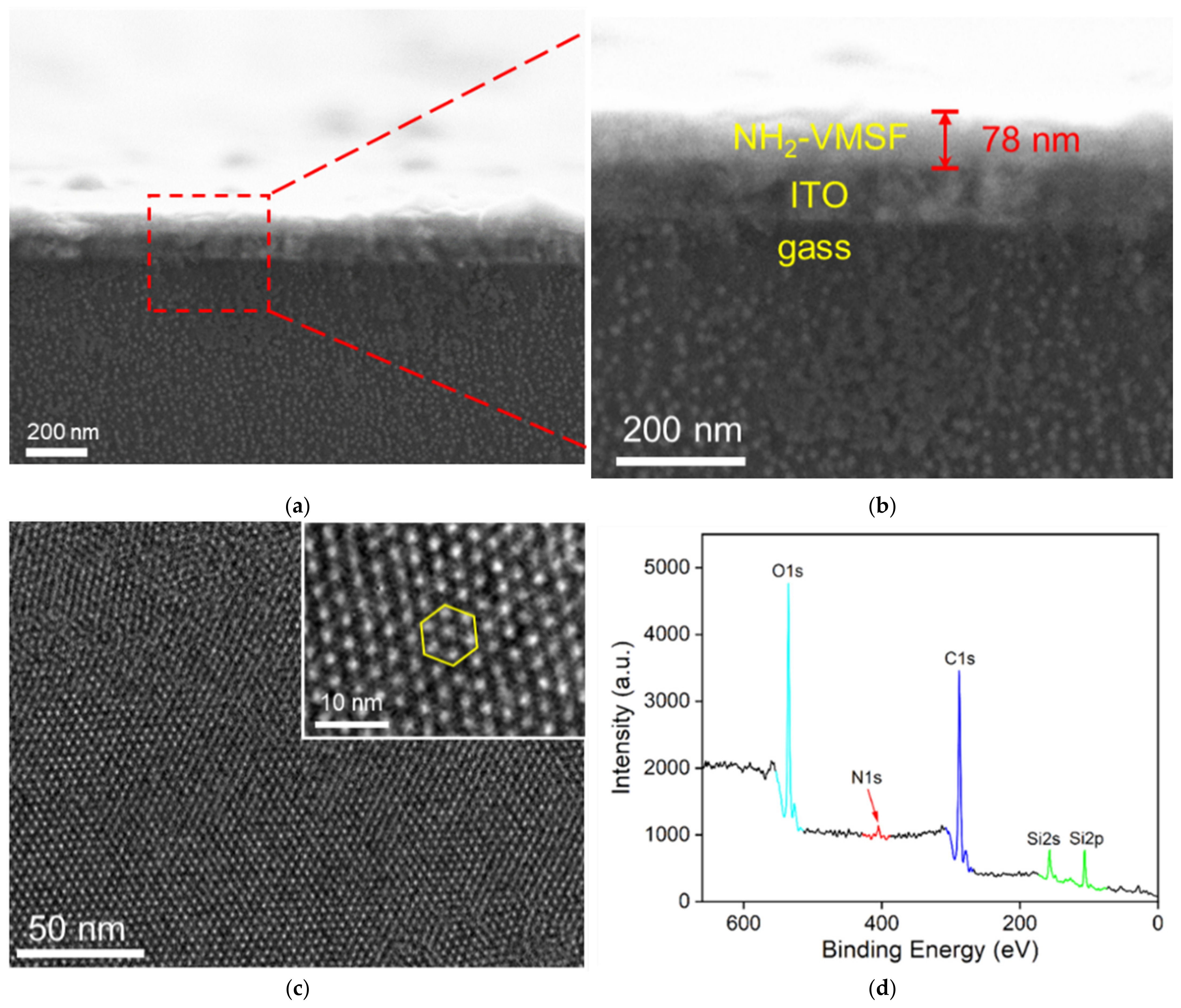
3.2. Characterization of Morphology and Structure of NH2-VMSF
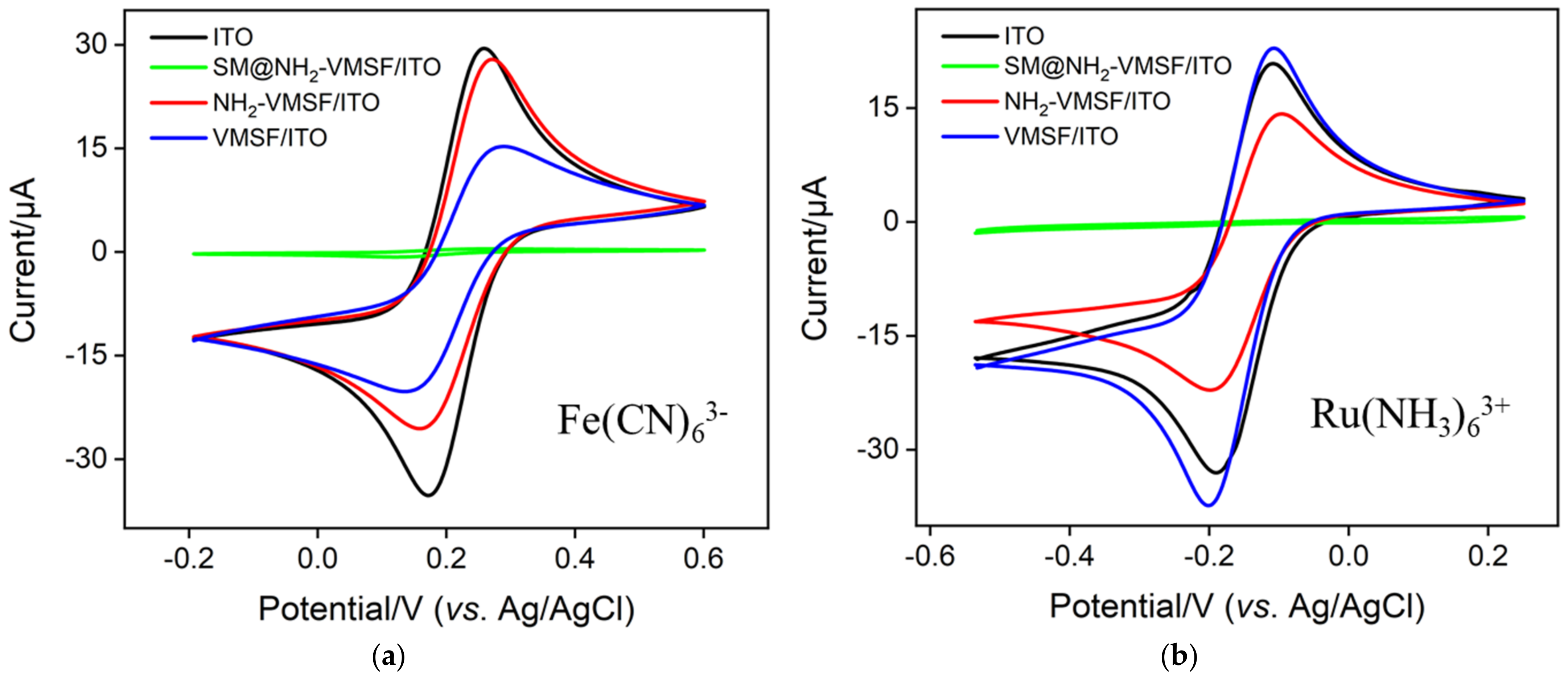
3.3. Charge-Based Selective Permeability or Redox Probe in NH2-VMSF
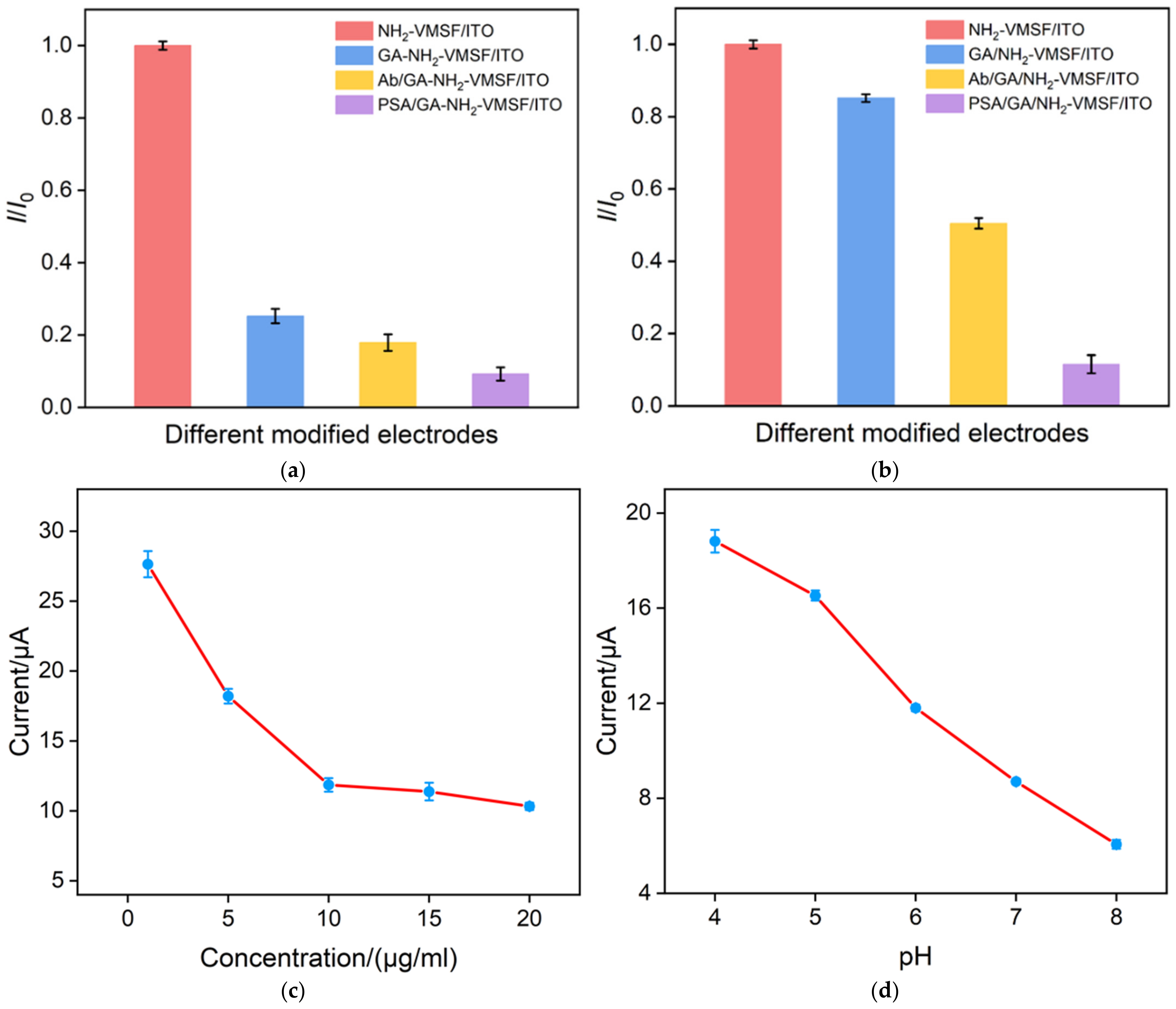
3.4. Electrochemical Characterization of the Fabrication Process of Immunosensor
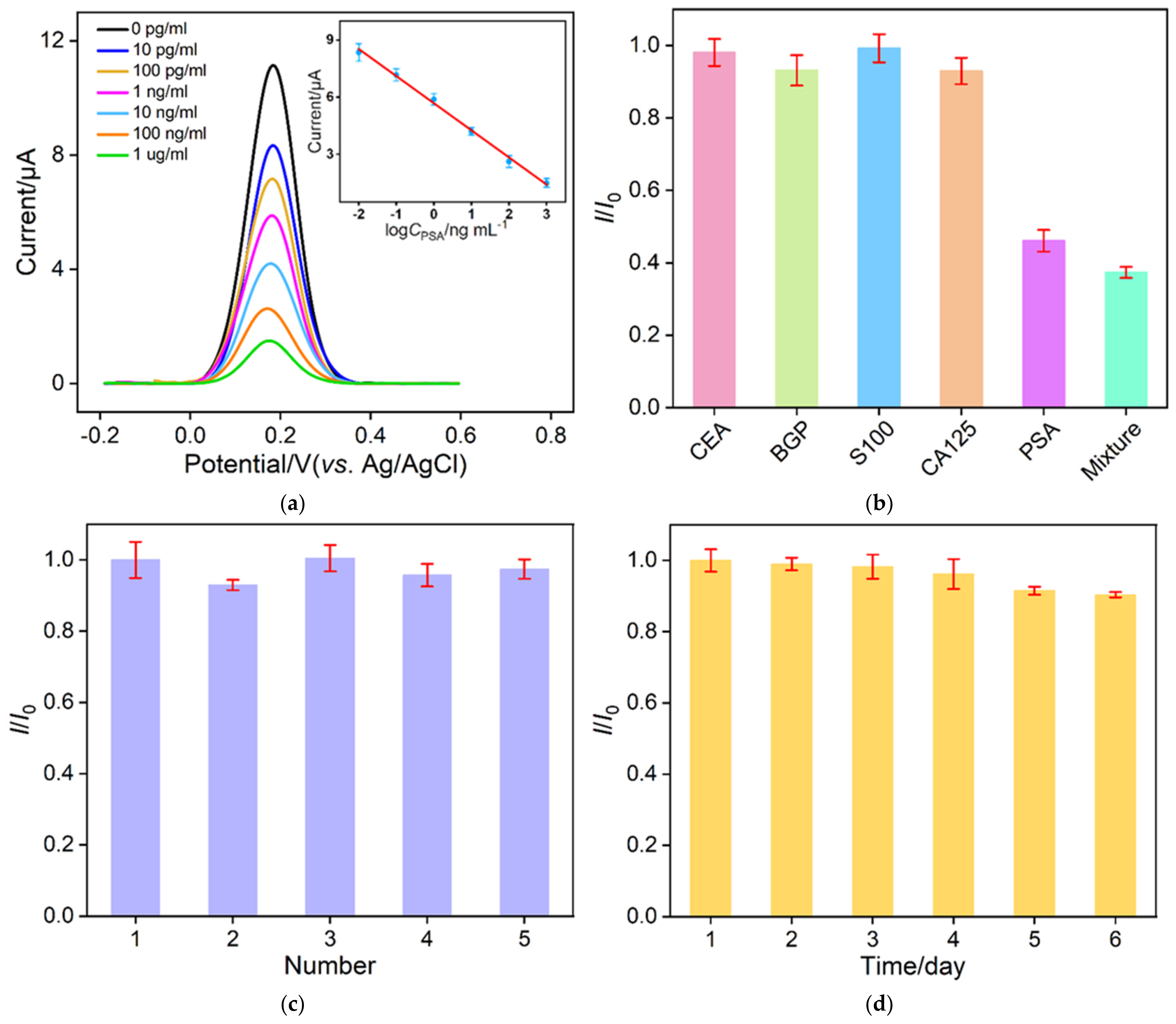
3.5. Label-Free Electrochemical Determination of PSA
3.6. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shokorlou, Y.M.; Heidarzadeh, H. Multispectral plasmonic biosensors based on a Penta-supercell metamaterial for detection of prostate-specific antigen: Ultrasensitive in LC resonance mode. Biosens. Bioelectron. 2022, 217, 114722. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kim, E.S.; Sharma, P.; Wang, Z.; Yang, S.; Kaushik, A.; Wang, C.; Li, Y.; Kim, N.Y. Tailored biofunctionalized biosensor for the label-free sensing of prostate-specific antigen. ACS Appl. Bio Mater. 2020, 3, 7821–7830. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, J. Plasmon-based colorimetric nanosensors for ultrasensitive molecular diagnostics. ACS Sens. 2017, 2, 857–875. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, M.; Yu, H.; Wang, G.; Ma, P.; Pang, S.; Jiao, Y.; Liu, A. Screening of peptide selectively recognizing prostate-specific antigen and its application in detecting total prostate-specific antigen. Sens. Actuators B Chem. 2022, 367, 132009. [Google Scholar] [CrossRef]
- Han, L.; Wang, D.; Yan, L.; Petrenko, V.; Liu, A. Specific phages-based electrochemical impedimetric immunosensors for label-free and ultrasensitive detection of dual prostate-specific antigens. Sens. Actuators B Chem. 2019, 297, 126727. [Google Scholar] [CrossRef]
- Dejous, C.; Krishnan, U. Sensors for diagnosis of prostate cancer: Looking beyond the prostate specific antigen. Biosens. Bioelectron. 2020, 173, 112790. [Google Scholar] [CrossRef]
- Ghorbani, F.; Abbaszadeh, H.; Dolatabadi, J.; Aghebati-Maleki, L.; Yousefi, M. Application of various optical and electrochemical aptasensors for detection of human prostate specific antigen: A review. Biosens. Bioelectron. 2019, 142, 111484. [Google Scholar] [CrossRef]
- Negahdary, M.; Sattarahmady, N.; Heli, H. Advances in prostate specific antigen biosensors-impact of nanotechnology. Clin. Chim. Acta 2020, 504, 43–55. [Google Scholar] [CrossRef]
- Ozyurt, C.; Uludag, I.; Ince, B.; Sezginturk, M. Biosensing strategies for diagnosis of prostate specific antigen. J. Pharm. Biomed. Anal. 2022, 209, 114535. [Google Scholar] [CrossRef]
- Zhou, H.; Dong, G.; Sailjoi, A.; Liu, J. Facile pretreatment of three-dimensional graphene through electrochemical polarization for improved electrocatalytic performance and simultaneous electrochemical detection of catechol and hydroquinone. Nanomaterials 2022, 12, 65. [Google Scholar] [CrossRef]
- Gong, J.; Tang, H.; Wang, M.; Lin, X.; Wang, K.; Liu, J. Novel three-dimensional graphene nanomesh prepared by facile electro-etching for improved electroanalytical performance for small biomolecules. Mater. Design 2022, 215, 110506. [Google Scholar] [CrossRef]
- Wang, K.; Yang, L.; Huang, H.; Lv, N.; Liu, J.; Liu, Y. Nanochannel array on electrochemically polarized screen printed carbon electrode for rapid and sensitive electrochemical determination of clozapine in human whole blood. Molecules 2022, 27, 2739. [Google Scholar] [CrossRef]
- Bao, C.; Zhang, R.; Qiao, Y.; Cao, X.; He, F.; Hu, W.; Wei, M.; Lu, W. Au nanoparticles anchored on cobalt boride nanowire arrays for the electrochemical determination of prostate-specific antigen. ACS Appl. Nano Mater. 2021, 4, 5707–5716. [Google Scholar] [CrossRef]
- Chen, M.; Han, R.; Li, Y.; Luo, X. Nonfouling and ratiometric electrochemical detection of prostate specific antigen in whole serum. Anal. Chim. Acta 2022, 1224, 340191. [Google Scholar] [CrossRef]
- He, Y.; Xie, S.; Yang, X.; Yuan, R.; Chai, Y. Electrochemical peptide biosensor based on in situ silver deposition for detection of prostate specific antigen. ACS Appl. Mater. Interfaces 2015, 7, 13360–13366. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Tan, C.; Chen, C.; Hu, C.; Bai, Y.; Ming, D. Electrochemical immunosensor based on hybrid MoS2/Pt@Au-nanoprism/PDA for simultaneous detection of free and total prostate specific antigen in serum. Sens. Actuators B Chem. 2022, 357, 131413. [Google Scholar] [CrossRef]
- Kabay, G.; Yin, Y.; Singh, C.; Ahmad, N.; Gunasekaran, S.; Mutlu, M. Disposable electrochemical immunosensor for prostate cancer detection. Sens. Actuators B Chem. 2022, 360, 131667. [Google Scholar] [CrossRef]
- Rong, S.; Zou, L.; Li, Y.; Guan, Y.; Guan, H.; Zhang, Z.; Zhang, Y.; Gao, H.; Yu, H.; Zhao, F.; et al. An ultrasensitive disposable sandwich-configuration electrochemical immunosensor based on OMC@AuNPs composites and AuPt-MB for alpha-fetoprotein detection. Bioelectrochemistry 2021, 141, 107846. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, T.; Dong, G.; Zhu, S.; Yan, F.; Liu, J. Direct and sensitive electrochemical detection of bisphenol a in complex environmental samples using a simple and convenient nanochannel-modified electrode. Front. Chem. 2022, 10, 900282. [Google Scholar] [CrossRef]
- Yan, F.; Chen, J.; Jin, Q.; Zhou, H.; Sailjoi, A.; Liu, J.; Tang, W. Fast one-step fabrication of a vertically-ordered mesoporous silica-nanochannel film on graphene for direct and sensitive detection of doxorubicin in human whole blood. J. Mater. Chem. C 2020, 8, 7113–7119. [Google Scholar] [CrossRef]
- Thunkhamrak, C.; Chuntib, P.; Ounnunkad, K.; Banet, P.; Aubert, P.; Saianand, G.; Gopalan, A.I.; Jakmunee, J. Highly sensitive voltammetric immunosensor for the detection of prostate specific antigen based on silver nanoprobe assisted graphene oxide modified screen printed carbon electrode. Talanta 2020, 208, 120389. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Deng, W.; Su, Y.; Zhu, X.; Peng, C.; Hu, H.; Peng, H.; Song, S.; Fan, C. Carbon nanotube-based ultrasensitive multiplexing electrochemical immunosensor for cancer biomarkers. Biosens. Bioelectron. 2011, 30, 93–99. [Google Scholar] [CrossRef]
- Choosang, J.; Khumngern, S.; Thavarungkul, P.; Kanatharana, P.; Numnuam, A. An ultrasensitive label-free electrochemical immunosensor based on 3D porous chitosan-graphene-ionic liquid-ferrocene nanocomposite cryogel decorated with gold nanoparticles for prostate-specific antigen. Talanta 2021, 224, 121787. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, C.; Xi, F. Disposable amperometric label-free immunosensor on chitosan–graphene-modified patterned ITO electrodes for prostate specific antigen. Molecules 2022, 27, 5895. [Google Scholar] [CrossRef] [PubMed]
- Demirbakan, B.; Sezgintürk, M. A novel electrochemical immunosensor based on disposable ITO-PET electrodes for sensitive detection of PAK 2 antigen. J. Electroanal. Chem. 2019, 848, 113304. [Google Scholar] [CrossRef]
- Yadav, A.; Verma, D.; Lakshmi, G.; Eremin, S.; Solanki, P. Fabrication of label-free and ultrasensitive electrochemical immunosensor based on molybdenum disulfide nanoparticles modified disposable ITO: An analytical platform for antibiotic detection in food samples. Food Chem. 2021, 363, 130245. [Google Scholar] [CrossRef]
- Chen, S.; Xu, L.; Sheng, K.; Zhou, Q.; Dong, B.; Bai, X.; Lu, G.; Song, H. A label-free electrochemical immunosensor based on facet-controlled Au nanorods/reduced graphene oxide composites for prostate specific antigen detection. Sens. Actuators B Chem. 2021, 336, 129748. [Google Scholar] [CrossRef]
- Jones, A.; Dhanapala, L.; Kankanamage, R.; Kumar, C.; Rusling, J. Multiplexed immunosensors and immunoarrays. Anal. Chem. 2020, 92, 345–362. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Pei, J.; Tian, Y.; Liu, J. A reagentless electrochemical immunosensor for sensitive detection of carcinoembryonic antigen based on the interface with redox probe-modified electron transfer wires and effectively immobilized antibody. Front. Chem. 2022, 10, 939736. [Google Scholar] [CrossRef]
- Lin, J.; Li, K.; Wang, M.; Chen, X.; Liu, J.; Tang, H. Reagentless and sensitive determination of carcinoembryonic antigen based on a stable Prussian blue modified electrode. RSC Adv. 2020, 10, 38316–38322. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, T.; Luo, T.; Luo, X.; Yan, F.; Tang, W.; Liu, J. Bipolar silica nanochannel array confined electrochemiluminescence for ultrasensitive detection of SARS-CoV-2 antibody. Biosens. Bioelectron. 2022, 215, 114563. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, X.; Sailjoi, A.; Zou, Y.; Lin, X.; Yan, F.; Su, B.; Liu, J. Vertical silica nanochannels supported by nanocarbon composite for simultaneous detection of serotonin and melatonin in biological fluids. Sens. Actuators B Chem. 2022, 353, 131101. [Google Scholar] [CrossRef]
- Yan, F.; Luo, T.; Jin, Q.; Zhou, H.; Sailjoi, A.; Dong, G.; Liu, J.; Tang, W. Tailoring molecular permeability of vertically-ordered mesoporous silica-nanochannel films on graphene for selectively enhanced determination of dihydroxybenzene isomers in environmental water samples. J. Hazard. Mater. 2021, 410, 124636. [Google Scholar] [CrossRef]
- Ma, X.; Liao, W.; Zhou, H.; Tong, Y.; Yan, F.; Tang, H.; Liu, J. Highly sensitive detection of rutin in pharmaceuticals and human serum using ITO electrodes modified with vertically-ordered mesoporous silica-graphene nanocomposite films. J. Mater. Chem. B 2020, 8, 10630–10636. [Google Scholar] [CrossRef]
- Walcarius, A. Electroinduced surfactant self-assembly driven to vertical growth of oriented mesoporous films. Acc. Chem. Res. 2021, 54, 3563–3575. [Google Scholar] [CrossRef]
- Zhou, H.; Ding, Y.; Su, R.; Lu, D.; Tang, H.; Xi, F. Silica nanochannel array film supported by ß-cyclodextrin-functionalized graphene modified gold film electrode for sensitive and direct electroanalysis of acetaminophen. Front. Chem. 2022, 9, 812086. [Google Scholar] [CrossRef]
- Zhu, X.; Xuan, L.; Gong, J.; Liu, J.; Wang, X.; Xi, F.; Chen, J. Three-dimensional macroscopic graphene supported vertically-ordered mesoporous silica-nanochannel film for direct and ultrasensitive detection of uric acid in serum. Talanta 2022, 238, 123027. [Google Scholar] [CrossRef]
- Liang, R.; Jiang, J.; Zheng, Y.; Sailjoi, A.; Chen, J.; Liu, J.; Li, H. Vertically oriented mesoporous silica film modified fluorine-doped tin oxide electrode for enhanced electrochemiluminescence detection of lidocaine in serum. RSC Adv. 2021, 11, 34669–34675. [Google Scholar] [CrossRef]
- Zheng, W.; Su, R.; Lin, X.; Liu, J. Nanochannel array modified three-dimensional graphene electrode for sensitive electrochemical detection of 2,4,6-trichlorophenol and prochloraz. Front. Chem. 2022, 10, 954802. [Google Scholar] [CrossRef]
- Zhou, P.; Yao, L.; Chen, K.; Su, B. Silica nanochannel membranes for electrochemical analysis and molecular sieving: A comprehensive review. Crit. Rev. Anal. Chem. 2019, 50, 424–444. [Google Scholar] [CrossRef]
- Xi, F.; Xuan, L.; Lu, L.; Huang, J.; Yan, F.; Liu, J.; Dong, X.; Chen, P. Improved adhesion and performance of vertically-aligned mesoporous silica-nanochannel film on reduced graphene oxide for direct electrochemical analysis of human serum. Sens. Actuators B Chem. 2019, 288, 133–140. [Google Scholar] [CrossRef]
- Wang, M.; Lin, J.; Gong, J.; Ma, M.; Tang, H.; Liu, J.; Yan, F. Rapid and sensitive determination of doxorubicin in human whole blood by vertically-ordered mesoporous silica film modified electrochemically pretreated glassy carbon electrodes. RSC Adv. 2021, 11, 9021–9028. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Zheng, Y.; An, L.; Liu, J. Ultrasensitive immunosensor for prostate-specific antigen based on enhanced electrochemiluminescence by vertically ordered mesoporous silica-nanochannel film. Front. Chem. 2022, 10, 851178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Guo, W.; Xu, L.; Yang, Q.; Su, B. Two orders-of-magnitude enhancement in the electrochemiluminescence of by vertically ordered silica mesochannels. Anal. Chim. Acta 2015, 886, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Yang, L.; Liu, J.; Liu, J. Electrochemical sensor nanoarchitectonics for sensitive detection of uric acid in human whole blood based on screen-printed carbon electrode equipped with vertically-ordered mesoporous silica-nanochannel film. Nanomaterials 2022, 12, 1157. [Google Scholar] [CrossRef]
- Zhou, L.; Hou, H.; Wei, H.; Yao, L.; Sun, L.; Yu, P.; Su, B.; Mao, L. In vivo monitoring of oxygen in rat brain by carbon fiber microelectrode modified with antifouling nanoporous membrane. Anal. Chem. 2019, 91, 3645–3651. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, T.; Chen, P.; Yan, F.; Liu, J. Bipolar silica nanochannel array for dual-mode electrochemiluminescence and electrochemical immunosensing platform. Sens. Actuators B Chem. 2022, 368, 132086. [Google Scholar] [CrossRef]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J. Electrochemically assisted self-assembly of mesoporous silica thin films. Nat. Mater. 2007, 6, 602–608. [Google Scholar] [CrossRef]
- Teng, Z.; Zheng, G.; Dou, Y.; Li, W.; Mou, C.; Zhang, X.; Asiri, A.; Zhao, D. Highly ordered mesoporous silica films with perpendicular mesochannels by a simple Stöber-solution growth approach. Angew. Chem. Int. Ed. 2012, 51, 2173–2177. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, X.; Xie, L.; Tang, H.; Yan, F. Vertically-ordered mesoporous silica films grown on boron nitride-graphene composite modified electrodes for rapid and sensitive detection of carbendazim in real samples. Front. Chem. 2022, 10, 939510. [Google Scholar] [CrossRef]
- Yan, F.; Ma, X.; Jin, Q.; Tong, Y.; Tang, H.; Lin, X.; Liu, J. Phenylboronic acid-functionalized vertically ordered mesoporous silica films for selective electrochemical determination of fluoride ion in tap water. Microchim. Acta 2020, 187, 470. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, Y.; Zhou, X.; Yan, F.; Ding, Z. Vertically-ordered mesoporous silica films for electrochemical detection of Hg(II) ion in pharmaceuticals and soil samples. Front. Chem. 2022, 10, 952936. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, T.; Tang, H.; Liu, J. Novel electrochemical and electrochemiluminescence dual-modality sensing platform for sensitive determination of antimicrobial peptides based on probe encapsulated liposome and nanochannel array electrode. Front. Nutr. 2022, 9, 962736. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, P.; Wang, A.; Luo, X.; Xue, Y.; Zhang, L.; Feng, J. A novel electrochemical immunosensor for highly sensitive detection of prostate-specific antigen using 3D open-structured PtCu nanoframes for signal amplification. Biosens. Bioelectron. 2019, 126, 187–192. [Google Scholar] [CrossRef]
- Li, L.; Wei, Y.; Zhang, S.; Chen, X.; Shao, T.; Feng, D. Electrochemical immunosensor based on metal ions functionalized CNSs@Au NPs nanocomposites as signal amplifier for simultaneous detection of triple tumor markers. J. Electroanal. Chem. 2021, 880, 114882. [Google Scholar] [CrossRef]
- Jeong, S.; Barman, S.; Yoon, H.; Park, J. A prostate cancer detection immunosensor based on Nafion/Reduced graphene oxide/aldehyde functionalized methyl pyridine composite electrode. J. Electrochem. Soc. 2019, 166, B920–B926. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, X.; Mei, L.; Zhang, L.; Wang, X.; Liao, X.; Qiao, X.; Hong, C. PSA detection electrochemical immunosensor based on MOF-235 nanomaterial adsorption aggregation signal amplification strategy. Microchem. J. 2021, 171, 106870. [Google Scholar] [CrossRef]
- Dai, L.; Li, Y.; Wang, Y.; Luo, X.; Wei, D.; Feng, R.; Yan, T.; Ren, X.; Du, B.; Wei, Q. A prostate-specific antigen electrochemical immunosensor based on Pd NPs functionalized electroactive Co-MOF signal amplification strategy. Biosens. Bioelectron. 2019, 132, 97–104. [Google Scholar] [CrossRef]
- Huang, B.; Liu, X.; Chen, J.; Mao, C.; Niu, H.; Jin, B. Electrochemiluminescence immunoassay for the prostate-specific antigen by using a CdS/chitosan/g-C3N4 nanocomposite. Microchim. Acta. 2020, 187, 155. [Google Scholar] [CrossRef]
- Paramasivam, S.; Veerapandian, M.; Kumar, S. Rational design of effective solid-state electrochemiluminescence platform of Gold@Polyluminol nanocomposite as an ultrasensitive immuno-probe for selective detection of prostate specific antigen. Anal. Chim. Acta. 2022, 1206, 339736. [Google Scholar] [CrossRef]


| Sample a | Added (ng/mL) | Found (ng/mL) | RSD (%, n = 3) | Recovery (%) |
|---|---|---|---|---|
| Human serum a | 0.0100 | 0.0103 | 2.7 | 103.2 |
| 0.100 | 0.0957 | 2.8 | 95.7 | |
| 10.0 | 10.1 | 3.7 | 101.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, L.; Xu, S.; Xi, F. Disposal Immunosensor for Sensitive Electrochemical Detection of Prostate-Specific Antigen Based on Amino-Rich Nanochannels Array-Modified Patterned Indium Tin Oxide Electrode. Nanomaterials 2022, 12, 3810. https://doi.org/10.3390/nano12213810
Yan L, Xu S, Xi F. Disposal Immunosensor for Sensitive Electrochemical Detection of Prostate-Specific Antigen Based on Amino-Rich Nanochannels Array-Modified Patterned Indium Tin Oxide Electrode. Nanomaterials. 2022; 12(21):3810. https://doi.org/10.3390/nano12213810
Chicago/Turabian StyleYan, Liang, Shuai Xu, and Fengna Xi. 2022. "Disposal Immunosensor for Sensitive Electrochemical Detection of Prostate-Specific Antigen Based on Amino-Rich Nanochannels Array-Modified Patterned Indium Tin Oxide Electrode" Nanomaterials 12, no. 21: 3810. https://doi.org/10.3390/nano12213810
APA StyleYan, L., Xu, S., & Xi, F. (2022). Disposal Immunosensor for Sensitive Electrochemical Detection of Prostate-Specific Antigen Based on Amino-Rich Nanochannels Array-Modified Patterned Indium Tin Oxide Electrode. Nanomaterials, 12(21), 3810. https://doi.org/10.3390/nano12213810







