Silk ProteinsEnriched Nanocomposite Hydrogels Based on Modified MMT Clay and Poly(2-hydroxyethyl methacrylate-co-2-acrylamido-2-methylpropane Sulfonic Acid) Display Favorable Properties for Soft Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis and Characterization
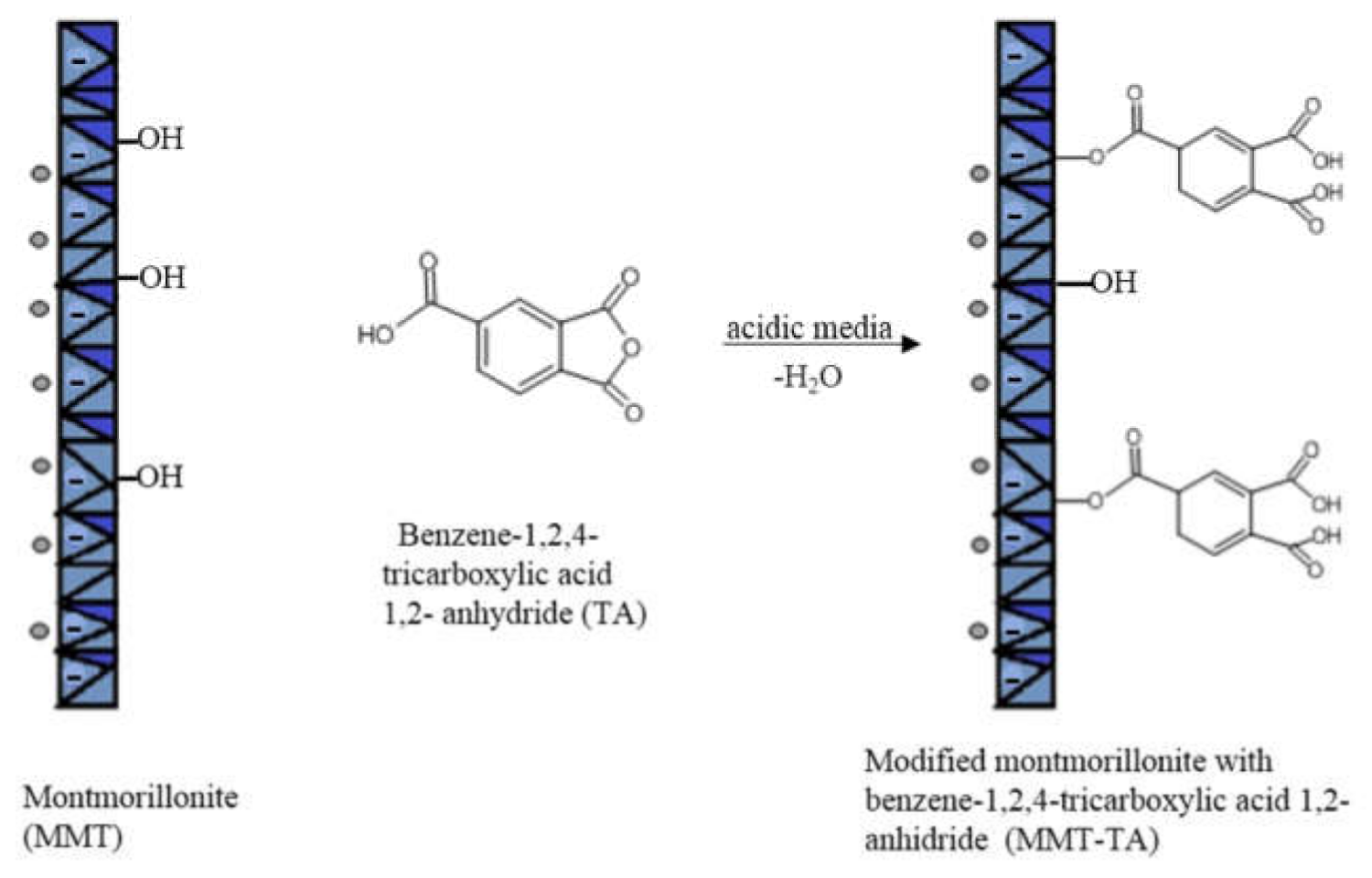
2.1.1. Synthesis of Modified MMT by Reaction with Benzene-1,2,4-tricarboxylic Acid 1,2-Anhydride (MMT-TA)
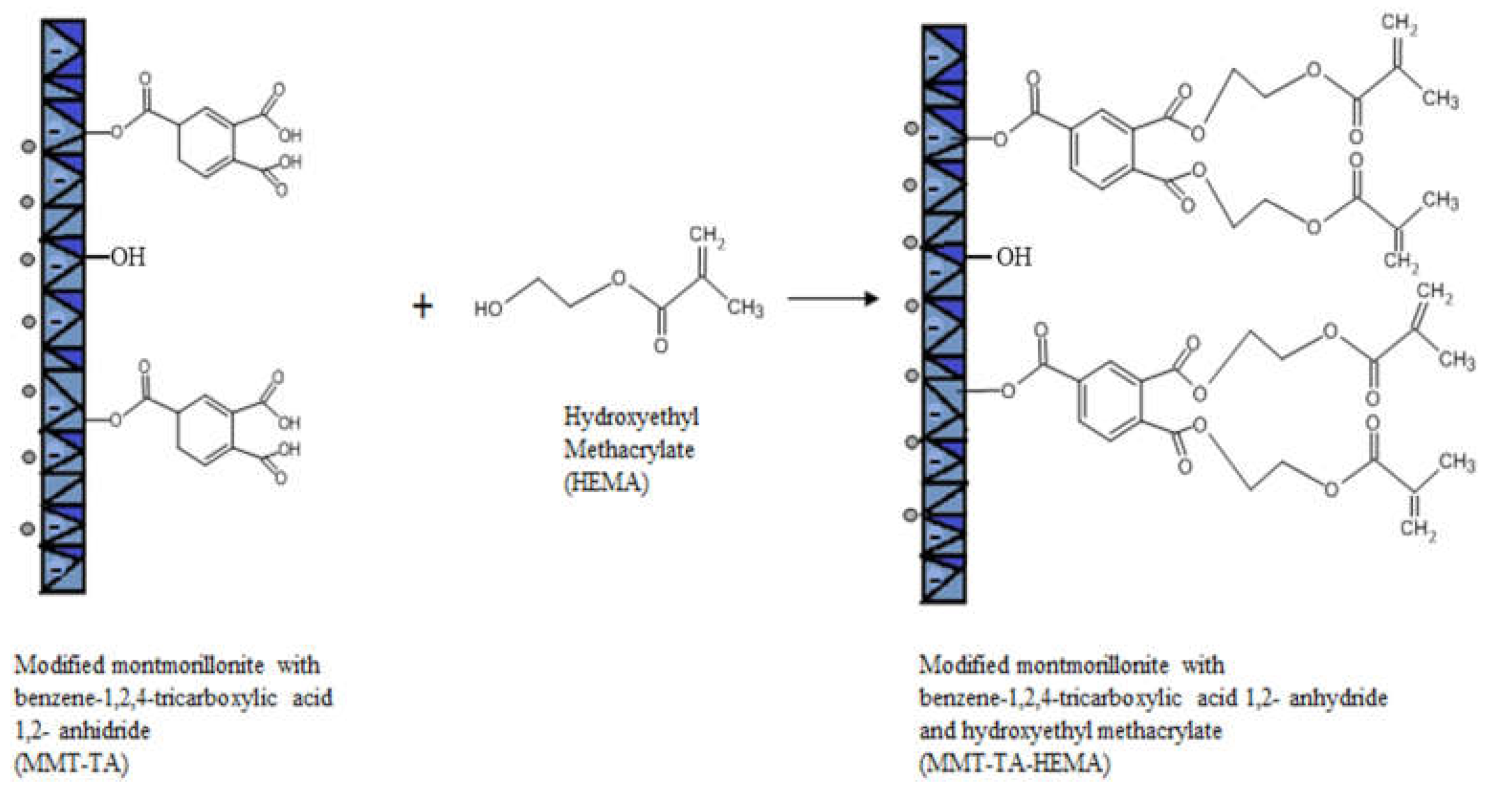
2.1.2. MMT-TA Modification by Reaction with 2-Hydroxyetylmetacrylaye (MMT-TA-HEMA)
2.1.3. Preparation of the Hydrogels from Modified Clay (MMT-TA-HEMA) and HE-MA/AMPSA Monomers
2.1.4. Fourier Transform Infrared Spectroscopy-Attenuated Total Reflectance (FTIR-ATR) Characterization
2.1.5. Morphological Investigation by Scanning Electron Microscopy (SEM)
2.1.6. Swelling Behavior and Rheological Properties
2.2. In Vitro Tests
2.2.1. Biocompatibility Assessment of the Materials
2.2.2. Biochemical Analysis
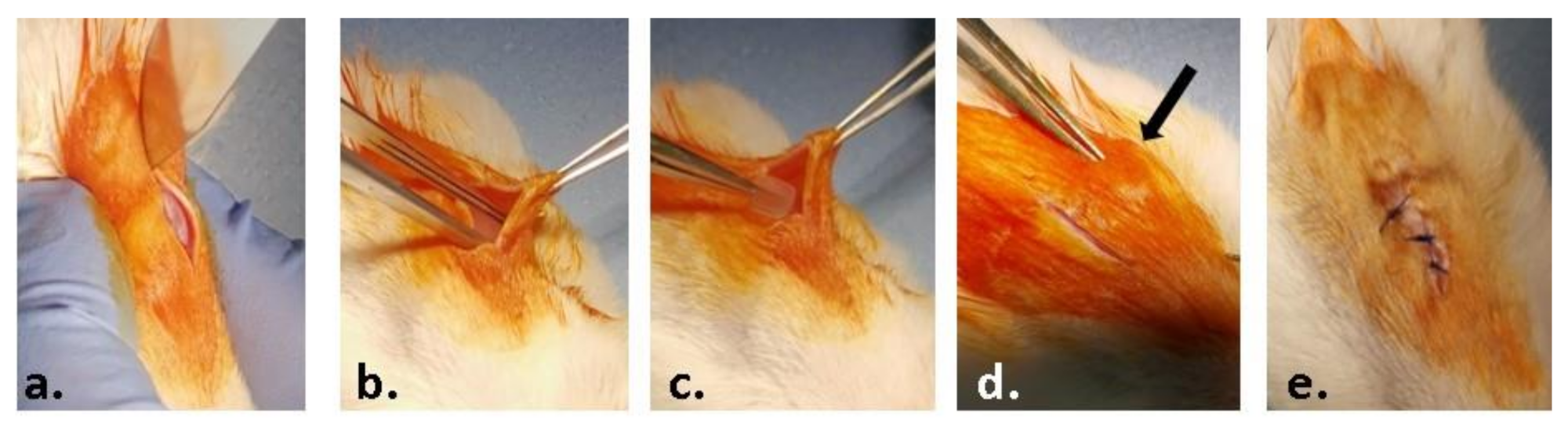
2.3. In Vivo Experiment
- -
- Group 1 (control group) without any material;
- -
- Group 2 (M1 group) implanted material containing MMT-5 mol/L-H95%-A5% with Sericin and Fibroin;
- -
- Group 3 (M2 group) implanted material containing MMT-5 mol/L-H97%-A3% with Sericin and Fibroin;
- -
- Group 4 (M3 group) implanted material containing MMT-5 mol/L-H95%-A5%;
- -
- Group 5 (M4 group) implanted material containing MMT-5 mol/L-H97%-A3%.
2.3.1. Biochemical Analysis
2.3.2. Histology and Immunohistochemistry
3. Results
3.1. Materials Synthesis and Characterization
3.1.1. MMT Modification Pathway
3.1.2. Physico-Chemical Investigation
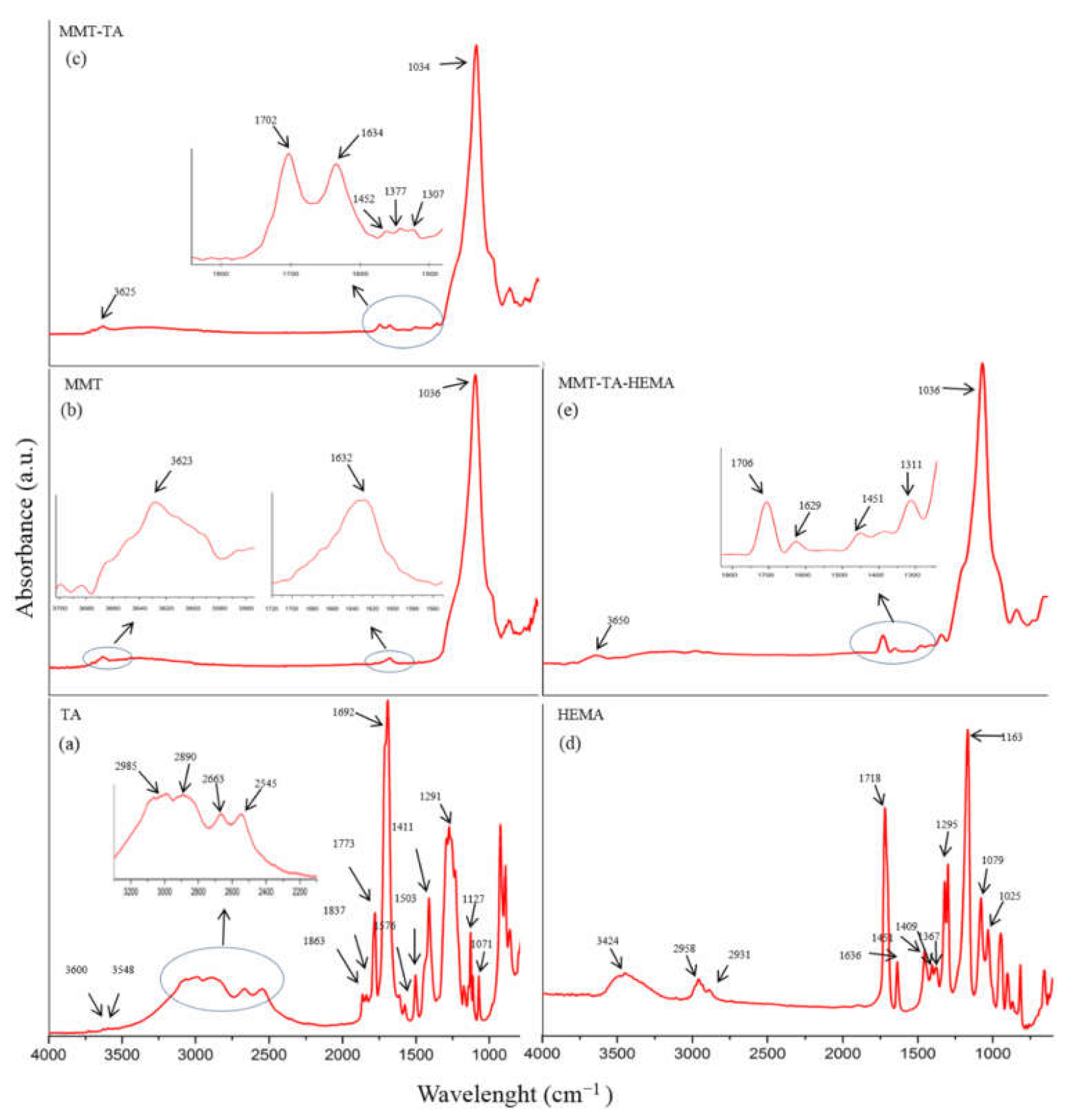
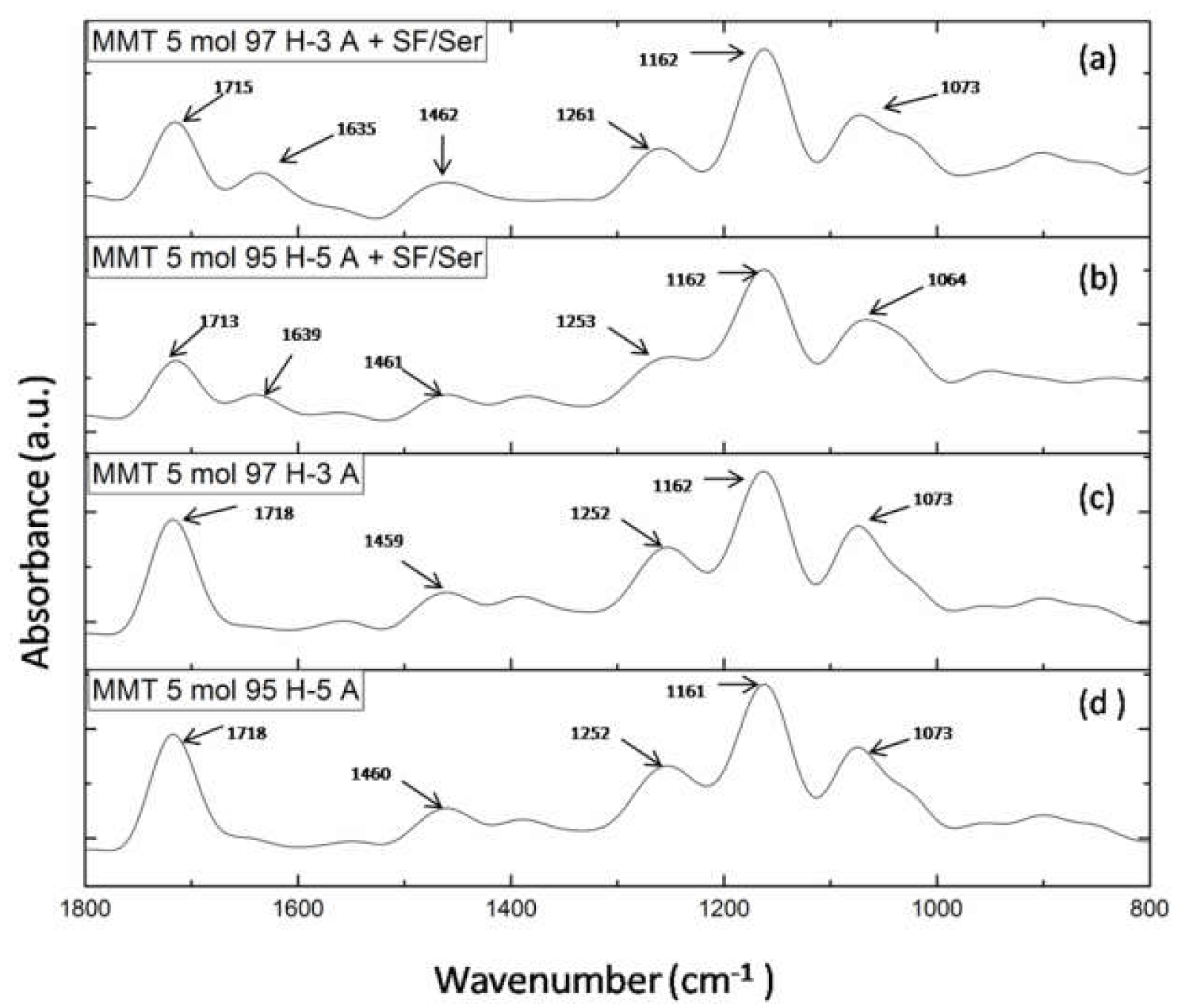
FTIR-ATR Analysis
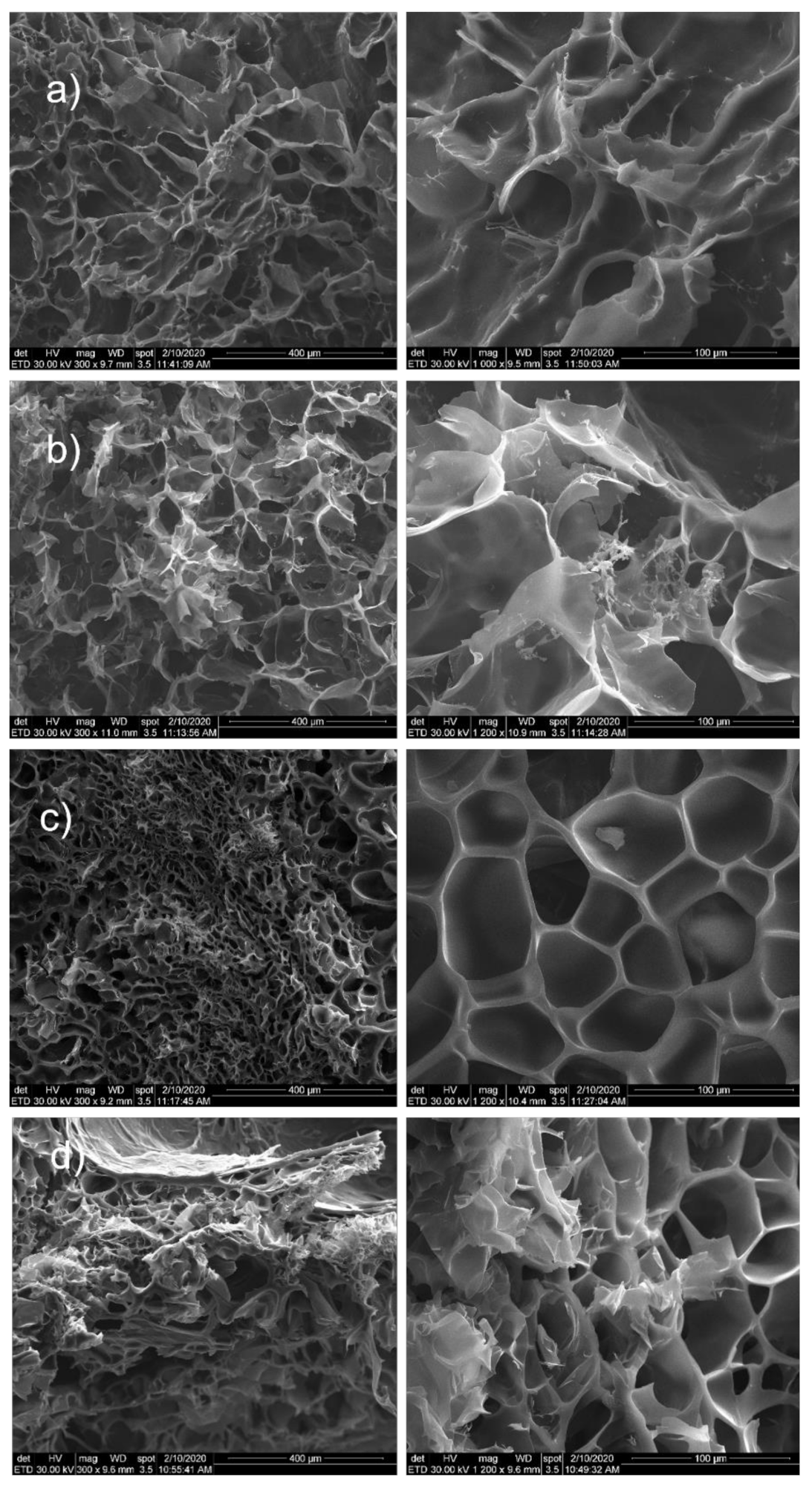
3.1.3. SEM Morphological Characterization
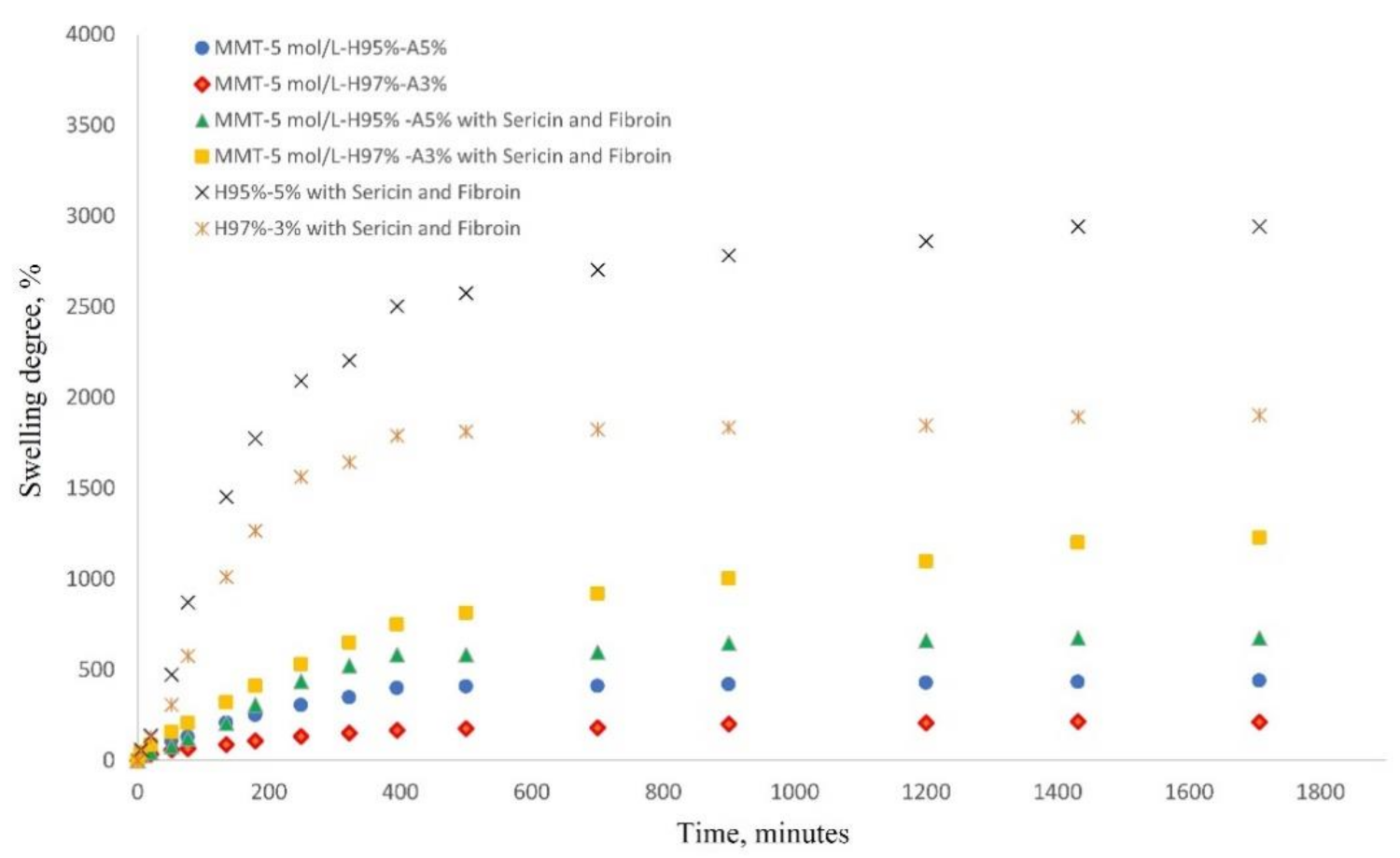
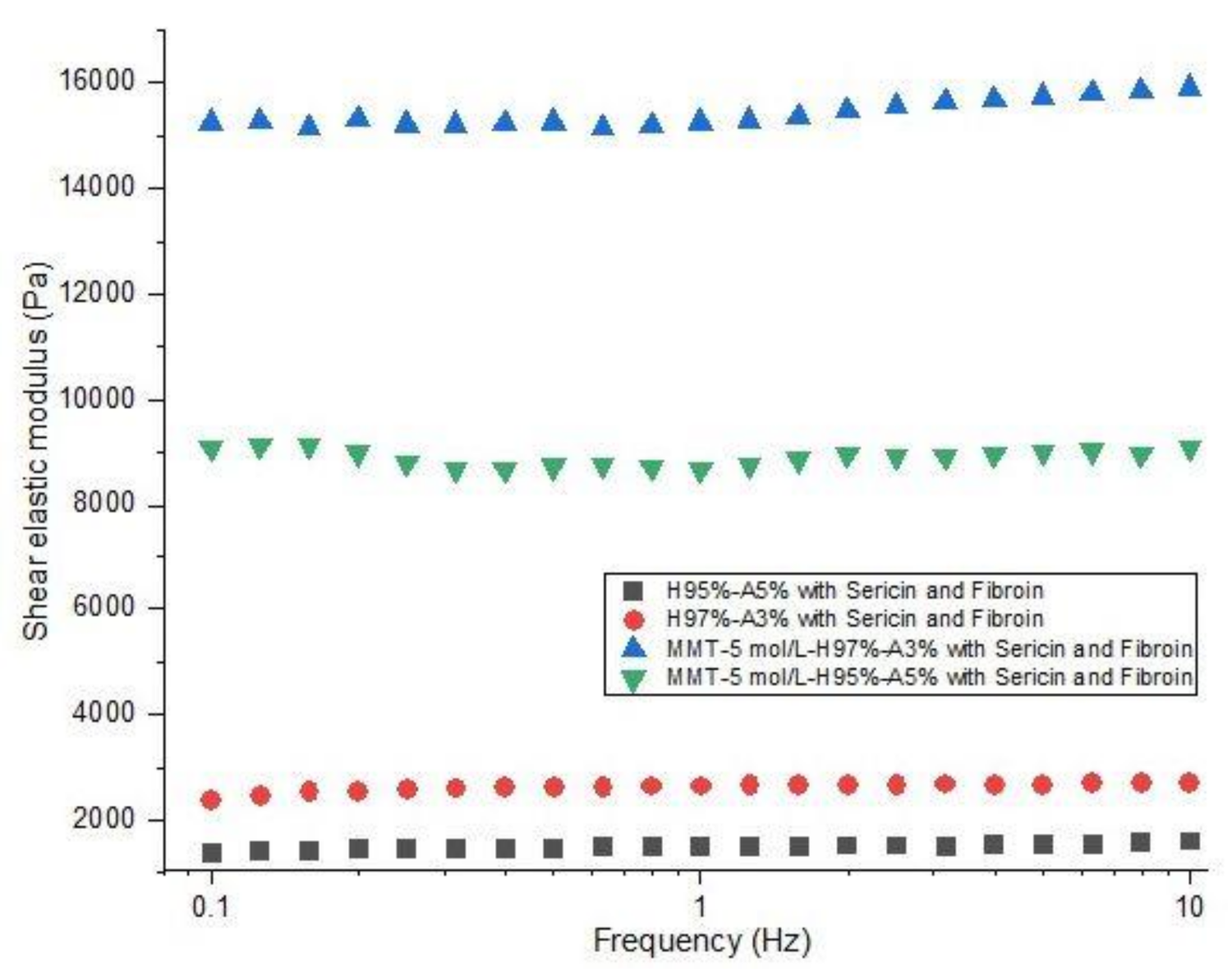
3.1.4. Swelling Behavior and Rheological Properties
3.2. In Vitro Tests
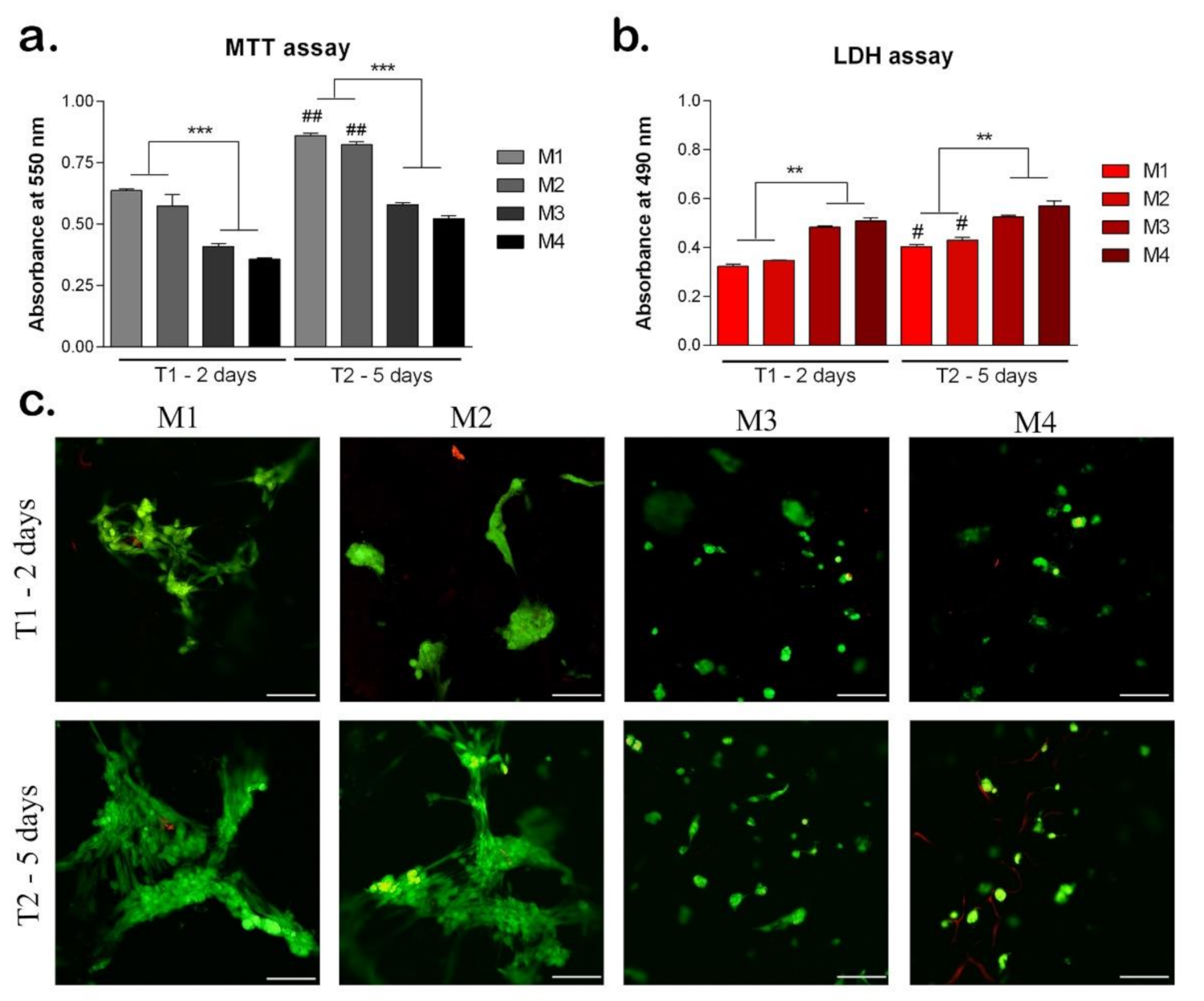
3.2.1. Biocompatibility Assessment of the Materials
3.2.2. Adipogenic Differentiation
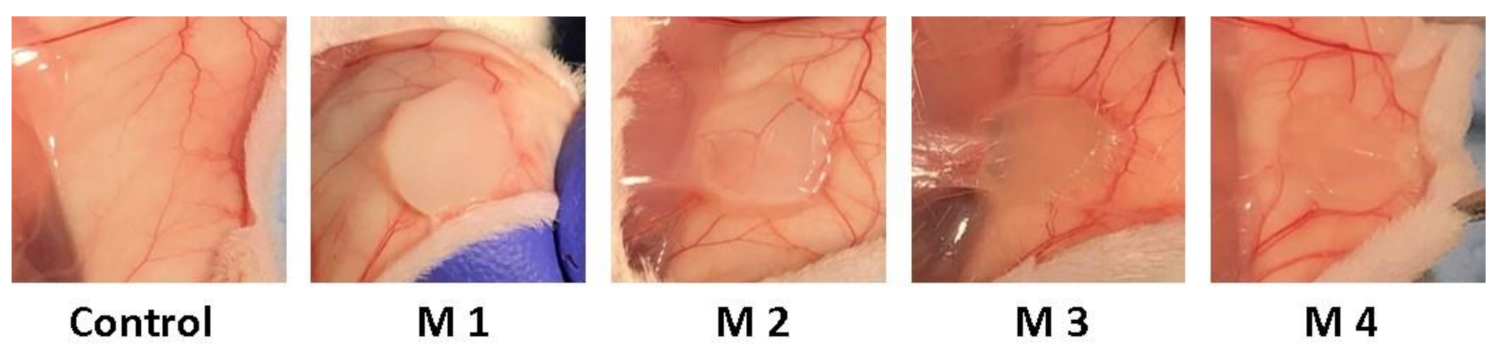
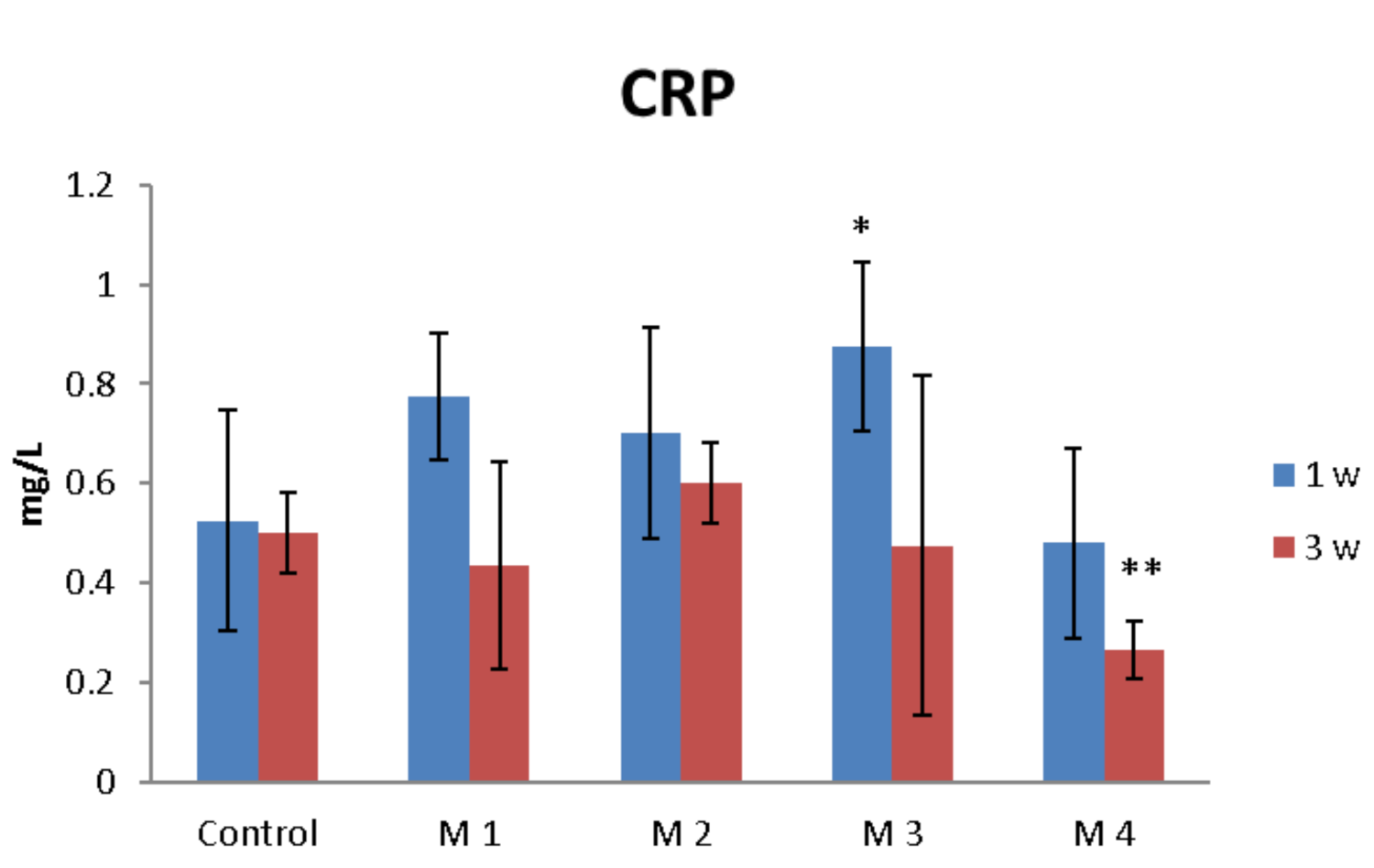
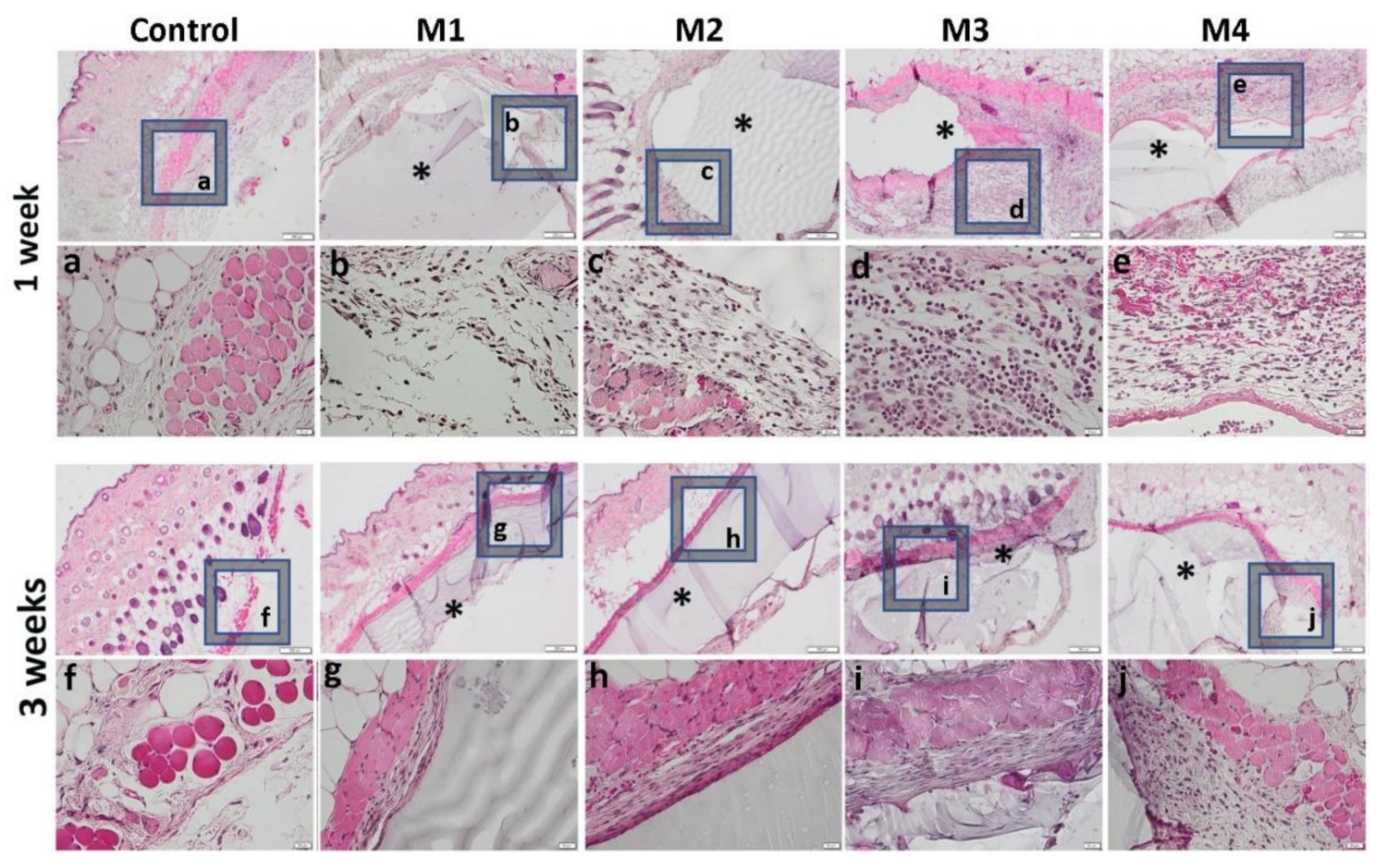
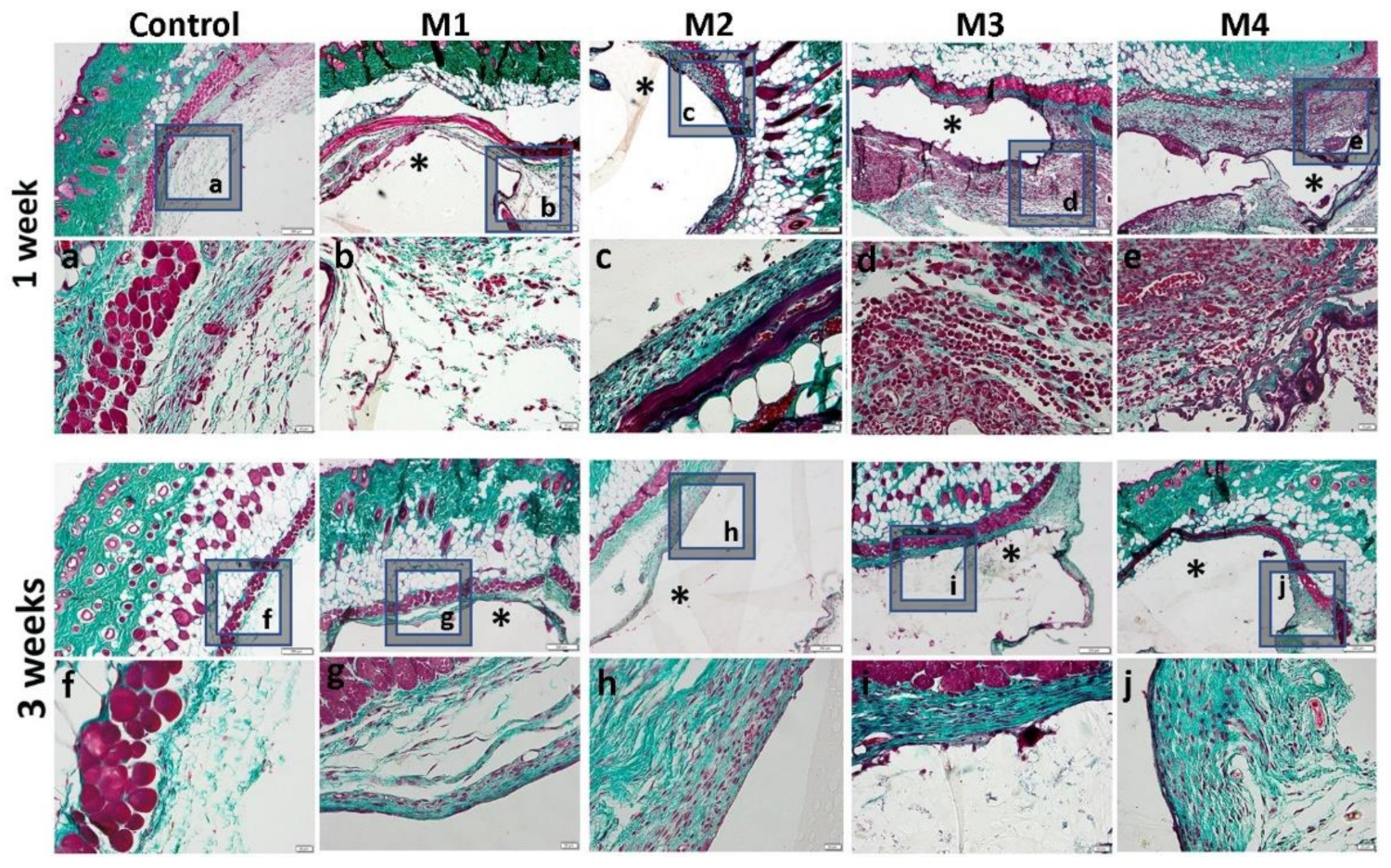
3.3. In Vivo Biocompatibility and Inflammatory Response
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, J.H.; Gimble, J.M.; Lee, K.; Marra, K.G.; Rubin, J.P.; Yoo, J.J.; Vunjak-Novakovic, G.; Kaplan, D.L. Adipose Tissue Engineering for Soft Tissue Regeneration. Tissue Eng. Part B Rev. 2010, 16, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.; Kumar, S.; Singh, A.; Kumar, A.; Kaur, N.; Sanyasi, S.; Chawla, S.; Goswami, C.; Goswami, L. Hydroxyethyl methacrylate grafted carboxy methyl tamarind (CMT-g-HEMA) polysaccharide based matrix as a suitable scaffold for skin tissue engineering. Carbohydr. Polym. 2018, 189, 87–98. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, D.M.; Pandit, A.S. Tissue-Engineering Approach to Regenerating the Intervertebral Disc. Tissue Eng. 2007, 13, 1927–1954. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, M.; Nouri Khorasani, S.; Zare, M.; Esmaeely Neisiany, R.; Davoodi, P. Advanced Hydrogels for Cartilage Tissue Engineering: Recent Progress and Future Directions. Polymers 2021, 13, 4199. [Google Scholar] [CrossRef]
- Kim, S.; Shin, B.; Yang, C.; Jeong, S.; Shim, J.; Park, M.; Choy, Y.; Heo, C.; Lee, K. Development of Poly(HEMA-Am) Polymer Hydrogel Filler for Soft Tissue Reconstruction by Facile Polymerization. Polymers 2018, 10, 772. [Google Scholar] [CrossRef] [Green Version]
- Macková, H.; Plichta, Z.; Proks, V.; Kotelnikov, I.; Kučka, J.; Hlídková, H.; Horák, D.; Kubinová, Š.; Jiráková, K. RGDS- and SIKVAVS-Modified Superporous Poly(2-hydroxyethyl methacrylate) Scaffolds for Tissue Engineering Applications. Macromol. Biosci. 2016, 16, 1621–1631. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M.; Dong, W.; Zhang, J. Fabrication of Salecan/poly(AMPS-co-HMAA) semi-IPN hydrogels for cell adhesion. Carbohydr. Polym. 2017, 174, 171–181. [Google Scholar] [CrossRef]
- Osman, A.F.; Fitri, M.T.F.; Rakibuddin, M.; Hashim, F.; Tuan Johari, S.A.T.; Ananthakrishnan, R.; Ramli, R. Pre-dispersed organo-montmorillonite (organo-MMT) nanofiller: Morphology, cytocompatibility and impact on flexibility, toughness and biostability of biomedical ethyl vinyl acetate (EVA) copolymer. Mater. Sci. Eng. C 2017, 74, 194–206. [Google Scholar] [CrossRef]
- Alexa, R.L.; Iovu, H.; Trica, B.; Zaharia, C.; Serafim, A.; Alexandrescu, E.; Radu, I.-C.; Vlasceanu, G.; Preda, S.; Ninciuleanu, C.M.; et al. Assessment of Naturally Sourced Mineral Clays for the 3D Printing of Biopolymer-Based Nanocomposite Inks. Nanomaterials 2021, 11, 703. [Google Scholar] [CrossRef]
- Kopeček, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, D.; Xu, D.; Gersappe, D.; Rafailovich, M.H. Hyaluronic Acid and Gelatin Clay Composite Hydrogels: Substrates for Cell Adhesion and Controlled Drug Delivery. J. Chem. Biol. Interfaces 2014, 2, 34–44. [Google Scholar] [CrossRef]
- Chang, C.-W.; van Spreeuwel, A.; Zhang, C.; Varghese, S. PEG/clay nanocomposite hydrogel: A mechanically robust tissue engineering scaffold. Soft Matter 2010, 6, 5157. [Google Scholar] [CrossRef]
- Sakr, M.A.; Mohamed, M.G.A.; Wu, R.; Shin, S.R.; Kim, D.; Kim, K.; Siddiqua, S. Development of bentonite-gelatin nanocomposite hybrid hydrogels for tissue engineering. Appl. Clay Sci. 2020, 199, 105860. [Google Scholar] [CrossRef]
- Takeno, H.; Nagai, S. Mechanical Properties and Structures of Clay-Polyelectrolyte Blend Hydrogels. Gels 2018, 4, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafieian, S.; Mirzadeh, H.; Mahdavi, H.; Masoumi, M.E. A review on nanocomposite hydrogels and their biomedical applications. Sci. Eng. Compos. Mater. 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Siavashani, A.Z.; Mohammadi, J.; Rottmar, M.; Senturk, B.; Nourmohammadi, J.; Sadeghi, B.; Huber, L.; Maniura-Weber, K. Silk fibroin/sericin 3D sponges: The effect of sericin on structural and biological properties of fibroin. Int. J. Biol. Macromol. 2020, 153, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, H.; Zhu, J.; Cui, X.; Gao, J.; Wang, X.; Xiong, J. 3-D mineralized silk fibroin/polycaprolactone composite scaffold modified with polyglutamate conjugated with BMP-2 peptide for bone tissue engineering. Colloids Surf. B Biointerfaces 2018, 163, 369–378. [Google Scholar] [CrossRef]
- Galateanu, B.; Radu, I.C.; Vasile, E.; Hudita, A.; Serban, M.V.; Costache, M.; Iovu, H.; Zaharia, C. Fabrication of Novel Silk Fibroin-LDHs Composite Arhitectures for Potential Bone Tissue Engineering. Mater. Plast. 2017, 54, 659–665. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X. Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2020, 149, 513–521. [Google Scholar] [CrossRef]
- Dinescu, S.; Galateanu, B.; Albu, M.; Cimpean, A.; Dinischiotu, A.; Costache, M. Sericin Enhances the Bioperformance of Collagen-Based Matrices Preseeded with Human-Adipose Derived Stem Cells (hADSCs). Int. J. Mol. Sci. 2013, 14, 1870–1889. [Google Scholar] [CrossRef] [Green Version]
- Kolaparthy, L.K.; Sanivarapu, S.; Moogla, S.; Kutcham, R.S. Adipose Tissue-Adequate, Accessible Regenerative Material. Int. J. Stem Cells 2015, 8, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignat, S.-R.; Lazăr, A.D.; Şelaru, A.; Samoilă, I.; Vlăsceanu, G.M.; Ioniţă, M.; Radu, E.; Dinescu, S.; Costache, M. Versatile Biomaterial Platform Enriched with Graphene Oxide and Carbon Nanotubes for Multiple Tissue Engineering Applications. Int. J. Mol. Sci. 2019, 20, 3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinescu, S.; Hermenean, A.; Costache, M. Human Adipose-Derived Stem Cells for Tissue Engineering Approaches: Current Challenges and Perspectives. In Stem Cells in Clinical Practice and Tissue Engineering; InTech: London, UK, 2018. [Google Scholar]
- Bertozzi, N.; Simonacci, F.; Grieco, M.P.; Grignaffini, E.; Raposio, E. The biological and clinical basis for the use of adipose-derived stem cells in the field of wound healing. Ann. Med. Surg. 2017, 20, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Nazarie (Ignat), S.-R.; Gharbia, S.; Hermenean, A.; Dinescu, S.; Costache, M. Regenerative Potential of Mesenchymal Stem Cells’ (MSCs) Secretome for Liver Fibrosis Therapies. Int. J. Mol. Sci. 2021, 22, 13292. [Google Scholar] [CrossRef] [PubMed]
- Radu, I.-C.; Biru, I.-E.; Damian, C.-M.; Ion, A.-C.; Iovu, H.; Tanasa, E.; Zaharia, C.; Galateanu, B. Grafting versus Crosslinking of Silk Fibroin-g-PNIPAM via Tyrosine-NIPAM Bridges. Molecules 2019, 24, 4096. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.Y.; Yoo, E.S.; Im, S.S. The synthesis of copolymers, blends and composites based on poly(butylene succinate). Polym. J. 2012, 44, 1179–1190. [Google Scholar] [CrossRef]
- Asgari, M.; Sundararaj, U. Silane functionalization of sodium montmorillonite nanoclay: The effect of dispersing media on intercalation and chemical grafting. Appl. Clay Sci. 2018, 153, 228–238. [Google Scholar] [CrossRef]
- Bullermann, J.; Spohnholz, R.; Friebel, S.; Salthammer, T. Synthesis and characterization of polyurethane ionomers with trimellitic anhydride and dimethylol propionic acid for waterborne self-emulsifying dispersions. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 680–690. [Google Scholar] [CrossRef]
- Cai, Z.; Ji, B.; Yan, K.; Zhu, Q. Investigation on Reaction Sequence and Group Site of Citric Acid with Cellulose Characterized by FTIR in Combination with Two-Dimensional Correlation Spectroscopy. Polymers 2019, 11, 2071. [Google Scholar] [CrossRef] [Green Version]
- Arjunan, V.; Raj, A.; Subramanian, S.; Mohan, S. Vibrational, electronic and quantum chemical studies of 1,2,4-benzenetricarboxylic-1,2-anhydride. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 110, 141–150. [Google Scholar] [CrossRef]
- Hermann, P.; Kästner, B.; Hoehl, A.; Kashcheyevs, V.; Patoka, P.; Ulrich, G.; Feikes, J.; Ries, M.; Tydecks, T.; Beckhoff, B.; et al. Enhancing the sensitivity of nano-FTIR spectroscopy. Opt. Express 2017, 25, 16574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; Dai, F.; Zhang, H.; Ni, B.; Zhou, W.; Yang, X.; Wu, Y. Preparation and characterization of silk fibroin as a biomaterial with potential for drug delivery. J. Transl. Med. 2012, 10, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamalha, E.; Zheng, Y.S.; Zeng, Y.C.; Fredrick, M.N. FTIR and WAXD Study of Regenerated Silk Fibroin. Adv. Mater. Res. 2013, 677, 211–215. [Google Scholar] [CrossRef]
- Gianak, O.; Pavlidou, E.; Sarafidis, C.; Karageorgiou, V.; Deliyanni, E. Silk Fibroin Nanoparticles for Drug Delivery: Effect of Bovine Serum Albumin and Magnetic Nanoparticles Addition on Drug Encapsulation and Release. Separations 2018, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- TSUBOUCHI, K.; IGARASHI, Y.; TAKASU, Y.; YAMADA, H. Sericin Enhances Attachment of Cultured Human Skin Fibroblasts. Biosci. Biotechnol. Biochem. 2005, 69, 403–405. [Google Scholar] [CrossRef]
- Chirila, T.V.; Suzuki, S.; Bray, L.J.; Barnett, N.L.; Harkin, D.G. Evaluation of silk sericin as a biomaterial: In vitro growth of human corneal limbal epithelial cells on Bombyx mori sericin membranes. Prog. Biomater. 2013, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Arimura, N.; Horiba, T.; Imagawa, M.; Shimizu, M.; Sato, R. The Peroxisome Proliferator-activated Receptor γ Regulates Expression of the Perilipin Gene in Adipocytes. J. Biol. Chem. 2004, 279, 10070–10076. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, K. Synthesis and properties of soft nanocomposite materials with novel organic/inorganic network structures. Polym. J. 2011, 43, 223–241. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Z. A constitutive model of nanocomposite hydrogels with nanoparticle crosslinkers. J. Mech. Phys. Solids 2016, 94, 127–147. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, G. Novel Nanocomposite Hydrogels Consisting of Layered Double Hydroxide with Ultrahigh Tensibility and Hierarchical Porous Structure at Low Inorganic Content. Adv. Mater. 2014, 26, 5950–5956. [Google Scholar] [CrossRef] [PubMed]
- Tanasa, E.; Zaharia, C.; Radu, I.-C.; Surdu, V.-A.; Vasile, B.S.; Damian, C.-M.; Andronescu, E. Novel Nanocomposites Based on Functionalized Magnetic Nanoparticles and Polyacrylamide: Preparation and Complex Characterization. Nanomaterials 2019, 9, 1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radu, I.C.; Vasile, E.; Damian, C.M.; Iovu, H.; Stanescu, P.O.; Zaharia, C. Influence of the Double Bond LDH Clay on the Exfoliation/Intercalation Mechanism of Polyacrylamide Nanocomposite Hydrogels. Mater. Plast. 2018, 55, 263–268. [Google Scholar] [CrossRef]
- Vasile, E.; Radu, I.-C.; Galateanu, B.; Rapa, M.; Hudita, A.; Jianu, D.; Stanescu, P.-O.; Cioflan, H.; Zaharia, C. Novel Nanocomposites Based on Bacterial Polyester/LDH-SDS Clay for Stem Cells Delivery in Modern Wound Healing Management. Materials 2020, 13, 4488. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guan, J.; Peng, H.; Shu, X.; Chen, L.; Guo, H. Tightly adhered silk fibroin coatings on Ti6Al4V biometals for improved wettability and compatible mechanical properties. Mater. Des. 2019, 175, 107825. [Google Scholar] [CrossRef]
- Nayak, S.; Dey, T.; Naskar, D.; Kundu, S.C. The promotion of osseointegration of titanium surfaces by coating with silk protein sericin. Biomaterials 2013, 34, 2855–2864. [Google Scholar] [CrossRef]
- Zeplin, P.; Berninger, A.-K.; Maksimovikj, N.; van Gelder, P.; Scheibel, T.; Walles, H. Verbesserung der Biokompatibilität von Silikonimplantaten durch Spinnenseidenbeschichtung: Immunhistochemische Untersuchungen zum Einfluss auf die Kapselbildung. Handchirurgie · Mikrochirurgie · Plast. Chir. 2014, 46, 336–341. [Google Scholar] [CrossRef]
- Borkner, C.B.; Wohlrab, S.; Möller, E.; Lang, G.; Scheibel, T. Surface Modification of Polymeric Biomaterials Using Recombinant Spider Silk Proteins. ACS Biomater. Sci. Eng. 2017, 3, 767–775. [Google Scholar] [CrossRef]
- Wen, D.-L.; Sun, D.-H.; Huang, P.; Huang, W.; Su, M.; Wang, Y.; Han, M.-D.; Kim, B.; Brugger, J.; Zhang, H.-X.; et al. Recent progress in silk fibroin-based flexible electronics. Microsystems Nanoeng. 2021, 7, 35. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, H.; Leow, W.R.; Cai, Y.; Loh, X.J.; Han, M.-Y.; Chen, X. Silk Fibroin for Flexible Electronic Devices. Adv. Mater. 2016, 28, 4250–4265. [Google Scholar] [CrossRef]
- Lazăr, A.D.; Dinescu, S.; Albu-Kaya, M.G.; Gharbia, S.; Hermenean, A.; Costache, M. Release of the Non-Steroidal Anti-Inflammatory Drug Flufenamic Acid by Multiparticulate Delivery Systems Promotes Adipogenic Differentiation of Adipose-Derived Stem Cells. Materials (Basel). 2020, 13, 1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinescu, S.; Galateanu, B.; Lungu, A.; Radu, E.; Nae, S.; Iovu, H.; Costache, M. Perilipin Expression Reveals Adipogenic Potential of hADSCs inside Superporous Polymeric Cellular Delivery Systems. Biomed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Xu, L.; Deng, Y.; Wang, G.; Wang, Z.; Wang, L. Sericin hydrogels promote skin wound healing with effective regeneration of hair follicles and sebaceous glands after complete loss of epidermis and dermis. Biomater. Sci. 2018, 6, 2859–2870. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Sun, L.; Zheng, J. In Vitro and In Vivo Characterization of a Silk Fibroin-Coated Polyester Vascular Prosthesis. Artif. Organs 2008, 32, 932–941. [Google Scholar] [CrossRef] [PubMed]


| Materials | Monomer Concentration (mol/L) | HEMA:AMPSA (%) | KPS * Molar | MMT (w/v %) | Sericin (w/v %) | 2% Fibroin Solution (v/v %) |
|---|---|---|---|---|---|---|
| M1 | 5 | 95:5 | 1% | 1% | 0.5% | 15% |
| M2 | 5 | 97:3 | 1% | 1% | 0.5% | 15% |
| M3 | 5 | 95:5 | 1% | 1% | - | - |
| M4 | 5 | 97:3 | 1% | 1% | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Șerban, M.V.; Nazarie, S.-R.; Dinescu, S.; Radu, I.-C.; Zaharia, C.; Istrătoiu, E.-A.; Tănasă, E.; Herman, H.; Gharbia, S.; Baltă, C.; et al. Silk ProteinsEnriched Nanocomposite Hydrogels Based on Modified MMT Clay and Poly(2-hydroxyethyl methacrylate-co-2-acrylamido-2-methylpropane Sulfonic Acid) Display Favorable Properties for Soft Tissue Engineering. Nanomaterials 2022, 12, 503. https://doi.org/10.3390/nano12030503
Șerban MV, Nazarie S-R, Dinescu S, Radu I-C, Zaharia C, Istrătoiu E-A, Tănasă E, Herman H, Gharbia S, Baltă C, et al. Silk ProteinsEnriched Nanocomposite Hydrogels Based on Modified MMT Clay and Poly(2-hydroxyethyl methacrylate-co-2-acrylamido-2-methylpropane Sulfonic Acid) Display Favorable Properties for Soft Tissue Engineering. Nanomaterials. 2022; 12(3):503. https://doi.org/10.3390/nano12030503
Chicago/Turabian StyleȘerban, Mirela Violeta, Simona-Rebeca Nazarie (Ignat), Sorina Dinescu, Ionuț-Cristian Radu, Cătălin Zaharia, Elena-Alexandra Istrătoiu, Eugenia Tănasă, Hildegard Herman, Sami Gharbia, Cornel Baltă, and et al. 2022. "Silk ProteinsEnriched Nanocomposite Hydrogels Based on Modified MMT Clay and Poly(2-hydroxyethyl methacrylate-co-2-acrylamido-2-methylpropane Sulfonic Acid) Display Favorable Properties for Soft Tissue Engineering" Nanomaterials 12, no. 3: 503. https://doi.org/10.3390/nano12030503
APA StyleȘerban, M. V., Nazarie, S.-R., Dinescu, S., Radu, I.-C., Zaharia, C., Istrătoiu, E.-A., Tănasă, E., Herman, H., Gharbia, S., Baltă, C., Hermenean, A., & Costache, M. (2022). Silk ProteinsEnriched Nanocomposite Hydrogels Based on Modified MMT Clay and Poly(2-hydroxyethyl methacrylate-co-2-acrylamido-2-methylpropane Sulfonic Acid) Display Favorable Properties for Soft Tissue Engineering. Nanomaterials, 12(3), 503. https://doi.org/10.3390/nano12030503









