Construction of Petal-Like Ag NWs@NiCoP with Three-Dimensional Core-Shell Structure for Overall Water Splitting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Three-Dimensional Core-Shell Petal-Like Ag NWs@Ni0.33Co0.67P
2.2. Characterizations
2.3. Electrochemical Measurements
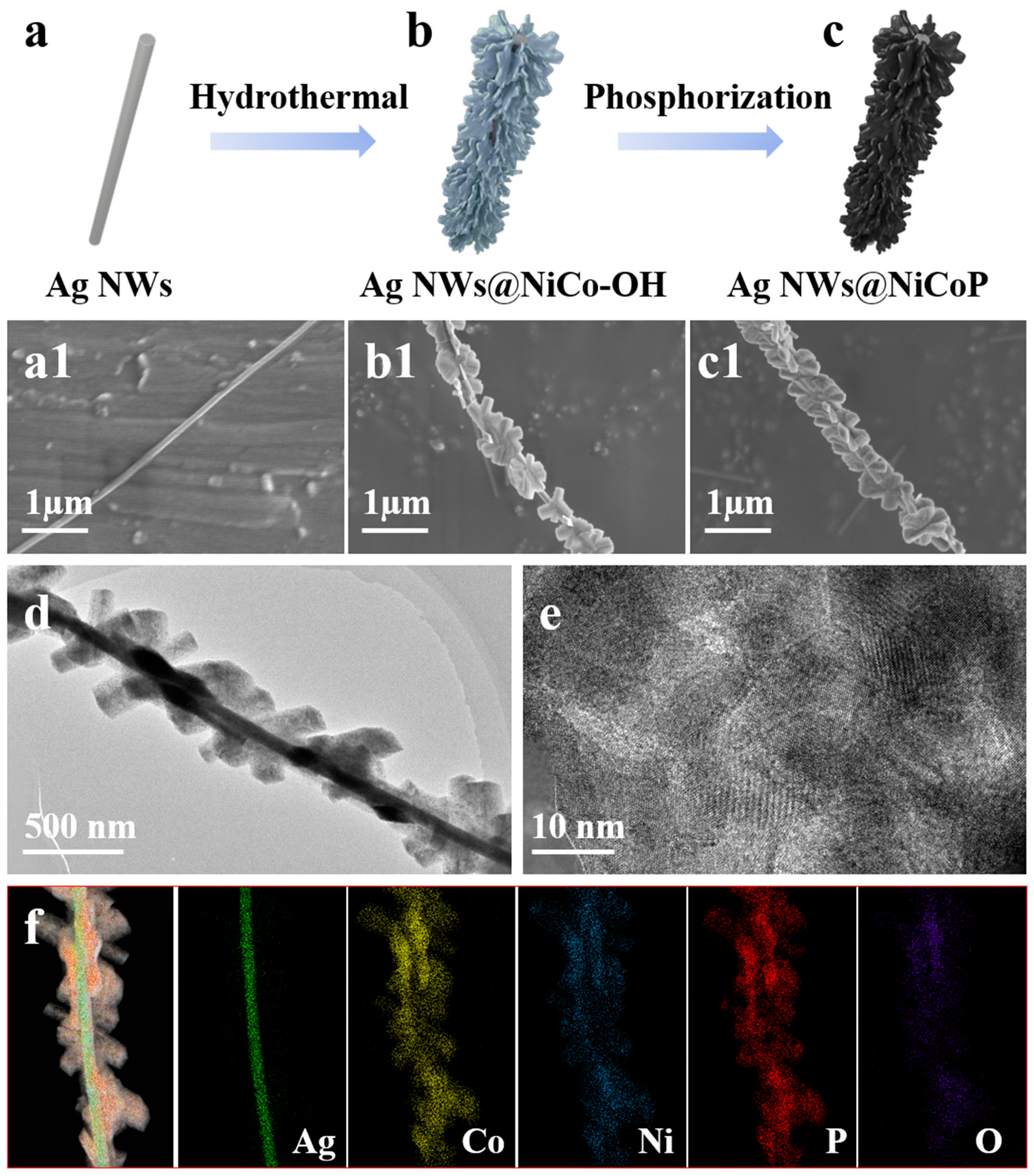
3. Results and Discussion
3.1. Structure of Three-Dimensional Core-Shell Petal-Like Ag NWs@Ni0.33Co0.67P
3.2. Electrocatalytic Performance of Three-Dimensional Core-Shell Petal-Like Ag NWs@Ni0.33Co0.67P
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, X.; Ji, P.; Wang, P.; Cheng, R.; Chen, D.; Lin, C.; Zhang, J.; He, J.; Shi, Z.; Li, N.; et al. Interface Engineering of Hierarchical Branched Mo-Doped Ni3S2/NixPy Hollow Heterostructure Nanorods for Efficient Overall Water Splitting. Adv. Energy Mater. 2020, 10, 1903891. [Google Scholar] [CrossRef]
- Meena, A.; Thangavel, P.; Nissimagoudar, A.S.; Singh, A.N.; Jana, A.; Jeong, D.S.; Im, H.; Kim, K.S. Bifunctional oxovanadate doped cobalt carbonate for high-efficient overall water splitting in alkaline-anion-exchange-membrane water-electrolyzer. Chem. Eng. J. 2022, 430, 132623. [Google Scholar] [CrossRef]
- Wang, N.; Ning, S.; Yu, X.; Chen, D.; Li, Z.; Xu, J.; Meng, H.; Zhao, D.; Li, L.; Liu, Q.; et al. Graphene composites with Ru-RuO2 heterostructures: Highly efficient Mott-Schottky-type electrocatalysts for pH-universal water splitting and flexible zinc-air batteries. Appl. Catal. B 2022, 302, 120838. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Luo, L.; Gong, S.; Wei, M.; Li, Y.; Gan, X.; Zhao, Y.; Zhu, Z.; Li, Z. Single-Atom and Bimetallic Nanoalloy Supported on Nanotubes as a Bifunctional Electrocatalyst for Ultrahigh-Current-Density Overall Water Splitting. ACS Catal. 2022, 12, 1167–1179. [Google Scholar] [CrossRef]
- Kenanakis, G.; Vernardou, D.; Dalamagkas, A.; Katsarakis, N. Photocatalytic and electrooxidation properties of TiO2 thin films deposited by sol-gel, Catal. Today 2015, 240, 146–152. [Google Scholar] [CrossRef]
- Zhang, H.; Maijenburg, A.W.; Li, X.; Schweizer, S.L.; Wehrspohn, R.B. Bifunctional Heterostructured Transition Metal Phosphides for Efficient Electrochemical Water Splitting. Adv. Funct. Mater. 2020, 30, 2003261. [Google Scholar] [CrossRef]
- Jiang, W.J.; Tang, T.; Zhang, Y.; Hu, J.S. Synergistic Modulation of Non-Precious-Metal Electrocatalysts for Advanced Water Splitting. Acc. Chem. Res. 2020, 53, 1111–1123. [Google Scholar] [CrossRef]
- Guo, L.; Liu, Q.; Liu, Y.; Chen, Z.; Jiang, Y.; Jin, H.; Zhou, T.; Yang, J.; Liu, Y. Self-supported tremella-like MoS2-AB particles on nickel foam as bifunctional electrocatalysts for overall water splitting. Nano Energy 2022, 92, 106707. [Google Scholar] [CrossRef]
- Ji, X.; Lin, Y.; Zeng, J.; Ren, Z.; Lin, Z.; Mu, Y.; Qiu, Y.; Yu, J. Graphene/MoS2/FeCoNi(OH)x and Graphene/MoS2/FeCoNiPx multilayer-stacked vertical nanosheets on carbon fibers for highly efficient overall water splitting. Nat. Commun. 2021, 12, 1380. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Zhang, F.; McElhenny, B.; Luo, D.; Karim, A.; Chen, S.; Ren, Z. Heterogeneous Bimetallic Phosphide Ni2P-Fe2P as an Efficient Bifunctional Catalyst for Water/Seawater Splitting. Adv. Funct. Mater. 2020, 31, 2006484. [Google Scholar] [CrossRef]
- Chala, S.A.; Tsai, M.C.; Su, W.N.; Ibrahim, K.B.; Thirumalraj, B.; Chan, T.S.; Lee, J.F.; Dai, H.; Hwang, B.J. Hierarchical 3D architectured Ag nanowires shelled with NiMn-layered double hydroxide as an efficient bifunctional oxygen electrocatalyst. ACS Nano 2020, 14, 1770–1782. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Y.; Zhao, Q.; Li, Q.; Pang, H. Facile synthesis of silver nanowire-zeolitic imidazolate framework 67 composites as high-performance bifunctional oxygen catalysts. Nanoscale 2018, 10, 15755–15762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, G.; Zuo, C.; Zhao, K.; Zeng, J.; Yin, J.; Chen, H.; Xie, S.; Qiu, Y. Core-Shell AgNWs@Ni(OH)2 Nanowires Anchored on Filter Paper for Efficient Hydrogen Evolution Reaction. J. Electrochem. Soc. 2020, 167, 116520. [Google Scholar] [CrossRef]
- Zhang, X.; Marianov, A.N.; Jiang, Y.; Cazorla, C.; Chu, D. Hierarchically constructed silver nanowire@nickel-iron layered double hydroxide nanostructures for electrocatalytic water splitting. ACS Appl. Nano Mater. 2019, 3, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Lin, S.; Yu, Y.; Liu, S.; Meng, F.; Du, G.; Xu, B. One-pot synthesis of N and P Co-doped carbon layer stabilized cobalt-doped MoP 3D porous structure for enhanced overall water splitting. J. Alloy. Compd. 2022, 895, 162595. [Google Scholar] [CrossRef]
- Wang, P.; Wang, T.; Qin, R.; Pu, Z.; Zhang, C.; Zhu, J.; Chen, D.; Feng, D.; Kou, Z.; Mu, S.; et al. Swapping Catalytic Active Sites from Cationic Ni to Anionic S in Nickel Sulfide Enables More Efficient Alkaline Hydrogen Generation. Adv. Energy Mater. 2022, 210, 3359. [Google Scholar] [CrossRef]
- Suryanto, B.H.R.; Wang, Y.; Hocking, R.K.; Adamson, W.; Zhao, C. Overall electrochemical splitting of water at the heterogeneous interface of nickel and iron oxide. Nat. Commun. 2019, 10, 5599. [Google Scholar] [CrossRef]
- Zhao, Y.; Dongfang, N.; Triana, C.A.; Huang, C.; Erni, R.; Wan, W.; Li, J.; Stoian, D.; Pan, L.; Zhang, P.; et al. Dynamics and control of active sites in hierarchically nanostructured cobalt phosphide/chalcogenide-based electrocatalysts for water splitting. Energy Environ. Sci. 2022. [CrossRef]
- Du, C.; Yang, L.; Yang, F.; Cheng, G.; Luo, W. Nest-like NiCoP for Highly Efficient Overall Water Splitting. ACS Catal. 2017, 7, 4131–4137. [Google Scholar] [CrossRef]
- Ji, L.; Wang, J.; Teng, X.; Meyer, T.J.; Chen, Z. CoP Nanoframes as Bifunctional Electrocatalysts for Efficient Overall Water Splitting. ACS Catal. 2019, 10, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Hu, Z.; Chen, C.; Liu, Y.; Yuan, Z. Integrated Ni2P nanosheet arrays on three-dimensional Ni foam for highly efficient water reduction and oxidation. J. Energy Chem. 2017, 26, 1196–1202. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Sun, K.; Liu, S.; Chen, X.; Cheng, Y.; Cheong, W.C.; Chen, Z.; Zheng, L.; Zhang, J.; Li, X.; et al. Construction of CoP/NiCoP Nanotadpoles Heterojunction Interface for Wide pH Hydrogen Evolution Electrocatalysis and Supercapacitor. Adv. Energy Mater. 2019, 9, 1901213. [Google Scholar] [CrossRef]
- Yang, T.; Ye, Q.; Liang, Y.; Wu, L.; Long, X.; Xu, X.; Wang, F. Graded holey Nickel Cobalt layered double hydroxide nanosheet array electrode with high mass loading for high-energy-density all-solid-state supercapacitors. J. Power Sources 2020, 449, 227590. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Xia, Z.; Luo, M.; Zhang, Q.; Qin, Y.; Tao, L.; Yin, K.; Chao, Y.; Gu, L.; et al. Exclusive Strain Effect Boosts Overall Water Splitting in PdCu/Ir Core/Shell Nanocrystals. Angew. Chem. Int. Ed. Engl. 2021, 60, 8243–8250. [Google Scholar] [CrossRef]
- Patil, S.J.; Chodankar, N.R.; Hwang, S.-K.; Shinde, P.A.; Raju, G.S.R.; Ranjith, K.S.; Huh, Y.S.; Han, Y.-K. Co-metal-organic framework derived CoSe2@MoSe2 core-shell structure on carbon cloth as an efficient bifunctional catalyst for overall water splitting. Chem. Eng. J. 2022, 429, 132379. [Google Scholar] [CrossRef]
- Jeong, S.; Mai, H.D.; Nam, K.H.; Park, C.M.; Jeon, K.J. Self-Healing Graphene-Templated Platinum-Nickel Oxide Heterostructures for Overall Water Splitting. ACS Nano 2022, 16, 930–938. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S.; et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 5106. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Chen, T.; Wang, H.; Bai, S.; Guo, X. One-pot rapid synthesis of high aspect ratio silver nanowires for transparent conductive electrodes. Mater. Res. Bull. 2018, 102, 79–85. [Google Scholar] [CrossRef]
- Liao, H.; Guo, X.; Hou, Y.; Liang, H.; Zhou, Z.; Yang, H. Construction of defect-rich Ni-Fe-doped K0.23MnO2 cubic nanoflowers via etching prussian blue analogue for efficient overall water splitting. Small 2020, 16, e1905223. [Google Scholar] [CrossRef]
- Wang, F.; Guo, X.; He, F.; Hou, Y.; Liu, F.; Zou, C.; Yang, H. Binder free construction of hollow hierarchical Mn-Co-P nanoarrays on nickel foam as an efficient bifunctional electrocatalyst for overall water splitting. Sustain. Energy Fuels 2022, 6, 851–860. [Google Scholar] [CrossRef]
- Bao, F.; Zhang, Z.; Guo, W.; Liu, X. Facile synthesis of three dimensional NiCo2O4@MnO2 core-shell nanosheet arrays and its supercapacitive performance. Electrochim. Acta 2015, 157, 31–40. [Google Scholar] [CrossRef]
- Jin, W.; Chen, J.; Wu, H.; Zang, N.; Li, Q.; Cai, W.; Wu, Z. Interface engineering of oxygen-vacancy-rich NiCo2O4/NiCoP heterostructure as an efficient bifunctional electrocatalyst for overall water splitting. Catal. Sci. Technol. 2020, 10, 5559–5565. [Google Scholar] [CrossRef]
- Liang, H.; Gandi, A.N.; Anjum, D.H.; Wang, X.; Schwingenschlogl, U.; Alshareef, H.N. Plasma-Assisted Synthesis of NiCoP for Efficient Overall Water Splitting. Nano Lett. 2016, 16, 7718–7725. [Google Scholar] [CrossRef]
- Boppella, R.; Tan, J.; Yang, W.; Moon, J. Homologous CoP/NiCoP Heterostructure on N-Doped Carbon for Highly Efficient and pH-Universal Hydrogen Evolution Electrocatalysis. Adv. Funct. Mater. 2018, 29, 1807976. [Google Scholar] [CrossRef]
- Ma, B.; Yang, Z.; Chen, Y.; Yuan, Z. Nickel cobalt phosphide with three-dimensional nanostructure as a highly efficient electrocatalyst for hydrogen evolution reaction in both acidic and alkaline electrolytes. Nano Res. 2018, 12, 375–380. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, R.; Dong, S.; Miao, X.; Zhang, Z.; Wang, C.; Yin, L. Alkali-induced 3D crinkled porous Ti3C2 MXene architectures coupled with NiCoP bimetallic phosphide nanoparticles as anodes for high-performance sodium-ion batteries. Energy Environ. Sci. 2019, 12, 2422–2432. [Google Scholar] [CrossRef]
- Lu, M.; Li, L.; Chen, D.; Li, J.; Klyui, N.I.; Han, W. MOF-derived nitrogen-doped CoO@CoP arrays as bifunctional electrocatalysts for efficient overall water splitting. Electrochim. Acta 2020, 330, 135210. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Hähnel, A.; Naumann, V.; Lin, C.; Azimi, S.; Schweizer, S.L.; Maijenburg, A.W.; Wehrspohn, R.B. Bifunctional Heterostructure Assembly of NiFe LDH Nanosheets on NiCoP Nanowires for Highly Efficient and Stable Overall Water Splitting. Adv. Funct. Mater. 2018, 28, 1706847. [Google Scholar] [CrossRef]
- Zhang, F.; Pei, Y.; Ge, Y.; Chu, H.; Craig, S.; Dong, P.; Cao, J.; Ajayan, P.M.; Ye, M.; Shen, J. Controlled synthesis of eutectic NiSe/Ni3Se2 self-supported on Ni foam: An excellent bifunctional electrocatalyst for overall water splitting. Adv. Mater. Interfaces 2018, 5, 1701507. [Google Scholar] [CrossRef]
- Xing, Y.; Li, D.; Li, L.; Tong, H.; Jiang, D.; Shi, W. Accelerating water dissociation kinetic in Co9S8 electrocatalyst by mn/N Co-doping toward efficient alkaline hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 7989–8001. [Google Scholar] [CrossRef]
- Xu, H.; Jia, H.; Fei, B.; Ha, Y.; Li, H.; Guo, Y.; Liu, M.; Wu, R. Charge transfer engineering via multiple heteroatom doping in dual carbon-coupled cobalt phosphides for highly efficient overall water splitting. Appl. Catal. B 2020, 268, 118404. [Google Scholar] [CrossRef]
- Liang, Z.; Zhou, W.; Gao, S.; Zhao, R.; Zhang, H.; Tang, Y.; Cheng, J.; Qiu, T.; Zhu, B.; Qu, C.; et al. Fabrication of hollow CoP/TiOx heterostructures for enhanced oxygen evolution reaction. Small 2020, 16, e1905075. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, M.; Zhou, X.; Huang, Z.-Q.; Xia, Z.; Chang, C.-R.; Ma, Y.; Qu, Y. Mechanistic Insights on Ternary Ni2-xCoxP for Hydrogen Evolution and Their Hybrids with Graphene as Highly Efficient and Robust Catalysts for Overall Water Splitting. Adv. Funct. Mater. 2016, 26, 6785–6796. [Google Scholar] [CrossRef]
- Qiu, B.; Cai, L.; Wang, Y.; Lin, Z.; Zuo, Y.; Wang, M.; Chai, Y. Fabrication of Nickel-Cobalt Bimetal Phosphide Nanocages for Enhanced Oxygen Evolution Catalysis. Adv. Funct. Mater. 2018, 28, 1706008. [Google Scholar] [CrossRef]
- Liu, F.; Guo, X.; Hou, Y.; Wang, F.; Zou, C.; Yang, H. Hydrothermal combined with electrodeposition construction of a stable Co9S8/Ni3S2@NiFe-LDH heterostructure electrocatalyst for overall water splitting. Sustain. Energy Fuels 2021, 5, 1429–1438. [Google Scholar] [CrossRef]
- Liu, X.; Wang, R.; He, Y.; Ni, Z.; Su, N.; Guo, R.; Zhao, Y.; You, J.; Yi, T. Construction of alternating layered quasi-three-dimensional electrode Ag NWs/CoO for water splitting: A discussion of catalytic mechanism. Electrochim. Acta 2019, 317, 468–477. [Google Scholar] [CrossRef]
- He, P.; Yu, X.Y.; Lou, X.W. Carbon-Incorporated Nickel-Cobalt Mixed Metal Phosphide Nanoboxes with Enhanced Electrocatalytic Activity for Oxygen Evolution. Angew. Chem. Int. Ed. Engl. 2017, 56, 3897–3900. [Google Scholar] [CrossRef]
- Hu, E.; Feng, Y.; Nai, J.; Zhao, D.; Hu, Y.; Lou, X.W. Construction of hierarchical Ni-Co-P hollow nanobricks with oriented nanosheets for efficient overall water splitting. Energy Environ. Sci. 2018, 11, 872–880. [Google Scholar] [CrossRef]
- Bao, J.; Liu, W.; Xie, J.; Xu, L.; Guan, M.; Lei, F.; Zhao, Y.; Huang, Y.; Xia, J.; Li, H. NixCo3−xO4 nanoneedle arrays grown on Ni foam as an efficient bifunctional electrocatalyst for full water splitting. Chem. Asian J. 2019, 14, 480–485. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Lim, A.C.; Roy, A.; Seo, J.G. Room-Temperature Ultrafast Synthesis of NiCo-Layered Double Hydroxide as an Excellent Electrocatalyst for Water Oxidation. ChemistrySelect 2019, 4, 2409–2415. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, X.; Wang, T.; Xiao, W.; Xi, P.; Wang, J.; Gao, D.; Wang, J. Bimetallic Nickel Cobalt Sulfide as Efficient Electrocatalyst for Zn-Air Battery and Water Splitting. Nanomicro Lett. 2019, 11, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, M.; Sun, D.; Zhang, L.; Ding, X. In situ assembly of metal-organic framework-derived N-doped carbon/Co/CoP catalysts on carbon paper for water splitting in alkaline electrolytes. Chin. J. Catal. 2020, 41, 242–248. [Google Scholar] [CrossRef]
- He, D.; Wu, X.; Liu, W.; Lei, C.; Yu, C.; Zheng, G.; Pan, J.; Lei, L.; Zhang, X. Co1−xS embedded in porous carbon derived from metal organic framework as a highly efficient electrocatalyst for oxygen evolution reaction. Chin. Chem. Lett. 2019, 30, 229–233. [Google Scholar] [CrossRef]
- Qin, J.-F.; Lin, J.-H.; Chen, T.-S.; Liu, D.-P.; Xie, J.-Y.; Guo, B.-Y.; Wang, L.; Chai, Y.-M.; Dong, B. Facile synthesis of V-doped CoP nanoparticles as bifunctional electrocatalyst for efficient water splitting. J. Energy Chem. 2019, 39, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Tolstoy, V.P.; Lobinsky, A.A.; Kaneva, M.V. Features of inorganic nanocrystals formation in conditions of successive ionic layers deposition in water solutions and the Co(II)Co(III) 2D layered double hydroxide synthesis. J. Mol. Liq. 2019, 282, 32–38. [Google Scholar] [CrossRef]
- Zou, W.; Dou, K.; Jiang, Q.; Xiang, J.; Kaun, C.-C.; Tang, H. Nearly spherical CoP nanoparticle/carbon nanosheet hybrids: A high-performance trifunctional electrocatalyst for oxygen reduction and water splitting. RSC Adv. 2019, 9, 39951–39957. [Google Scholar] [CrossRef] [Green Version]
- Tie, J.; Han, J.; Diao, G.; Liu, J.; Xie, Z.; Cheng, G.; Sun, M.; Yu, L. Controllable synthesis of hierarchical nickel cobalt sulfide with enhanced electrochemical activity. Appl. Surf. Sci. 2018, 435, 187–194. [Google Scholar] [CrossRef]
- Yan, J.; Chen, L.; Liang, X. Co9S8 nanowires@NiCo LDH nanosheets arrays on nickel foams towards efficient overall water splitting. Sci. Bull. 2019, 64, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Li, Z. MOF-derived Co, Ni, Mn co-doped N-enriched hollow carbon for efficient hydrogen evolution reaction catalysis. J. Solid State Chem. 2021, 295, 121912. [Google Scholar] [CrossRef]
- Sun, X.; Shao, Q.; Pi, Y.; Guo, J.; Huang, X. A general approach to synthesise ultrathin NiM (M = Fe, Co, Mn) hydroxide nanosheets as high-performance low-cost electrocatalysts for overall water splitting. J. Mater. Chem. A 2017, 5, 7769–7775. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Gao, G.; Liu, D.; Liu, R.; Fan, R.; Gan, S.; Wang, Y.; Wang, Y. 3D metallic Ti@Ni0.85Se with triple hierarchy as high-efficiency electrocatalyst for overall water splitting. ChemSusChem 2019, 12, 2271–2277. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Tian, R.; Guo, X.; Hou, Y.; Zou, C.; Yang, H. Construction of Petal-Like Ag NWs@NiCoP with Three-Dimensional Core-Shell Structure for Overall Water Splitting. Nanomaterials 2022, 12, 1205. https://doi.org/10.3390/nano12071205
Wang F, Tian R, Guo X, Hou Y, Zou C, Yang H. Construction of Petal-Like Ag NWs@NiCoP with Three-Dimensional Core-Shell Structure for Overall Water Splitting. Nanomaterials. 2022; 12(7):1205. https://doi.org/10.3390/nano12071205
Chicago/Turabian StyleWang, Fan, Rui Tian, Xingzhong Guo, Yang Hou, Chang Zou, and Hui Yang. 2022. "Construction of Petal-Like Ag NWs@NiCoP with Three-Dimensional Core-Shell Structure for Overall Water Splitting" Nanomaterials 12, no. 7: 1205. https://doi.org/10.3390/nano12071205





