Enhanced Tribocatalytic Degradation of Organic Pollutants by ZnO Nanoparticles of High Crystallinity
Abstract
:1. Introduction
2. Materials and Methods
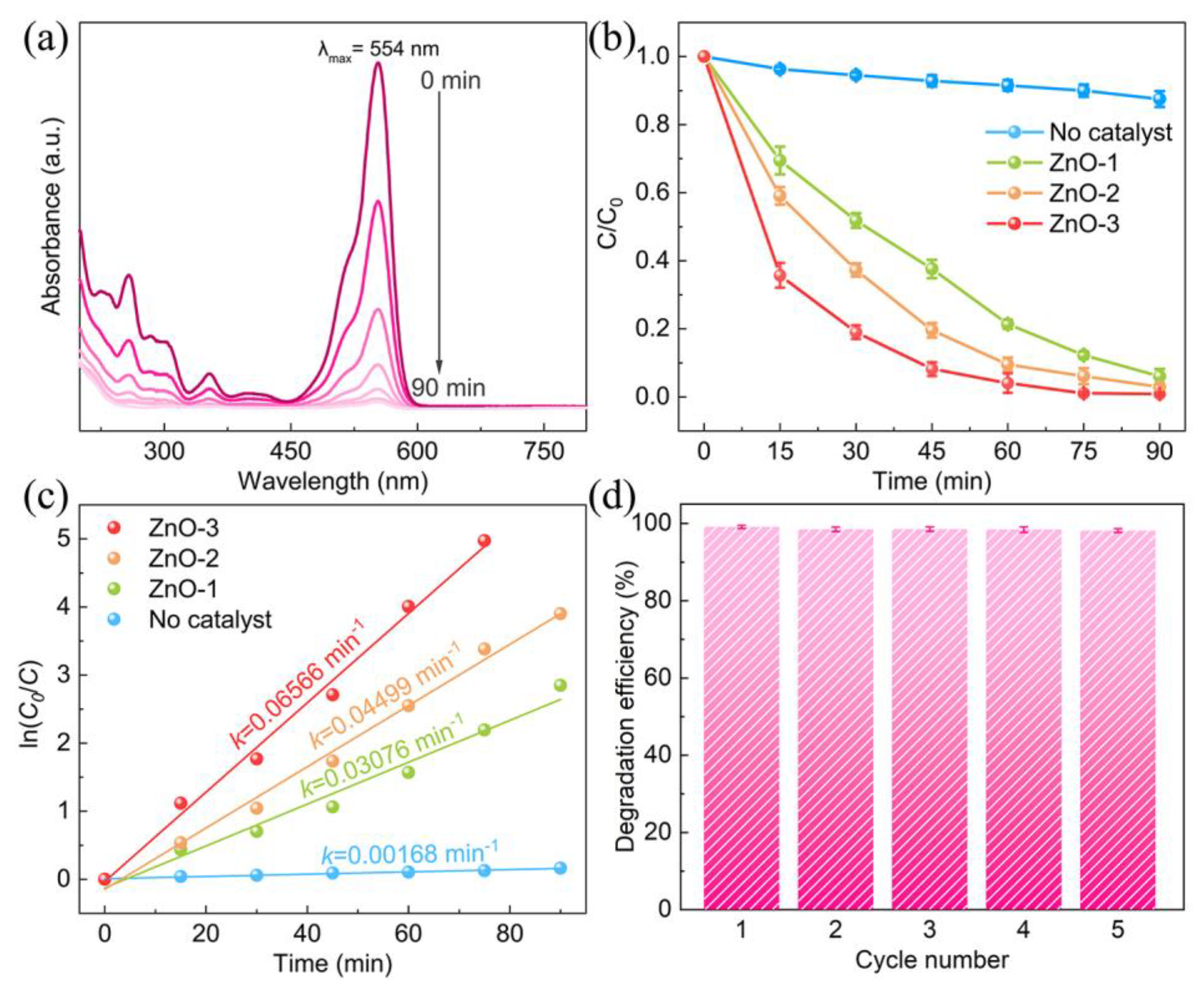
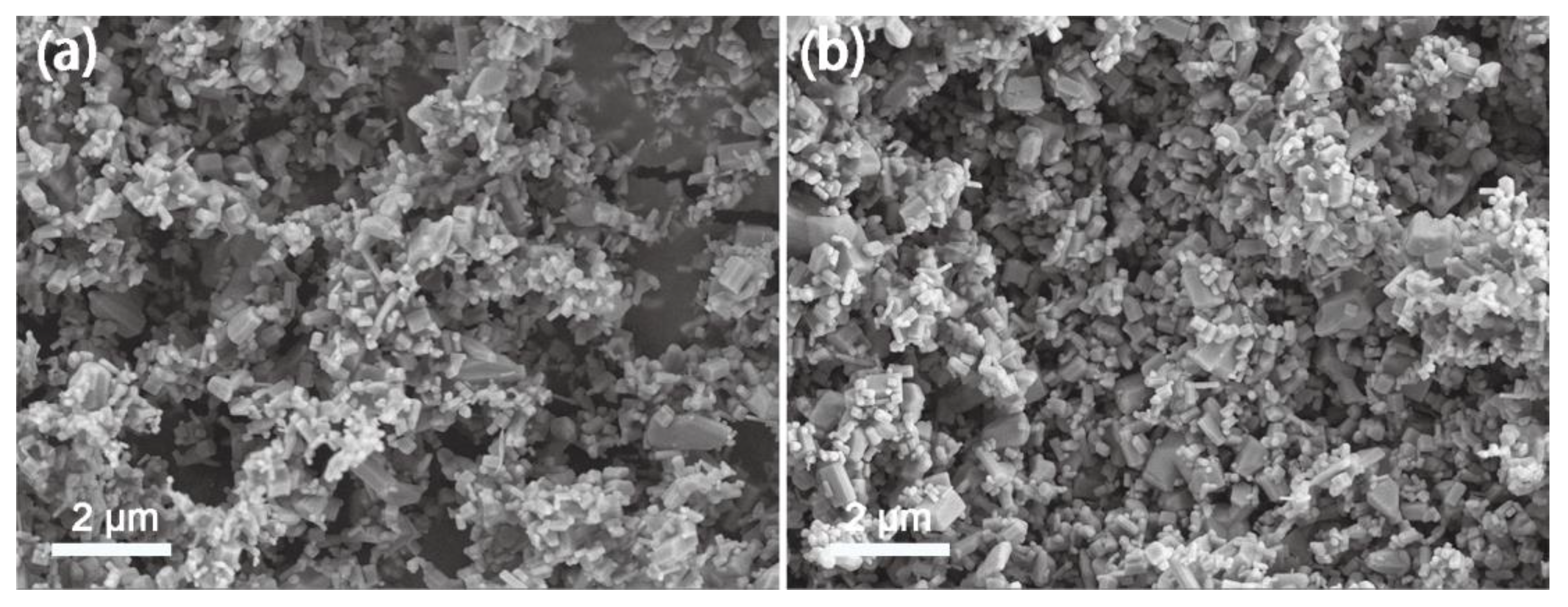
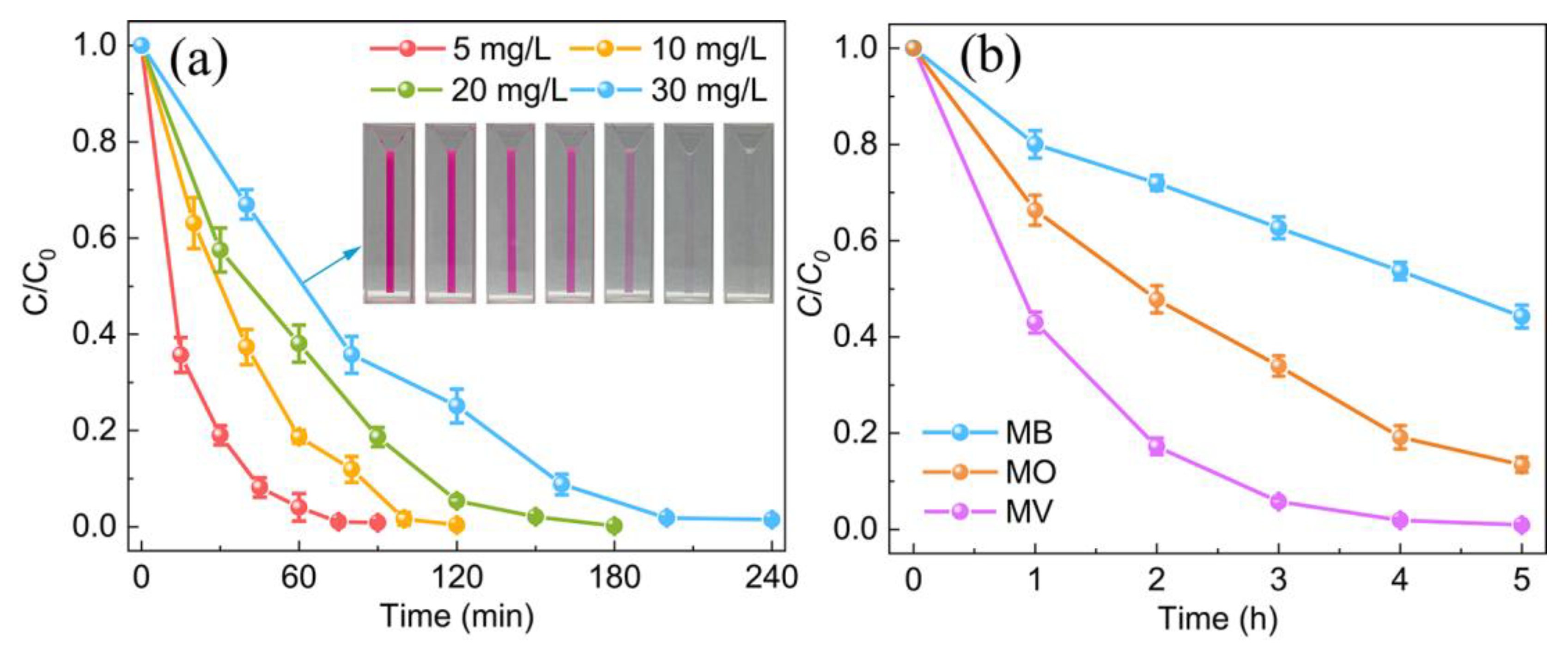
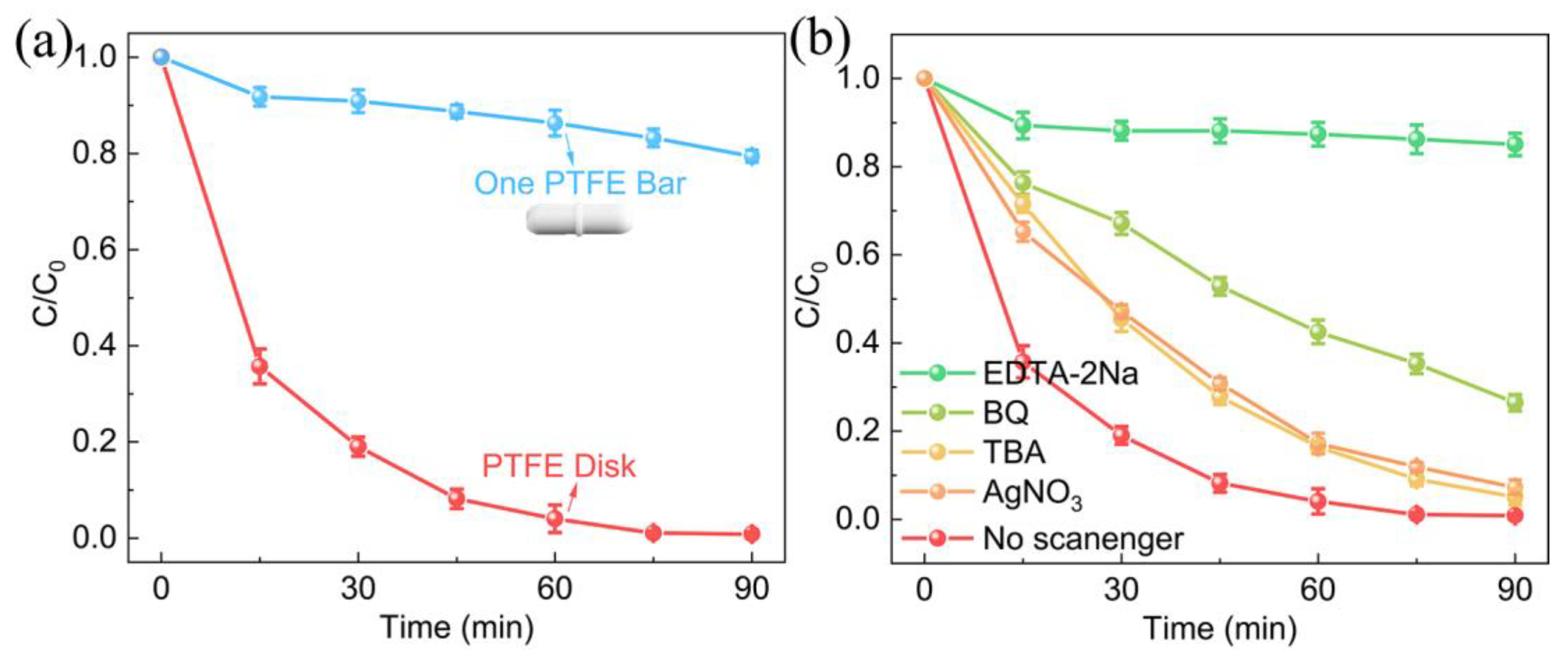
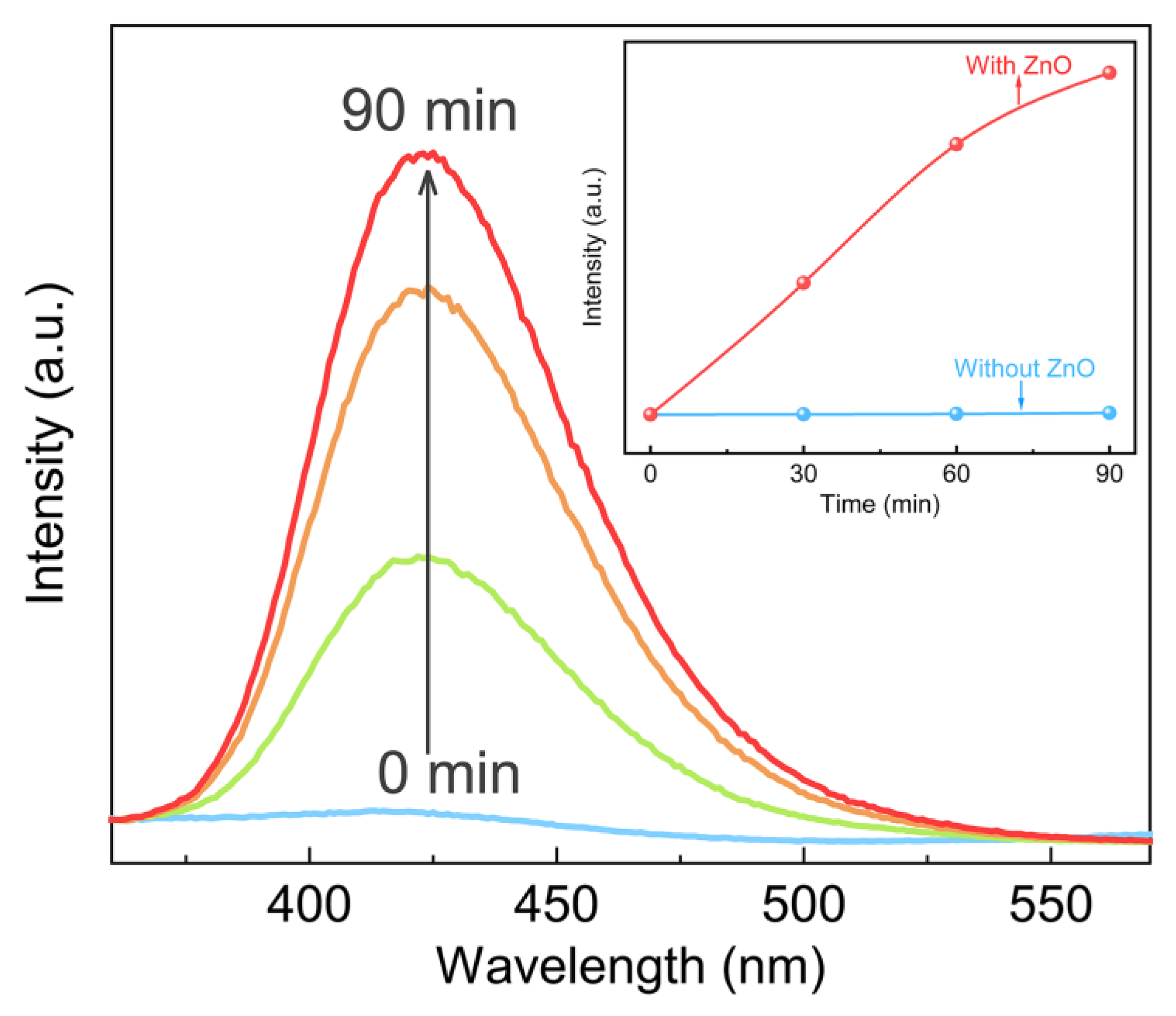
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gong, P.; Xu, H.; Wang, C.; Chen, Y.; Guo, L.; Wang, X. Persistent organic pollutant cycling in forests. Nat. Rev. Earth Environ. 2021, 2, 182–197. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhang, H.; Xiang, Q. Porous graphitic carbon nitride for solar photocatalytic applications. Nanoscale Horiz. 2020, 5, 765–786. [Google Scholar] [CrossRef]
- Liang, Z.; Yan, C.F.; Rtimi, S.; Bandara, J. Piezoelectric materials for catalytic/photocatalytic removal of pollutants: Recent advances and outlook. Appl. Catal. B Environ. 2019, 241, 256–269. [Google Scholar] [CrossRef]
- Yadav, A.A.; Kang, S.W.; Hunge, Y.M. Photocatalytic degradation of Rhodamine B using graphitic carbon nitride photocatalyst. J. Mater. Sci. Mater. Electron. 2021, 32, 15577–15585. [Google Scholar] [CrossRef]
- Brody, J.G.; Rudel, R.A. Environmental pollutants and breast cancer. Environ. Health Perspect. 2003, 111, 1007–1019. [Google Scholar] [CrossRef] [Green Version]
- Vrijheid, M.; Casas, M.; Gascon, M.; Valvi, D.; Nieuwenhuijsen, M. Environmental pollutants and child health—A review of recent concerns. Int. J. Hyg. Environ. Health. 2016, 219, 331–342. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef]
- Gibson, L.T. Mesosilica materials and organic pollutant adsorption: Part A removal from air. Chem. Soc. Rev. 2014, 43, 5163–5172. [Google Scholar] [CrossRef]
- Silva, A.R.; Soares, O.S.G.; Pereira, M.F.R.; Alves, M.M.; Pereira, L. Tailoring carbon nanotubes to enhance their efficiency as electron shuttle on the biological removal of acid orange 10 under anaerobic conditions. Nanomaterials 2020, 10, 2496. [Google Scholar] [CrossRef]
- Gaur, N.; Narasimhulu, K.; PydiSetty, Y. Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment. J. Clean. Prod. 2018, 198, 1602–1631. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, A.; Ciacchi, C.L.; Wei, G. Recent advances in nanoporous membranes for water purification. Nanomaterials 2018, 8, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Maqdi, K.A.; Elmerhi, N.; Athamneh, K.; Bilal, M.; Alzamly, A.; Ashraf, S.S.; Shah, I. Challenges and Recent Advances in Enzyme-Mediated Wastewater Remediation—A Review. Nanomaterials 2021, 11, 3124. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, S.; Xia, L.; Wang, Z.; Suo, N.; Chen, H.; Long, Y.; Zhou, B.; Yu, Y. In-situ ion exchange electrocatalysis biological coupling (i-IEEBC) for simultaneously enhanced degradation of organic pollutants and heavy metals in electroplating wastewater. J. Hazard. Mater. 2019, 364, 562–570. [Google Scholar] [CrossRef]
- Cagnetta, G.; Robertson, J.; Huang, J.; Zhang, K.; Yu, G. Mechanochemical destruction of halogenated organic pollutants: A critical review. J. Hazard. Mater. 2016, 313, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Kleine, T.; Buendia, J.; Bolm, C. Mechanochemical degradation of lignin and wood by solvent-free grinding in a reactive medium. Green Chem. 2013, 15, 160–166. [Google Scholar] [CrossRef]
- Cagnetta, G.; Huang, J.; Wang, B.; Deng, S.; Yu, G. A comprehensive kinetic model for mechanochemical destruction of persistent organic pollutants. Chem. Eng. J. 2016, 291, 30–38. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, J.; Zhang, W.; Yu, Y.; Deng, S.; Wang, B.; Yu, G. Coupling the dechlorination of aqueous 4-CP with the mechanochemical destruction of solid PCNB using Fe-Ni-SiO2. J. Hazard. Mater. 2013, 250–251, 175–180. [Google Scholar] [CrossRef]
- Cagnetta, G.; Zhang, Q.; Huang, J.; Lu, M.; Wang, B.; Wang, Y.; Deng, S.; Yu, G. Mechanochemical destruction of perfluorinated pollutants and mechanosynthesis of lanthanum oxyfluoride: A Waste-to-Materials process. Chem. Eng. J. 2017, 316, 1078–1090. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, J.; Zhang, W.; Zhang, K.; Deng, S.; Yu, G. Mechanochemical destruction of mirex co-ground with iron and quartz in a planetary ball mill. Chemosphere 2013, 90, 1729–1735. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; Li, W.; Lu, Y.; Meng, H.; Li, C. Efficient destruction of hexachlorobenzene by calcium carbide through mechanochemical reaction in a planetary ball mill. Chemosphere 2017, 166, 275–280. [Google Scholar] [CrossRef]
- Rowlands, S.A.; Hall, A.K.; McCormick, P.G.; Street, R.; Hart, R.J.; Ebell, G.F.; Donecker, P. Destruction of toxic materials. Nature 1994, 367, 223. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, H. Degradation of methyl red by mechanochemical methods. J. Nanjing Univ. Technol. 2007, 29, 62–66. [Google Scholar]
- Hu, J.; Huang, Z.; Yu, J. Highly-effective mechanochemical destruction of hexachloroethane and hexachlorobenzene with Fe/Fe3O4 mixture as a novel additive. Sci. Total. Environ. 2019, 659, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Zhao, J.; Sun, W.; Li, X.; Li, J.; Zhang, J.; Jing, Z. NaOH-activated persulfate-assisted mechanochemical mechanism and removal of lindane from contaminated soil. J. Environ. Chem. Eng. 2021, 9, 105391. [Google Scholar] [CrossRef]
- Fan, G.; Liu, X.; Li, X.; Lin, C.; He, M.; Ouyang, W. Mechanochemical treatment with CaO-activated PDS of HCB contaminated soils. Chemosphere 2020, 257, 127207. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Wu, Z.; Jia, Y.; Ma, J.; Chen, W.; Zhang, L.; Yang, J.; Liu, Y. Strong tribocatalytic dye decomposition through utilizing triboelectric energy of barium strontium titanate nanoparticles. Nano Energy 2019, 63, 103832. [Google Scholar] [CrossRef]
- Lei, H.; Wu, M.; Mo, F.; Ji, S.; Dong, X.; Wu, Z.; Gao, J.; Yang, Y.; Jia, Y. Tribo-catalytic degradation of organic pollutants through bismuth oxyiodate triboelectrically harvesting mechanical energy. Nano Energy 2020, 78, 105290. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Guo, X.; Wang, L.; Xu, T.; Bian, J.; Yang, Y.; Liu, Q.; Du, Y.; Lou, X. Enhanced tribocatalytic degradation using piezoelectric CdS nanowires for efficient water remediation. J. Mater. Chem. C 2020, 8, 14845–14854. [Google Scholar] [CrossRef]
- Wu, M.; Lei, H.; Chen, J.; Dong, X. Friction energy harvesting on bismuth tungstate catalyst for tribocatalytic degradation of organic pollutants. J. Colloid Interface Sci. 2021, 587, 883–890. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Yang, Y.; Wang, L.; Bian, J.; Liu, Q.; Lou, X. Insights into the tribo-/pyro-catalysis using Sr-doped BaTiO3 ferroelectric nanocrystals for efficient water remediation. Chem. Eng. J. 2021, 416, 128986. [Google Scholar] [CrossRef]
- Sun, C.; Guo, X.; Ji, R.; Hu, C.; Liu, L.; Fang, L.; Cheng, Z.; Luo, N. Strong tribocatalytic dye degradation by tungsten bronze Ba4Nd2Fe2Nb8O30. Ceram. Int. 2021, 47, 5038–5043. [Google Scholar] [CrossRef]
- Cui, X.; Li, P.; Lei, H.; Tu, C.; Wang, D.; Wang, Z.; Chen, W. Greatly enhanced tribocatalytic degradation of organic pollutants by TiO2 nanoparticles through efficiently harvesting mechanical energy. Sep. Purif. Technol. 2022, 289, 120814. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, R.; Zhang, Y.; Zhou, B.; Sun, P.; Dong, X. Unveiling the Mechanism of Frictional Catalysis in Water by Bi12TiO20: A Charge Transfer and Contaminant Decomposition Path Study. Langmuir 2022, 38, 14153–14161. [Google Scholar] [CrossRef]
- Ruan, L.; Jia, Y.; Guan, J.; Xue, B.; Huang, S.; Wang, Z.; Fu, Y.; Wu, Z. Tribo-electro-catalytic dye degradation driven by mechanical friction using MOF-derived NiCo2O4 double-shelled nanocages. J. Clean. Prod. 2022, 345, 131060. [Google Scholar] [CrossRef]
- Sun, C.; Guo, X.; Hu, C.; Liu, L.; Fang, L.; Cheng, Z.; Luo, N. Tribocatalytic degradation of dyes by tungsten bronze ferroelectric Ba2.5Sr2.5Nb8Ta2O30 submicron particles. RSC Adv. 2021, 11, 13386–13395. [Google Scholar] [CrossRef]
- Tang, M.; Lu, S.; He, L.; Zhu, X.; Feng, W.; Zhang, W. Preparation, Characterization of ZnTiO3/ZnO Composite Materials and Their Photocatalytic Performance. Nanomaterials 2022, 12, 1345. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.W.; Kim, H. Facile synthesis of multitasking composite of Silver nanoparticle with Zinc oxide for 4-nitrophenol reduction, photocatalytic hydrogen production, and 4-chlorophenol degradation. J. Alloy. Comp. 2022, 928, 167133. [Google Scholar] [CrossRef]
- Ma, J.; Ren, J.; Jia, Y.; Wu, Z.; Chen, L.; Haugen, N.O.; Huang, H.; Liu, Y. High efficiency bi-harvesting light/vibration energy using piezoelectric zinc oxide nanorods for dye decomposition. Nano Energy 2019, 62, 376–383. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.W.; Lim, S.J.; Kim, H. Visible light activated MoS2/ZnO composites for photocatalytic degradation of ciprofloxacin antibiotic and hydrogen production. J. Photochem. Photobiol. A Chem. 2023, 434, 114250. [Google Scholar] [CrossRef]
- Hunge, Y.M. Photoelectrocatalytic degradation of methylene blue using spray deposited ZnO thin films under UV illumination. MO J. Polym. Sci. 2017, 1, 00020. [Google Scholar]
- Hu, J.; Ma, W.; Pan, Y.; Chen, Z.; Zhang, Z.; Wan, C.; Sun, Y.; Qiu, C. Resolving the Tribo-catalytic reaction mechanism for biochar regulated Zinc Oxide and its application in protein transformation. J. Colloid Interface Sci. 2022, 607, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ma, W.; Pan, Y.; Cheng, Z.; Yu, S.; Gao, J.; Zhang, Z.; Wan, C.; Qiu, C. Insights on the mechanism of Fe doped ZnO for tightly-bound extracellular polymeric substances tribo-catalytic degradation: The role of hydration layers at the interface. Chemosphere 2021, 276, 130170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, L.; Luo, W.; Li, H.; Wu, Z.; Xu, Z.; Zhang, Y.; Zhang, H.; Yuan, G.; Gao, J.; et al. Strong tribo-catalysis of zinc oxide nanorods via triboelectrically-harvesting friction energy. Ceram. Int. 2020, 46, 25293–25298. [Google Scholar] [CrossRef]
- Lei, H.; Wu, M.; Liu, Y.; Mo, F.; Chen, J.; Ji, S.; Zou, Y.; Dong, X. Built-in piezoelectric field improved photocatalytic performance of nanoflower-like Bi2WO6 using low-power white LEDs. Chin. Chem. Lett. 2021, 32, 2317–2321. [Google Scholar] [CrossRef]
- Wan, C.; Pan, Y.; Chen, Z.; Hu, J.; Zhang, Z.; Sun, Y.; Ma, W. The action of enhanced reactive oxygen species production through the dopant of Al2O3/GO in piezoelectric ZnO. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127148. [Google Scholar] [CrossRef]
- Watanabe, T.; Takizawa, T.; Honda, K. Photocatalysis through Excitation to Adsorbates. 1. Highly Efficient/V-Deethylation of Rhodamine B Adsorbed to CdS. J. Phys. Chem. 1977, 81, 1845–1851. [Google Scholar] [CrossRef]
- Li, P.; Tang, C.; Xiao, X.; Jia, Y.; Chen, W. Flammable gases produced by TiO2 nanoparticles under magnetic stirring in water. Friction 2022, 10, 1127–1133. [Google Scholar] [CrossRef]
- Nie, Q.; Xie, Y.; Ma, J.; Wang, J.; Zhang, G. High piezo–catalytic activity of ZnO/Al2O3 nanosheets utilizing ultrasonic energy for wastewater treatment. J. Clean. Prod. 2020, 242, 118532. [Google Scholar] [CrossRef]
- Park, J.Y.; Salmeron, M. Fundamental aspects of energy dissipation in friction. Chem. Rev. 2014, 114, 677–711. [Google Scholar] [CrossRef]
- Enesca, A.; Andronic, L. UV-Vis Activated Cu2O/SnO2/WO3 Heterostructure for Photocatalytic Removal of Pesticides. Nanomaterials 2022, 12, 2648. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, H.; Cui, X.; Jia, X.; Qi, J.; Wang, Z.; Chen, W. Enhanced Tribocatalytic Degradation of Organic Pollutants by ZnO Nanoparticles of High Crystallinity. Nanomaterials 2023, 13, 46. https://doi.org/10.3390/nano13010046
Lei H, Cui X, Jia X, Qi J, Wang Z, Chen W. Enhanced Tribocatalytic Degradation of Organic Pollutants by ZnO Nanoparticles of High Crystallinity. Nanomaterials. 2023; 13(1):46. https://doi.org/10.3390/nano13010046
Chicago/Turabian StyleLei, Hua, Xiaodong Cui, Xuchao Jia, Jianquan Qi, Zhu Wang, and Wanping Chen. 2023. "Enhanced Tribocatalytic Degradation of Organic Pollutants by ZnO Nanoparticles of High Crystallinity" Nanomaterials 13, no. 1: 46. https://doi.org/10.3390/nano13010046



