DNA-Directed Protein Anchoring on Oligo/Alkanethiol-Coated Gold Nanoparticles: A Versatile Platform for Biosensing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterisation of AuNPs
2.3. ss-DNA/TOEG6@AuNPs Functionalization
2.4. Assessing the Conformation of DNA Strands on AuNP Surface
2.5. Stability of ss-DNA/TOEG6@AuNPs in a Biological Environment
2.6. Biofunctionalization of AuNPs with Anti-HER2 Nanobodies
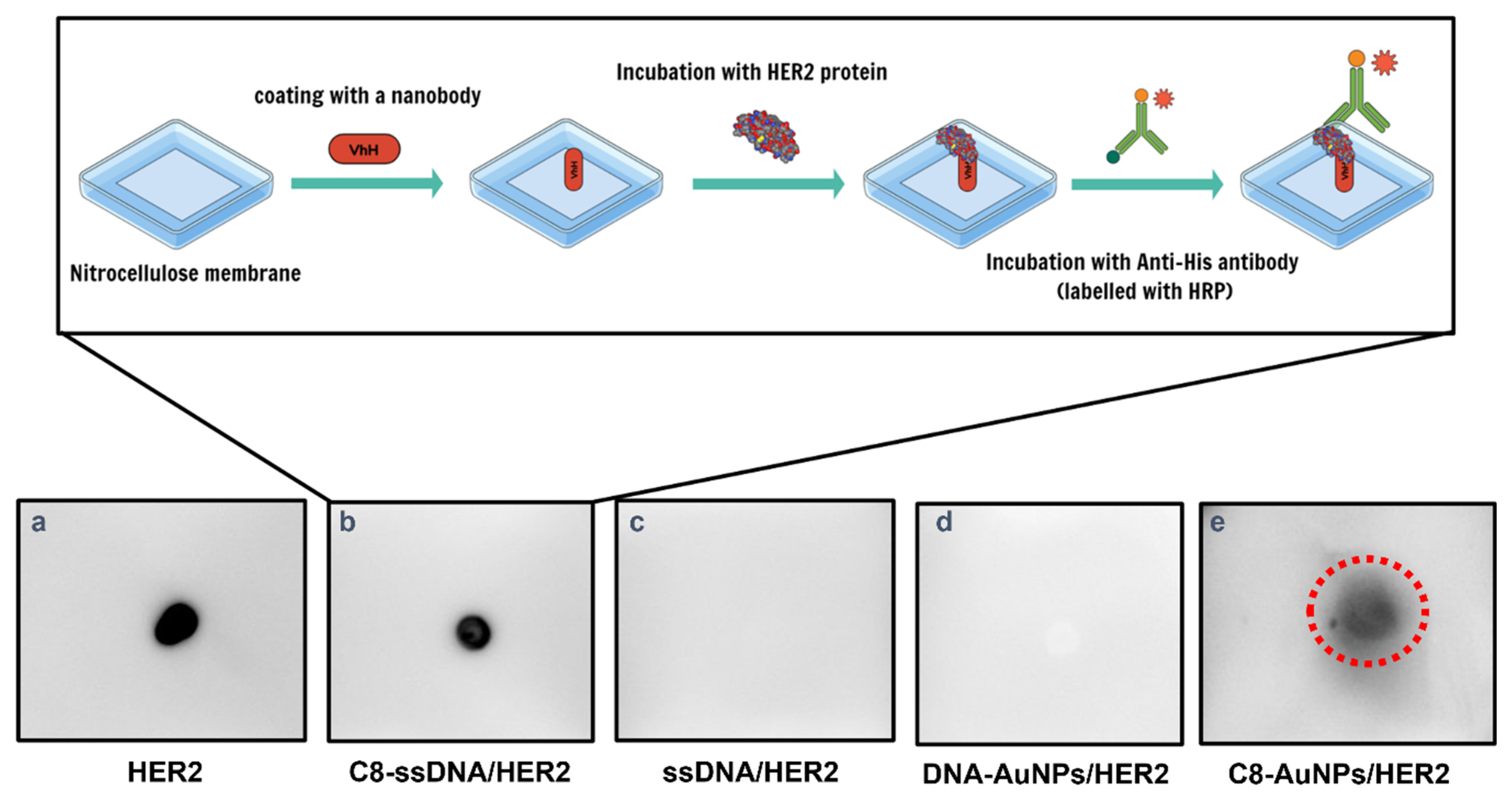
2.7. Dot Blot Immunological Assay for Detection of HER2 Antigen
2.8. Detection of HER2 Using Online TLS Coupled with MGEC
3. Results and Discussion
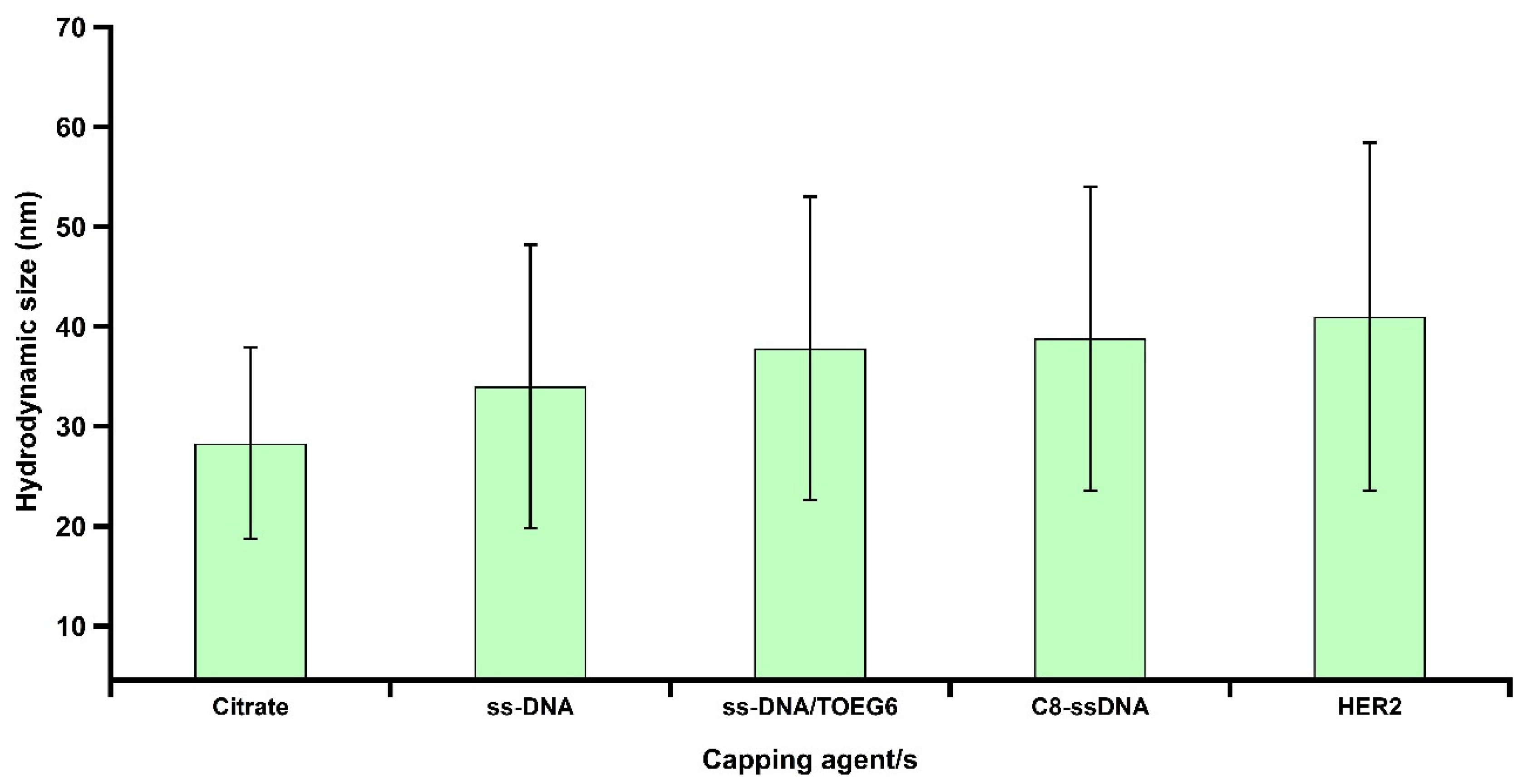
3.1. Characterization of Synthesized AuNPs
3.2. Mixed-SAM Formation on the AuNP Surface
3.3. Assessing the Stability of ssDNA/TOEG6@AuNPs in a Physiological Environment
3.4. AuNP Functionalization and ECD-HER2 Detection
3.5. Novel Detection: MGEC-TLS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rana, S.; Yeh, Y.-C.; Rotello, V.M. Engineering the nanoparticle–protein interface: Applications and possibilities. Curr. Opin. Chem. Biol. 2010, 14, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L. Interesting developments at the nanoparticle–protein interface: Implications for next generation drug delivery. Future Sci. 2016, 7, 513–516. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, J.M.; Lindberg, J.; Sundberg, M.; Uhlen, M.; Nilsson, P. Determination of Binding Specificities in Highly Multiplexed Bead-based Assays for Antibody Proteomics* S. Mol. Cell. Proteom. 2007, 6, 125–132. [Google Scholar] [CrossRef]
- Bano, F.; Fruk, L.; Sanavio, B.; Glettenberg, M.; Casalis, L.; Niemeyer, C.M.; Scoles, G. Toward multiprotein nanoarrays using nanografting and DNA directed immobilization of proteins. Nano Lett. 2009, 9, 2614–2618. [Google Scholar] [CrossRef]
- Arrabito, G.; Reisewitz, S.; Dehmelt, L.; Bastiaens, P.I.; Pignataro, B.; Schroeder, H.; Niemeyer, C.M. Biochips for cell biology by combined Dip-Pen nanolithography and DNA-directed protein immobilization. Small 2013, 9, 4243–4249. [Google Scholar] [CrossRef]
- Yang, Z.; Kasprzyk-Hordern, B.; Goggins, S.; Frost, C.G.; Estrela, P. A novel immobilization strategy for electrochemical detection of cancer biomarkers: DNA-directed immobilization of aptamer sensors for sensitive detection of prostate specific antigens. Analyst 2015, 140, 2628–2633. [Google Scholar] [CrossRef]
- Tort, N.; Salvador, J.-P.; Marco, M.-P. Multimodal plasmonic biosensing nanostructures prepared by DNA-directed immobilization of multifunctional DNA-gold nanoparticles. Biosens. Bioelectron. 2017, 90, 13–22. [Google Scholar] [CrossRef]
- Deka, J.; Mojumdar, A.; Parisse, P.; Onesti, S.; Casalis, L. DNA-conjugated gold nanoparticles based colorimetric assay to assess helicase activity: A novel route to screen potential helicase inhibitors. Sci. Rep. 2017, 7, 44358. [Google Scholar] [CrossRef]
- Asbaghi, B.A.N.; Alsadig, A.; Cabrera, H. Online electrophoretic nanoanalysis using miniaturized gel electrophoresis and thermal lens microscopy detection. J. Chromatogr. A 2021, 1657, 462596. [Google Scholar] [CrossRef]
- Asbaghi, B.A.N.; Alsadig, A.; Casalis, L.; Parisse, P.; Niemela, J.; Bellucci, S.; Cabrera, H. An electrophoresis approach with online thermal lens detection to monitoring DNA surface coatings on gold nanoparticles. Microchem. J. 2022, 173, 106961. [Google Scholar] [CrossRef]
- Djender, S.; Schneider, A.; Beugnet, A.; Crepin, R.; Desrumeaux, K.E.; Romani, C.; Moutel, S.; Perez, F.; de Marco, A. Bacterial cytoplasm as an effective cell compartment for producing functional VHH-based affinity reagents and Camelidae IgG-like recombinant antibodies. Microb. Cell Factories 2014, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.A.; Fortuna, S.; De Marco, A.; Laio, A. Binding affinity prediction of nanobody–protein complexes by scoring of molecular dynamics trajectories. Phys. Chem. Chem. Phys. 2018, 20, 3438–3444. [Google Scholar] [CrossRef]
- Soler, M.A.; Medagli, B.; Semrau, M.S.; Storici, P.; Bajc, G.; De Marco, A.; Laio, A.; Fortuna, S. A consensus protocol for the in silico optimisation of antibody fragments. Chem. Commun. 2019, 55, 14043–14046. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Alsadig, A.; Vondracek, H.; Pengo, P.; Pasquato, L.; Posocco, P.; Parisse, P.; Casalis, L. Label-Free, Rapid and Facile Gold-Nanoparticles-Based Assay as a Potential Spectroscopic Tool for Trastuzumab Quantification. Nanomaterials 2021, 11, 3181. [Google Scholar] [CrossRef]
- Podesta, A.; Imperadori, L.; Colnaghi, W.; Finzi, L.; Milani, P.; Dunlap, D. Atomic force microscopy study of DNA deposited on poly L-ornithine-coated mica. J. Microsc. 2004, 215, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Deka, J.; Mech, R.; Ianeselli, L.; Amenitsch, H.; Cacho-Nerin, F.; Parisse, P.; Casalis, L. Surface passivation improves the synthesis of highly stable and specific DNA-functionalized gold nanoparticles with variable DNA density. ACS Appl. Mater. Interfaces 2015, 7, 7033–7040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, Z.; Liu, D. DNA discrete modified gold nanoparticles. Nanoscale 2011, 3, 4015–4021. [Google Scholar] [CrossRef] [PubMed]
- Ravan, H.; Kashanian, S.; Sanadgol, N.; Badoei-Dalfard, A.; Karami, Z. Strategies for optimizing DNA hybridization on surfaces. Anal. Biochem. 2014, 444, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Seferos, D.S.; Prigodich, A.E.; Giljohann, D.A.; Patel, P.C.; Mirkin, C.A. Polyvalent DNA nanoparticle conjugates stabilize nucleic acids. Nano Lett. 2009, 9, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.M.; Dabrowiak, J.C.; Maye, M.M. Investigation of the drug binding properties and cytotoxicity of DNA-capped nanoparticles designed as delivery vehicles for the anticancer agents doxorubicin and actinomycin D. Bioconjugate Chem. 2012, 23, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Guo, Y.; Roebuck, D.; Chen, C.; Yang, M.; Yang, Z.; Sreedharan, S.; Glover, C.; Thomas, J.A.; Liu, D. Terminal PEGylated DNA–gold nanoparticle conjugates offering high resistance to nuclease degradation and efficient intracellular delivery of DNA binding agents. ACS Appl. Mater. Interfaces 2015, 7, 18707–18716. [Google Scholar] [CrossRef]
- Ravelli, A.; Reuben, J.M.; Lanza, F.; Anfossi, S.; Cappelletti, M.R.; Zanotti, L.; Gobbi, A.; Senti, C.; Brambilla, P.; Milani, M. Breast cancer circulating biomarkers: Advantages, drawbacks, and new insights. Tumor Biol. 2015, 36, 6653–6665. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.N.; Mathur, R.; Farooque, A.; Verma, A.; Dwarakanath, B. Cancer biomarkers-current perspectives. Indian J. Med. Res. 2010, 132, 129–149. [Google Scholar] [PubMed]
- Tsé, C.; Gauchez, A.-S.; Jacot, W.; Lamy, P.-J. HER2 shedding and serum HER2 extracellular domain: Biology and clinical utility in breast cancer. Cancer Treat. Rev. 2012, 38, 133–142. [Google Scholar] [CrossRef]
- Lam, L.; McAndrew, N.; Yee, M.; Fu, T.; Tchou, J.C.; Zhang, H. Challenges in the clinical utility of the serum test for HER2 ECD. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2012, 1826, 199–208. [Google Scholar] [CrossRef]
- Tafe, L.J.; Tsongalis, G.J. The human epidermal growth factor receptor 2 (HER2). Clin. Chem. Lab. Med. 2012, 50, 23–30. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.a.; Robinson, G.; Hammers, C.; Songa, E.B.; Bendahman, N.; Hammers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Revets, H.; De Baetselier, P.; Muyldermans, S. Nanobodies as novel agents for cancer therapy. Expert Opin. Biol. Ther. 2005, 5, 111–124. [Google Scholar] [CrossRef]
- Van de Broek, B.; Devoogdt, N.; D’Hollander, A.; Gijs, H.-L.; Jans, K.; Lagae, L.; Muyldermans, S.; Maes, G.; Borghs, G. Specific cell targeting with nanobody conjugated branched gold nanoparticles for photothermal therapy. ACS Nano 2011, 5, 4319–4328. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh-Ghassabeh, G.; Devoogdt, N.; De Pauw, P.; Vincke, C.; Muyldermans, S. Nanobodies and their potential applications. Nanomedicine 2013, 8, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, H.; Korte, D.; Franko, M. Mode-mismatched confocal thermal-lens microscope with collimated probe beam. Rev. Sci. Instrum. 2015, 86, 053701. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, H.; Goljat, L.; Korte, D.; Marín, E.; Franko, M. A multi-thermal-lens approach to evaluation of multi-pass probe beam configuration in thermal lens spectrometry. Anal. Chim. Acta 2020, 1100, 182–190. [Google Scholar] [CrossRef]
- Patris, S.; De Pauw, P.; Vandeput, M.; Huet, J.; Van Antwerpen, P.; Muyldermans, S.; Kauffmann, J.-M. Nanoimmunoassay onto a screen printed electrode for HER2 breast cancer biomarker determination. Talanta 2014, 130, 164–170. [Google Scholar] [CrossRef]
- Ravalli, A.; da Rocha, C.G.; Yamanaka, H.; Marrazza, G. A label-free electrochemical affisensor for cancer marker detection: The case of HER2. Bioelectrochemistry 2015, 106, 268–275. [Google Scholar] [CrossRef]
- Emami, M.; Shamsipur, M.; Saber, R.; Irajirad, R. An electrochemical immunosensor for detection of a breast cancer biomarker based on antiHER2–iron oxide nanoparticle bioconjugates. Analyst 2014, 139, 2858–2866. [Google Scholar] [CrossRef]
- Al-Khafaji, Q.; Harris, M.; Tombelli, S.; Laschi, S.; Turner, A.; Mascini, M.; Marrazza, G. An electrochemical immunoassay for HER2 detection. Electroanalysis 2012, 24, 735–742. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsadig, A.; Abbasgholi-NA, B.; Vondracek, H.; Medagli, B.; Fortuna, S.; Posocco, P.; Parisse, P.; Cabrera, H.; Casalis, L. DNA-Directed Protein Anchoring on Oligo/Alkanethiol-Coated Gold Nanoparticles: A Versatile Platform for Biosensing Applications. Nanomaterials 2023, 13, 78. https://doi.org/10.3390/nano13010078
Alsadig A, Abbasgholi-NA B, Vondracek H, Medagli B, Fortuna S, Posocco P, Parisse P, Cabrera H, Casalis L. DNA-Directed Protein Anchoring on Oligo/Alkanethiol-Coated Gold Nanoparticles: A Versatile Platform for Biosensing Applications. Nanomaterials. 2023; 13(1):78. https://doi.org/10.3390/nano13010078
Chicago/Turabian StyleAlsadig, Ahmed, Behnaz Abbasgholi-NA, Hendrik Vondracek, Barbara Medagli, Sara Fortuna, Paola Posocco, Pietro Parisse, Humberto Cabrera, and Loredana Casalis. 2023. "DNA-Directed Protein Anchoring on Oligo/Alkanethiol-Coated Gold Nanoparticles: A Versatile Platform for Biosensing Applications" Nanomaterials 13, no. 1: 78. https://doi.org/10.3390/nano13010078
APA StyleAlsadig, A., Abbasgholi-NA, B., Vondracek, H., Medagli, B., Fortuna, S., Posocco, P., Parisse, P., Cabrera, H., & Casalis, L. (2023). DNA-Directed Protein Anchoring on Oligo/Alkanethiol-Coated Gold Nanoparticles: A Versatile Platform for Biosensing Applications. Nanomaterials, 13(1), 78. https://doi.org/10.3390/nano13010078






