Elaboration of Silicon Nanostructures with Vapor-Phase Silver Assisted Chemical Etching: Correlation between Structural and Optical Properties
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
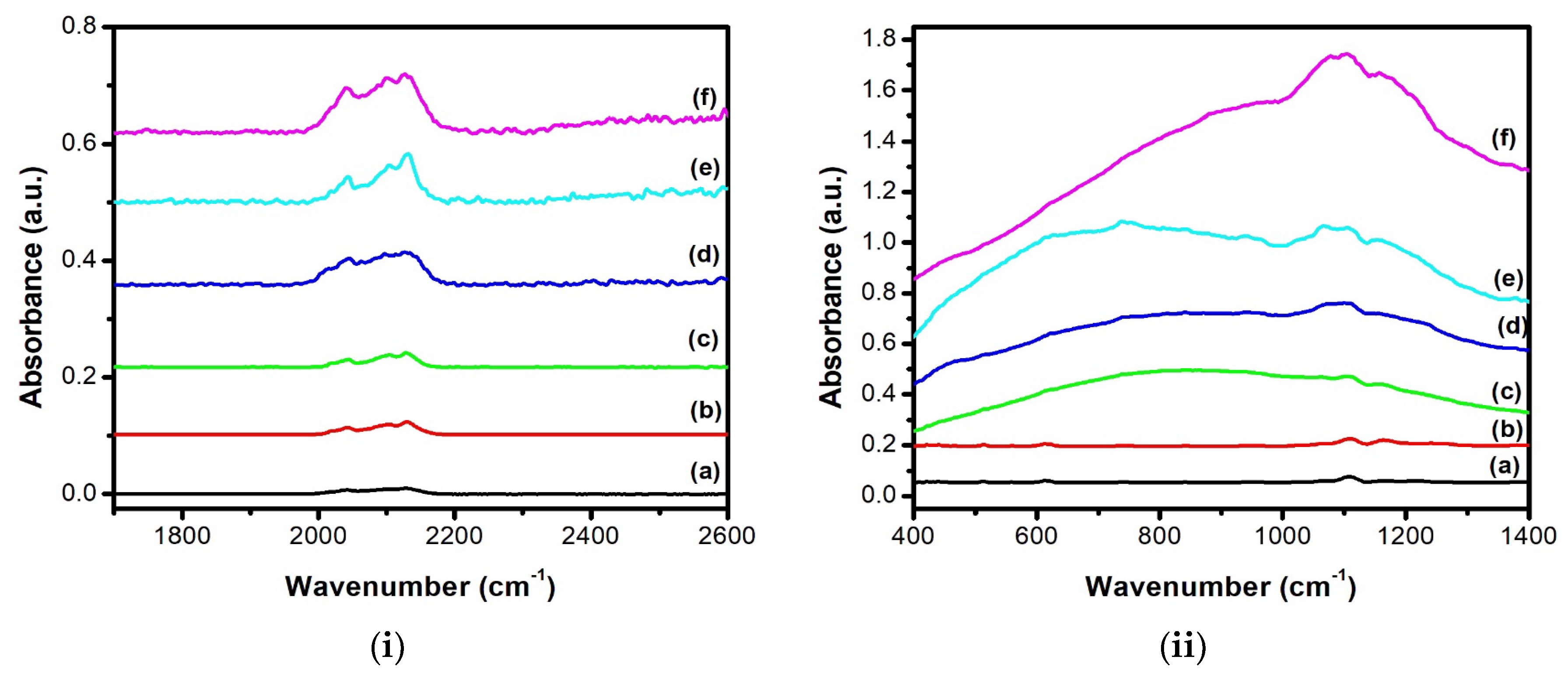
3.1. SEM Analysis
3.2. Total Reflectivity
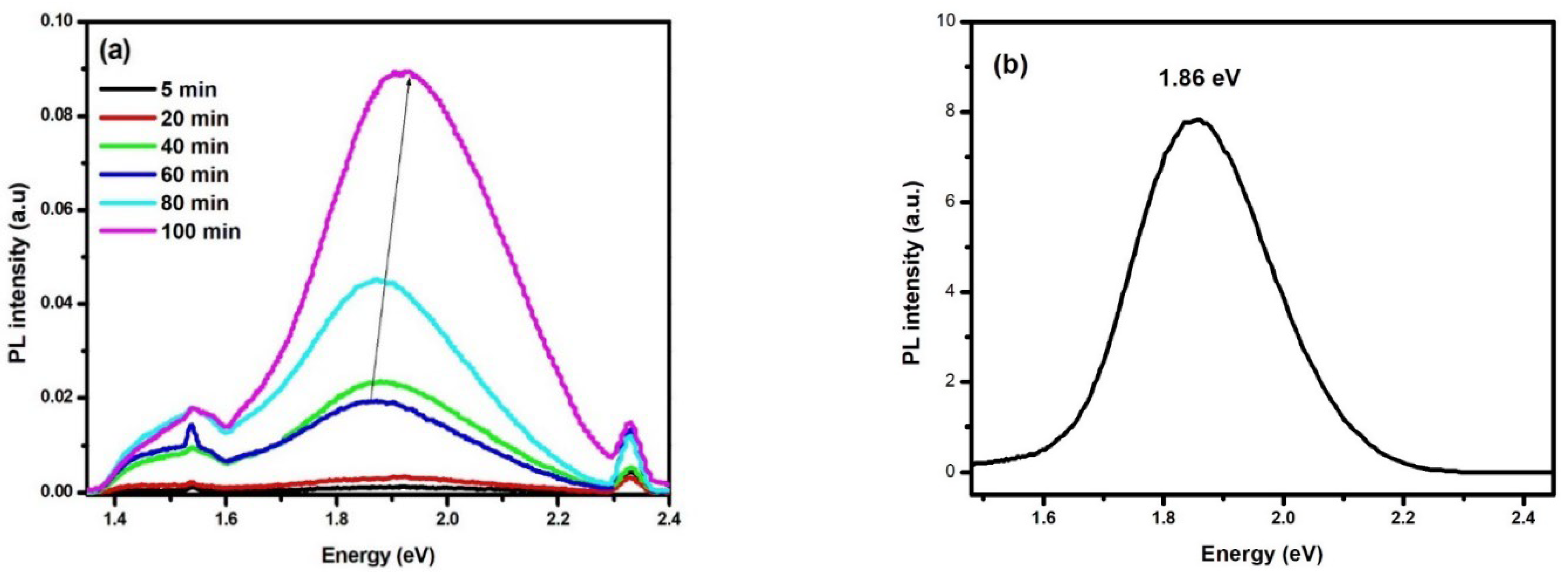
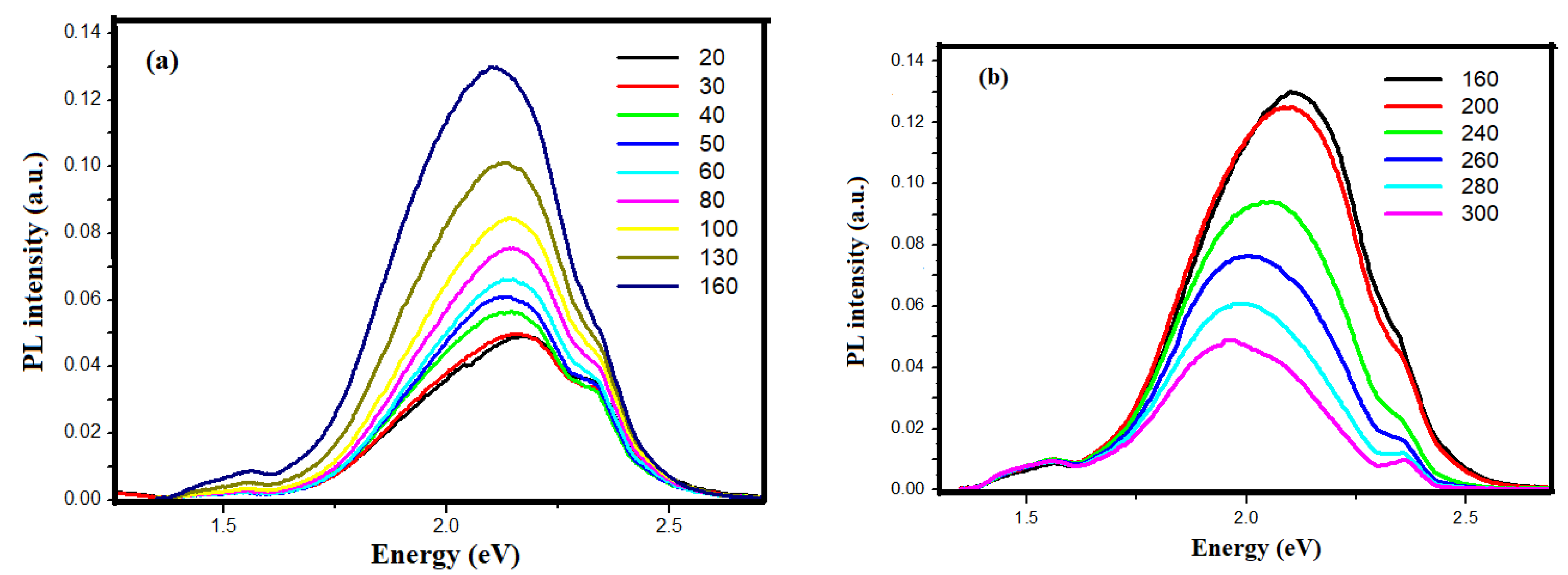
3.3. PL Spectroscopic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canham, L.T. Silicon quantum wire array fabrication by electrochemical and chemical dissolution of wafers. Appl. Phys. Lett. 1990, 57, 1046–1048. [Google Scholar] [CrossRef]
- Lo Faro, M.J.; Leonardi, A.A.; Priolo, F.; Fazio, B.; Miritello, M.; Irrera, A. Erbium Emission in Er:Y2O3 Decorated Fractal Arrays of Silicon Nanowires. Sci. Rep. 2020, 10, 12854. [Google Scholar] [CrossRef]
- Najar, A.; Charrier, J.; Ajlani, H.; Lorrain, N.; Haesaert, S.; Oueslati, M.; Haji, L. Optical gain at 1.53 μm in Er3+–Yb3+ co-doped porous silicon waveguides. Mater. Sci. Eng. B 2008, 146, 260–263. [Google Scholar] [CrossRef]
- Seredin, P.V.; Goloshchapov, D.L.; Lenshin, A.S.; Kasatkin, I.A. Effect of combination of etching modes on the design, structural and optical properties of the compliant substrates based on porous silicon. Opt. Mater. 2021, 119, 111358. [Google Scholar] [CrossRef]
- Amri, C.; Ouertani, R.; Hamdi, A.; Ezzaouia, H. Effect of acid vapor etching on morphological and opto-electric properties of flat silicon and silicon nanowire arrays: A comparative study. Superlattices Microstruct. 2016, 91, 278–289. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Lo Faro, M.J.; Irrera, A. Silicon Nanowires Synthesis by Metal-Assisted Chemical Etching: A Review. Nanomaterials 2021, 11, 383. [Google Scholar] [CrossRef] [PubMed]
- Gonchar, K.A.; Moiseev, D.V.; Bozhev, I.V.; Osminkina, L.A. Influence of H2O2 concentration on the structural and photoluminescent properties of porous silicon nanowires fabricated by metal-assisted chemical etching. Mater. Sci. Semicond. Process. 2021, 125, 105644. [Google Scholar] [CrossRef]
- Hildreth, O.J.; Schmidt, D.R. Vapor Phase Metal-Assisted Chemical Etching of Silicon. Adv. Funct. Mater. 2014, 24, 3827–3833. [Google Scholar] [CrossRef]
- Amri, C.; Ouertani, R.; Hamdi, A.; Ezzaouia, H. Effect of Silver-Assisted Chemical Vapor Etching on morphological properties and silicon solar cell performance. Mater. Sci. Semicond. Process. 2017, 63, 176–183. [Google Scholar] [CrossRef]
- Kubota, W.; Yamaoka, R.; Utsunomiya, T.; Ichii, T.; Sugimura, H. Vapor-Phase Chemical Etching of Silicon Assisted by Graphene Oxide for Microfabrication and Microcontact Printing. ACS Appl. Nano Mater. 2022, 5, 11707–11714. [Google Scholar] [CrossRef]
- Lo Faro, M.J.; Leonardi, A.A.; D’Andrea, C.; Morganti, D.; Musumeci, P.; Vasi, C.; Priolo, F.; Fazio, B.; Irrera, A. Low Cost Synthesis of Silicon Nanowires for Photonic Applications. J. Mater. Sci. Mater. Electron. 2020, 31, 34–40. [Google Scholar] [CrossRef]
- Najar, A.; Charrier, J.; Lorrain, N.; Haji, L.; Oueslati, M. Optical gain measurements in porous silicon planar waveguides codoped by erbium and ytterbium ions at 1.53 μm. Appl. Phys. Lett. 2007, 91, 121120. [Google Scholar] [CrossRef]
- Cullis, A.G.; Canham, L.T.; Calcott, P.D.J. The structural and luminescence properties of porous silicon. J. Appl. Phys. 1997, 82, 909–965. [Google Scholar] [CrossRef]
- Kanemitsu, Y. Light emission from porous silicon and related materials. Phys. Rep. 1995, 263, 1–91. [Google Scholar] [CrossRef]
- Amri, C.; Ouertani, R.; Hamdi, A.; Chtourou, R.; Ezzaouia, H. Effect of porous layer engineered with acid vapor etching on optical properties of solid silicon nanowire arrays. Mater. Des. 2016, 111, 394–404. [Google Scholar] [CrossRef]
- de la Mora, M.B.; Bornacelli, J.; Nava, R.; Zanella, R.; Reyes-Esqueda, J.A. Porous silicon photoluminescence modification by colloidal gold nanoparticles: Plasmonic, surface and porosity roles. J. Lumin. 2014, 146, 247–255. [Google Scholar] [CrossRef]
- Canham, L. Routes of Formation for Porous Silicon. In Handbook of Porous Silicon; Canham, L., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 3–11. [Google Scholar]
- Sohn, H. Refractive Index of Porous Silicon. In Handbook of Porous Silicon; Canham, L., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 231–243. [Google Scholar]
- Uddin, S.; Hashim, M.R.; Pakhuruddin, M.Z. Broadband light absorption enhancement in nanoporous black silicon synthesized by aluminium-catalyzed chemical etching. Opt. Mater. 2022, 134, 113111. [Google Scholar] [CrossRef]
- Gautier, G.; Defforge, T.; Desplobain, S.; Billoué, J.; Capelle, M.; Povéda, P.; Vanga, K.; Lu, B.; Bardet, B.; Lascaud, J.; et al. Porous Silicon in Microelectronics: From Academic Studies to Industry. ECS Trans. 2015, 69, 123. [Google Scholar] [CrossRef]
- Amri, C.; Ezzaouia, H.; Ouertani, R. Photoluminescence origin of lightly doped silicon nanowires treated with acid vapor etching. Chin. J. Phys. 2020, 63, 325–336. [Google Scholar] [CrossRef]
- He, H.; Liu, C.; Sun, L.; Ye, Z. Temperature-dependent photoluminescence properties of porous silicon nanowire arrays. Appl. Phys. Lett. 2011, 99, 123106. [Google Scholar] [CrossRef]
- Lin, L.H.; Sun, X.Z.; Tao, R.; Li, Z.C.; Feng, J.Y.; Zhang, Z.J. Photoluminescence origins of the porous silicon nanowire arrays. J. Appl. Phys. 2011, 110, 073109. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Liu, Z.; Zheng, J.; Xue, C.; Zuo, Y.; Cheng, B.; Wang, Q. Enhanced photoluminescence from porous silicon nanowire arrays. Nanoscale Res. Lett. 2013, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.; Bandyopadhyay, N.R.; Ghanta, U.; Klie, R.F.; Pramanick, A.K.; Das, S.; Ray, S.K.; Hossain, S.M. Temperature dependent photoluminescence from porous silicon nanostructures: Quantum confinement and oxide related transitions. J. Appl. Phys. 2011, 110, 094309. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Smyntyna, V.; Pavlenko, N.; Sviridova, O. Peculiarities of Photoluminescence in Porous Silicon Prepared by Metal-Assisted Chemical Etching. ISRN Opt. 2012, 2012, 958412. [Google Scholar] [CrossRef]
- Parida, B.; Choi, J.; Lim, G.; Park, S.; Kim, K. Formation of Nanotextured Surfaces on Microtextured Si Solar Cells by Metal-Assisted Chemical Etching Process. J. Nanosci. Nanotechnol. 2014, 14, 9224–9231. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, J.W.; Leem, J.Y.; Kim, J.S.; Kang, S.K.; Son, J.S.; Kang, H.B.; Mun, Y.H.; Lee, D.K.; Kim, D.H.; et al. Strong and stable red photoluminescence from porous silicon prepared by Fe-contaminated silicon. J. Cryst. Growth 2004, 260, 394–399. [Google Scholar] [CrossRef]
- Podhorodecki, A.; Zatryb, G.; Misiewicz, J.; Gourbilleau, F.; Dufour, C. Temperature Dependent Emission Quenching for Silicon Nanoclusters. J. Nanosci. Nanotechnol. 2010, 10, 5648–5652. [Google Scholar] [CrossRef]
- Ghosh, R.; Giri, P.K.; Imakita, K.; Fujii, M. Origin of visible and near-infrared photoluminescence from chemically etched Si nanowires decorated with arbitrarily shaped Si nanocrystals. Nanotechnology 2014, 25, 045703. [Google Scholar] [CrossRef]
- Prokes, S.M.; Carlos, W.E.; Glembocki, O.J. Defect-based model for room-temperature visible photoluminescence in porous silicon. Phys. Rev. B 1994, 50, 17093–17096. [Google Scholar] [CrossRef]
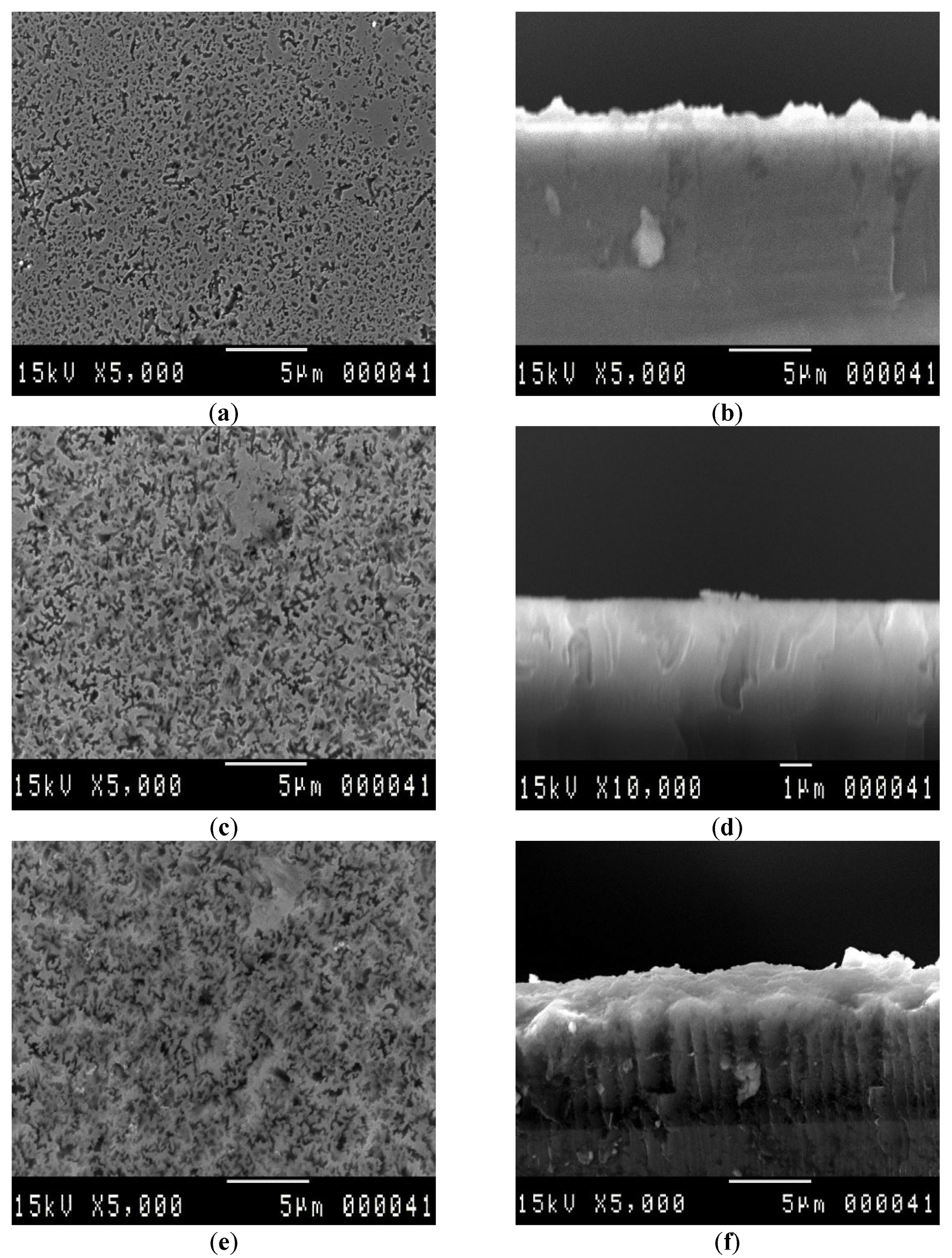
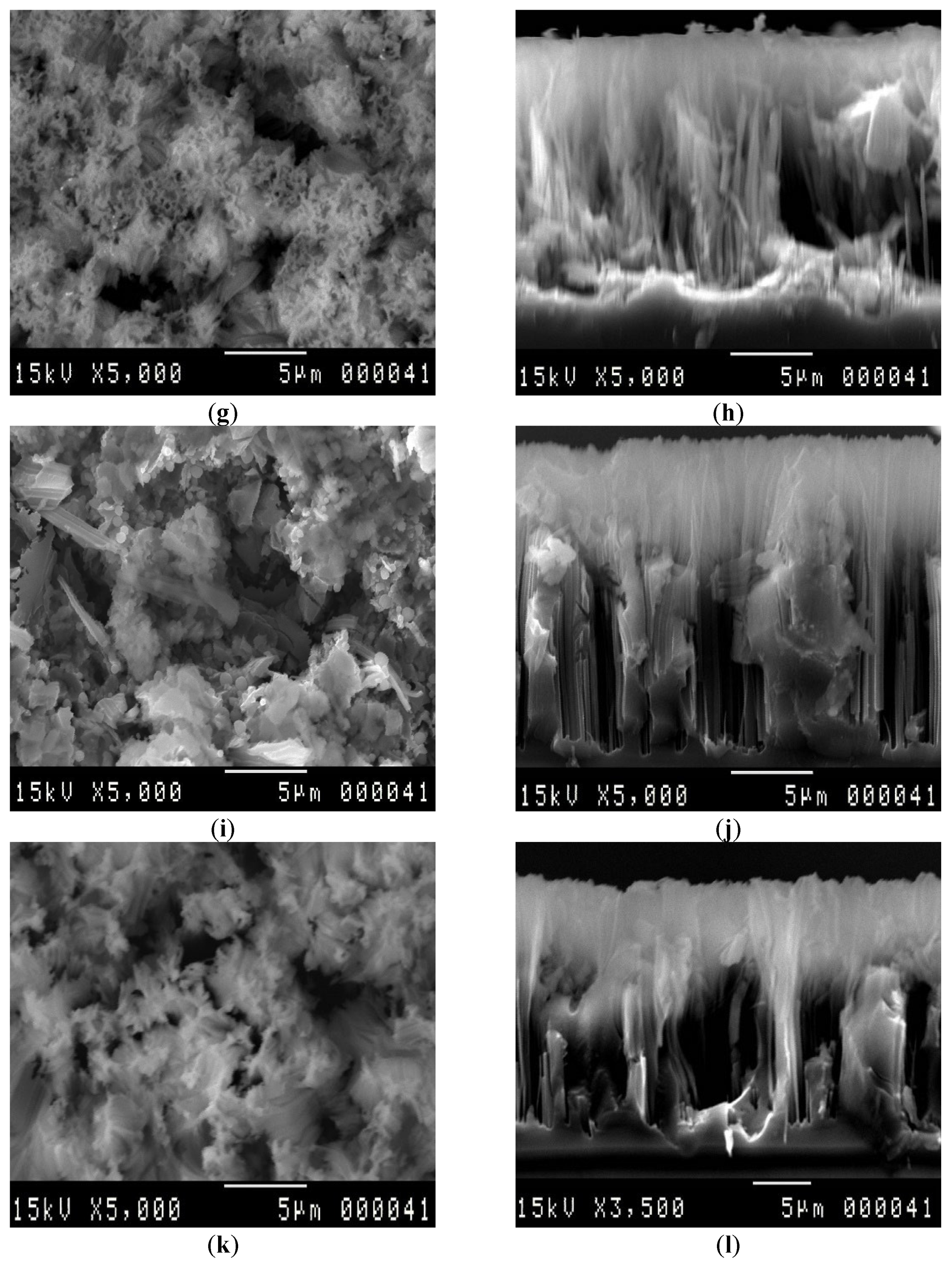
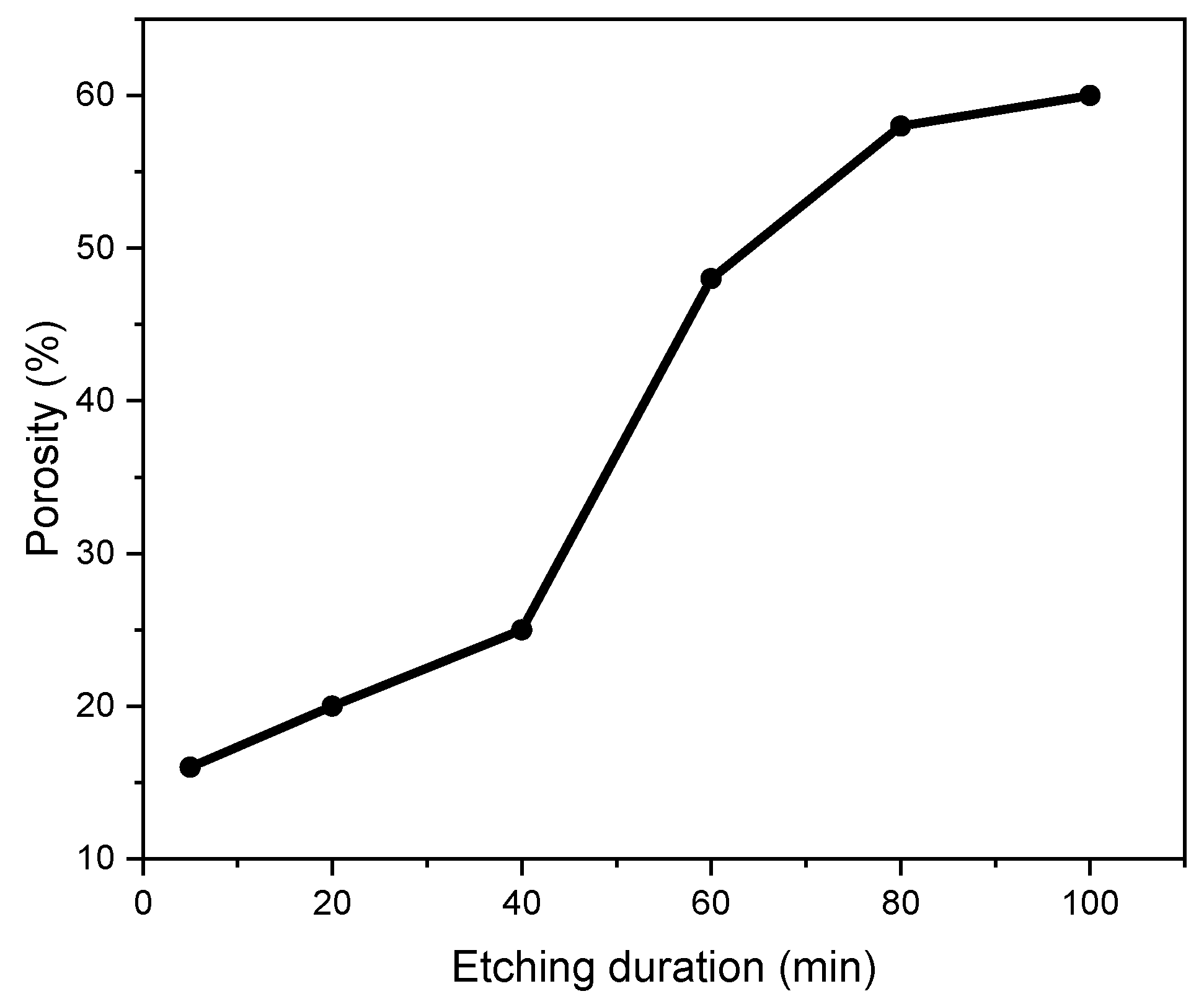

| Etching Duration (min) | Pore Diameter (µm) | Pore Thickness (µm) | LER (µm/min) | VER (µm/min) |
|---|---|---|---|---|
| 5 | 0.5 | 0.5 | 0.1 | 0.1 |
| 20 | 1 | 3 | 0.05 | 0.15 |
| 40 | 1.5 | 7 | 0.0375 | 0.175 |
| 60 | 3 | 12 | 0.05 | 0.2 |
| 80 | 4 | 15 | 0.05 | 0.187 |
| 100 | 5 | 18 | 0.05 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amri, C.; Liu, S.; Najar, A. Elaboration of Silicon Nanostructures with Vapor-Phase Silver Assisted Chemical Etching: Correlation between Structural and Optical Properties. Nanomaterials 2023, 13, 1602. https://doi.org/10.3390/nano13101602
Amri C, Liu S, Najar A. Elaboration of Silicon Nanostructures with Vapor-Phase Silver Assisted Chemical Etching: Correlation between Structural and Optical Properties. Nanomaterials. 2023; 13(10):1602. https://doi.org/10.3390/nano13101602
Chicago/Turabian StyleAmri, Chohdi, Shengzhong (Frank) Liu, and Adel Najar. 2023. "Elaboration of Silicon Nanostructures with Vapor-Phase Silver Assisted Chemical Etching: Correlation between Structural and Optical Properties" Nanomaterials 13, no. 10: 1602. https://doi.org/10.3390/nano13101602





