Construction of an Amethyst-like MoS2@Ni9S8/Co3S4 Rod Electrocatalyst for Overall Water Splitting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the ZIF-67/NF Precursor
2.3. Synthesis of Amethyst-like MoS2@Ni9S8/Co3S4 Rods
2.4. Material Characterization
2.5. Electrochemical Measurements
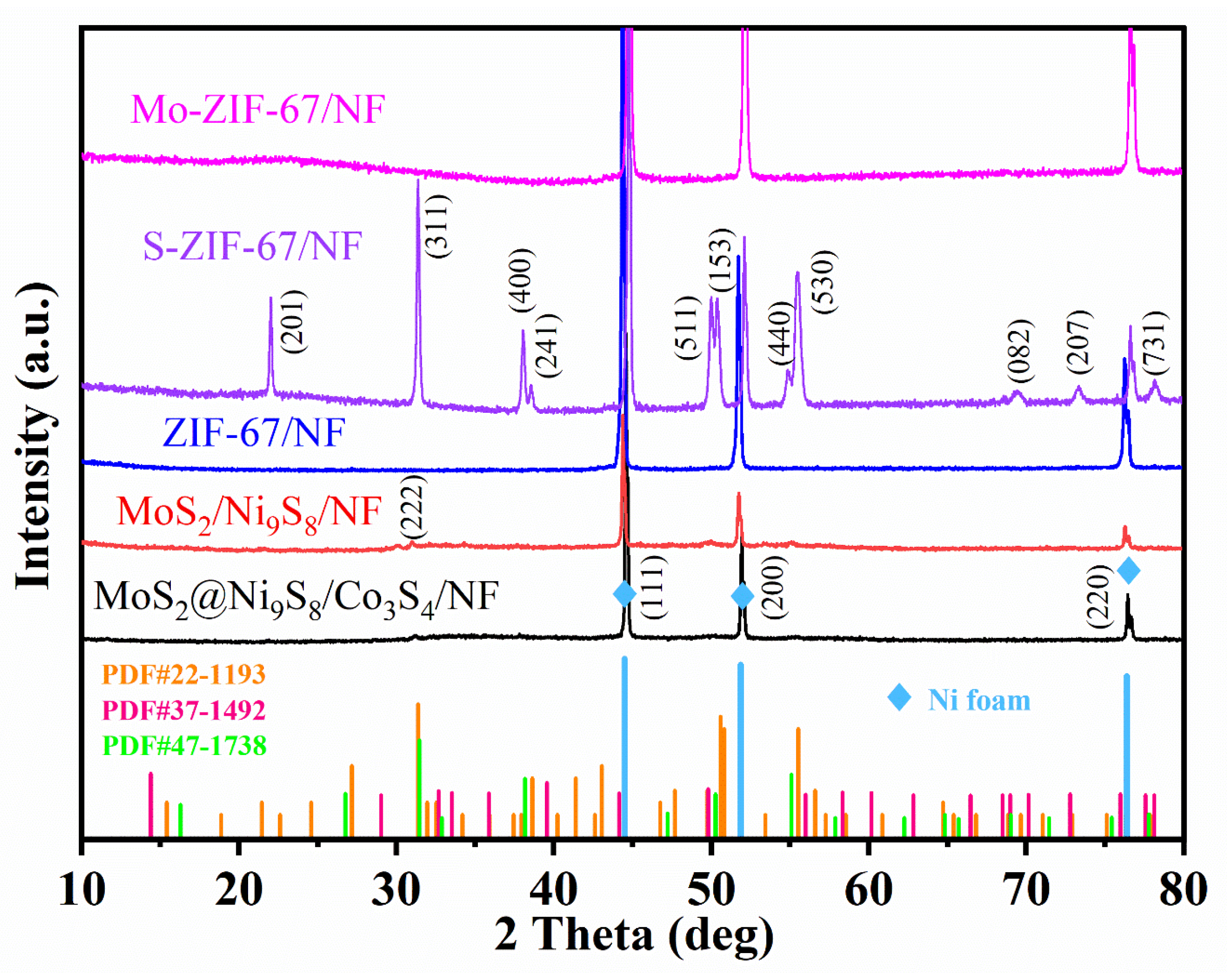
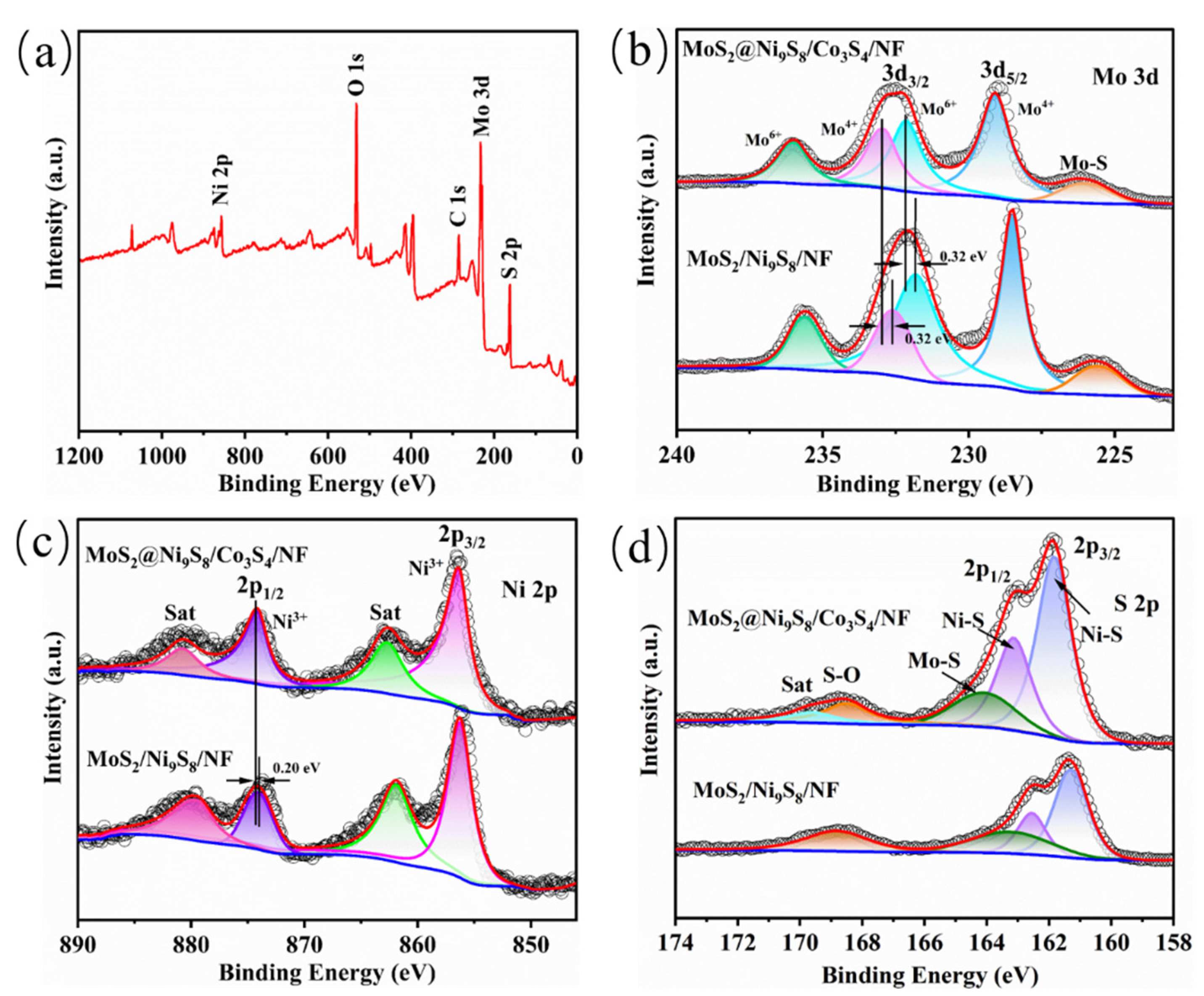
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; An, Y.; Guo, K.; Ren, X.; Jiang, B. Nitrogen doped FeCoNiS nanoparticles on N, S-co-doped vertical graphene as bifunctional electrocatalyst for water splitting. Int. J. Hydrogen Energy 2023, 48, 4143–4157. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef]
- Huang, C.Q.; Yu, L.; Zhang, W.; Xiao, Q.; Zhou, J.Q.; Zhang, Y.L.; An, P.F.; Zhang, J.; Yu, Y. N-doped Ni-Mo based sulfides for high-efficiency and stable hydrogen evolution reaction. Appl. Catal. B Environ. 2020, 276, 119137. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Rui, Y.; Wang, R.; Li, X. Recent advances in non-precious metal electrocatalysts for pH-universal hydrogen evolution reaction. Green Energy Environ. 2021, 6, 458–478. [Google Scholar] [CrossRef]
- El-Refaei, S.M.; Russo, P.A.; Pinna, N. Recent Advances in Multimetal and Doped Transition-Metal Phosphides for the Hydrogen Evolution Reaction at Different pH values. ACS Appl. Mater. Interfaces 2021, 13, 22077–22097. [Google Scholar] [CrossRef]
- Chang, Z.; Zhu, L.; Zhao, J.; Chen, P.; Chen, D.; Gao, H. NiMo/Cu-nanosheets/Ni-foam composite as a high performance electrocatalyst for hydrogen evolution over a wide pH range. Int. J. Hydrogen Energy 2021, 46, 3493–3503. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. Metal Sulfide Photocatalysts for Hydrogen Generation: A Review of Recent Advances. Catalysts 2022, 12, 1316. [Google Scholar] [CrossRef]
- Qian, L.-H.; Dong, W.-W.; Li, H.-B.; Song, X.; Ding, Y. Fe-doped NiCo2S4 catalyst derived from ZIF-67 towards efficient hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 8820–8828. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Duan, Y.; Feng, X.Y.; Yu, X.; Gao, M.R.; Yu, S.H. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater 2021, 33, 2007100. [Google Scholar] [CrossRef]
- Guo, Y.; Park, T.; Yi, J.W.; Henzie, J.; Kim, J.; Wang, Z.; Jiang, B.; Bando, Y.; Sugahara, Y.; Tang, J.; et al. Nanoarchitectonics for Transition-Metal-Sulfide-Based Electrocatalysts for Water Splitting. Adv. Mater. 2019, 31, 1807134. [Google Scholar] [CrossRef]
- Yu, F.; Yu, L.; Mishra, I.K.; Yu, Y.; Ren, Z.F.; Zhou, H.Q. Recent developments in earth-abundant and non-noble electrocatalysts for water electrolysis. Mater. Today Phys. 2018, 7, 121–138. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Huang, Y.; Fu, J. Recent advances in electrocatalysts for neutral and large-current-density water electrolysis. Nano Energy 2021, 80, 105545. [Google Scholar] [CrossRef]
- Peng, W.; Zheng, G.; Wang, Y.; Cao, S.; Ji, Z.; Huan, Y.; Zou, M.; Yan, X. Zn doped ZIF67-derived porous carbon framework as efficient bifunctional electrocatalyst for water splitting. Int. J. Hydrogen Energy 2019, 44, 19782–19791. [Google Scholar] [CrossRef]
- Li, Y.; Peng, C.K.; Hu, H.; Chen, S.Y.; Choi, J.H.; Lin, Y.G.; Lee, J.M. Interstitial boron-triggered electron-deficient Os aerogels for enhanced pH-universal hydrogen evolution. Nat. Commun. 2022, 13, 1143. [Google Scholar] [CrossRef]
- Pi, C.; Huang, C.; Yang, Y.; Song, H.; Zhang, X.; Zheng, Y.; Gao, B.; Fu, J.; Chu, P.K.; Huo, K. In situ formation of N-doped carbon-coated porous MoP nanowires: A highly efficient electrocatalyst for hydrogen evolution reaction in a wide pH range. Appl. Catal. B Environ. 2020, 263, 118358. [Google Scholar] [CrossRef]
- Shao, L.; Qian, X.; Wang, X.; Li, H.; Yan, R.; Hou, L. Low-cost and highly efficient CoMoS4/NiMoS4-based electrocatalysts for hydrogen evolution reactions over a wide pH range. Electrochim. Acta 2016, 213, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.H.; Yang, Y.C.; Lin, J.W.; Lin, Y.C.; Chen, P.T.; Dong, C.L.; Lin, H.M.; Chan, K.M.; Kao, Y.T.; Suenaga, K.; et al. Bifunctional Monolayer WSe2/Graphene Self-Stitching Heterojunction Microreactors for Efficient Overall Water Splitting in Neutral Medium. ACS Nano 2022, 16, 18274–18283. [Google Scholar] [CrossRef]
- Wei, D.; Tang, W.; Wang, Y. Hairy sphere-like Ni9S8/CuS/Cu2O composites grown on nickel foam as bifunctional electrocatalysts for hydrogen evolution and urea electrooxidation. Int. J. Hydrogen Energy 2021, 46, 20950–20960. [Google Scholar] [CrossRef]
- Pi, Y.; Shao, Q.; Wang, P.; Guo, J.; Huang, X. General Formation of Monodisperse IrM (M = Ni, Co, Fe) Bimetallic Nanoclusters as Bifunctional Electrocatalysts for Acidic Overall Water Splitting. Adv. Funct. Mater. 2017, 27, 1700886. [Google Scholar] [CrossRef]
- Zhao, D.; Dai, M.; Liu, H.; Chen, K.; Zhu, X.; Xue, D.; Wu, X.; Liu, J. Sulfur-Induced Interface Engineering of Hybrid NiCo2O4@NiMo2S4 Structure for Overall Water Splitting and Flexible Hybrid Energy Storage. Adv. Mater. Interfaces 2019, 6, 1901308. [Google Scholar] [CrossRef]
- Tian, R.; Wang, F.; Zou, C.; Pei, Z.; Guo, X.; Yang, H. Modulating organic ligands to construct 2D–3D-hybrid porous P-doped metal-organic frameworks electrocatalyst for overall water splitting. J. Alloys Compd. 2023, 933, 167670. [Google Scholar] [CrossRef]
- Wang, F.; Guo, X.; He, F.; Hou, Y.; Liu, F.; Zou, C.; Yang, H. Binder free construction of hollow hierarchical Mn–Co–P nanoarrays on nickel foam as an efficient bifunctional electrocatalyst for overall water splitting. Sustain. Energy Fuels 2022, 6, 851–860. [Google Scholar] [CrossRef]
- Su, H.; Du, X.; Zhang, X. NiCoP coated on NiCo2S4 nanoarrays as electrode materials for hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 30910–30916. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, L.; Jin, Q.; Yun, J.; Liang, X. MoS2–Co3S4 hollow polyhedrons derived from ZIF-67 towards hydrogen evolution reaction and hydrodesulfurization. Int. J. Hydrogen Energy 2019, 44, 24246–24255. [Google Scholar] [CrossRef]
- Jayabal, S.; Saranya, G.; Wu, J.; Liu, Y.; Geng, D.; Meng, X. Understanding the high-electrocatalytic performance of two-dimensional MoS2 nanosheets and their composite materials. J. Mater. Chem. A 2017, 5, 24540–24563. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; He, Y.; Zhu, H. Recent advances in transition-metal-sulfide-based bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2021, 9, 5320–5363. [Google Scholar] [CrossRef]
- Chen, L.; Deng, Z.; Chen, Z.; Wang, X. Building Ni9S8/MoS2 Nanosheets Decorated NiMoO4 Nanorods Heterostructure for Enhanced Water Splitting. Adv. Mater. Interfaces 2021, 8, 2101483. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Zhang, J.; Wan, S.; Guo, S.; Lu, G.; Yao, J.; Huang, X. Precise tuning in platinum-nickel/nickel sulfide interface nanowires for synergistic hydrogen evolution catalysis. Nat. Commun. 2017, 8, 14580. [Google Scholar] [CrossRef]
- Wang, D.Y.; Gong, M.; Chou, H.L.; Pan, C.J.; Chen, H.A.; Wu, Y.; Lin, M.C.; Guan, M.; Yang, J.; Chen, C.W.; et al. Highly active and stable hybrid catalyst of cobalt-doped FeS2 nanosheets-carbon nanotubes for hydrogen evolution reaction. J. Am. Chem. Soc. 2015, 137, 1587–1592. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, H.; Yu, Z.; Islam, S.M.; He, H.; Yuan, M.; Yue, Y.; Xu, K.; Hao, W.; Sun, G.; et al. Hierarchical Nanoassembly of MoS2/Co9S8/Ni3S2/Ni as a Highly Efficient Electrocatalyst for Overall Water Splitting in a Wide pH Range. J. Am. Chem. Soc. 2019, 141, 10417–10430. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Song, B.-Y. A Novel Phosphide Derived From Metal-Organic Frameworks as Cost-Effective Electrocatalyst for Oxygen Evolution Reaction. J. Electrochem. Energy Convers. Storage 2022, 19, 010907. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Bandal, H.A.; Ramakrishna, S.; Kim, H. Critical Review, Recent Updates on Zeolitic Imidazolate Framework-67 (ZIF-67) and Its Derivatives for Electrochemical Water Splitting. Adv. Mater. 2022, 34, e2107072. [Google Scholar] [CrossRef]
- Wu, Q.; Dong, A.; Yang, C.; Ye, L.; Zhao, L.; Jiang, Q. Metal-organic framework derived Co3O4@Mo-Co3S4-Ni3S2 heterostructure supported on Ni foam for overall water splitting. Chem. Eng. J. 2021, 413, 127482. [Google Scholar] [CrossRef]
- Zhao, L.; Gong, C.; Chen, X.; He, X.; Chen, H.; Du, X.; Wang, D.; Fang, W.; Zhang, H.; Li, W. ZIF-67 derived Mo-CoS2 nanoparticles embedded in hierarchically porous carbon hollow sphere for efficient overall water splitting. Appl. Surf. Sci. 2023, 623, 157030. [Google Scholar] [CrossRef]
- Chen, P.; Duan, X.; Li, G.; Qiu, X.; Wang, S.; Huang, Y.; Stavitskaya, A.; Jiang, H. Construction of ZIF-67/MIL-88(Fe, Ni) catalysts as a novel platform for efficient overall water splitting. Int. J. Hydrogen Energy 2023, 48, 7170–7180. [Google Scholar] [CrossRef]
- Wang, J.; Chaemchuen, S.; Chen, C.; Heynderickx, P.M.; Roy, S.; Verpoort, F. N-functionalized hierarchical carbon composite derived from ZIF-67 and carbon foam for efficient overall water splitting. J. Ind. Eng. Chem. 2022, 105, 222–230. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S.; Bu, X.; Dai, Y.; Wang, J.; Bao, X.; Wang, T. Hollow ZIF-67 derived porous cobalt sulfide as an efficient bifunctional electrocatalyst for overall water splitting. N. J. Chem. 2021, 45, 17313–17319. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Kong, X.; Liang, Y.; Li, Z.; Wu, S.; Chang, C.; Luo, S.; Cui, Z. ZIF-67 derived Co@NC/g-C3N4 as a photocatalyst for enhanced water splitting H(2) evolution. Environ. Res. 2021, 197, 111002. [Google Scholar] [CrossRef]
- Qin, J.F.; Xie, J.Y.; Wang, N.; Dong, B.; Chen, T.S.; Lin, Z.Y.; Liu, Z.Z.; Zhou, Y.N.; Yang, M.; Chai, Y.M. Surface construction of loose Co(OH)(2) shell derived from ZIF-67 nanocube for efficient oxygen evolution. J. Colloid. Interface Sci. 2020, 562, 279–286. [Google Scholar] [CrossRef]
- Liu, H.; Jin, M.; Zhan, D.; Wang, J.; Cai, X.; Qiu, Y.; Lai, L. Stacking faults triggered strain engineering of ZIF-67 derived Ni-Co bimetal phosphide for enhanced overall water splitting. Appl. Catal. B Environ. 2020, 272, 118951. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Qian, H.; Wang, Z.; Yamauchi, Y. One-Pot Synthesis of Zeolitic Imidazolate Framework 67-Derived Hollow Co3S4@MoS2 Heterostructures as Efficient Bifunctional Catalysts. Chem. Mater. 2017, 29, 5566–5573. [Google Scholar] [CrossRef]
- Peng, W.; Yang, X.; Mao, L.; Jin, J.; Yang, S.; Zhang, J.; Li, G. ZIF-67-derived Co nanoparticles anchored in N doped hollow carbon nanofibers as bifunctional oxygen electrocatalysts. Chem. Eng. J. 2021, 407, 127157. [Google Scholar] [CrossRef]
- Ma, F.; Jin, S.; Li, Y.; Feng, Y.; Tong, Y.; Ye, B.-C. Pyrolysis-derived materials of Mn-doped ZIF-67 for the electrochemical detection of o-nitrophenol. J. Electroanal. Chem. 2022, 904, 115932. [Google Scholar] [CrossRef]
- Jiao, L.; Zhou, Y.X.; Jiang, H.L. Metal-organic framework-based CoP/reduced graphene oxide: High-performance bifunctional electrocatalyst for overall water splitting. Chem. Sci. 2016, 7, 1690–1695. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.Y.; Feng, Y.; Jeon, Y.; Guan, B.; Lou, X.W.; Paik, U. Formation of Ni-Co-MoS(2) Nanoboxes with Enhanced Electrocatalytic Activity for Hydrogen Evolution. Adv. Mater. 2016, 28, 9006–9011. [Google Scholar] [CrossRef]
- Gao, H.; Zang, J.; Wang, Y.; Zhou, S.; Tian, P.; Song, S.; Tian, X.; Li, W. One-step preparation of cobalt-doped NiS@MoS2 core-shell nanorods as bifunctional electrocatalyst for overall water splitting. Electrochim. Acta 2021, 377, 138051. [Google Scholar] [CrossRef]
- Ge, Y.C.; Chu, H.; Chen, J.Y.; Zhuang, P.Y.; Feng, Q.Y.; Smith, W.R.; Dong, P.; Ye, M.X.; Shen, J.F. Ultrathin MoS2 Nanosheets Decorated Hollow CoP Heterostructures for Enhanced Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2019, 7, 10105–10111. [Google Scholar] [CrossRef]
- Wang, Z.C.; Xu, W.J.; Chen, X.K.; Peng, Y.H.; Song, Y.Y.; Lv, C.X.; Liu, H.L.; Sun, J.W.; Yuan, D.; Li, X.Y.; et al. Defect-Rich Nitrogen Doped Co3O4/C Porous Nanocubes Enable High-Efficiency Bifunctional Oxygen Electrocatalysis. Adv. Funct. Mater. 2019, 29, 1902875. [Google Scholar] [CrossRef]
- Kuang, P.; Tong, T.; Fan, K.; Yu, J. In Situ Fabrication of Ni–Mo Bimetal Sulfide Hybrid as an Efficient Electrocatalyst for Hydrogen Evolution over a Wide pH Range. ACS Catal. 2017, 7, 6179–6187. [Google Scholar] [CrossRef]
- Tan, X.; Duan, Z.; Liu, H.; Wu, X.; Cho, Y.-R. Core-shell structured MoS2/Ni9S8 electrocatalysts for high performance hydrogen and oxygen evolution reactions. Mater. Res. Bull. 2022, 146, 111626. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Li, J.; Cao, L.; Lu, Z.; Zhu, D. Structure Engineering of MoS2 via Simultaneous Oxygen and Phosphorus Incorporation for Improved Hydrogen Evolution. Small 2020, 16, e1905738. [Google Scholar] [CrossRef]
- Yan, Y.; Li, A.; Lu, C.; Zhai, T.; Lu, S.; Li, W.; Zhou, W. Double-layered yolk-shell microspheres with NiCo2S4-Ni9S8-C hetero-interfaces as advanced battery-type electrode for hybrid supercapacitors. Chem. Eng. J. 2020, 396, 125316. [Google Scholar] [CrossRef]
- Liu, F.; Guo, X.; Hou, Y.; Wang, F.; Zou, C.; Yang, H. Hydrothermal combined with electrodeposition construction of a stable Co9S8/Ni3S2@NiFe-LDH heterostructure electrocatalyst for overall water splitting. Sustain. Energy Fuels 2021, 5, 1429–1438. [Google Scholar] [CrossRef]
- Khani, H.; Wipf, D.O. Iron Oxide Nanosheets and Pulse-Electrodeposited Ni-Co-S Nanoflake Arrays for High-Performance Charge Storage. ACS Appl. Mater. Interfaces 2017, 9, 6967–6978. [Google Scholar] [CrossRef]
- Yang, Y.J.; Chen, S.; Jiang, C.; Wang, N.; Liu, M.; Yang, P.; Cheng, Y. Assembly of Co3S4 nanoporous structure on Ni foam for binder-free high-performance supercapacitor electrode. J. Solid State Electrochem. 2023, 27, 1107–1118. [Google Scholar] [CrossRef]
- Yuan, F.; Liu, Z.; Qin, G.; Ni, Y. Fe-Doped Co-Mo-S microtube: A highly efficient bifunctional electrocatalyst for overall water splitting in alkaline solution. Dalton Trans. 2020, 49, 15009–15022. [Google Scholar] [CrossRef]
- Tang, S.; Wang, X.; Zhang, Y.; Courte, M.; Fan, H.J.; Fichou, D. Combining Co3S4 and Ni:Co3S4 nanowires as efficient catalysts for overall water splitting: An experimental and theoretical study. Nanoscale 2019, 11, 2202–2210. [Google Scholar] [CrossRef]
- Dai, Z.; Geng, H.; Wang, J.; Luo, Y.; Li, B.; Zong, Y.; Yang, J.; Guo, Y.; Zheng, Y.; Wang, X.; et al. Hexagonal-Phase Cobalt Monophosphosulfide for Highly Efficient Overall Water Splitting. ACS Nano 2017, 11, 11031–11040. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, W.; Xiao, Y.; Shi, Z.; Cao, X.; Tang, Y.; Gao, Q. CoNiSe2 heteronanorods decorated with layered-double-hydroxides for efficient hydrogen evolution. Appl. Catal. B Environ. 2019, 242, 132–139. [Google Scholar] [CrossRef]
- Wu, C.; Du, Y.; Fu, Y.; Feng, D.; Li, H.; Xiao, Z.; Liu, Y.; Yang, Y.; Wang, L. Mo, Co co-doped NiS bulks supported on Ni foam as an efficient electrocatalyst for overall water splitting in alkaline media. Sustain. Energy Fuels 2020, 4, 1654–1664. [Google Scholar] [CrossRef]
- Narasimman, R.; Waldiya, M.K.J.; Vemuri, S.K.; Mukhopadhyay, I.; Ray, A. Self-standing, hybrid three-dimensional-porous MoS2/Ni3S2 foam electrocatalyst for hydrogen evolution reaction in alkaline medium. Int. J. Hydrogen Energy 2021, 46, 7759–7771. [Google Scholar] [CrossRef]
- Zhai, Z.; Li, C.; Zhang, L.; Wu, H.-C.; Zhang, L.; Tang, N.; Wang, W.; Gong, J. Dimensional construction and morphological tuning of heterogeneous MoS2/NiS electrocatalysts for efficient overall water splitting. J. Mater. Chem. A 2018, 6, 9833–9838. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Z.; Qin, T.; Tian, R.; Ou, Y.; Guo, X. Construction of an Amethyst-like MoS2@Ni9S8/Co3S4 Rod Electrocatalyst for Overall Water Splitting. Nanomaterials 2023, 13, 2302. https://doi.org/10.3390/nano13162302
Pei Z, Qin T, Tian R, Ou Y, Guo X. Construction of an Amethyst-like MoS2@Ni9S8/Co3S4 Rod Electrocatalyst for Overall Water Splitting. Nanomaterials. 2023; 13(16):2302. https://doi.org/10.3390/nano13162302
Chicago/Turabian StylePei, Zhen, Tengteng Qin, Rui Tian, Yangxin Ou, and Xingzhong Guo. 2023. "Construction of an Amethyst-like MoS2@Ni9S8/Co3S4 Rod Electrocatalyst for Overall Water Splitting" Nanomaterials 13, no. 16: 2302. https://doi.org/10.3390/nano13162302




